Abstract
Aims/Introduction
The safety and efficacy of insulin‐to‐liraglutide switch in type 2 diabetes has not been studied adequately. Here, we retrospectively characterize clinical parameters that might predict insulin‐to‐liraglutide treatment switch without termination due to hyperglycemia, and examine the effects of switching the therapies on glycated hemoglobin (HbA1c) and bodyweight in Japanese type 2 diabetes.
Materials and Methods
Japanese type 2 diabetes patients who underwent the switch of therapy were evaluated for their clinical data including β‐cell function‐related indices, such as increment of serum C‐peptide during glucagon stimulation test (GST‐ΔCPR). HbA1c and bodyweight were analyzed in patients continuing with liraglutide after switching from insulin for 12 weeks.
Results
Of 147 patients, 28 failed in the switch due to hyperglycemia, nine failed because of other reasons and 110 continued with liraglutide for the 12‐week period. Patients failing in the switch due to hyperglycemia showed longer duration and higher daily insulin dose, as well as lower GST‐ΔCPR. Receiver–operating characteristic analysis showed that GST‐ΔCPR of 1.34 ng/mL is a cut‐off point for insulin‐to‐liraglutide switch without termination due to hyperglycemia. In patients continuing liraglutide for 12 weeks, the switch significantly reduced HbA1c and bodyweight with no severe hypoglycemia, irrespective of sulfonylurea co‐administration, body mass index, duration and total daily insulin dose. The switch also significantly reduced the percentage of body fat and visceral fat areas.
Conclusions
Insulin‐to‐liraglutide switch can improve glycemic control and reduce bodyweight in Japanese type 2 diabetes patients. However, caution must be taken with the switch in patients with reduced insulin secretory capacity as predicted by GST‐ΔCPR.
Keywords: Glucagon stimulation test, Glucagon‐like peptide‐1 receptor agonist, β‐Cell function
Introduction
Type 2 diabetes is a heterogeneous disease characterized by β‐cell dysfunction, as well as insulin resistance1. As a result of a progressive decline in β‐cell function3, antidiabetic treatment regimens must be adjusted over time based on estimates of the remaining insulin secretory capacity. Conventional antidiabetic drugs that compensate for reduced insulin secretion include insulin and sulfonylurea (SU), both of which have been shown to maintain good glycemic control and to prevent progression of diabetes‐related micro‐ and macrovascular complications6. However, insulin and SU are often associated with varying degrees of hypoglycemia and weight gain3. Treatment regimens that improve β‐cell function without hypoglycemia and bodyweight gain have long been sought.
Glucagon‐like peptide‐1 (GLP‐1) receptor agonists are emerging antidiabetic drugs that enhance insulin secretion glucose‐dependently, as well as suppress glucagon secretion and slow gastric emptying, thereby improving glycemic control and reducing bodyweight in patients with type 2 diabetes9. Clinical trials of the two initially‐launched GLP‐1 receptor agonists, liraglutide and exenatide, that compared their efficacy and safety with those of insulin or SU showed the capability of GLP‐1 receptor agonists to achieve appropriate glycemic control with reduced risk of hypoglycemia, as well reduced bodyweight10. Although these lines of evidence encourage the use of GLP‐1 receptor agonists over insulin or SU in management of type 2 diabetes, caution must be taken when switching from insulin to GLP‐1 receptor agonists as replacement for insulin. In Japan, several cases of severe hyperglycemia in patients with type 2 diabetes after initiation of the GLP‐1 receptor agonist, liraglutide, as replacement for insulin were reported15. Although investigation of the these limited cases suggested that the remaining insulin secretory capacity might be a critical determinant for a successful switch from insulin to GLP‐1 receptor agonists, further investigation is required to determine the clinical features of patients who might show severe hyperglycemia after the switch.
To assess the remaining insulin secretory capacity in patients with type 2 diabetes in clinical settings, several indices using serum C‐peptide immunoreactivity (CPR) are used: glucagon‐stimulated changes of CPR16, fasting and postprandial C‐peptide index (CPI)19, and secretory units of islets in transplantation19. It has been shown that these CPR‐related indices for β‐cell function can predict the success of insulin therapy in patients with type 2 diabetes19, but it is not known whether these indices can predict the success of GLP‐1 receptor agonist therapy.
Here, we report in Japanese patients with type 2 diabetes: (i) characteristics of the clinical parameters that might be relevant to successful insulin‐to‐liraglutide treatment switch without termination of liraglutide due to hyperglycemia; and (ii) effects of insulin‐to‐liraglutide switch on glycated hemoglobin (HbA1c) level and bodyweight.
