Abstract
Aims/Introduction
Type 2 diabetes mellitus is a progressive disease that frequently requires patients to use more than one oral antihyperglycemic agent to achieve adequate glycemic control. The present multicenter, randomized study assessed the efficacy and safety of the addition of sitagliptin to ongoing voglibose monotherapy (0.2–0.3 mg three times daily) in Japanese patients with type 2 diabetes mellitus who had inadequate glycemic control (glycated hemoglobin ≥6.9% and <10.5%).
Materials and Methods
The present study had an initial 12‐week, double‐blind treatment period in which patients were randomized (1:1) to sitagliptin 50 mg/day (n = 70) or placebo (n = 63), followed by a 40‐week, open‐label treatment period during which all patients received sitagliptin 50 mg/day, that could have been increased to 100 mg/day for patients meeting predefined glycemic criteria.
Results
After 12 weeks, treatment with sitagliptin resulted in placebo‐subtracted mean changes from baseline in glycated hemoglobin (the primary end‐point), fasting plasma glucose and 2‐h postmeal glucose of –0.9%, –22.5 mg/dL and –51.3 mg/dL, respectively (all, P < 0.001). During the double‐blind period, adverse experiences were reported with similar frequency in both treatment groups, and the occurrences of hypoglycemia and gastrointestinal adverse experiences were low. In the open‐label period, sustained improvements in glycemic parameters were observed with sitagliptin treatment, and sitagliptin was generally well tolerated.
Conclusions
Sitagliptin added on to ongoing voglibose monotherapy provided significant improvements in glycemic parameters and was well tolerated in Japanese patients with type 2 diabetes mellitus who had inadequate glycemic control. This trial was registered with ClinicalTrails.gov (no. NCT00837577).
Keywords: Incretins, Sitagliptin, Voglibose
Introduction
Treatment of hyperglycemia reduces the risk of complications associated with type 2 diabetes mellitus1. Patients with type 2 diabetes mellitus frequently do not achieve glycemic goals with single oral antihyperglycemic agents (OHAs), and require treatment with combination therapy4. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors, a class of drugs that target the incretin axis for the treatment of type 2 diabetes mellitus5, stabilize the intact forms of the incretin hormones, glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP)7. Incretins are released by the intestine in response to a meal, but are rapidly degraded by the peptidase enzyme DPP‐4. Through multiple physiological actions, including stimulation of insulin secretion (GLP‐1 and GIP) and suppression of glucagon secretion (GLP‐1), intact GLP‐1 and GIP play an important role in glycemic homeostasis9.
Sitagliptin is an oral, highly‐selective DPP‐4 inhibitor for the treatment of type 2 diabetes mellitus that has been shown to provide significant improvements in key glycemic parameters relative to placebo, and to be generally well tolerated, when used as monotherapy or in combination with other OHAs10.
Voglibose is an α‐glucosidase inhibitor (α‐GI) widely prescribed in Japan for the treatment of patients with type 2 diabetes mellitus. Voglibose primarily inhibits α‐glucosidase enzymes in the brush border of the proximal small intestine, reducing the breakdown of disaccharides and other carbohydrates in the gut, and delaying the absorption of monosaccharides (principally glucose and fructose), resulting in smaller postprandial excursions of glucose and insulin. Voglibose has also been shown to increase total GLP‐1 levels in patients with type 2 diabetes mellitus20.
As sitagliptin and voglibose work through different mechanisms, the glucose‐lowering actions of these agents can be complementary. The present study assessed the efficacy and safety of adding once‐daily sitagliptin to ongoing voglibose therapy in Japanese patients with type 2 diabetes mellitus.
Materials and Methods
Patients
Men and women aged ≥20 years with type 2 diabetes mellitus receiving voglibose treatment (0.2–0.3 mg three times daily) as monotherapy or in combination with other OHAs were eligible to participate in the study. The main exclusion criteria included: type 1 diabetes, recent treatment with insulin or pioglitazone, presence of progressive diabetic complications, unstable cardiovascular disease or uncontrolled severe hypertension, increased serum creatinine (>1.5 mg/dL in men, >1.3 mg/dL in women) or increased alanine aminotransferase or aspartate aminotransferase more than twofold the upper limit of normal, hemoglobin <11.0 g/dL in men or <10.0 g/dL in women, or body mass index (BMI) <18 kg/m2 or >40 kg/m2.
Study Design and Procedures
The present randomized clinical trial was carried out at 26 sites in Japan. After an initial 12‐week, placebo‐controlled, double‐blind treatment period that assessed the primary efficacy hypothesis, patients entered a 40‐week, open‐label treatment period during which all patients received sitagliptin.
