Abstract
Aims/Introduction
To realize the effectiveness of a novel system for measuring glucose area under the curve (AUC) using minimally invasive interstitial fluid extraction technology (MIET), outpatients undergoing oral glucose tolerance tests (OGTT) were investigated for the efficacy of screening for glucose intolerance using this system.
Materials and Methods
Fifty outpatients scheduled to undergo a 75‐g OGTT for medical reasons were recruited to the study. An area of skin on the forearm was pretreated with microneedle arrays before the application of hydrogels for interstitial fluid extraction. Plasma glucose (PG) levels were measured every 30 min for 2 h to calculate reference (actual) AUC. The AUC was predicted by MIET on the basis of glucose extracted by the hydrogel using sodium ion levels as the internal standard.
Results
Good correlation between MIET‐predicted and reference AUCs obtained using PG levels was confirmed for a wide AUC range. By introducing a threshold level for AUC to separate glucose intolerance with peak glucose ≥180 mg/dL from normal glucose tolerance, the system was demonstrated to provide better screening accuracy compared with conventional methods that use HbA1c and fasting PG levels. The results of a questionnaire‐based survey administered to the subjects suggested that this system was readily accepted by the majority as a painless monitoring method.
Conclusions
The findings suggest that our glucose AUC measurement system using MIET would be useful for screening of glucose intolerance. In the future, this system may prove to be a useful aid as a screen for glucose intolerance before performing an OGTT for diagnosis.
Keywords: Glucose area under the curve, Glucose monitoring, Screening
Introduction
The incidence of type 2 diabetes is rapidly increasing worldwide1, and this is problematic because of its associated complications. Although the detection of diabetes at an early stage is crucial in order to prevent the onset and progression of complications, detection of early stage diabetes is difficult because neither HbA1c nor fasting plasma glucose (FPG) levels, which are used as part of routine health check‐ups, are sufficiently sensitive. Recently, the International Expert Committee2 and American Diabetes Association3 recommended that HbA1c levels should be used for the diagnosis of diabetes; this recommendation has provoked controversy in relation to HbA1c screening‐ and diagnosis‐related issues, such as sensitivity in the detection of impaired glucose tolerance (IGT)4, appropriate criteria5, differences between diagnoses obtained on the basis of HbA1c levels and those obtained on the basis of an oral glucose tolerance test (OGTT)7, and the reliability of measurements8 among other issues9. At present, the use of HbA1c levels for precise screening or diagnosis of IGT has its limitations. Although an OGTT, which is the gold standard for diagnosis, is useful in the detection of IGT10, such tests are troublesome for both patients and medical staff because of the requirement for frequent blood sampling, especially at primary care hospitals.
The availability of a system to estimate postprandial glucose excursion without the need for blood sampling would be beneficial11. For this purpose, we investigated and developed a system for the measurement of postprandial glucose excursion estimated as a glucose area under the curve (AUC) value using minimally invasive interstitial fluid extraction technology (MIET). This corresponds to the total increase in postprandial glucose levels; therefore, consideration of the timing of blood sampling is unnecessary. With this technology, the glucose AUC value after a glucose load can be analyzed by placing a hydrogel patch on pretreated skin for a predefined period to accumulate interstitial fluid glucose (IG). This system enables easy measurement of glucose AUC, which acts as a surrogate for postprandial hyperglycemia.
The feasibility of our technology has been reported by Sato et al.11, who showed that, in healthy subjects, the IG AUC correlated strongly with glucose AUC as determined by postprandial self‐monitoring of blood glucose (SMBG). The accuracy of this measurement system was then evaluated by performing an OGTT in patients with and without diabetes, and comparing IG AUC with reference plasma glucose (PG) AUC for a wide range of AUCs during rapid changes in glucose12.
In the present study, we investigated the efficacy of this system to assess its feasibility as a screening tool for glucose intolerance in outpatients scheduled to undergo an OGTT. The effectiveness of this screening test was compared with conventional tests, including tests that use HbA1c, FPG, and 2‐h post‐load glucose levels.