Materials and Methods
Participants
A total of 184 patients with type 2 diabetes who underwent an insulin‐to‐liraglutide switch at Kansai Electric Power Hospital between June 2010 and February 2012 were retrospectively analyzed in the current study. None of these patients had type 1 diabetes, pancreatic disease, liver disease, renal disease, diabetogenic medication, malignancy or were pregnant. In accord with the institutional safety regulations regarding insulin‐to‐liraglutide switch, all patients were judged as insulin independent before the switch by the physicians in charge based on preprandial or postprandial serum levels of CPR or serum CPR levels after glucagon stimulation test. All patients in the study received physical and laboratory evaluation including HbA1c before and every 6 weeks after the insulin‐to‐liraglutide switch. The estimated duration of type 2 diabetes in the present study was defined as years after diagnosis of the disease according to the criteria of the Japan Diabetes Society21. Of the original 184 patients, 36 were excluded from the analysis because they did not visit the hospital on 6 ± 1 and/or 12 ± 1 weeks after the switch. One patient with a creatinine clearance rate (CCr; Cockcroft–Gault equation) of <30 was excluded because such serum CPR elevation is likely due to decreased renal function22, and the frequency of gastrointestinal adverse effects associated with liraglutide is increased by severe renal dysfunction23. The baseline clinical profiles of the remaining 147 patients are shown in Table 1. Of these patients, 28 are referred to as ‘discontinued due to hyperglycemia’, because the physician in charge independently judged that they should be switched back to insulin, usually when their average daily glucose levels measured by self‐monitoring of blood glucose showed worse glycemic control than before the switch. Nine patients were withdrawn within 12 weeks after the insulin‐to‐liraglutide switch because of nausea (n = 4), switching to oral antidiabetic drugs (n = 2), skin rash (n = 1), serum gamma‐glutamyl transpeptidase elevation (n = 1) and hypotension (n = 1), and are referred to as ‘discontinued due to other reasons’. After the switch, patients received liraglutide with or without the SUs glimepiride or gliclazide; no other antidiabetic drugs (e.g., metformin and pioglitazone) were used, as combination of liraglutide with oral antidiabetic agents other than sulfonylurea is not approved in Japan. The dosage adjustments of liraglutide and SU were decided by the physician in charge. In patients who continued liraglutide for 12 weeks after the switch, the mean daily liraglutide, gliclazide and glimepiride doses at week 12 were as follows: liraglutide (n = 110) 0.77 ± 0.01 mg; gliclazide (n = 27) 34.1 ± 3.1 mg and glimepiride (n = 23) 1.0 ± 0.1 mg. Severe hypoglycemia was defined as requiring assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions24. Non‐severe hypoglycemia was not evaluated in the current study, as its definition varied among physicians in charge.
Table 1. Characteristics of patients with type 2 diabetes undergoing insulin‐to‐liraglutide switch.
| Total | Continued | Discontinued/hyperglycemia | Discontinued/other reasons | |
|---|---|---|---|---|
| n | 147 | 110 | 28 | 9 |
| Male (%) | 65.5 | 64.5 | 71.4 | 66.7 |
| Age (years) | 64.3 ± 1.0 | 63.9 ± 1.1 | 64.5 ± 2.8 | 67.7 ± 3.1 |
| Duration (years) | 14.4 ± 0.8 | 12.3 ± 0.8 | 21.2 ± 1.9* | 19.7 ± 4.2 |
| BMI (kg/m2) | 25.7 ± 0.4 | 25.9 ± 0.4 | 26.0 ± 0.9 | 22.7 ± 0.8 |
| Baseline HbA1c (%) | 7.8 ± 0.1 | 7.7 ± 0.1 | 8.4 ± 0.2* | 7.7 ± 0.3 |
| Systolic BP (mmHg) | 131.3 ± 1.4 | 131.0 ± 1.7 | 133.2 ± 3.4 | 129.1 ± 2.5 |
| Diastolic BP (mmHg) | 76.7 ± 0.9 | 77.5 ± 1.0 | 74.4 ± 2.1 | 73.8 ± 4.1 |
| Total daily insulin (unit per day per kg bodyweight) | 0.38 ± 0.01 | 0.34 ± 0.02 | 0.53 ± 0.04* | 0.36 ± 0.05 |
| Daily insulin injections (%) | ||||
| Once per day | 29 | 31 | 17 | 33 |
| Twice per day | 22 | 19 | 36 | 11 |
| Three times per day | 12 | 11 | 11 | 33 |
| More than four times per day | 37 | 39 | 36 | 22 |
| Oral antidiabetic drug usage (%) | ||||
| Sulfonylureas | 22 | 25 | 7 | 22 |
| Metformin | 20 | 17 | 32 | 22 |
| Glinides | 16 | 15 | 25 | 11 |
| α‐Glycosidase inhibitors | 6 | 5 | 7 | 11 |
| Pioglitazone | 5 | 4 | 7 | 11 |
| DPP‐4 inhibitors | 1 | 1 | 4 | 0 |
BMI, body mass index; BP, blood pressure; DPP‐4, dipeptidyl peptidase‐4; HbA1c, glycated hemoglobin. Each value represents the mean ± standard error of the mean. *P < 0.05 in Mann–Whitney U‐test (vs continued).
Measurements
HbA1c was measured using high performance liquid chromatography with cation‐exchange resins that separate the stable form of β‐N1‐mono‐deoxyfructosyl Hb; values are shown in National Glycohemoglobin Standardization Program values as recommended by the Japan Diabetes Society25. The glucagon stimulation test was carried out after an overnight fast by measuring serum CPR at fasting or 6 min after intravenous injection of 1 mg glucagon (CPR‐0 min and CPR‐6 min, respectively)22. Insulin injections were continued to avoid hyperglycemia until the night before measuring fasting and/or glucagon‐stimulated levels of serum C‐peptide in the morning, and were stopped until the end of the glucagon stimulation test. The glucagon stimulation test was carried out when fasting plasma glucose levels reached ≥80 mg/dL. Increments of CPR after glucagon stimulation test (GST‐ΔCPR) and C‐peptide index (CPI) were calculated as follows: GST‐ΔCPR (ng/mL), (CPR‐6 min) – (CPR‐0 min); CPI, (fasting CPR)/(fasting plasma glucose) × 100. Serum C‐peptide was measured using lumipulse presto C‐peptide (Fujirebio Inc., Tokyo, Japan). Antidiabetic drugs and insulin were stopped for the glucagon test, but were maintained until 1 day before to prevent hyperglycemia during the test. Percentage of body fat and visceral fat areas were estimated using multifrequency bioelectrical impedance scales (InBody S20; BioSpace Col. Ltd., Tokyo, Japan). The accuracy of the scale was shown in previous studies26. Other laboratory measurements including plasma glucose were measured by standard assays.
