Abstract
Higher morbidity and mortality following infections, particularly influenza, is observed in the elderly population. Because of this, people over 65 years old are often targeted for preventive immunization. Many vaccines, however, are not as effective in generating protective antibodies in older individuals. CD4+ T cells, through their B cell helper functions, play a central role in the humoral response. Aging has deleterious effects on the immune system, and understanding how aging impairs CD4+ T cell functions is of critical importance to design new immunization and treatment strategies targeted to the elderly population. In this paper, we review some of the qualitative and quantitative changes in the CD4+ T cell compartment that arise with aging. We also summarize the age-related intrinsic defects that impact naïve, memory and regulatory CD4+ T cell functions.
Keywords: CD4+ T cells, Age-associated defects
INTRODUCTION
The mean age of the population is increasing worldwide. In the United States, the proportion of people over 65 years old went from 12.4% in 2000 to 12.9% in 2009 and is expected to reach 19% by 2030 [1]. This population is much more susceptible to infections, particularly influenza and bacterial pneumonia, which also results in higher morbidity and mortality in the elderly [2–4]. Because of their higher susceptibility to infection, the elderly are often targeted for vaccination. But this is problematic since the efficacy of vaccines for influenza or Streptococcus pneumonia, for example, is greatly reduced in people over 65 years of age [5,6]. Similarly, old mice show a decreased antibody response following vaccination for a variety of antigens [7,8]. This poor response to immunization, as well as the increased susceptibility to infections in the elderly, reflects the decline in the immune system functions with aging. Since immunization remains the favored method to protect people against influenza and pneumonia, finding ways to improve the immune response of the elderly to vaccines is an important social health goal. To do so, research efforts have been put forward to determine how aging impacts the immune system and how these defects can be overcome.
The decreased antibody response in the elderly has been associated with reduced germinal center formation, somatic hypermutations and high affinity antibody production [9,10]. Although defects in the B cell compartment can partly account for these defects (see Dunn-Walters and Blomberg article of this issue), impairment of CD4+ T cell functions with age most certainly plays an important role in the reduced humoral response seen in older individuals. Indeed, through their B cell helper functions, CD4+ T cells play a major role in the immune response, and it is now well recognize that CD4+ T cells are absolutely required for germinal center formation and high affinity antibody production by B cells [11]. Any changes that modify how CD4+ T cells interact with other cells, are activated or differentiate into effectors could have a major impact on the humoral response and hence, on the efficacy of vaccination. This review thus focuses on the changes arising in the CD4+ T cell compartment with aging.
The dwindling of the CD4+ T cell population is an important characteristic of aging. The declining CD4+ T cell population also progressively switches from a mostly naïve to a mainly memory phenotype, with an increased proportion of cells expressing a regulatory phenotype. In the first part of this review, we summarize these age-associated qualitative and quantitative changes and their possible causes, and briefly discuss their consequences. We dedicate the second part of this review to the intrinsic defects acquired by CD4+ T cells with aging. Interestingly, CD4+ T cells do not necessarily acquire the same defects whether these cells express a naïve, memory or regulatory phenotype. We therefore separately describe the intrinsic defects acquired by naïve, memory and regulatory CD4+ T cells.
AGE-ASSOCIATED QUANTITATIVE AND QUALITATIVE CHANGES IN THE CD4+ T CELL COMPARTMENT
The observation that older people and animals have defective immune responses has been known and investigated for some time. Early studies demonstrated, using limiting dilution assays, that older animals had fewer responding cells [12]. Further characterization of the modifications in the composition of the CD4+ T cell compartment revealed a shift in the ratio of cells expressing naïve and memory phenotype.
Whereas the CD4+ T cell pool in young individuals is mainly composed of naïve cells, the proportion of memory cells increases with age in both mice and humans [13–15]. This shift is thought to mainly result from thymic involution, which is the shrinking in size and function of the thymus that leads to the reduced output of new naïve T cells toward the periphery [16]. Measurement of T-cell receptor excision circle molecules (TREC) expression has been shown to be a reliable marker to assess thymus function [17]. By assessing TREC expression on human peripheral blood mononuclear cells, Sempowski and co-workers showed that the number of naïve T cells drops by 3 orders of magnitude over an 80 year lifespan [18]. In mice, the thymus reduces its production of naïve T cells from 2×106 cells per day in 1 month-old mice to 105 cells per day in 6 month-old mice [19]. These results therefore clearly demonstrate the profound impact of thymus involution on the reduction of naïve CD4+ T cells in the periphery with age.
Even though the thymus output of new T cells lessens with aging, the proportion of naïve CD4+ T cells in the periphery does not decrease accordingly and the total CD4+ T cell pool remains stable for some time before declining. This implies that thymus-independent homeostatic mechanisms occur in the periphery to maintain the CD4+ T cell pool (reviewed in [20]). The homeostatic regulation of naïve and memory T cells differs greatly. Indeed, various studies using labeling with the thymidine analog bromodeoxyuridine (BrdU) or injections of fluoresceinisothiocyanate (FITC) into the thymus revealed that naïve CD4+ T cells scarcely, if ever, proliferate in the periphery, whereas memory CD4+ T cell turnover regularly [21,22]. Under lymphopenic conditions, however, naïve CD4+ T cells do proliferate in a IL-7/self-peptide MHC-dependent mechanism [23]. During this so-called lymphopenia-induced proliferation (LIP), naïve CD4+ T cells irreversibly acquire a memory-like phenotype [23]. Considering the diminished thymic output and slow decrease in total T cells in aging, one could argue that the aged environment is somewhat lymphopenic and promotes LIP. In this context, even though new naïve T cells exit the thymus in old individuals, some could be induced to proliferate and acquire a memory phenotype instead of remaining naïve. This would then contribute to the higher proportion of memory cells seen in the aged T cell pool. Supporting this hypothesis is the demonstration by Timm and collaborators that the aged microenvironment promotes a memory-enriched population when CD4+ depleted aged mice are reconstituted with naïve CD4+ T cells [24]. Noteworthy, homeostatic proliferation of naïve CD4+ T cells has been reported in humans [25,26] without loss of the naïve phenotype, suggesting that the homeostasis of naïve CD4+ T cells differs between human and mice.