Materials and Methods
Clinical Evaluation Protocol
IG AUC was measured using MIET during the administration of an OGTT to 50 outpatients with suspected glucose intolerance. Interstitial fluid (ISF) was collected from the skin of the forearm. The collection site was wiped with an antiseptic, and microneedle arrays were then stamped at two sites using a microneedle applicator. Two hydrogel patches were placed on each pretreated area to absorb ISF, and a third hydrogel patch was placed on an untreated area for sweat monitoring. Venous blood was sampled for measurement of PG and insulin levels. After glucose consumption (TRELAN‐G75; Ajinomoto Pharma, Tokyo, Japan), PG levels were measured every 30 min for 2 h. Before the final PG measurement, the hydrogel patches were collected to analyze ISF composition. Immediately after ISF extraction and 1 day after measurement, patients were requested to complete a questionnaire and provide feedback on the system.
Insulin, PG, and HbA1c (NGSP) levels13 were measured using a conventional clinical laboratory system that is routinely calibrated.
The present study was performed in accordance with the latest version of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Osaka Police Hospital (Osaka, Japan) and all participants provided written informed consent.
Apparatus and Materials
The polycarbonate microneedle arrays covered approximately 50 mm2 and comprised 305 needles (length 0.3 mm). The applicator for microneedle stamping of the skin was a spring‐action hand‐held system with a stamping speed of approximately 6 m/s. The hydrogel patch comprised a polyvinyl alcohol hydrogel (2% KCl) and an adhesive tape (KP; Nichiban, Tokyo, Japan).
The reagent for glucose analysis comprised four enzymes and one dye in phosphate buffer solution. Each 0.1 mL phosphate buffer solution contained 2.6 U glucose oxidase (Wako Pure Chemical Industries, Osaka, Japan), 0.023 U mutarotase (Wako Pure Chemical Industries), 0.25 U peroxidase (Wako Pure Chemical Industries), 0.49 U ascorbic acid oxidase (Wako Pure Chemical Industries), and 0.016 mL Amplex red solution (Molecular Probes, Eugene, OR, USA).
Procedures for Glucose and Sodium Ion Analyses
The hydrogel was separated from the adhesive tape prior to analysis of the extract and was then placed in 0.3 mL pure water overnight to extract glucose and sodium. For glucose measurement, 0.1 mL of a 12‐fold diluted sample solution was mixed with 0.1 mL reagent. After 60 min incubation, the fluorescence intensity of Amplex red was measured using a fluorescence plate reader (GENios; TECAN Japan, Kawasaki, Japan). Sodium ion concentrations were analyzed using the DX‐500 ion chromatography system (Dionex, Bannockburn, IL, USA).
Data Analysis Methods
Reference PG AUCs were calculated by trapezoidal approximation of PG levels measured every 30 min. Defining the PG value at x min as PG(x), the reference PG AUC was calculated as follows:
The IG AUC was calculated on the basis of the measured mass of glucose Mglu (in nmol) and sodium ions MNa (in nmol) in the hydrogel. The Mglu level was corrected by the sodium level in the hydrogel for sweat monitoring. The details and principles of data analysis have been described previously10. PG AUC and IG AUC data with time delay for blood and ISF sampling were corrected by sampling time to obtain 2‐h equivalent values for the evaluation of screening effectiveness.
The insulinogenic index (II), homeostasis model assessment of β‐cell function (HOMA‐β), homeostasis model assessment of insulin resistance (HOMA‐IR), Matsuda index, and disposition index (DI) were calculated from PG and insulin data obtained during the OGTT14.
Classification of glucose tolerance by the OGTT was based on World Health Organization criteria.16 Impaired fasting glucose (IFG) with IGT was classified as IGT.
Pearson's product–moment correlation coefficients were analyzed for statistical analysis of the correlation between two parameters.