Statistical Analysis
Patient characteristics and results are reported as mean ± standard error of the mean unless otherwise stated. Statistical analysis was carried out using IBM spss Statistics version 20 (SAS Institute Inc., Cary, NC, USA), including two‐way anova for repeated measures with post‐hoc analysis to analyze time‐course curves. Clinical parameters among the two groups at single time‐points were compared by Mann–Whitney U‐test. Clinical parameters in the same group at single time‐points were compared by paired t‐test. P‐values <0.05 were considered statistically significant. Histograms and receiver–operating characteristic (ROC) curve were constructed for CPR‐0 min, CPR‐6 min, GST‐ΔCPR and CPI. Sensitivity, specificity, cut‐off point, area under the ROC curve (AUC) and the likelihood ratios were calculated.
Results
Baseline characteristics of the patients who were followed closely for the 12‐week period are shown in Table 1. Despite the fact that unnoticed lifestyle changes might significantly influence the failure rate later, the safety of the therapy switch might well be apparent within this period. Of these 147 patients, 28 failed in the switch due to hyperglycemia within 12 weeks, nine failed due to other reasons indicated in Materials and Methods, and 110 continued on liraglutide for the 12‐week period. The logistic analysis showed that estimated duration, baseline HbA1c and total daily insulin dose were significantly associated with the failure due to hyperglycemia (Table S1). In addition, withdrawal of non‐SU antidiabetic drugs was not significantly associated with failure due to hyperglycemia. The proportion of patients who failed in the insulin‐to‐liraglutide switch due to hyperglycemia was higher in patients with increased baseline HbA1c, longer duration of type 2 diabetes and higher total daily insulin dose, but was not affected by BMI or frequency of insulin injections (Figure S1). The indices related to β‐cell function were significantly reduced in patients who failed in the insulin‐to‐liraglutide switch due to hyperglycemia (Figure 1a). ROC analyses were carried out for CPR‐0 min, CPR‐6 min, GST‐ΔCPR and CPI to characterize their relevance to the success of the insulin‐to‐liraglutide switch. GST‐ΔCPR showed the largest AUC (0.812, 95% confidence interval [CI] 0.703–0.920), with a cut‐off point estimated to be 1.34 ng/mL with 59% sensitivity and 95% specificity (Figure 1b and Table 2).
Figure 1.
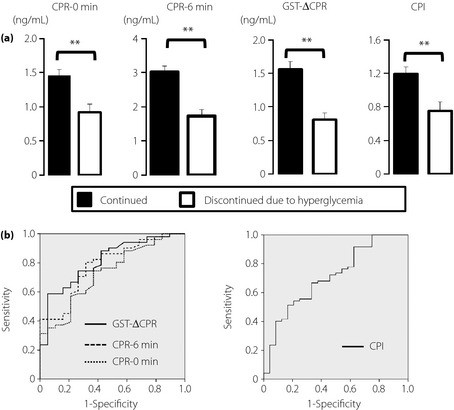
Predictors for insulin‐to‐liraglutide switch without liraglutide termination due to hyperglycemia. (a) Indices related to β‐cell function were compared in patients who failed in the switch due to hyperglycemia within 12 weeks (n = 28) and patients who continued liraglutide for 12 weeks after the switch (n = 110). The increment of serum C‐peptide levels before and 6 min after i.v. administration of 1 mg glucagon (GST‐ΔCPR), and C‐peptide index (CPI) defined as (fasting CPR [ng/mL]) / (fasting plasma glucose [(mg/dL)]) × 100 are compared. Each value represents mean ± standard error of the mean. **P < 0.01 in unpaired t‐test (vs patients with liraglutide continued for 12 weeks after the switch). (b) Receiver–operating characteristic curves of serum C‐peptide immunoreactivity (CPR)‐0 min, CPR‐6 min and GST‐ΔCPR to predict insulin‐to‐liraglutide switch without termination of liraglutide due to hyperglycemia. Areas under the curve for CPR‐0 min, CPR‐6 min and GST‐ΔCPR were 0.730, 0.784 and 0.812, respectively. Cut‐off points for CPR‐0 min, CPR‐6 min, GST‐ΔCPR, and CPI were determined as 1.07, 1.94, 1.34 ng/mL and 0.93, respectively.
Table 2. Receiver–operating characteristics analysis of β‐cell function‐related indices for insulin‐to‐liraglutide switch without liraglutide termination due to hyperglycemia.
| CPR‐0 min | CPR‐6 min | GST‐ΔCPR | CPI | |
|---|---|---|---|---|
| AUC | 0.730 | 0.784 | 0.812 | 0.710 |
| 95% CI | 0.602–0.857 | 0.668–0.901 | 0.703–0.920 | 0.591–0.830 |
| Cut‐off point | 1.07 | 1.95 | 1.34 | 0.93 |
| Values at cut‐off points | ||||
| Sensitivity (%) | 75 | 80 | 59 | 51 |
| Specificity (%) | 63 | 68 | 95 | 83 |
| Likelihood ratio | 2.0 | 2.5 | 11.8 | 3.0 |
AUC, area under the curve; CI, confidence interval; CPI, C‐peptide index; CPR, C‐peptide immunoreactivity; CPR‐0 min, C‐peptide immunoreactivity at fasting; CPR‐6 min, C‐peptide immunoreactivity at 6 min after intravenous injection of glucagon; GST‐ΔCPR, increment of C‐peptide immunoreactivity after glucagon stimulation test.