Finally, growing evidence demonstrates that the number and proportion of regulatory CD4+ T cells, defined as forkhead box p3(Foxp3)+CD4+, increases with age in both mice and humans [27–32], although some studies report no differences in the frequency of that population between young and aged individuals [33]. Regulatory CD4+ T cells modulate the immune response through the production of various cytokines such as IL-4, IL-10 and TGF-β, and by inhibiting T cell functions (reviewed in [34]). An increase in the frequency and number of regulatory T cells with aging would likely contribute to the impaired immunity observed in the elderly. Multiple examples are found in the literature supporting this idea, including a recent study which demonstrated that the higher frequency of regulatory T cells in aged mice was associated with the reactivation of chronic Leishmania major infections [29].
The reduction of naïve T cell output from the thymus, the increase in memory T cells from multiple antigenic encounters and homeostatic proliferation, as well as the increase in regulatory T cells, are profound qualitative and quantitative changes that occur with age (Fig. 1). As a consequence, there are fewer naïve T cells in the periphery to respond to newly encountered antigens, and more regulatory T cells to inhibit T cell functions, which likely lead to the delayed and decreased response in the elderly. In addition to these global changes in the CD4+ T cell pool with age, CD4+ T cells also develop intrinsic defects that impair their functions, and therefore impact on the immune response of older individuals. These intrinsic defects will be reviewed in the next sections.
Fig. 1.
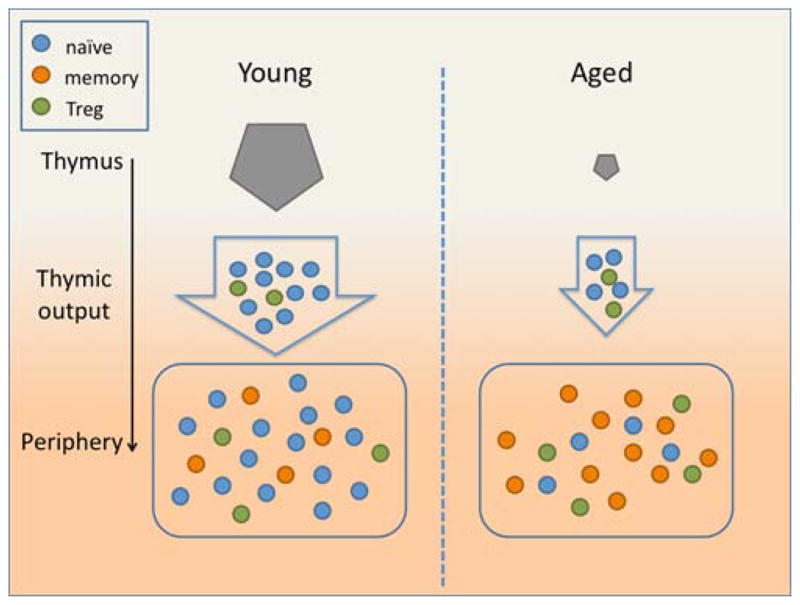
In young age (left panel), the thymus generates a large number of naïve CD4+ T cells every day. These cells reach the periphery where they form the majority of the CD4+ T cell pool, with fewer memory and regulatory CD4+ T cells. In old age (right panel), thymic involution leads to a much reduced output of naïve CD4+ T cells and a relatively increased output of regulatory T cells. Very few naïve CD4+ T cells therefore reach the periphery where memory CD4+ T cells, generated through antigen encounters throughout life and other factors (see text), accumulate.
AGE-ASSOCIATED INTRINSIC DEFECTS IN CD4+ T CELL FUNCTIONS
Naïve CD4+ T Cells
Naïve CD4+ T cells respond to newly encountered antigens and, once activated, provide help to cognate B cells. As mentioned in the introduction, these interactions were shown to be essential to germinal center formation and high affinity antibody generation [11]. Our group has therefore been interested in studying how aging affects the cognate functions of CD4+ T cells. In order to study the impact of age on the CD4+ T cells without the putative impact of age on the environment, we took advantage of an adoptive transfer model in which we transferred identical numbers of naïve TCR transgenic CD4+ T cells from young or aged donor mice into young CD4+ deficient hosts. In this model, the only help provided to B cells comes from the transferred cells since the hosts do not have any endogenous CD4+ T cells. Following immunization, mice that received aged CD4+ T cells had a 3 log decrease in antigen-specific antibody response compared to mice that received young CD4+ T cells [35]. This corresponded with very low germinal center formation, thus supporting a major impact of age on intrinsic CD4+ helper functions.