Patients on a stable regimen of diet/exercise for at least 6 weeks and a stable dose of voglibose for at least 10 weeks (final 6 or more weeks as monotherapy), and who met all eligibility criteria including a glycated hemoglobin (HbA1c) value ≥6.9% and <10.5% (presented as HbA1c [National Glycohemoglobin Standardization Program (NGSP)] by conversion from HbA1c [Japan Diabetes Society (JDS)] that was originally used in the study with the formula HbA1c [NGSP] [%] = 1.02 × HbA1c [JDS] [%] + 0.25%)21, could directly enter a 2‐week, single‐blind, placebo run‐in period. Otherwise, patients on combination therapy with voglibose for at least 4 weeks and other OHAs, with HbA1c values ≥6.4% and ≤9.4%, and who met all other eligibility criteria, could enter the placebo run‐in period after a 6‐week wash‐off of non‐voglibose OHAs. This ensured that all patients received at least 8 weeks of diet/exercise therapy and at least 12 weeks of voglibose therapy at a stable dose (final 8 or more weeks as monotherapy) before randomization. All patients were instructed not to change their diet and exercise program for the duration of the study.
Patients with a HbA1c ≥6.9% and <10.5%, and a fasting plasma glucose (FPG) of ≤270 mg/dL just before initiating the placebo run‐in, and ≥75% treatment compliance during the placebo run‐in, were randomized (1:1) to sitagliptin 50 mg/day or matching placebo for 12 weeks in a double‐blind fashion, using a computer‐generated allocation schedule.
On completion of the double‐blind period, patients entered a 40‐week, open‐label period. Patients who received sitagliptin during the double‐blind period continued to do so in the open‐label period (S/S group). Patients who received a placebo in the double‐blind period were started on sitagliptin 50 mg/day on entry to the open‐label period (P/S group). All patients, regardless of treatment group, were to have their sitagliptin dose increased to 100 mg/day if FPG was ≥140 mg/dL on or after week 16, or if HbA1c was ≥7.4% on or after week 24; the dose of sitagliptin was to remain stable after week 40 and until study end. Patients were to be considered for discontinuation from the study for consistent (on two consecutive determinations) FPG levels >270 mg/dL at any time during the study, or for consistent HbA1c levels >8.4% at or after week 40.
Meal tolerance tests were carried out at weeks 0, 12 and 52, starting 30 min after administration of sitagliptin or the placebo (at week 0 patients received a dose of matching placebo), and just after administration of voglibose with blood samples drawn at 0, 0.5, 1 and 2 h after beginning the meal; the test meal contained approximately 500 kcal (60% carbohydrate, 15% protein, 25% fat).
The present study was designed and carried out in accordance with the guidelines on good clinical practice and ethical principles stated in the Declaration of Helsinki. The study protocol was approved by the institutional review board at each study site, and all patients provided written informed consent.
Study End‐points
A change from baseline in HbA1c at week 12 was the primary efficacy end‐point, and changes from baseline in FPG and 2‐h postmeal glucose (2‐h PMG) at week 12 were secondary end‐points. In the open‐label period, HbA1c, FPG and 2‐h PMG were assessed as exploratory end‐points. Additionally, fasting 1,5‐anhydroglucitol (1,5‐AG), insulin, homeostasis model assessment of β‐cell function (HOMA‐β), homeostasis model assessment of insulin resistance (HOMA‐IR)22, 2‐h postmeal insulin and C‐peptide, PMG area under the concentration‐versus‐time curve (AUC), insulin AUC, C‐peptide AUC and insulinogenic index23 were assessed as exploratory end‐points at weeks 12 and 52. The proportions of patients with HbA1c values <7.4% and <6.9% (corresponding to 7.0 and 6.5% in HbA1c [JDS], respectively), were also assessed at weeks 12 and 52. In addition, AUCs of postmeal total and active GLP‐1 were assessed at weeks 12 and 52.
Adverse experiences (AEs) were monitored throughout the study up to 2 weeks post‐treatment, and were rated by investigators as to their intensity and relationship to the study drug. Hypoglycemia and selected gastrointestinal (GI) AEs (nausea, vomiting, diarrhea) were predefined for additional analyses. Hypoglycemia was diagnosed by the investigators based on their assessment of patients' reports. Safety and tolerability were also assessed by physical examination, monitoring of vital signs, electrocardiogram and safety laboratory tests that included hematology, serum chemistry and urinalysis.
All laboratory assays were carried out at one central laboratory (Mitsubishi Chemical Medience Corporation, Tokyo, Japan).
Statistical Analysis
Efficacy
Efficacy was assessed on the full analysis set (FAS) population that included all randomized patients who took at least one dose of the study drug and had a baseline measurement or at least one measurement post‐randomization. The treatment groups were compared for continuous efficacy parameters using a constrained longitudinal data analysis (cLDA) model24, focusing on a change from baseline at week 12, with treatment group, time (categorical variable), interaction of time by treatment, prior OHA status other than voglibose (on or not on), and interaction of prior OHA status by treatment as covariates and with a restriction of the same baseline mean across treatment groups (due to randomization). Missing values were not explicitly imputed. The between‐group difference in least squares (LS) mean and 95% confidence intervals (CIs) were estimated with an alpha level of <0.05 (two‐sided) considered statistically significant. The proportions of patients with HbA1c values <7.4% and <6.9% at week 12 were analyzed using a logistic regression model that included treatment group, prior OHA status and baseline HbA1c as covariates. For a logistic regression analysis, measurements at baseline and post‐randomization (at least one) were required.