In the 110 patients continuing liraglutide for 12 weeks, the insulin‐to‐liraglutide switch reduced the mean HbA1c significantly at 6 and 12 weeks after the switch (compared with baseline; Figure 2a). Mean HbA1c levels were improved from 7.69 ± 0.11% (week 0) to 7.11 ± 0.07% (week 12). The proportion of patients who achieved HbA1c levels of <7% was increased from 22.7% at week 0 to 46.4% at week 12. No severe hypoglycemic events were reported. The switch also reduced bodyweight as well as percentage of body fat and visceral fat area estimated using multifrequency bioelectrical impedance scales (Figure 2b–d).
Figure 2.
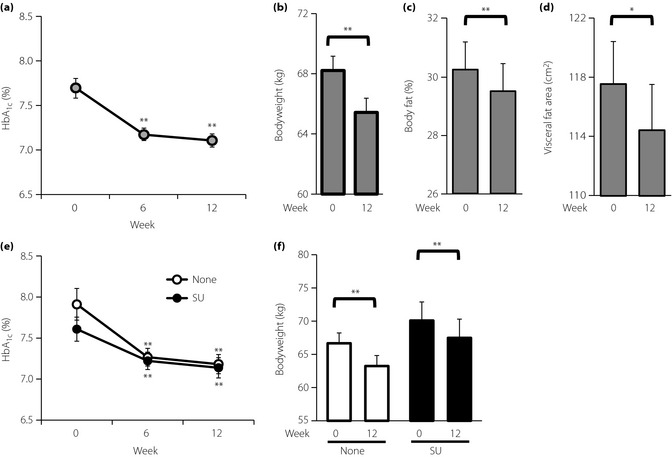
Effect of insulin‐to‐liraglutide switch on glycated hemoglobin (HbA1c) and bodyweight as well as percentage of body fat and estimated visceral fat area in patients who continued liraglutide for 12 weeks after the switch. Changes of (a) HbA1c and (b) bodyweight in patients whose insulin injections were switched to liraglutide (n = 110). Changes of (c) percentage of body fat and (d) visceral fat area estimated using multifrequency scales in patients whose insulin injections were switched to liraglutide. (e,f) Comparison of HbA1c and bodyweight changes in patients who initiated liraglutide without (None; n = 60) or with sulfonylureas (SU; n = 50). Each value represents the mean ± standard error of the mean. *P < 0.05 and **P < 0.01 in paired t‐test (vs 0 week).
Because combination of liraglutide with oral antidiabetic agents other than sulfonylurea is not approved in Japan, liraglutide was initiated with and without SU. Patients who initially received liraglutide without SU (n = 60) and those receiving liragultide with SU (n = 50) showed a similarly significant reduction in mean HbA1c after the switch (Figure 2e), despite their varied baseline characteristics (Table S2). The proportion of patients who achieved HbA1c <7% was increased in these two groups: from 21.7% (week 0) to 45.0% in those initially receiving liraglutide without SU (week 12), and from 24.0% (week 0) to 48.0% (week 12) in those initially receiving liraglutide with SU. Bodyweight also was significantly reduced in the two groups (Figure 2f). Changes in SU doses did not significantly influence changes in HbA1c levels and bodyweight during the 12‐week observation period (Figure S2). Withdrawal of non‐SU antidiabetic drugs did not significantly affect changes in HbA1c levels and bodyweight (Figure S3).
Subgroup analyses of HbA1c and bodyweight changes were carried out in patients continuing liraglutide for 12 weeks for baseline HbA1c, estimated duration of type 2 diabetes, daily insulin dose and BMI (Figure 3). Patients with baseline HbA1c ≥8.0% (n = 36) and those with baseline HbA1c ≥7.0 and <8.0% (n = 49) showed a significant reduction of mean HbA1c after the switch (Figure 3a). Mean HbA1c remained similar to baseline in patients with baseline HbA1c <7.0% (n = 25). The proportion of patients who achieved HbA1c <7.0% were changed as follows: baseline HbA1c ≥8.0%, 0% (week 0) to 25.0% (week 12); ≥7.0 and <8.0%, 0% (week 0) to 42.9% (week 12); and <7.0%, 100% (week 0) to 84.0% (week 12). Bodyweight was significantly reduced in the three groups (Figure 3b).
Figure 3.