But how does aging affect naïve CD4+ T cells? As mentioned previously, the reduction in the number of naïve CD4+ T cells in the periphery is not as dramatic as the reduction of the thymic output of new naïve cells with age [20]. Since naïve CD4+ T cells do not undergo homeostatic proliferation in the periphery [21, 22], they would have to have a longer lifespan to compensate for the reduced input from the thymus. Tsukamoto and collaborators recently showed that the longevity of naïve CD4+ T cells in the periphery was in fact increased in older mice [36]. The increased lifespan of the naïve T cells correlated with a decreased expression of Bim, a pro-apoptotic molecule of the Bcl family. Using bone marrow chimeras of Bim+/+ and Bim+/−, they showed that the reduced expression of Bim by naïve CD4+ T cells in old mice accounted for that increased longevity [37]. We have hypothesized that the aging of the cells themselves likely provokes intrinsic defects in the “old” naïve CD4+ T cells [38]. If this were true, then newly generated naïve CD4+ T cells in an old mouse would not show the defects associated with age. To address this question, we used two different approaches to promote the release of new naïve CD4+ T cells in aged mice: 1) we depleted the CD4+ T cells from young or aged mice using an anti-CD4 antibody; 2) we transferred bone marrow from young or aged mice into irradiated young or aged hosts. We then allowed the mice to reconstitute their CD4+ T cell pool and determined whether or not the new naïve CD4+ T cells still showed functional defects by measuring their ability to proliferate in response to antigen or to produce cytokines ex vivo. In these experiments, naïve CD4+ T cells that have spent a short period of time in the periphery proliferated and produced IL-2 as efficiently whether they originated from young or aged mice [39]. These results strongly support that longer longevity promotes functional defects in aged individuals.
The fact that naïve CD4+ T cells from older individuals have limited proliferative capacity and reduced IL-2 production in both mice and humans were amongst the first age-associated defects identified [40]. It was also shown that this defective IL-2 production is in fact the cause of the diminished proliferation observed in the aged cultures. Indeed, IL-2 is an essential T cell growth factor mainly produced by activated CD4+ T cells [41], and exogenous addition of IL-2 restored the proliferation of aged cells in culture to levels equivalent to those of young CD4+ T cells [42,43]. Similarly, the reduced proliferation of peripheral blood mononuclear cells from elderly subjects stimulated in vitro correlated with the proportion of naïve CD4+ T cells present in the cultures as well as the amount of IL-2 produced [44].
Major defects in intracellular signaling of T cells develop with aging. These defects, which were recently reviewed [45], likely contribute to the various functional impairments observed in naïve CD4+ T cells. For example, defective immunological synapse formation, an essential event of naïve CD4+ T cell activation and cognate helper functions, was observed in aged CD4+ T cells [46,47]. Moreover, TCR signaling is also weakened with aging. It was indeed shown that ZAP-70 activation in response to CD3-TCR stimulation is reduced in naïve CD4+ T cells from elderly humans and leads to decreased tyrosine phosphorylation of the ζ-chains [48]. In fact, the impaired ZAP-70 activation with aging is independent of the CD4+ phenotype and occurs in memory as well as naïve CD4+ T cells [48]. Downstream events of ZAP-70 activation include cytoskeleton remodeling, an important aspect of multiple essential functions of T cells such as cell migration, cytokine secretion, receptor endocytosis and many more. Impairment in multiple steps of the signaling pathways associated to the cytoskeleton arises with age and therefore compromises those important cellular functions (reviewed in [49]). Another downstream event of TCR signaling, Raf-1 activation, is reduced in aged naïve CD4+ T cells compared to young CD4+ T cells [50]. This decrease likely contributes to the reduced production of IL-2 by CD4+ T cells from old mice as Raf-1 activates the ERK/MAPK pathway that leads to IL-2 transcription. Additionally, the activation of the transcription factors NF-AT and AP-1, which are implicated in IL-2 transcription, is also decreased with age [51,52].
Death by apoptosis is an important control mechanism used to prevent autoimmune diseases and other immune disorders by eliminating autoreactive T cells during positive selection in the thymus and superfluous effector T cells at the end of an immune response. There are two major pathways of apoptosis: one receptor-mediated pathway through CD95 (Fas) or tumor-necrosis factor receptor (TNFR), for example; and one mitochondrial pathway controlled by members of the Bcl-2 family (reviewed in [53]). Any change in the balance of the pro- and anti-apoptotic signals modifies the susceptibility of the cells to apoptosis and therefore has an important impact in the control of the immune response. Changes in the expression of proteins involved in both the receptor- and mitochondrial-mediated pathways have been reported in aging. Compared to naïve CD4+ T cells of young people, naïve CD4+ T cells from aged humans have an increased expression of the pro-apoptotic TNFRI, TNFR-associated death domain protein (TRADD), Fas, Fas ligand and bax along with a decreased expression of the anti-apoptotic TNFRII, TNFR-associated factor 2 (TRAF-2) and Bcl-2 at both the protein and mRNA levels [54,55]. These modifications render old naïve CD4+ T cells more susceptible to TNF- and Fas-induced cell death, which putatively contribute to the reduced proliferation of these cells.
Finally, once activated, CD4+ T cells become effector cells that produce different arrays of cytokines according to the helper subset they differentiate into. For example, T helper 1 (Th1) cells mainly secrete IL-2 and IFN-γ, whereas Th2 cells produce IL-4, IL-5 and IL-10 [56]. The poor capacity of aged CD4+ T cells to produce IL-2 impairs their ability to fully differentiate to Th1/Th2 effectors [42,57]. The defect in effector differentiation by aged CD4+ T cells could be fully restored by the exogenous addition of IL-2. Ex vivo stimulation of purified CD4+ T cells from the peripheral blood of young and aged individuals showed that there was an increased proportion of cells able to produce IFNγ in the elderly subjects compared to the young [58]. The authors concluded that CD4+ T cells from the elderly preferentially commit to the Th1 subset comparatively to the Th2 subset. Work performed in our lab [59], and others [60–62], showed that aged CD4+ T cells readily differentiate into the Th1 7 subset, a subset more recently identified by the capacity of those cells to generate cytokines of the IL-17 family. This imbalance toward this Th17 polarization is thought to account for the general pro-inflammatory state and autoimmune response in the elderly [61,63].