For longer‐term assessment of efficacy, summary statistics for efficacy end‐points were provided by treatment group (P/S or S/S) at each time‐point in which the end‐point was measured up to week 52; missing values were not imputed. At week 52, the within‐group mean change from baseline (i.e., week 0) for all efficacy end‐points was assessed using a paired t‐test. For P/S, the comparisons versus baseline (week 0) were carried out post‐hoc.
The effect of increasing the dose of sitagliptin to 100 mg/day was assessed (post‐hoc). For patients whose sitagliptin dose was increased and whose HbA1c value at the time of uptitration was ≥7.4%, the proportion of patients with HbA1c values <7.4% 12 weeks after uptitration was tabulated. Additionally, for patients whose sitagliptin dose was increased and who completed the study, the proportion of patients with HbA1c values <7.4% at week 52 was also assessed.
For assessment of GLP‐1 in the double‐blind period, the AUCs (calculated by linear trapezoidal rule) during the meal tolerance test were analyzed using the cLDA model described earlier. Summary statistics of AUCs were provided by treatment group at each time‐point up to week 52.
Safety
Safety and tolerability were assessed on the all‐patients‐as‐treated (APaT) population, which included randomized patients who received at least one dose of the double‐blind study drug. In the double‐blind period, between‐group comparisons using Fisher's exact test were carried out for occurrences of overall (one or more) AEs, drug‐related AEs, hypoglycemia and prespecified GI AEs. The analysis of change from baseline in bodyweight was carried out (post‐hoc) using a cLDA model similar to that used for the efficacy end‐points. For laboratory tests, vital signs and bodyweight up to week 12, summary statistics were provided and within‐group changes from baseline were assessed using a paired t‐test.
Longer‐term safety was assessed in all patients who received at least one dose of sitagliptin in the open‐label portion of the study (i.e., from weeks 12 to 52). The occurrences of overall AEs, drug‐related AEs, hypoglycemia and selected GI AEs were summarized. For laboratory tests and vital signs, summary statistics were generated up to week 52 for each group individually. Within‐group mean change from baseline in bodyweight was also assessed using a paired t‐test at week 52.
Results
Of the 187 patients screened, 133 were randomized (sitagliptin 70, placebo 63; Figure 1). Demographic, anthropometric and disease characteristics were generally similar in both treatment groups, except for a greater proportion of females in the sitagliptin group (40%) compared with the placebo group (29%) (Table S1). At baseline, patients had a mean HbA1c of 7.9%, a mean FPG of 152.1 mg/dL, the average duration of known diabetes was 7.2 years and the mean BMI was 24.1 kg/m2.
Figure 1.
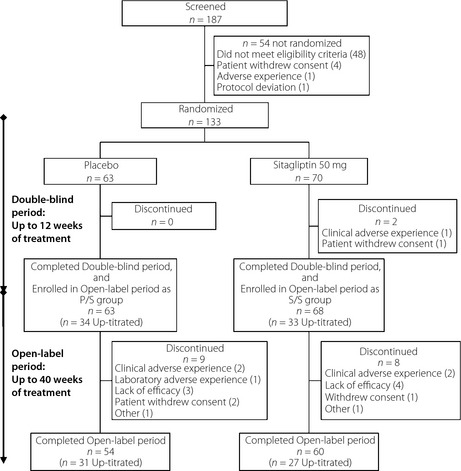
Patient disposition. Patients who received placebo during the double‐blind period and open‐label sitagliptin in the open‐label period (P/S group). Patients who received sitagliptin in both periods (S/S group).
A total of 131 patients completed the double‐blind period and entered the open‐label period; of those, 114 patients subsequently completed the open‐label period (Figure 1). All randomized patients were included in both the FAS and the APaT populations for analysis.
Efficacy
Double‐Blind Period (Weeks 0 Through 12)
Sitagliptin as add‐on to voglibose therapy resulted in a significant (P < 0.001) reduction from baseline in HbA1c compared with the placebo at week 12 (Table 1, Figure 2), with a between‐group difference in LS‐mean, −0.9%. At week 12, a significantly greater proportion of patients in the sitagliptin group relative to the placebo group had HbA1c values <7.4% (79.7 vs 30.2%, respectively; P < 0.001) and <6.9% (47.8 vs 11.1%, respectively; P < 0.001). Patients with baseline HbA1c values ≤8.4% and >8.4% had between‐group mean HbA1c changes from baseline of −0.8 and −1.4%, respectively.