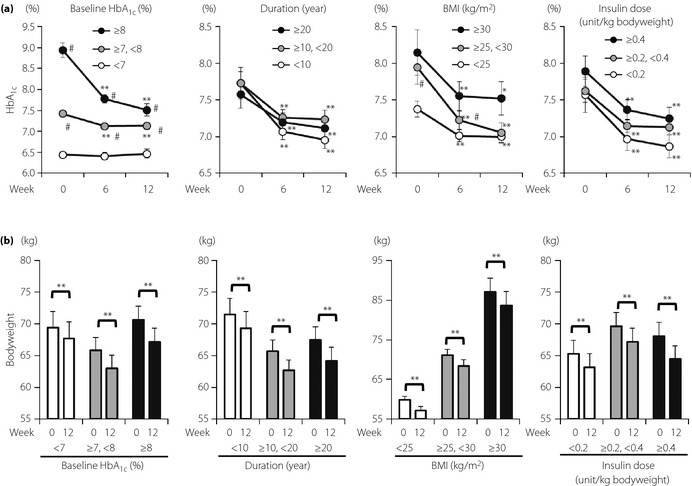
Subgroup analyses of insulin‐to‐liraglutide switch on (a) glycated hemoglobin (HbA1c) and (b) bodyweight compared for baseline HbA1c, estimated duration of diabetes, body mass index (BMI) and total daily insulin dose. Patients who continued liraglutide for 12 weeks were divided by baselines of HbA1c (%) of ≥8.0 (n = 36), ≥7.0 and < 8.0 (n = 49), and < 7.0 (n = 25); estimated duration of type 2 diabetes (years) of ≥20 (n = 22), ≥10 and < 20 (n = 47), and <10 (n = 41); BMI (kg/m2) of ≥30 (n = 19), ≥25 and < 30 (n = 36), and < 25 (n = 55); total daily insulin dose (unit per kg bodyweight) of ≥0.4 (n = 24), ≥0.2 and < 0.4 (n = 52), and < 0.2 (n = 34). Each value represents mean ± standard error of the mean. *P < 0.05 and **P < 0.01 in paired t‐test (vs 0 week). #P < 0.05 in Mann–Whitney U‐test (vs baseline HbA1c < 7.0%, duration <10 years, BMI < 25 kg/m2 or insulin dose < 0.2 unit/kg bodyweight).
Patients with duration ≥20 years (n = 22), as well as those with duration ≥10 and <20 years (n = 47), and those with duration <10 years (n = 41) showed a significant reduction of mean HbA1c after the switch (Figure 3a). Proportions of patients who achieved HbA1c <7.0% were improved as follows: duration ≥20 years, 18.2% (week 0) to 40.9% (week 12); ≥10 and <20 years, 19.1% (week 0) to 38.3% (week 12); and <10 years, 29.3% (week 0) to 58.5% (week 12). Bodyweight was significantly reduced in the three groups (Figure 3b).
Patients with BMI ≥30 kg/m2 (n = 19), those with BMI ≥25 and <30 kg/m2 (n = 36), and those with BMI <25 kg/m2 (n = 55) all showed a significant reduction of mean HbA1c after the switch (Figure 3a). The proportions of patients who achieved HbA1c <7.0% were improved as follows: BMI ≥30 kg/m2, 21.1% (week 0) to 42.1% (week 12); ≥25 and <30 kg/m2, 22.2% (week 0) to 47.2% (week 12); and <25 kg/m2, 23.6% (week 0) to 47.3% (week 12). Bodyweight was significantly reduced in the three groups (Figure 3b).
Patients with daily insulin dose ≥0.4 units per kg bodyweight (n = 24), those with insulin dose ≥0.2 and <0.4 units per kg bodyweight (n = 52), and those with insulin dose <0.2 units per kg bodyweight (n = 34) all showed a significant reduction of mean HbA1c after the switch (Figure 3a). The proportions of patients who achieved HbA1c <7.0% were improved as follows: daily insulin dose ≥0.4 units per kg, 20.6% (week 0) to 38.2% (week 12); ≥0.2 and <0.4 units per kg bodyweight, 25.0% (week 0) to 46.2% (week 12); and <0.2 units per kg bodyweight, 20.8% (week 0) to 58.3% (week 12). Bodyweight was significantly reduced in the three groups (Figure 3b).
Discussion
The current report shows that failure of insulin‐to‐liraglutide switch can partially be explained by reduced β‐cell function. Among the several indices related to β‐cell function examined, GST‐ΔCPR seems to be the most valuable parameter to avoid termination of liraglutide due to hyperglycemia after the switch. Additionally, the present study showed that liraglutide significantly reduces HbA1c and bodyweight in Japanese patients with type 2 diabetes who had previously been receiving insulin therapy in the presence and absence of various oral antidiabetic drugs.
It has been shown that intensive glycemic control by insulin therapy is effective in preventing micro‐ and macrovascular complications, as well as death from any cause of type 2 diabetes6. However, insulin therapy is often associated with bodyweight gain and hypoglycemia3, and antidiabetic therapies that circumvent these problems have long been sought. GLP‐1 receptor agonists, such as liraglutide, are gaining attention because they can ameliorate glycemic control with lower risk of hypoglycemia, and have the ability to reduce bodyweight as well28. Head‐to‐head trials comparing GLP‐1 receptor agonists with insulin glargine or biphasic insulin demonstrated that GLP‐1 receptor agonists show similar or slightly improved HbA1c reduction with significant bodyweight reduction11. However, little is known regarding the adverse consequences of switching from insulin to GLP‐1 receptor agonists. The current study strongly suggests that switching treatment regimens from insulin to liraglutide in relatively poorly controlled type 2 diabetes patients improves HbA1c and reduces bodyweight together with visceral fat reduction without any severe hypoglycemia. Furthermore, insulin‐to‐liraglutide switch liberates patients from tedious multiple injections and concerns about hypoglycemia, as judged by the results of questionnaires on patient quality of life (Yokota, K., Yabe, D., Kurose, T., and Seino, Y. unpublished observation, 2011). However, rigorous caution must be taken when insulin is switched to liraglutide, as there are several reported cases of severe hyperglycemia, two of which were insulin‐dependent and resulted in death due to ketoacidosis within a few days after switching the therapies15. As shown in the current study, the proportion of failure of insulin‐to‐liraglutide switch due to hyperglycemia is increased in patients with longer duration of diabetes, more total insulin dose and reduced β‐cell function. Importantly, switching has caused hyperglycemia even in patients who received only a single injection of insulin per day. Therefore, insulin‐to‐liraglutide switch should be carried out ideally in inpatient wards, with β‐cell function evaluated by experienced diabetologists before the switch.