Knowing what are the defects acquired by naïve CD4+ T cells with aging (summarized in Table 1) will certainly help to develop better vaccines destined to the elderly population.
Table 1.
Intrinsic Defects Acquired by Naïve and Memory CD4+ T Cells with Aging
| Defects | Naïve CD4+ T cells | Memory CD4+ T cells1 |
|---|---|---|
| Impaired helper functions | Yes | Yes |
| Increased longevity (reduced Bim expression) | Yes | Not determined |
| Reduced cytokine production | Yes | Yes |
| Reduced proliferation | Yes | Yes |
| Impaired immunological synapse formation | Yes | Yes |
| Impaired intracellular signaling | Yes | Yes |
| Increased susceptibility to apoptosis | Yes | Yes |
| Preferential differentiation to Th17 compared to Th1/Th2 effector subsets | Yes | Not determined |
| Increased expression of inhibitory receptors (ICOS, CTLA-4, PD-1) | No | Yes |
| Increased proportion of hyporesponsive cells (pgp+) | No | Yes |
These defects refer to newly generated memory cells in an old mouse. Memory CD4+ T cells generated in a young mouse function well in old age (see text in the Memory CD4+ T cells section).
Memory CD4+ T cells
The establishment of an efficient memory immune response is essential to ensure faster clearance of recurring infections. Once a memory response is generated, it can last for a lifetime [64]. After naïve CD4+ T cells encounter their cognate antigens, they proliferate and acquire an effector phenotype. Following antigen clearance, most of those effector cells die and only a small proportion of antigen specific memory CD4+ T cells remains [65]. Despite the critical importance of memory CD4+ T cells in the immune response, very little is known on the impact of aging on these cells. Kang and co-workers monitored the immune response of elderly subjects three months post-influenza immunization and observed that these people mounted a good initial memory response but the memory cells were not maintained [66]. This putatively resulted from lower circulating IL-7 concentration, which was impeding memory cell homeostasis. Work performed using young and aged TCR transgenic mice has shown that naïve CD4+ T cells from aged mice generate fewer memory cells, both in vitro and in vivo, when compared to naïve CD4+ T cells from young mice [67]. This was attributed to increased cell death following stimulation of aged CD4+ T cells compared to young CD4+ T cells [67]. It has been proposed that the strength and duration of the TCR signal at the time of antigen encounter by naïve CD4+ T cells contribute to the development of memory cells (reviewed in [68]). As mentioned earlier, TCR signaling is impaired in aged CD4+ T cells, which may contribute to the impaired transition to memory [45].
Aside from this defect in generating memory in aged individuals, intrinsic functional defects in the memory CD4+ T cell compartment with aging have also been demonstrated in different models. In a first model, we transferred Th1 or Th2 effectors that had been generated from antigen-specific CD4+ T cells isolated from young or aged mice into young hosts and allowed them to rest for a month to generate memory cells [69,70]. The memory cells that were generated from aged mice showed reduced proliferation and cytokine production (IL-2 for Th1, IL-4 and IL-5 for Th2) [69,70] upon restimulation compared to the memory cells that were generated from young mice. Moreover, memory cells generated from aged mice also had poor cognate helper functions compared to memory cells generated from young mice. Indeed, mice that have received memory cells from aged mice developed approximately 10 times fewer germinal center B cells (PNAhighCD38low) and over 2 logs fewer hapten-specific IgG1 in response to immunization than mice that have received memory cells from young mice [70]. Similar results were obtained in another model in which a polyclonal memory CD4+ T cell population was generated in vivo by immunizing mice with maleylatedovalbumin in complete Freund’s adjuvant and allowing them to rest for 7, 28 or 45 days [67]. In this model, the cells harvested from the draining lymph node of aged mice produced less IFNγ after ex vivo restimulation than the cells from the draining lymph nodes of young mice. The cognate helper functions of the aged memory cells, however, were not evaluated in this study. Interestingly, memory cells generated from young effector CD4+ T cells are perfectly functional even 12 month post-adoptive transfer and provide strong help to B cells [69]. Taken together, these results suggest that in older individuals, memory cells would display divergent functional capacity depending on when they were generated. In depth analysis of the progression of memory CD4+ T cells isolated from young (2–6 months old) and aged (>20 months old) C57BL/6 into the cell cycle following αCD3 (±αCD28) antibody stimulation supports this idea [71]. Indeed, even though total memory CD4+ T cells isolated from aged mice showed significantly lower proliferation than memory cells from young mice, an important proportion of those memory cells progressed normally into the post-G0 stages of the cell-cycle. Since some memory cells function normally in old mice, while others exhibit functional defects, this suggests that memory cells in aged individuals are quite heterogeneous.