Results
Glucose Profiles
Baseline patient information, OGTT results, and analyses of OGTT‐derived data classified by diagnostic criteria are given in Table 1. According to these criteria, there were 15 cases of normal glucose tolerance (NGT), four of IFG, 16 of IGT, and 13 of diabetes. The IGT subclasses depending on peak glucose levels are discussed in detail in the following section. One subject whose hydrogel patches were collected 1 h later than the predefined time and one whose HbA1c levels could not be used were excluded from all analyses.
Table 1. Subject characteristics and oral glucose tolerance test results according to glucose tolerance.
| NGT | IFG | IGT | DM | |||
|---|---|---|---|---|---|---|
| Total | Peak PG <180 mg/dL | Peak PG ≥180 mg/dL | ||||
| n | 15 | 4 | 16 | 4 | 12 | 13 |
| Age (years) | 53.5 ± 17.5 | 62.8 ± 7.5 | 59.6 ± 13.1 | 67.0 ± 5.9 | 57.1 ± 14.0 | 62.5 ± 6.6 |
| BMI (kg/m2) | 24.6 ± 4.0 | 22.7 ± 2.9 | 25.4 ± 3.5 | 24.6 ± 0.9 | 25.7 ± 4.0 | 26.7 ± 7.6 |
| HbA1c (%) | 5.6 ± 0.4 | 6.1 ± 0.2 | 5.9 ± 0.4 | 5.5 ± 0.2 | 6.0 ± 0.4 | 6.6 ± 0.4 |
| PG (mg/dL) | ||||||
| Fasting | 95 ± 9 | 112 ± 2 | 105 ± 12 | 96 ± 9 | 108 ± 11 | 130 ± 14 |
| 0.5 h | 145 ± 32 | 174 ± 31 | 179 ± 36 | 129 ± 24 | 195 ± 20 | 217 ± 17 |
| 1 h | 152 ± 41 | 185 ± 30 | 202 ± 58 | 112 ± 21 | 232 ± 25 | 266 ± 31 |
| 2 h | 108 ± 22 | 113 ± 15 | 159 ± 17 | 144 ± 4 | 164 ± 17 | 215 ± 62 |
| AUC | 267 ± 53 | 301 ± 48 | 345 ± 74 | 234 ± 23 | 381 ± 39 | 454 ± 45 |
| Insulin (mU/L) | ||||||
| Fasting | 7.5 ± 5.0 | 9.0 ± 4.0 | 8.7 ± 3.9 | 8.6 ± 2.5 | 8.8 ± 4.4 | 7.5 ± 3.5 |
| 0.5 h | 48.8 ± 23.7 | 46.1 ± 21.8 | 51.7 ± 44.0 | 73.8 ± 58.7 | 44.3 ± 38.3 | 22.4 ± 11.2 |
| 1 h | 62.0 ± 36.3 | 79.9 ± 42.6 | 70.8 ± 54.5 | 33.0 ± 20.9 | 83.5 ± 56.9 | 42.6 ± 26.3 |
| 2 h | 38.0 ± 21.8 | 37.2 ± 25.0 | 72.3 ± 51.1 | 54.5 ± 36.6 | 78.2 ± 55.2 | 45.5 ± 25.8 |
| AUC | 94.3 ± 44.7 | 103.7 ± 30.5 | 115.7 ± 74.1 | 81.9 ± 40.8 | 127.0 ± 80.5 | 72.8 ± 30.7 |
| Insulinogenic index | 1.2 ± 1.4 | 0.6 ± 0.1 | 1.1 ± 2.0 | 3.3 ± 3.4 | 0.4 ± 0.4 | 0.2 ± 0.1 |
| HOMA‐β (%) | 84 ± 48 | 65 ± 26 | 83 ± 52 | 98 ± 32 | 79 ± 57 | 41 ± 20 |
| HOMA‐IR | 1.8 ± 1.2 | 2.5 ± 1.2 | 2.2 ± 0.9 | 2.0 ± 0.7 | 2.3 ± 1.0 | 2.4 ± 1.2 |
| Matsuda index | 6.8 ± 4.7 | 4.0 ± 1.6 | 4.2 ± 1.9 | 5.7 ± 2.3 | 3.7 ± 1.6 | 4.5 ± 2.6 |
| Disposition index | 8.4 ± 11.2 | 2.3 ± 0.7 | 6.3 ± 16.5 | 21.7 ± 30.7 | 1.2 ± 0.6 | 0.7 ± 0.5 |
NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; PG, plasma glucose; DM, diabetes mellitus; BMI, body mass index; AUC, area under the curve; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance.