Type 2 diabetes is characterized partly by progressive loss of β‐cell function4. Previous studies reported indices related to β‐cell function (e.g., fasting and postprandial serum levels of CPR, fasting and postprandial CPI, and GST‐ΔCPR) as predictors for insulin therapy19. Based on the data of 201 Japanese patients with type 2 diabetes, Funakoshi et al. suggested that GST‐ΔCPR 2.25 ng/mL and CPI 1.1 ng/mg were cut‐off points for choosing insulin therapy to achieve good glycemic control19. The current study shows that GST‐ΔCPR 1.34 ng/mL and CPI 0.93 represent cut‐off points for insulin‐to‐liraglutide switch without liraglutide termination due to hyperglycemia, levels that are lower than those recommended by the Funakoshi study. This discrepancy could be related to the following considerations: (i) liraglutide per se improves β‐cell function and reduces glucagon secretion as well33, reducing the degree of improvement in β‐cell function required; (ii) fasting and/or glucagon‐stimulated C‐peptide levels are sufficiently suppressed by lowered glucose levels from exogenously injected insulin continued until the night before the glucagon stimulation test36; and (iii) the current investigation for cut‐off points of GST‐ΔCPR and CPI for insulin‐to‐liraglutide switch without liraglutide termination due to hyperglycemic episodes within 12 weeks differs from an investigation for cut‐off points above which the switch might be expected to yield long‐term glycemic control. When HbA1c changes were stratified in the 70 remaining patients over a 36‐week period, they remained significantly reduced in patients with GST‐ΔCPR >1.34 ng/mL, but not in patients with GST‐ΔCPR ≤1.34 ng/mL (Figure S4). This longer‐term observation suggests >1.34 ng/mL GST‐ΔCPR as the cut‐off for switching to liraglutide. Further prospective studies are required to corroborate cut‐off points of β‐cell function‐related indices that predict long‐term glycemic control after insulin‐to‐liraglutide switch. Despite the high specificity (95%), the relatively low sensitivity (59%) for the GST‐ΔCPR cut‐off point suggests that some patients with GST‐ΔCPR ≤1.34 ng/mL might nevertheless succeed in switching to liraglutide. Patients with GST‐ΔCPR ≤1.34 ng/mL might yet achieve better glycemic control by liraglutide by compensating for lesser β‐cell function by beneficial lifestyle modifications, for example. However, in our data, patients with GST‐ΔCPR ≤1.34 ng/mL were more frequently switched back to insulin and did not show significant improvement in HbA1c after the insulin‐to‐liraglutide switch (Figure S4).
In the present study, liraglutide with or without SU similarly reduced HbA1c as well as bodyweight significantly. Synergy of SU and GLP‐1 receptor agonists has been suggested in several clinical studies of type 2 diabetes38. GLP‐1 receptor agonists exert their insulinotropic action by mechanisms distinct from that of SU40. In addition, it has also been shown recently that SU and GLP‐1 receptor signaling potentiate insulin secretion through the same signaling molecule in β‐cells called Epac2, an exchange factor directly activated by adenosine 3′,5′‐cyclic monophosphate40. The current study was not able to compare the effects of liraglutide with or without SU, because the baseline characteristics of the patients who received liraglutide without or with SU were varied (Figure 2 and Table S2). However, it will be interesting to learn if SU and GLP‐1 receptor agonists exert synergistic effects in prospective studies.
Stratified analyses of patients continuing liraglutide for 12 weeks show that changes of HbA1c were significantly greater in those with higher baseline HbA1c. However, fewer patients with higher baseline HbA1c achieved target HbA1c <7.0%, showing the inadequacy of baseline HbA1c to predict a successful switch to liraglutide. Stratified analyses also show that insulin‐to‐liraglutide switch improves HbA1c and bodyweight regardless of BMI (Figure 3). These results are consistent with previous observations that BMI might not serve as a determinant for efficacy of liraglutide therapy10, which suggests the possibility of wider liraglutide use in the management of type 2 diabetes. Although patients in the current study who failed insulin‐to‐liraglutide switch showed longer duration and higher daily insulin dose (Figure S1), duration and insulin dose did not affect efficacy in stratified analyses of patients continuing with liraglutide (Figure 3). In addition, ROC analyses did not suggest cut‐off values of duration or insulin dose (data not shown). These results show that the duration and insulin dose might not influence insulin‐to‐liraglutide failure directly, but rather impact failure through reduced β‐cell function, which can be more precisely assessed by GST‐ΔCPR.
There were some limitations in the present study. First, this was a retrospective and single‐center analysis, with a limited sample size. Prospective and multicenter analyses with an adequate sample size are necessary to confirm the current findings. Second, patients with severe renal impairment (CCr <30 mL/min) were excluded from the present study due to possible increased gastrointestinal adverse effects related to liraglutide23, as well as to potential elevation in serum CPR levels22. Third, the protocols for starting an insulin‐to‐liraglutide switch and discontinuing liraglutide due to hyperglycemia or other reasons were not strictly defined in the present study. However, the decisions as to starting or discontinuing liraglutide were confirmed retrospectively by three independent diabetologists certified by the Japan Diabetes Society as described in Materials and Methods to have been made according to the treatment guide for diabetes of the Japan Diabetes Society. Fourth, approximately 20% of patients who received the insulin‐to‐liraglutide switch were excluded as they did not visit hospital 6 ± 1 and/or 12 ± 1 weeks after the switch due to their personal schedules or being transferred to general practitioners. However, as most of these patients are likely to have continued liraglutide for at least 12 weeks, the effectiveness of insulin‐to‐liraglutide might be underestimated in the current study.