Table 1. Results for fasting and postmeal glycemic end‐points at week 12.
| n | Week 0 mean (SD) | Week 12 mean (SD) | Change from week 0 –12 (LS‐mean [95% CI]) | Between‐group difference (LS‐mean [95% CI]) | |
|---|---|---|---|---|---|
| HbA1c (%) | |||||
| Placebo | 63 | 7.9 (0.9) | 8.0 (1.0) | 0.2 (0.0, 0.3) | −0.9 (−1.1, −0.8)*** |
| Sitagliptin | 70 | 7.9 (0.8) | 7.1 (0.7) | −0.8 (−0.9, −0.6) | |
| Fasting plasma glucose (mg/dL) | |||||
| Placebo | 63 | 151.5 (30.6) | 153.4 (34.0) | −0.1 (‐6.4, 6.2) | −22.5 (−30.0, −15.0)*** |
| Sitagliptin | 70 | 152.7 (37.1) | 131.8 (27.7) | −22.6 (−28.5, −16.8) | |
| 1,5‐Anhydroglucitol (μg/mL) | |||||
| Placebo | 63 | 11.2 (7.7) | 11.6 (8.6) | 0.1 (−1.1, 1.3) | 6.1 (4.6, 7.6)*** |
| Sitagliptin | 70 | 10.5 (6.7) | 16.9 (8.7) | 6.2 (5.1, 7.3) | |
| Fasting insulin (μU/mL) | |||||
| Placebo | 63 | 5.7 (3.2) | 5.6 (3.6) | −0.1 (−0.8, 0.5) | 0.2 (−0.6, 1.0) |
| Sitagliptin | 70 | 5.4 (3.7) | 5.4 (3.6) | 0.0 (‐0.6, 0.6) | |
| HOMA‐IR | |||||
| Placebo | 63 | 2.1 (1.3) | 2.2 (1.7) | 0.0 (−0.3, 0.3) | −0.3 (−0.6, 0.1) |
| Sitagliptin | 70 | 2.0 (1.3) | 1.8 (1.2) | −0.2 (−0.5, 0.1) | |
| HOMA‐β | |||||
| Placebo | 63 | 25.6 (17.1) | 24.1 (15.2) | −2.9 (−6.4, 0.6) | 9.0 (4.6, 13.3)*** |
| Sitagliptin | 70 | 24.5 (20.3) | 31.6 (23.2) | 6.1 (2.8, 9.3) | |
| 2‐h Postmeal glucose (mg/dL) | |||||
| Placebo | 63 | 209.4 (52.4) | 210.1 (49.7) | −4.0 (−13.5, 5.5) | −51.3 (−62.3, −40.2)*** |
| Sitagliptin | 70 | 218.0 (53.2) | 164.4 (40.3) | −55.3 (−64.2, −46.3) | |
| 2‐h Postmeal insulin (μU/mL) | |||||
| Placebo | 63 | 25.4 (13.9) | 26.0 (11.7) | 1.9 (−1.2, 5.0) | −2.8 (−6.5, 0.9) |
| Sitagliptin | 70 | 26.9 (18.1) | 24.4 (16.3) | −0.9 (−3.8, 2.0) | |
| 2‐h Postmeal C‐peptide (ng/mL) | |||||
| Placebo | 63 | 4.3 (1.5) | 4.1 (1.3) | –0.0 (−0.3, 0.3) | −0.1 (−0.5, 0.2) |
| Sitagliptin | 70 | 4.2 (1.7) | 3.9 (1.4) | −0.1 (−0.4, 0.1) | |
| Glucose AUC (mg h/dL) | |||||
| Placebo | 63 | 387.7 (75.4) | 388.4 (77.6) | −5.1 (−19.1, 8.9) | −78.0 (−94.5, −61.5)*** |
| Sitagliptin | 70 | 395.5 (83.9) | 316.4 (60.6) | −83.1 (−96.2, −70.0) | |
| Insulin AUC (μU h/mL) | |||||
| Placebo | 63 | 40.1 (20.3) | 40.1 (18.0) | 1.5 (−2.0, 5.1) | 0.3 (−4.1, 4.6) |
| Sitagliptin | 70 | 39.9 (26.0) | 40.2 (24.9) | 1.8 (−1.5, 5.1) | |
| C‐peptide AUC (ng h/mL) | |||||
| Placebo | 63 | 6.2 (2.2) | 6.0 (1.9) | −0.0 (−0.3, 0.3) | 0.1 (−0.2, 0.5) |
| Sitagliptin | 70 | 6.0 (2.3) | 5.9 (2.1) | 0.1 (−0.2, 0.4) | |
| Insulinogenic index (μU/mL)/(mg/dL) | |||||
| Placebo | 63 | 0.4 (0.3) | 0.6 (0.9) | 0.2 (−0.0, 0.4) | −0.0 (−0.3, 0.3) |
| Sitagliptin | 70 | 0.4 (0.5) | 0.6 (0.8) | 0.2 (−0.0, 0.4) | |
| Active GLP‐1 AUC (pmol h/L) | |||||
| Placebo | 62 | 15.8 (0.4) | 14.2 (0.4) | 0.9 (0.8, 1.0)[Link] | 2.5 (2.2, 2.8)[Link],*** |
| Sitagliptin | 68 | 18.6 (0.4) | 39.2 (0.5) | 2.2 (2.0, 2.4)[Link] | |
| Total GLP‐1 AUC (pmol h/L) | |||||
| Placebo | 62 | 26.1 (0.4) | 25.8 (0.4) | 1.0 (0.9, 1.1)[Link] | 0.8 (0.7, 0.9)[Link],*** |
| Sitagliptin | 68 | 28.5 (0.4) | 21.9 (0.4) | 0.8 (0.7, 0.8)[Link] | |
*P < 0.05, **P < 0.01, ***P < 0.001. †Geometric least squares (LS) mean ratio. ‡Ratio of geometric LS mean ratios. For the parameters related to glucagon‐like peptide‐1 (GLP‐1), the values were log‐transformed before analysis and the back‐transformed results were reported. AUC, total area under the concentration‐versus‐time curve; CI, confidence interval; HbA1c, glycated hemoglobin; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; SD, standard deviation.