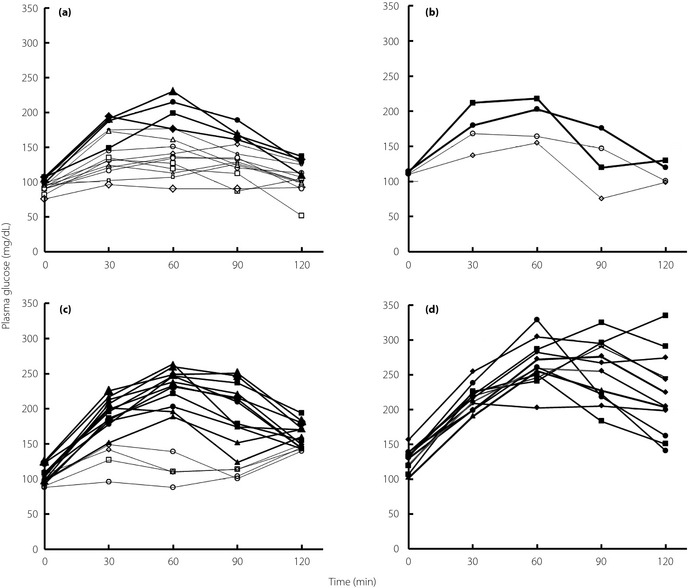
Figure 1 shows the PG profiles classified according to the diagnostic criteria. Of the 15 profiles for NGT, four showed peak glucose ≥180 mg/dL. There were two and four profiles for IFG and IGT, respectively, with peak glucose <180 mg/dL. As shown in Figure 1, times for peak PG were class dependent and occurred mainly at 30–60 min in cases of NGT and IFG, at 60 min in cases of IGT, and at 60–120 min in cases of diabetes. Furthermore, IGT profiles showed wide variability at 30–90 min, in contrast with the variation in diabetes profiles that were observed at 120 min.
Figure 1.
Plasma glucose profiles indicative of (a) normal glucose tolerance, (b) impaired fasting glucose, (c) impaired glucose tolerance, and (d) diabetes mellitus after the oral glucose tolerance test. Open symbols, peak plasma glucose <180 mg/dL; filled symbols, peak plasma glucose ≥180 mg/dL.
Measurement of IG AUC and Its Usefulness in Screening for Glucose Intolerance
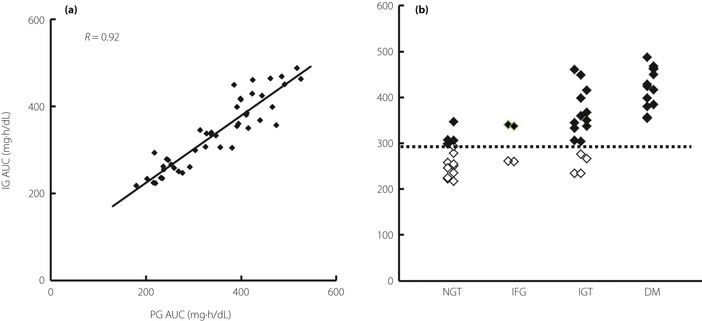
Figure 2a shows the correlation between IG AUC (mean of two measurements) and reference PG AUC. The correlation coefficient was sufficiently high (R = 0.92) for a wide range of PG AUC (180–526 mg h/dL). The reproducibility of two simultaneous IG AUC measurements was 8.3% and the mean percentage error from the regression line was 11.6%; these results are consistent with those from a previous study12. High accuracy among patients aged 27–77 years and those with a body mass index of 19.8–48.5 kg/m2 indicated that MIET is suitable for use in a variety of patients.