Changes in the phenotype of memory CD4+ T cells that potentially impact their functions arise with aging. For example, several groups have reported the increased expression of inhibitory receptors in aged mice including inducible co-stimulatory molecule (ICOS), programmed death (PD)-1 and cytotoxic T-lymphocyte-associated protein (CTLA)-4 on memory and regulatory T cells [72–74]. Additionally, a higher proportion of cells within the memory and regulatory T cell pool express ICOS, PD-1 and CTLA-4 in older individuals [72]. Whether this increased expression of inhibitory receptors has a functional impact on aged memory cells, however, is still object of debate. On one side, PD-1−CD4+ T cells from old mice proliferated more extensively in response to αCD3 antibody than the PD-1+CD4+ T cells from the same mice [73,74]. On the other hand, treatment of aged PD-1+ CD4+ T cells with antibodies directed against PD-1 or its ligands PD-L1 and PD-L2 in vitro did not restore their proliferative capacity or modify their cytokine production [74]. To provide inhibitory signals, PD-1 likely has to interact with either PD-L1 or PD-L2, expression of which was shown to be increased in different type of dendritic cells with age [74]. Using the different blocking antibodies in a more complex system implicating the interaction of PD-1 with its ligands might provide further insights in the role of the increased expression of these molecules with aging. The increased expression of PD-L1 and PD-L2 on dendritic cells and other immune cells with aging suggests that the aging environment also provides negative signals that might impact the immune functions of memory CD4+ T cells. In line with this hypothesis, Mittler and Lee observed that the clonal expansion of young memory CD4+ T cells was reduced following re-stimulation when those cells were adoptively transferred into aged hosts compared to young hosts [75]. When these adoptively transferred cells were isolated from their hosts and re-stimulated in vitro, however, no defect in proliferation was observed. This suggests that the aged microenvironment may indeed impair memory T cells that would otherwise function normally.
The expression and/or activity of the multidrug transporter p-glycoprotein (pgp) encoded by the mdr1 gene was also shown to be increased on aged CD4+ T cells [76–78], although a decreased activity or expression of the protein with age was also reported [79, 80]. The precise role of pgp in lymphocyte functions is still not well understood, but work using mdr1a−/− mice showed that it was not required for proliferation, cytokine secretion or cytotoxic functions [81]. CD4+ T cells expressing high levels of pgp were shown to be mostly of the memory (CD44high) phenotype [78]. The pgp+ cells also correspond to memory cells with reduced calcium mobilization [82], IL-2-induced IL-4 release and proliferation [83]. The recruitment of the adaptor protein linker for activation of T cells (LAT) and the protein kinase (PK) Cθ to the immunological synapse was also reduced when these pgp+ memory CD4+ T cells were incubated ex vivo with live anti-CD3 ε hybridoma (2C11) [84]. This defect in immunological synapse formation is not exclusive to the pgp+ subset and pgplow aged memory CD4+ T cells showed a similar defect compared to young memory CD4+ T cells [84]. Moreover, F-actin accumulation to the immunological synapse was slightly reduced in the aged compared to the young memory CD4+ T cells [84]. Additionally, much like naïve CD4+ T cells, memory CD4+ T cells were shown to have decreased ZAP-70 activation (reduced ζ-chain phosphorylation) in response to TCR/CD3 stimulation [48]. Taken together, these studies suggest that the impaired response of memory CD4+ T cells with aging results from a combination of factors that include the accumulation of hyporesponsive (pgp+ cells) as well as the development of intracellular signaling defects and altered immunological synapse formation.
Finally, the susceptibility of memory CD4+ T cells to programmed cell death is modified with aging. As described earlier for naïve CD4+ T cells, aged human memory CD4+ T cells have increased susceptibility to both Fas- and TNF-mediated apoptosis [54,55]. This increased susceptibility to apoptosis might explain the reduced establishment of a sustained memory response in aged individuals [66].
Although many of the defects acquired by memory CD4+ T cells with age are similar to those acquired by naïve CD4+ T cells (reduced proliferation and cytokine production, reduced helper functions, impaired intracellular signaling and immunological synapse formation and increased susceptibility to apoptosis); some age-associated changes are specific to CD4+ T cells of the memory phenotype (increased expression of inhibitory molecules and increased proportion of hyporesponsive cells). These changes are summarized in Table 1. More work is however needed to further characterize these defects and understand their impact on memory CD4+ T cells activation in aged individuals.
Regulatory CD4+ T cells
Regulatory CD4+T cells play a major role in controlling immune responses: they help to prevent autoimmunity by tempering T cell responses and promote immune tolerance (reviewed in [85]). Because of their ability to inhibit or reduce effector T cell functions (through direct interaction or cytokine secretion), the regulatory T cell response has to be precisely balanced to maintain protective immunity against infection while tolerating non-pathogenic antigens. Changes occurring in the regulatory CD4+ T cell compartment with aging could therefore negatively impact multiple aspects of the immune response. As mentioned in the first section, the proportion of regulatory CD4+ T cells is generally considered to increase with age. Reports using various animal models demonstrated that this increase in regulatory T cells with aging correlates with impairment of the immune response. For example, old Balb/c mice were shown to have increased frequencies of regulatory T cells in both their spleen and lymph nodes, which was associated with a decreased ability to reject the tumor cell line BM-185 [27]. Similarly, aged C57BL/6 mice also had a higher frequency of Foxp3+CD4+ T cells that correlated with the spontaneous reactivation of a chronic infection with Leishmania major [29]. In both cases, the elimination of the regulatory T cells using an αCD25 antibody improved the immune function of the aged animals that were then able to reject the tumor cells [27], or had reduced disease severity following Leishmania major infection [29]. Noteworthy, some of these inhibitory effects of regulatory T cells in aged mice are mediated through the impairment of optimal antigen presentation by dendritic cells. The increase in Foxp3+ CD4+ T cells was indeed associated with reduced expression of co-stimulatory molecules by lymph node dendritic cells, and inactivation of regulatory T cells using an αCD25 antibody restored CD40 and CD86 expression to levels similar to those of young mice [30].