Figure 2.
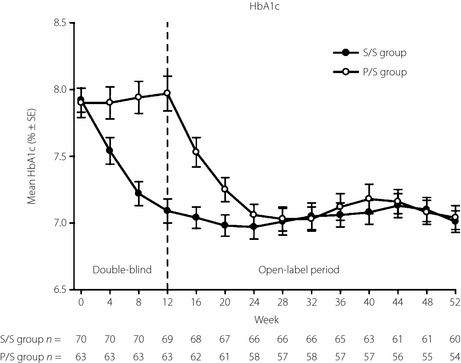
Time course of glycated hemoglobin (HbA1c) in Japanese patients with type 2 diabetes mellitus treated with double‐blind sitagliptin 50 mg/day or the placebo added to voglibose for the first 12 weeks and open‐label sitagliptin 50 or 100 mg/d added to voglibose for the subsequent 40 weeks. The data are values for mean ± standard error (SE), and results from the patients who received the placebo during the double‐blind period and open‐label sitagliptin in the open‐label period (P/S group) and patients who received sitagliptin in both periods (S/S group) treatment groups are shown with open and closed circles, respectively. Statistics for HbA1c were calculated using at each time‐point the data available for that specific time‐point. The sample sizes at each time‐point are shown beneath the plots.
Significant improvements (P < 0.001) in both FPG and 2‐h PMG were also observed with sitagliptin treatment at week 12, with between‐group differences in LS‐mean changes from baseline of −22.5 and −51.3 mg/dL, respectively (Table 1).
Consistent results were observed in analyses of changes from baseline in other efficacy parameters at week 12 that were supportive of the primary and secondary findings, including significant improvement with sitagliptin treatment in HOMA‐β and 1,5‐AG (Table 1).
Sitagliptin increased active GLP‐1, decreased total GLP‐1 compared with the placebo at week 12 (Table 1).
Open‐Label Period (Weeks 12 Through 52)
In the open‐label period, HbA1c, FPG and 2‐h PMG improved from baseline through week 52 in the S/S and P/S groups (all P < 0.001; Table 2, Figures S1–S3). Similarly, in both the S/S and P/S groups at week 52, significant changes (P < 0.05) from baseline were observed in other efficacy parameters (i.e. 1,5‐AG, HOMA‐β, fasting‐insulin, postmeal AUC of glucose, postmeal AUC of insulin and the insulinogenic index; Table 2).
Table 2. Results for fasting and postmeal glycemic end‐points at week 52.
| n | Week 0 Mean (SD) | Week 52 mean (SD) | Change from week 0 (baseline) to week 52 (mean [95% CI]) | |
|---|---|---|---|---|
| HbA1c (%) | ||||
| P/S | 54 | 7.8 (0.8) | 7.0 (0.7) | −0.8 (−1.0, −0.6)*** |
| S/S | 60 | 7.8 (0.7) | 7.0 (0.7) | −0.8 (−1.0,−0.7)*** |
| Fasting plasma glucose (mg/dL) | ||||
| P/S | 54 | 150.5 (31.2) | 135.6 (25.1) | −14.9 (−20.6, −9.3)*** |
| S/S | 60 | 149.9 (34.3) | 128.0 (20.9) | −21.9 (−28.0, −15.7)*** |
| 1,5‐anhydroglucitol (μg/mL) | ||||
| P/S | 54 | 11.6 (8.0) | 16.8 (9.1) | 5.1 (3.7, 6.5)*** |
| S/S | 60 | 11.0 (6.9) | 17.1 (9.2) | 6.1 (4.6, 7.5)*** |
| Fasting insulin (μU/mL) | ||||
| P/S | 54 | 5.4 (3.1) | 6.5 (3.9) | 1.0 (0.4, 1.7)** |
| S/S | 60 | 5.2 (3.5) | 6.2 (4.0) | 1.0 (0.4, 1.6)** |
| HOMA‐IR | ||||
| P/S | 54 | 2.0 (1.2) | 2.2 (1.5) | 0.2 (−0.1, 0.4) |
| S/S | 60 | 1.9 (1.3) | 2.0 (1.3) | 0.1 (−0.2, 0.3) |
| HOMA‐β | ||||
| P/S | 54 | 25.0 (17.6) | 34.1 (19.0) | 9.2 (5.2, 13.1)*** |
| S/S | 60 | 23.7 (17.4) | 37.0 (26.8) | 13.3 (9.5, 17.1)*** |
| 2‐h Postmeal glucose (mg/dL) | ||||
| P/S | 54 | 207.1 (51.3) | 164.3 (41.3) | −42.9 (−52.8, −33.0)*** |
| S/S | 60 | 210.2 (48.8) | 154.7 (34.2) | −55.5 (−66.0, −44.9)*** |