Figure 2.
Usefulness of the interstitial fluid glucose (IG) area under the curve (AUC) for glucose intolerance screening. (a) Correlation between IG AUC and plasma glucose (PG) AUC. (b) IG AUC levels in subjects with normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and diabetes mellitus (DM). (◇), peak PG <180 mg/dL; (◆), peak PG ≥180 mg/dL.
Using measured IG AUC, IGT and diabetes were separated from NGT and IFG (Figure 2b). The threshold level for separation was approximately 290 mg h/dL. In Figure 2b, markers were characterized by peak glucose levels for NGT, IFG, and IGT. The larger glucose excursions after glucose load (NGT, IFG, or IGT with peak glucose ≥180 mg/dL) and diabetes were completely separated from the smaller glucose excursions (peak glucose <180 mg/dL in NGT, IFG, or IGT).
The PG profiles for IGT as shown in Figure 1c show the large difference between PG AUCs for IGT with low and high peak PG levels. The mean PG AUC for IGT with peak PG <180 mg/dL (234 mg h/dL) was similar to that for NGT (267 mg h/dL), and the mean PG AUC for IGT with peak PG level ≥180 mg/dL (381 mg h/dL) was similar to that for diabetes (454 mg h/dL). The small glucose excursions in the low peak PG subclass suggest that this subclass need not be classified as IGT. In contrast, the high peak PG subclass, which showed large glucose excursions, requires classification as early stage diabetes. Insulin‐related data support this hypothesis (Table 1). Values for II and DI associated with IGT with low peak PG levels were sufficiently high, but the level of IGT with high peak PG levels was closer to that of diabetes, suggesting that the ability to secrete insulin and insulin resistance differ between IGT subclasses (Table 1).
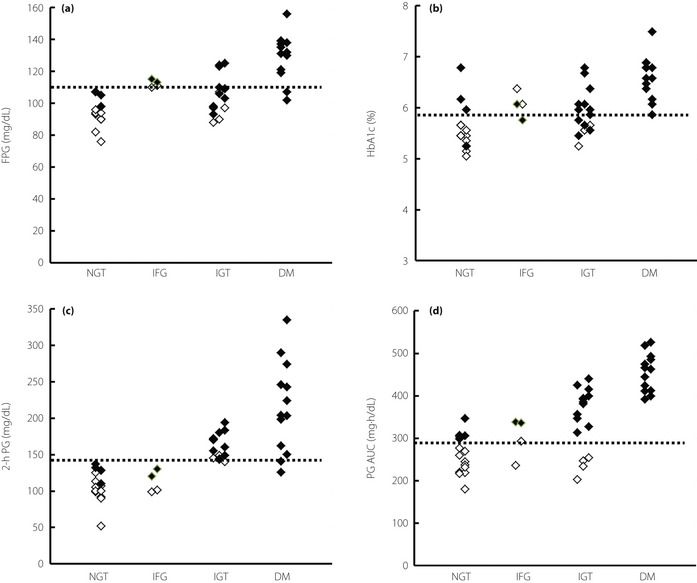
Screening Performance of Conventional Indices
Figure 3 shows FPG (Figure 3a), HbA1c (Figure 3b), 2‐h PG (Figure 3c), and PG AUC (Figure 3d) levels categorized by glucose tolerance. Most IGT and some diabetes cases were judged as NGT according to FPG and HbA1c levels. A degree of overlap between NGT and IGT/diabetes makes it difficult to define a suitable threshold level while screening for glucose intolerance. Although classification by 2‐h PG levels improved separation, no high peak PG levels were found with NGT or IFG in addition to the false‐positive results associated with IGT with low peak PG levels. There was good correlation between PG AUC and IG AUC (Figure 2a); therefore, classification by PG AUC was similar to that by IG AUC (Figure 3d).