To our knowledge, only one study directly attempted to correlate the increased number of regulatory T cells in aging with disease occurrence in humans [31]. Since neurodegenerative disorders are most prevalent in the elderly population, Rosenkranz and collaborators hypothesized that the increased proportion of regulatory T cells in aging caused Alzheimer and Parkinson diseases. Although they confirmed that older people (51–87 years old) had an increased frequency and number of Foxp3+CD4+ T cells compared to young people (23–40 years old), they found no differences between the diseased and healthy groups [31]. These findings however do not rule out that the increased proportion of regulatory T cells in aging is a contributing factor, amongst others, to disease establishment. Further studies directly addressing these questions are needed to definitely correlate diseases in aging humans with regulatory T cell frequency and/or function.
Aside from the number of regulatory T cells, changes in the function of these cells could also impact disease prevalence, outcome or gravity. For exemple, regulatory T cells isolated from patients during the acute phase of toxic epidermal necrolysis showed important defects in their ability to inhibit CD4+CD25− T cell proliferation in vitro as measured by [3H]thymidine incorporation [86]. In some autoimmune diseases such as psoriatic arthritis and systemic lupus erythematosus, low levels of circulating regulatory T cells were associated with increased disease activity or poor prognosis (reviewed in [87]). Changes in regulatory T cell functions combined with the increased frequency of these cells in aging could therefore greatly affect the immune response of older individuals. Whether aging correlates with modifications in regulatory T cell functions has however been subject of debate. The principal way to assess regulatory T cell functions in vitro has been to study their ability to inhibit CD4+CD25− T cell proliferation, which was shown to depend on the inhibition of IL-2 production [88] and to require cell-cell contacts [89]. While it was reported that the capacity of regulatory T cells to inhibit CD4+CD25− proliferation was reduced with aging [89], others showed that it was increased [31] or unchanged [28,33,90]. Different results were however obtained when other regulatory functions were assessed. In two of the studies reporting no change in the faculty of old regulatory T cells to inhibit the proliferation of CD4+CD25− compared to young regulatory T cell, the old CD4+CD25+ T cells had an increased ability to inhibit IL-2 [28] or IL-10 [33] liberation by these cells, and reduced capacity to inhibit the T cell-mediated delayed-type hypersensitivity (DTH) response in vivo [28]. Taken together, these results suggest that regulatory T cell functions are altered with aging. The nature of those alterations, however, depends on the immunological context in which the regulatory T cell functions are evaluated.
Multiple factors could explain the apparent discrepancy in the impact of age on regulatory T cell functions. First, differences in the stimuli used (phytohaemagglutinin, αCD3, αCD3+αCD28, alloantigen) might contribute to the contrasting results obtained since the target cells likely respond differently to those stimuli. Interestingly, Kozlowska and collaborators showed that the ability of old regulatory T cells to inhibit lymph node T cell proliferation differed whether those target cells were from an old (~5% inhibition) or a young mouse (~25% inhibition) [32]. They thus suggested that the responsiveness of the target cells to the inhibitory signals rather than the regulatory T cell themselves changed with age. It was indeed demonstrated that CD4+CD25− T cells from old mice were hyporesponsive [91,92]. In addition to the target cell responses that potentially varies according to the stimulus used, alterations in the regulatory T cell repertoire in old mice, as measured by their Vβ usage, also suggest that they would respond differently to various antigens [28].
Second, differences in the criteria used to define and sort regulatory T cells for the functional assays most certainly impacted the result obtained. When first described, regulatory T cells were identified as CD4+ T cells expressing high levels of CD25 [93,94]. The definition of “high” is however somewhat suggestive and divergences in gating strategies could lead to the identification of populations with different functional results. Moreover, since activated CD4+ T cells also up-regulate CD25, the sorted cells most likely include non-regulatory T cells. The identification of the transcription factor Foxp3 as an additional marker for regulatory T cells has become a helpful tool to study regulatory T cell functions in immunity [95–97]. This marker also allowed the discrimination of two distinct regulatory CD4+ T cells subsets that were CD25− and CD25+ as well as a CD8+ subset [97]. The need to stain intracellularly to detect Foxp3, however, prevents the use of this marker to sort cells. The development of mice expressing a Foxp3-GFP [98], as well as the identification of other surface markers specific for regulatory T cells such as GARP [99] should help to clearly determine how age impact regulatory T cell functions.
Finally, the phenotype of the regulatory T cells changes with age and regulatory T cells from old mice might [100] or might not [28] express high levels of CD44, which is typical of the memory phenotype. As we discussed earlier, the impact of aging on naïve and memory CD4+ T cells is not necessarily similar. Moreover, naïve and memory cells do not respond the same way to similar challenges. The comparison of regulatory T cell functions between young and aged individuals without accounting for these phenotypic, and pre-sumably functional, changes would certainly provide an inaccurate picture of the impact of aging on regulatory T cell.
CONCLUSION
Changes in the CD4+ T cell compartment due to aging result in reduced responses to vaccination and infection. These changes impact both the naïve and memory CD4+ T cell compartments and are due to both T cell intrinsic and extrinsic factors. Importantly, understanding the multiple changes that occur in the immune system with aging is challenging but is of major importance to design better vaccines or treatment aimed for the elderly.
Acknowledgments
Dr Haynes receives funding from the National Institute of Aging (AG021600) and Dr Lefebvre receives a postdoctoral fellowship from the Fonds de la Recherche en Santé du Québec (FRSQ).