| 2‐h Postmeal insulin (μU/mL) | ||||
| P/S | 54 | 25.4 (14.4) | 27.6 (14.5) | 2.1 (−1.4, 5.7) |
| S/S | 60 | 26.6 (18.2) | 26.8 (15.8) | 0.2 (−2.6, 3.0) |
| 2‐h C‐peptide (ng/mL) | ||||
| P/S | 54 | 4.3 (1.6) | 4.2 (1.6) | −0.1 (−0.4, 0.3) |
| S/S | 60 | 4.2 (1.7) | 3.9 (1.4) | −0.3 (−0.6, −0.1)* |
| Glucose AUC (mg h/dL) | ||||
| P/S | 54 | 384.7 (73.9) | 323.6 (61.5) | −61.1 (−75.8, −46.4)*** |
| S/S | 60 | 383.6 (77.4) | 301.7 (52.0) | −81.8 (−96.7, −66.9)*** |
| Insulin AUC (μU h/mL) | ||||
| P/S | 54 | 39.5 (20.5) | 47.5 (25.1) | 7.9 (3.5, 12.4)*** |
| S/S | 60 | 39.8 (26.2) | 43.7 (26.3) | 3.9 (0.3, 7.6)* |
| C‐peptide AUC (ng h/mL) | ||||
| P/S | 54 | 6.2 (2.3) | 6.3 (2.5) | 0.1 (−0.3, 0.5) |
| S/S | 60 | 5.9 (2.3) | 5.7 (2.1) | −0.2 (−0.5, 0.1) |
| Insulinogenic index (μU/mL)/(mg/dL) | ||||
| P/S | 54 | 0.4 (0.3) | 0.7 (1.0) | 0.3 (0.1, 0.5)** |
| S/S | 60 | 0.5 (0.5) | 0.8 (0.7) | 0.3 (0.1, 0.5)** |
| Active GLP‐1 AUC (pmol h/L) | ||||
| P/S | 54 | 16.0 (0.4) | 33.8 (0.5) | 2.1 (1.9, 2.3)[Link],*** |
| S/S | 59 | 18.0 (0.4) | 37.3 (0.5) | 2.1 (1.9, 2.3)[Link],*** |
| Total GLP‐1 AUC (pmol h/L) | ||||
| P/S | 54 | 26.2 (0.4) | 20.4 (0.4) | 0.8 (0.7, 0.8)[Link],*** |
| S/S | 59 | 27.7 (0.4) | 22.5 (0.4) | 0.8 (0.7, 0.9)[Link],*** |
*P < 0.05, **P < 0.01, ***P < 0.001. †Geometric mean ratio. For parameters related to glucagon‐like peptide‐1 (GLP‐1), the values were log‐transformed before analysis and the back‐transformed results were reported. AUC, total area under the concentration‐versus‐time curve; CI, confidence interval; HbA1c, glycated hemoglobin; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; P/S, patients who received placebo during the double‐blind period and open‐label sitagliptin in the open‐label period; SD, standard deviation, S/S, patients who received sitagliptin during the double‐blind period and the open‐label period.
In order to provide additional glycemic efficacy, dose titration of sitagliptin to 100 mg was allowed after week 16 for patients meeting predefined glycemic parameters. The sitagliptin dose was up‐titrated in 33 (52.4%) patients in the S/S group and 34 (50.0%) patients in the P/S group. HbA1c values 12 weeks post‐escalation were obtained from a total of 66 patients (S/S group 32 patients, P/S group 34 patients). Overall, 21.2% (7/33) of patients with an HbA1c ≥7.4% before uptitration had HbA1c <7.4% at 12 weeks after uptitration. Additionally, among all patients whose dose was uptitrated, 58 patients completed the study; 62.1% (36/58) of the patients had a HbA1c <7.4% at week 52.
Similarly to week 12, sitagliptin increased active GLP‐1 and decreased total GLP‐1 in both the P/S and S/S treatment groups at week 52.
Safety
Double‐Blind Period (Weeks 0 Through 12)
In the double‐blind period, the occurrences of clinical AEs, laboratory AEs, drug‐related AEs and serious AEs (SAEs) were similar in both treatment groups (Table 3). No SAEs were reported for patients in the sitagliptin group. One patient in the sitagliptin group discontinued because of a clinical AE of urticaria considered by the investigator to be of moderate intensity and related to the study drug. This AE resolved after discontinuation of the study drug.