Figure 3.
Screening performance of conventional indices: (a) fasting plasma glucose (FPG); (b) HbA1c; (d) plasma glucose (PG) 2 h after glucose loading; and (d) PG area under the curve (AUC). The dashed line shows the threshold level (upper limit) of normal glucose tolerance for each index. (◇), peak PG <180 mg/dL; (◆), peak PG ≥180 mg/dL. NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; DM, diabetes mellitus.
Correlation between IG AUC and Peak PG Levels
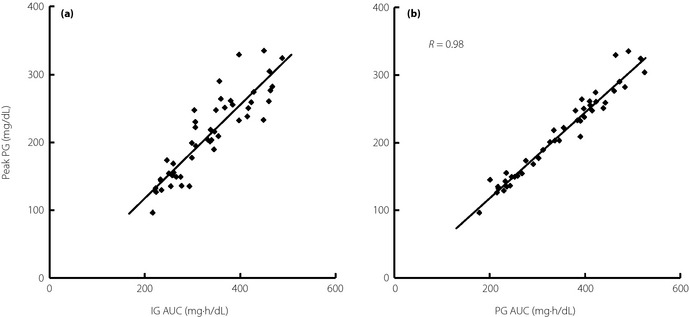
As demonstrated previously12, there is a good correlation between IG AUC and peak PG levels, and this was further confirmed in the present study (Figure 4a), which suggests the feasibility of detecting peak glucose levels using MIET. If peak PG level is selected as a screening marker, IG AUC would represent a good surrogate marker for peak PG levels. A higher correlation between PG AUC and peak PG levels suggests that PG AUC may act as a surrogate marker for peak PG levels for 2 h during an OGTT (Figure 4b).
Figure 4.
Correlation between (a) peak plasma glucose (PG) levels and interstitial fluid glucose (IG) area under the curve (AUC), and (b) peak PG levels and PG AUC.
Sampling Complications and Questionnaire Results
No bleeding was observed after stamping of the microneedle array or during accumulation of ISF. Slight erythema occurred at the stamped area, but this disappeared within a few days. Most patients reported neither pain nor discomfort associated with stamping in their responses to the questionnaire.
Discussion
The results of the present study suggest that glucose AUC measurement using MIET may provide satisfactory screening results for glucose intolerance without the requirement for blood sampling. Below we discuss the similarities and differences between PG AUC and IG AUC, focusing on the implications of screening markers in the application of MIET.
PG AUC for screening of glucose intolerance
Both glucose AUC and PG AUC have been used as markers of postprandial glucose excursion. For example, the glycemic index, which is used to select an appropriate diet for patients with diabetes, is calculated by comparing the glucose AUC of the planned meal with that of a standard meal17. Changes in PG AUC are used to evaluate the efficacy of medicines such as α‐glucosidase inhibitors or nateglinide for postprandial hyperglycemia18. This trend is also apparent in the evaluation of recently developed medicines, such as incretin‐related agents and sodium‐glucose cotransporter type 2 inhibitors, both of which act by suppressing increases in postprandial glucose levels20.
Nevertheless, there are no definitive guidelines in regard to PG AUC criteria for glucose intolerance screening or diagnosis. Only the study by Zhou et al.22 has demonstrated the effectiveness of 3‐h PG AUC as a screening index for IGT or diabetes compared with other indices used during an OGTT. Most studies have used 2‐h PG levels after OGTT, but issues such as low reproducibility23 and the importance of 1‐h or peak glucose measurements24 have been highlighted. The use of PG AUC can solve these issues. A high correlation between PG AUC and peak PG levels suggests that PG AUC would be a good surrogate marker of peak PG levels, which corresponds to the glucose spike level. Furthermore, PG AUC is expected to be robust compared with 2‐h PG because PG AUC embraces total glucose excursion after glucose intake, whereas 2‐h PG is an instant value obtained during PG fluctuation.