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Administration on Aging [homepage on the internet] Washington, DC: Department of Health & Human Services; [updated 2010 June 30; cited 2011 Aug 1]; Available from: http://www.aoa.gov/AoARoot/Aging_Statistics/index.aspx. [Google Scholar]
- 2.Cabre M. Pneumonia in the elderly. Curr Opin Pulm Med. 2009;15:223–9. doi: 10.1097/MCP.0b013e328326f571. [DOI] [PubMed] [Google Scholar]
- 3.Dao CN, Kamimoto L, Nowell M, Reingold A, Gershman K, Meek J, et al. Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis. 2010;202:881–8. doi: 10.1086/655904. [DOI] [PubMed] [Google Scholar]
- 4.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–88. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–9. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simioni PU, Costa EH, Tamashiro WM. Aging reduces the primary humoral response and the in vitro cytokine production in mice. Braz J Med Biol Res. 2007;40:1111–20. doi: 10.1590/s0100-879x2006005000140. [DOI] [PubMed] [Google Scholar]
- 8.Gahring LC, Weigle WO. The effect of aging on the induction of humoral and cellular immunity and tolerance in two long-lived mouse strains. Cell Immunol. 1990;128:142–51. doi: 10.1016/0008-8749(90)90013-h. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–70. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 11.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 12.Miller RA. Age-associated decline in precursor frequency for different T cell-mediated reactions, with preservation of helper or cytotoxic effect per precursor cell. J Immunol. 1984;132:63–8. [PubMed] [Google Scholar]
- 13.Kovaiou RD, Weiskirchner I, Keller M, Pfister G, Cioca DP, Grubeck-Loebenstein B. Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. Int Immunol. 2005;17:1359–66. doi: 10.1093/intimm/dxh314. [DOI] [PubMed] [Google Scholar]
- 14.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol. 1989;19:977–82. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 15.Jackola DR, Ruger JK, Miller RA. Age-associated changes in human T cell phenotype and function. Aging (Milano) 1994;6:25–34. doi: 10.1007/BF03324210. [DOI] [PubMed] [Google Scholar]
- 16.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–9. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dion ML, Sekaly RP, Cheynier R. Estimating thymic function through quantification of T-cell receptor excision circles. Methods Mol Biol. 2007;380:197–213. doi: 10.1007/978-1-59745-395-0_12. [DOI] [PubMed] [Google Scholar]
- 18.Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, et al. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164:2180–7. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 19.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–8. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 20.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39:2088–94. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 21.Sprent J, Schaefer M, Hurd M, Surh CD, Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991;174:717–28. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Timm JA, Thoman ML. Maturation of CD4+ lymphocytes in the aged microenvironment results in a memory-enriched population. J Immunol. 1999;162:711–7. [PubMed] [Google Scholar]
- 25.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–17. [PubMed] [Google Scholar]
- 26.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–55. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–94. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 29.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–48. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128:618–27. doi: 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, et al. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol. 2007;188:117–27. doi: 10.1016/j.jneuroim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Kozlowska E, Biernacka M, Ciechomska M, Drela N. Age-related changes in the occurrence and characteristics of thymic CD4(+) CD25(+) T cells in mice. Immunology. 2007;122:445–53. doi: 10.1111/j.1365-2567.2007.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130:509–17. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–22. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–22. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–8. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. 2010;185:4535–44. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–8. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–51. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rea IM, Stewart M, Campbell P, Alexander HD, Crockard AD, Morris TC. Changes in lymphocyte subsets, interleukin 2, and soluble interleukin 2 receptor in old and very old age. Gerontology. 1996;42:69–78. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
- 41.Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8:1142–8. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–24. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beckman I, Dimopoulos K, Xu XN, Ahern M, Bradley J. Age-related changes in the activation requirements of human CD4+ T-cell subsets. Cell Immunol. 1991;132:17–25. doi: 10.1016/0008-8749(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 44.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Proliferative responses of blood mononuclear cells (BMNC) in a cohort of elderly humans: role of lymphocyte phenotype and cytokine production. Clin Exp Immunol. 2000;119:433–40. doi: 10.1046/j.1365-2249.2000.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–8. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–7. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 47.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–51. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 48.Whisler RL, Chen M, Liu B, Newhouse YG. Age-related impairments in TCR/CD3 activation of ZAP-70 are associated with reduced tyrosine phosphorylations of zeta-chains and p59fyn/p56lck in human T cells. Mech Ageing Dev. 1999;111:49–66. doi: 10.1016/s0047-6374(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 49.Garcia GG, Miller RA. Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev. 2011;10:26–34. doi: 10.1016/j.arr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirk CJ, Miller RA. Analysis of Raf-1 activation in response to TCR activation and costimulation in murine T-lymphocytes: effect of age. Cell Immunol. 1998;190:33–42. doi: 10.1006/cimm.1998.1382. [DOI] [PubMed] [Google Scholar]
- 51.Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169:185–95. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- 52.Whisler RL, Chen M, Beiqing L, Carle KW. Impaired induction of c-fos/c-jun genes and of transcriptional regulatory proteins binding distinct c-fos/c-jun promoter elements in activated human T cells during aging. Cell Immunol. 1997;175:41–50. doi: 10.1006/cimm.1996.1048. [DOI] [PubMed] [Google Scholar]