Table 3. Safety and tolerability results.
| Weeks 0 –12[Link] (double‐blind) | Weeks 12–52 (open‐label) | |||
|---|---|---|---|---|
| Placebo (n = 63) | Sitagliptin (n = 70) | P/S (n = 63) | S/S (n = 68) | |
| No. patients (n [%]) who had one or more | ||||
| Clinical AE | 21 (33.3) | 28 (40.0) | 52 (82.5) | 54 (79.4) |
| Drug‐related[Link] clinical AE | 3 (4.8) | 6 (8.6) | 5 (7.9) | 4 (5.9) |
| Serious clinical AE | 1 (1.6) | 0 | 3 (4.8) | 5 (7.4) |
| Serious drug‐related‡ clinical AE | 0 | 0 | 0 | 0 |
| Number of patients (n [%]) who: | ||||
| Discontinued due to a clinical AE | 0 | 1 (1.4) | 2 (3.2) | 1 (1.5) |
| Discontinued due to a drug‐related‡ clinical AE | 0 | 1 (1.4) | 0 | 0 |
| Discontinued due to a serious clinical AE | 0 | 0 | 1 (1.6) | 1 (1.5) |
| Died | 0 | 0 | 0 | 0 |
| Number of patients (n [%]) who had: | ||||
| Hypoglycemia | 0 | 1 (1.4) | 0 | 1 (1.5) |
| Nausea, vomiting, or diarrhea | 1 (1.6) | 1 (1.4) | 3 (4.8) | 2 (2.9) |
| Number of patients (n [%]) who had one or more: | ||||
| Laboratory AE | 5 (7.9) | 3 (4.3) | 11 (17.5) | 16 (23.5) |
| Serious laboratory AE | 0 | 0 | 0 | 0 |
| Drug‐related‡ laboratory AE | 1 (1.6) | 1 (1.4) | 4 (6.3) | 1 (1.5) |
| Number of patients (n [%]) who: | ||||
| Discontinued due to a laboratory AE | 0 | 0 | 0 | 0 |
| Number of patients (n [%]) who had: | ||||
| Clinical AE§ | ||||
| Nasopharyngitis | 4 (6.3) | 5 (7.1) | 19 (30.2) | 21 (30.9) |
| Cataract | 0 | 2 (2.9) | 1 (1.6) | 5 (7.4) |
| Erosive gastritis | 0 | 0 | 0 | 4 (5.9) |
| Back pain | 1 (1.6) | 1 (1.4) | 4 (6.3) | 4 (5.9) |
| Eczema | 2 (3.2) | 1 (1.4) | 2 (3.2) | 4 (5.9) |
| Laboratory AE§ | ||||
| Blood triglycerides increased | 0 | 1 (1.4) | 3 (4.8) | 5 (7.4) |
| Blood creatine phosphokinase increased | 1 (1.6) | 1 (1.4) | 1 (1.6) | 4 (5.9) |
†Fisher's exact test was used to test the significance of differences in weeks 0 –12 between numbers of patients in the sitagliptin and placebo groups reported to have one or more clinical (or laboratory) adverse experience (AE) overall, drug‐related clinical (or laboratory) AE, occurrence of hypoglycemia or prespecified gastrointestinal AE (nausea, vomiting and diarrhea). All between‐group differences were non‐significant. ‡Considered to be possibly, probably or definitely treatment‐related by the study investigators. §Specific AEs for which there was a ≥5% occurrence in either the sitagliptin or placebo group in the double‐blind period (from week 0 to 12), patients who received placebo during the double‐blind period and open‐label sitagliptin in the open‐label period (P/S) or patients who received sitagliptin during the double‐blind period and the open‐label period (S/S) group in the open‐label period (from week 12 to 52).
One episode of hypoglycemia was reported for one patient in the sitagliptin group and none in the placebo group. This event was mild in intensity, lasted 20 min and did not lead to discontinuation. Predefined GI AEs were also reported at low and similar occurrences in both groups.
Nasopharyngitis was the only specific AE reported with a frequency ≥5% in either treatment group (7.1% in the sitagliptin group and 6.3% in the placebo group), regardless of causality (Table 3). All events in both groups were mild in intensity and resolved while the patient was on study medication.
The addition of sitagliptin or the placebo to ongoing therapy with voglibose resulted in mean changes from baseline in bodyweight of −0.4 and −0.5 kg, respectively. No meaningful changes in other safety parameters were observed in either treatment group.
Open‐Label Period (Weeks 12 Through 52)
Consistent with the longer period of observation in a patient population with type 2 diabetes mellitus, one or more clinical AEs were reported by most patients in both the S/S and P/S groups during the open‐label period (Table 3). Clinical AEs reported with an occurrence ≥5% in either the S/S or P/S group included nasopharyngitis, cataract, erosive gastritis, back pain and eczema. Drug‐related clinical AEs were reported in 5.9 and 7.9% of patients in the S/S and P/S groups, respectively; with the exception of vertigo (2 patients, 1.5%; mild or moderate in intensity) in the P/S group, none of these events were reported to occur in more than one patient.
SAEs were reported for five patients in the S/S group and three patients in the P/S group; all were considered not drug‐related by the investigator, and no specific SAE was reported to occur in more than one patient. The same patient in the S/S group for whom an AE of mild hypoglycemia was reported during the double‐blind period was reported to have an episode of hypoglycemia during the open‐label period, which was considered mild in intensity and did not lead to discontinuation. No other patients reported episodes of hypoglycemia throughout the study. The occurrence of GI AEs remained low through 52 weeks.