In conclusion, an insulin‐to‐liraglutide switch can improve glycemic control and reduce bodyweight in Japanese type 2 diabetes. However, caution must be taken with the switch in patients with reduced insulin secretory capacity as predicted by GST‐ΔCPR to avoid termination of liraglutide due to hyperglycemia.
Supplementary Material
Figure S1 | Proportions of patients who failed insulin‐to‐liraglutide switch due to hyperglycemia within 12 weeks after the switch stratified by baseline glycated hemoglobin (HbA1c), estimated duration of diabetes, body mass index (BMI), total daily insulin dose and frequency of insulin injections.
Figure S2 | Subgroup analyses of insulin‐to‐liraglutide switch on glycated hemoglobin (HbA1c) and bodyweight compared for changes in sulfonylurea dose at the time of the switch.
Figure S3 | Subgroup analysis of insulin‐to‐liraglutide switch on glycated hemoglobin (HbA1c) and bodyweight compared for use of non‐sulfonylurea (SU) antidiabetic drugs before the switch.
Figure S4 | Glycated hemoglobin (HbA1c) changes in patients with increment of serum C‐peptide during glucagon stimulation test (GST‐ΔCPR) ≤1.34 in comparison to those in patients with GST‐ΔCPR >1.34 over a 36‐week period.
Table S1 | Logistic regression analysis of baseline characteristics predicting failure of insulin‐to‐liraglutide switch due to hyperglycemia.
Table S2 | Characteristics of patients with type 2 diabetes undergoing insulin‐to‐liraglutide switch: Comparison of patients initiating liraglutide without or with sulfonylureas.
Acknowledgments
The authors thank Shimpei Fujimoto of Kochi University for generous advice on the glucagon stimulation test, and Kenta Murotani of Translational Research Informatics Center for discussion on statistics. We thank Takeshi Murakami, Ikuro Yamaguchi, Shinobu Shimizu and Hiroaki Eto of Kansai Electric Power Hospital for technical support. We also thank Aya Sanagi at Kansai Electric Power Hospital for secretarial assistance. This study was partly supported by grants from the Japan Diabetes Foundation and the Diabetes Masters Conference. YS reports receiving consulting and/or speaker fees from Eli Lilly, MSD, Novartis, Novo Nordisk, Sanofi‐Aventis and Takeda. KT and DY report receiving speaker fees from Eli Lilly, MSD, Sanofi‐Aventis, Novo Nordisk and Takeda. No other potential conflict of interest relevant to this article is reported.
(J Diabetes Invest, doi: 10.1111/jdi.12111, 2013)
References
- 1.DeFronzo RA. The triumvirate: beta‐cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687 [DOI] [PubMed] [Google Scholar]
- 2.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333–1346 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 4.Funakoshi S, Fujimoto S, Hamasaki A, et al Analysis of factors influencing pancreatic β‐cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract 2008; 82: 353–358 [DOI] [PubMed] [Google Scholar]
- 5.Funakoshi S, Fujimoto S, Hamasaki A, et al Analysis of factors influencing postprandial C‐peptide levels in Japanese patients with type 2 diabetes: comparison with C‐peptide levels after glucagon load. J Diabetes Invest 2012; 2: 429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1999; 44: 1249–1258 [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 8.Best JD, Drury PL, Davis TM, et al Glycemic control over 5 years in 4,900 people with type 2 diabetes: real‐world diabetes therapy in a clinical trial cohort. Diabetes Care 2012; 35: 1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck MA. Incretin‐based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 2011; 124: S3–S18 [DOI] [PubMed] [Google Scholar]
- 10.Garber A, Henry R, Ratner R, et al Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481 [DOI] [PubMed] [Google Scholar]
- 11.Russell‐Jones D, Vaag A, Schmitz O, et al Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck M, Frid A, Hermansen K, et al Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heine RJ, Van Gaal LF, Johns D, et al Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143: 559–569 [DOI] [PubMed] [Google Scholar]
- 14.Seino Y, Rasmussen MF, Nishida T, et al Efficacy and safety of the once‐daily human GLP‐1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin 2010; 26: 1013–1022 [DOI] [PubMed] [Google Scholar]
- 15.NovoNordiskPharmaLtd . Safety Information Letter: Ketoacidosis and hyperglycemia after switching from insulin therapy to human GLP‐1 analogue Victoza 18 mg. In; 2010.