In the present study, 290 mg h/dL was proposed as the appropriate threshold level when this system was used for the screening of glucose intolerance. Calculation of maximum AUC for NGT using diagnostic criteria at 0, 1, and 2 h gave a value of approximately 305 mg h/dL, which nearly corresponds to the value used in the present study. Further studies are required to confirm the adequacy of the threshold level of PG AUC by comparing it with conventional indices and related prognoses.
IG AUC as a Screening Marker
We showed that there was a strong correlation between IG AUC predicted by MIET and reference PG AUC. Screening using PG AUC is rare because of difficulties associated with its measurement. However, our painless and easy‐to‐use system can be used for glucose intolerance screening as part of a routine health check‐up or at primary care hospitals before a diagnostic OGTT is performed. Although a 75‐g glucose load with MIET is recommended for precise evaluation, a test meal would be appropriate for subjects undergoing a routine health check‐up as prescreening for glucose intolerance.
Application of the IG AUC Measurement System
The IG AUC measurement system can easily be applied for longer measurement periods by simply leaving the hydrogel patch attached for the period desired. If placed for 4 h, the AUC would reflect the total increment in postprandial glucose levels attributable to diabetes25. Glucose AUC divided by accumulation time corresponds to the average glucose level; therefore, if the patch is placed for 10–12 or 24 h, average daytime, night‐time, or 1‐day glucose levels can be determined without the need for blood sampling. This average glucose concept can be used as an alternative for the measurement of HbA1c levels, which is interpreted as the average glucose level over several months26. Average glucose levels measured by MIET can reflect glucose excursion over 1 day, which can then be applied to drug efficacy monitoring.
In addition, this system can potentially be used as a tool for disease management and patient education. Currently, SMBG is mainly used for this purpose, but the pain and inconvenience associated with SMBG prevent patients from using it effectively27. Glucose measurement by MIET would enable patients to measure total postprandial glucose excursion without the need for blood sampling and would also show changes over time. Therefore, MIET can play a role in self‐monitoring of glucose excursion at home.
Study Limitations
The sample size of the present study was not sufficiently large to show a powered result for screening performance using the receiver operating characteristic (ROC) curve. The usefulness of PG AUC as a screening marker can be discussed using the volume of OGTT data without MIET. The relevance of subclasses depending on the peak glucose levels and their importance should be discussed at the same time.
The accuracy of AUC measurement was evaluated over 2 h only; therefore, it should be tested in future studies for longer periods of time.
Conclusions
In summary, the results of the present study suggest that our recently developed system for glucose AUC measurement using MIET would be useful in screening for glucose intolerance. Potential benefits of postprandial glucose excursion measurement in daily clinical practice should be evaluated in extended studies.
Acknowledgements
This study was performed by the Minimally Invasive Interstitial Fluid Extraction Technology (MIET) study group, which was sponsored by Sysmex Corporation, Japan. K Sakamoto, FK, KY, K Sakaguchi, KT, HK, HM, and KK received research funding from Sysmex. TS is an employee of Sysmex. No other potential conflicts of interest relevant to this article are reported.
(J Diabetes Invest, doi: 10.1111/jdi.12096, 2013)
Parts of this study were presented at the 8th International Diabetes Federation Western Pacific Region Congress and the 54th General Conference of the Japan Diabetes Society.