- 53.Kurtulus S, Tripathi P, Opferman JT, Hildeman DA. Contracting the ‘mus cells’--does down-sizing suit us for diving into the memory pool? Immunol Rev. 2010;236:54–67. doi: 10.1111/j.1600-065X.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aggarwal S, Gollapudi S, Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol. 1999;162:2154–61. [PubMed] [Google Scholar]
- 55.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–37. [PubMed] [Google Scholar]
- 56.Mucida D, Cheroutre H. The many face-lifts of CD4 T helper cells. Adv Immunol. 2010;107:139–52. doi: 10.1016/B978-0-12-381300-8.00005-8. [DOI] [PubMed] [Google Scholar]
- 57.Haynes L, Eaton SM, Swain SL. The defects in effector generation associated with aging can be reversed by addition of IL-2 but not other related gamma(c)-receptor binding cytokines. Vaccine. 2000;18:1649–53. doi: 10.1016/s0264-410x(99)00501-0. [DOI] [PubMed] [Google Scholar]
- 58.Sakata-Kaneko S, Wakatsuki Y, Matsunaga Y, Usui T, Kita T. Altered Th1/Th2 commitment in human CD4+ T cells with ageing. Clin Exp Immunol. 2000;120:267–73. doi: 10.1046/j.1365-2249.2000.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–35. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–50. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 61.Ouyang X, Yang Z, Zhang R, Arnaboldi P, Lu G, Li Q, et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol. 2011;266:208–17. doi: 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, et al. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tesar BM, Du W, Shirali AC, Walker WE, Shen H, Goldstein DR. Aging augments IL-17 T-cell alloimmune responses. Am J Transplant. 2009;9:54–63. doi: 10.1111/j.1600-6143.2008.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 65.Gerlach C, van Heijst JW, Schumacher TN. The descent of memory T cells. Ann N Y Acad Sci. 2011;1217:139–53. doi: 10.1111/j.1749-6632.2010.05830.x. [DOI] [PubMed] [Google Scholar]
- 66.Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–81. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 67.Mattoo H, Faulkner M, Kandpal U, Das R, Lewis V, George A, et al. Naive CD4 T cells from aged mice show enhanced death upon primary activation. Int Immunol. 2009;21:1277–89. doi: 10.1093/intimm/dxp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim C, Williams MA. Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool. Immunology. 2010;131:310–7. doi: 10.1111/j.1365-2567.2010.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–8. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. J Immunol. 2008;181:4825–31. doi: 10.4049/jimmunol.181.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hale TJ, Richardson BC, Sweet LI, McElligott DL, Riggs JE, Chu EB, et al. Age-related changes in mature CD4+ T cells: cell cycle analysis. Cell Immunol. 2002;220:51–62. doi: 10.1016/s0008-8749(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 72.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130:709–12. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol. 2009;44:517–22. doi: 10.1016/j.exger.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell. 2010;9:785–98. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mittler JN, Lee WT. Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment. II. The memory T cell response is diminished. Mech Ageing Dev. 2004;125:59–68. doi: 10.1016/j.mad.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Aggarwal S, Tsuruo T, Gupta S. Altered expression and function of P-glycoprotein (170 kDa), encoded by the MDR 1 gene, in T cell subsets from aging humans. J Clin Immunol. 1997;17:448–54. doi: 10.1023/a:1027363525408. [DOI] [PubMed] [Google Scholar]
- 77.Bining N, Miller RA. Cytokine production by subsets of CD4 memory T cells differing in P-glycoprotein expression: effects of aging. J Gerontol A Biol Sci Med Sci. 1997;52:B137–B145. doi: 10.1093/gerona/52a.3.b137. [DOI] [PubMed] [Google Scholar]
- 78.Witkowski JM, Miller RA. Increased function of P-glycoprotein in T lymphocyte subsets of aging mice. J Immunol. 1993;150:1296–306. [PubMed] [Google Scholar]
- 79.Machado CG, Calado RT, Garcia AB, Falcao RP. Age-related changes of the multidrug resistance P-glycoprotein function in normal human peripheral blood T lymphocytes. Braz J Med Biol Res. 2003;36:1653–7. doi: 10.1590/s0100-879x2003001200006. [DOI] [PubMed] [Google Scholar]
- 80.Pilarski LM, Paine D, McElhaney JE, Cass CE, Belch AR. Multi-drug transporter P-glycoprotein 170 as a differentiation antigen on normal human lymphocytes and thymocytes: modulation with differentiation stage and during aging. Am J Hematol. 1995;49:323–35. doi: 10.1002/ajh.2830490411. [DOI] [PubMed] [Google Scholar]
- 81.Eisenbraun MD, Miller RA. mdr1a-encoded P-glycoprotein is not required for peripheral T cell proliferation, cytokine release, or cytotoxic effector function in mice. J Immunol. 1999;163:2621–7. [PubMed] [Google Scholar]
- 82.Witkowski JM, Miller RA. Calcium signal abnormalities in murine T lymphocytes that express the multidrug transporter P-glycoprotein. Mech Ageing Dev. 1999;107:165–80. doi: 10.1016/s0047-6374(98)00147-x. [DOI] [PubMed] [Google Scholar]
- 83.Witkowski JM, Li SP, Gorgas G, Miller RA. Extrusion of the P glycoprotein substrate rhodamine-123 distinguishes CD4 memory T cell subsets that differ in IL-2-driven IL-4 production. J Immunol. 1994;153:658–65. [PubMed] [Google Scholar]
- 84.Eisenbraun MD, Tamir A, Miller RA. Altered composition of the immunological synapse in an anergic, age-dependent memory T cell subset. J Immunol. 2000;164:6105–12. doi: 10.4049/jimmunol.164.12.6105. [DOI] [PubMed] [Google Scholar]
- 85.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182:8071–9. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 87.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 90.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 92.Shimizu J, Moriizumi E. CD4+CD25- T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–82. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- 93.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 95.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 96.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 97.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 98.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 99.Probst-Kepper M, Geffers R, Kroger A, Viegas N, Erck C, Hecht HJ, et al. GARP: a key receptor controlling FOXP3 in human regulatory T cells. J Cell Mol Med. 2009;13:3343–57. doi: 10.1111/j.1582-4934.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han GM, Zhao B, Jeyaseelan S, Feng JM. Age-associated parallel increase of Foxp3(+)CD4(+) regulatory and CD44(+)CD4(+) memory T cells in SJL/J mice. Cell Immunol. 2009;258:188–96. doi: 10.1016/j.cellimm.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