- 16.Faber OK, Binder C. C‐peptide response to glucagon. A test for the residual beta‐cell function in diabetes mellitus. Diabetes 1977; 26: 605–610 [DOI] [PubMed] [Google Scholar]
- 17.Hendriksen C, Faber OK, Drejer J, et al Prevalence of residual B‐cell function in insulin‐treated diabetics evaluated by the plasma C‐peptide response to intravenous glucagon. Diabetologia 1977; 13: 615–619 [DOI] [PubMed] [Google Scholar]
- 18.Gjessing HJ, Matzen LE, Froland A, et al Correlations between fasting plasma C‐peptide, glucagon‐stimulated plasma C‐peptide, and urinary C‐peptide in insulin‐treated diabetics. Diabetes Care 1987; 10: 487–490 [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi S, Fujimoto S, Hamasaki A, et al Utility of indices using C‐peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patientes with type 2 diaebtes. J Diabetes Invest 2011; 2: 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saisho Y, Kou K, Tanaka K, et al Postprandial serum C‐peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J 2011; 58: 315–322 [DOI] [PubMed] [Google Scholar]
- 21.Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajinuma H, Kanazawa Y, Sando H, et al Human plasma C‐peptide immunoreactivity: its correlation with immunoreactive insulin in diabetes, and chronic liver and renal diseases. Endocrinol Jpn 1979; 26: 65–73 [DOI] [PubMed] [Google Scholar]
- 23.Davidson JA, Brett J, Falahati A, et al Mild renal impairment has no effect on the efficacy and safety of liraglutide. Endocr Pract 2011; 17: 345–355 [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Workgroup on Hypoglycemia . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249 [DOI] [PubMed] [Google Scholar]
- 25.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedogni G, Malavolti M, Severi S, et al Accuracy of an eight‐point tactile‐electrode impedance method in the assessment of total body water. Eur J Clin Nutr 2002; 56: 1143–1148 [DOI] [PubMed] [Google Scholar]
- 27.Malavolti M, Mussi C, Poli M, et al Cross‐calibration of eight‐polar bioelectrical impedance analysis versus dual‐energy X‐ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21‐82 years. Ann Hum Biol 2003; 30: 380–391 [DOI] [PubMed] [Google Scholar]
- 28.Monami M, Dicembrini I, Marchionni N, et al Effects of glucagon‐like peptide‐1 receptor agonists on bodyweight: a meta‐analysis. Exp Diabetes Res 2012; 2012: 672658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once‐daily human glucagon‐like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin Ther 2009; 31: 2472–2488 [DOI] [PubMed] [Google Scholar]
- 30.Diamant M, Van Gaal L, Stranks S, et al Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet 2010; 375: 2234–2243 [DOI] [PubMed] [Google Scholar]
- 31.Madsbad S, Krarup T, McNair P, et al Practical clinical value of the C‐peptide response to glucagon stimulation in the choice of treatment in diabetes mellitus. Acta Med Scand 1981; 210: 153–156 [DOI] [PubMed] [Google Scholar]
- 32.Goto A, Takaichi M, Kishimoto M, et al Body mass index, fasting plasma glucose levels, and C‐peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr J 2010; 57: 237–244 [DOI] [PubMed] [Google Scholar]
- 33.Vilsboll T, Brock B, Perrild H, et al Liraglutide, a once‐daily human GLP‐1 analogue, improves pancreatic B‐cell function and arginine‐stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med 2008; 25: 152–156 [DOI] [PubMed] [Google Scholar]
- 34.Degn KB, Juhl CB, Sturis J, et al One week's treatment with the long‐acting glucagon‐like peptide 1 derivative liraglutide (NN2211) markedly improves 24‐h glycemia and alpha‐ and beta‐cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004; 53: 1187–1194 [DOI] [PubMed] [Google Scholar]
- 35.Seino Y, Rasmussen MF, Clauson P, et al The once‐daily human glucagon‐like peptide‐1 analog, liraglutide, improves β‐cell function in Japanese patients with type 2 diabetes. J Diabetes Invest 2012; 3: 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albareda M, Rigla M, Rodriguez‐Espinosa J, et al Influence of exogenous insulin on C‐peptide levels in subjects with type 2 diabetes. Diabetes Res Clin Pract 2005; 68: 202–206 [DOI] [PubMed] [Google Scholar]
- 37.Lindstrom T, Arnqvist HJ, Ludvigsson J, et al C‐peptide profiles in patients with non‐insulin‐dependent diabetes mellitus before and during insulin treatment. Acta Endocrinol (Copenh) 1992; 126: 477–483 [DOI] [PubMed] [Google Scholar]
- 38.Gutniak MK, Juntti‐Berggren L, Hellstrom PM, et al Glucagon‐like peptide I enhances the insulinotropic effect of glibenclamide in NIDDM patients and in the perfused rat pancreas. Diabetes Care 1996; 19: 857–863 [DOI] [PubMed] [Google Scholar]
- 39.Kaku K, Rasmussen MF, Clauson P, et al Improved glycaemic control with minimal hypoglycaemia and no weight change with the once‐daily human GLP‐1 analogue liraglutide as add‐on to sulfonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 341–347 [DOI] [PubMed] [Google Scholar]
- 40.Seino S. Cell signalling in insulin secretion: the molecular targets of ATP, cAMP and sulfonylurea. Diabetologia 2012; 55: 2096–2108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Proportions of patients who failed insulin‐to‐liraglutide switch due to hyperglycemia within 12 weeks after the switch stratified by baseline glycated hemoglobin (HbA1c), estimated duration of diabetes, body mass index (BMI), total daily insulin dose and frequency of insulin injections.
Figure S2 | Subgroup analyses of insulin‐to‐liraglutide switch on glycated hemoglobin (HbA1c) and bodyweight compared for changes in sulfonylurea dose at the time of the switch.
Figure S3 | Subgroup analysis of insulin‐to‐liraglutide switch on glycated hemoglobin (HbA1c) and bodyweight compared for use of non‐sulfonylurea (SU) antidiabetic drugs before the switch.
Figure S4 | Glycated hemoglobin (HbA1c) changes in patients with increment of serum C‐peptide during glucagon stimulation test (GST‐ΔCPR) ≤1.34 in comparison to those in patients with GST‐ΔCPR >1.34 over a 36‐week period.
Table S1 | Logistic regression analysis of baseline characteristics predicting failure of insulin‐to‐liraglutide switch due to hyperglycemia.
Table S2 | Characteristics of patients with type 2 diabetes undergoing insulin‐to‐liraglutide switch: Comparison of patients initiating liraglutide without or with sulfonylureas.