References
- 1.International Diabetes Federation . Diabetes Atlas, 5th edn International Diabetes Federation, Brussels, 2009 [Google Scholar]
- 2.Gillet MJ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl): S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson DE, Rhee MK, Herrick K, et al Screening for diabetes and pre‐diabetes with proposed A1C‐based diagnostic criteria. Diabetes Care 2010; 33: 2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Kim TH, Lim S, et al Hemoglobin A1c as a diagnostic tool for diabetes screening and new‐onset diabetes prediction: a 6‐year community‐based prospective study. Diabetes Care 2011; 34: 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Gregg EW, Williamson DF, et al A1C level and future risk of diabetes: a systematic review. Diabetes Care 2010; 33: 1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen DL, Witte DR, Kaduka L, et al Moving to an A1C‐based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 2010; 33: 580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haliassos A, Drakopoulos I, Katritsis D, et al Measurement of glycated hemoglobin (HbA1c) with an automated POCT instrument in comparison with HPLC and automated immunochemistry method: evaluation of the influence of hemoglobin variants. Clin Chem Lab Med 2006; 44: 223–227 [DOI] [PubMed] [Google Scholar]
- 9.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 2011; 34(Suppl 2): S184–S190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association . Standards of medical care in diabetes – 2012. Diabetes Care 2012; 35(Suppl): S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, Okada S, Hagino K, et al A novel technique of glucose area under the curve monitoring with interstitial fluid. Diabetes Technol Ther 2011; 13: 1194–1200 [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi K, Hirota Y, Hashimoto N, et al A minimally invasive system for glucose area under the curve measurement using interstitial fluid extraction technology; evaluation of the accuracy and usefulness with oral glucose tolerance tests in subject with and without diabetes. Diabetes Technol Ther 2012; 14: 485–491 [DOI] [PubMed] [Google Scholar]
- 13.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470 [DOI] [PubMed] [Google Scholar]
- 15.Retnakaran R, Qi Y, Goran MI, et al Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009; 26: 1198–1203 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. WHO Document Production Services, Geneva, 2006 [Google Scholar]
- 17.Standards Australia . Australian Standard Glycemic Index of Food 2007. Standards Australia, Sydney, 2007 [Google Scholar]
- 18.Rosenstock J, Hassman DR, Madder RD, et al Repaglinide Versus Nateglinide Comparison Study Group. Repaglinide versus nateglinide monotherapy: a randomized, multicenter study. Diabetes Care 2004; 27: 1265–1270 [DOI] [PubMed] [Google Scholar]
- 19.Shimabukuro M, Higa N, Chinen I, et al Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover study. J Clin Endocrinol Metab 2006; 91: 837–842 [DOI] [PubMed] [Google Scholar]
- 20.Pattzi HM, Pitale S, Alpizar M, et al Dutogliptin, a selective DPP4 inhibitor, improves glycaemic control in patients with type 2 diabetes: a 12‐week, double‐blind, randomized, placebo‐controlled, multicentre trial. Diabetes Obes Metab 2010; 12: 348–355 [DOI] [PubMed] [Google Scholar]
- 21.Hussey EK, Clark RV, Amin DM, et al Single‐dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy volunteers and patients with type 2 diabetes mellitus. J Clin Pharmacol 2010; 56: 623–635 [DOI] [PubMed] [Google Scholar]
- 22.Zhou W, Gu Y, Li H, et al Assessing 1‐h plasma glucose and shape of the glucose curve during oral glucose tolerance test. Eur J Endocrinol 2006; 155: 191–197 [DOI] [PubMed] [Google Scholar]
- 23.Libman IM, Barinas‐Mitchell E, Bartucci A, et al Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008; 93: 4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito K, Ciotola M, Carleo D, et al Postmeal glucose peaks at home associate with carotid initima–media thickness in type 2 diabetes. J Clin Endocrin Metab 2008; 93: 1345–1350 [DOI] [PubMed] [Google Scholar]
- 25.Ugi S, Morino K, Nishio Y, et al Evaluation of a novel glucose area under the curve (AUC) monitoring system: comparison to AUC measured by CGM. J Diabetes Invest 2012; 3(Suppl): S247(Abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan DM, Kuenen J, Borg R, et al A1c‐Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31: 1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerci B, Drouin P, Grangé V, et al Self‐monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto‐Surveillance Intervention Active (ASIA) study. Diabetes Metab 2003; 29: 587–594 [DOI] [PubMed] [Google Scholar]