Laboratory AEs were reported in 23.5% of patients in the S/S and in 17.5% of patients in the P/S group; none was serious, none led to discontinuation and few were considered to be drug related by the investigator (Table 3).
The occurrences of both clinical and laboratory AEs did not change with uptitration of sitagliptin. The overall occurrence of clinical AE was similar between patients whose sitagliptin dose was uptitrated to 100 mg/day (n = 67) and the total patients (n = 131). The occurrences of laboratory AE were similar as well. Of the patients who had uptitration of sitagliptin dose to 100 mg/day, only one patient (S/S group) was discontinued from the study due to a SAE of colon cancer, which was considered to be not drug related by the investigator. In addition, other SAEs of gastric cancer and cataract were reported for one patient each (both in the S/S group) during the post uptitration phase; both were considered to be not drug related by the investigator.
At week 52, the observed mean changes from baseline in bodyweight were 0.03 kg (P = 0.887) in the S/S group and 0.2 kg (P = 0.425) in the P/S group. Overall, there were no meaningful changes from baseline in vital signs and laboratory values in either treatment group during the long‐term safety assessment.
Discussion
The addition of sitagliptin for 12 weeks provided significant reductions in HbA1c relative to the placebo in patients with type 2 diabetes mellitus receiving voglibose monotherapy. At week 12, the proportions of patients with HbA1c values <7.4 and <6.9% with sitagliptin treatment were 2.6‐ and 4.3‐fold larger, respectively, compared with the placebo. Additionally, changes observed in FPG, 2‐h PMG and other efficacy parameters were also significant, supporting the primary efficacy hypotheses. The improvements in HOMA‐β are suggestive of improved insulin secretion in the fasting state.
The efficacy of sitagliptin as shown by changes in HbA1c, FPG and 2‐h PMG remained stable through the 52‐week study period. Furthermore, improvements in insulin secretion over 52 weeks were reflected by within‐group changes in HOMA‐β, insulin AUC and insulinogenic index. The improvement in glycemic parameters with sitagliptin for up to 52 weeks as add‐on to voglibose monotherapy is consistent with results from prior studies of sitagliptin as monotherapy,10 and as add‐on to metformin11, pioglitazone16 and glimepiride12 in both in Japanese and non‐Japanese patients. The present study also showed that sitagliptin increased active GLP‐1, while decreasing total GLP‐1, potentially due to the negative feedback by the enhancement of the active form. These results are consistent with findings from previous studies of sitagliptin10. However, as α‐GIs enhance total GLP‐1 secretion, complementary effects on GLP‐1 might exist when sitagliptin is added to voglibose.
The addition of sitagliptin to voglibose monotherapy was generally well tolerated during the double‐blind and open label study periods. Prior studies have shown that DPP‐4 inhibitors and α‐GIs are associated with a low risk of hypoglycemia and generally neutral effects on bodyweight6. Unlike DPP‐4 inhibitors, GI AEs are known to occur at a high frequency after treatment with α‐GIs25. In the present study, sitagliptin added to ongoing therapy with an α‐GI was not associated with an increase of GI AEs. Additionally, consistent with the glucose‐dependent action of incretins9, hypoglycemia was reported in a single patient in the 52 weeks of study treatment. The changes from baseline in bodyweight at week 12 and week 52 were not statistically significant and not considered to be clinically meaningful.
In patients with type 2 diabetes mellitus inadequately controlled with diet, exercise and ongoing voglibose therapy, the addition of sitagliptin 50 mg/day after 12 weeks of treatment resulted in a significant improvement in glycemic parameters relative to the placebo, consistent with the observed improvements in active GLP‐1 with sitagliptin therapy. These improvements remained stable throughout 52 weeks of treatment. In addition, the findings in the present study suggest that sitagliptin uptitration to 100 mg/day provides further opportunity for patients to meet glycemic goals. The addition of sitagliptin to voglibose was generally well tolerated, with a low occurrence of hypoglycemia and GI AEs, and with no clinically meaningful effect on bodyweight.
Supplementary Material
Figure S1 | Time course of fasting plasma glucose (FPG).
Figure S2 | Time course of meal tolerance results in P/S group.
Figure S3 | Time course of meal tolerance results in S/S group.
Table S1 | Baseline patient characteristics of randomized patients.
Appendix S1 | MK‐0431/ONO5435, P104 Primary Investigators listing.
Acknowledgments
The authors wish to thank the study investigators (Appendix S1) for their contributions to the execution of this study, and Christine McCrary Sisk and Jennifer Rotonda (employees of Merck Sharp and Dohme Corp.) for editorial assistance.
This study was sponsored by MSD KK, a subsidiary of Merck & Co., Inc., the manufacturer of sitagliptin, and by Ono Pharmaceutical Co. Ltd.
N Tajima and T Kadowaki were advisory board members for this study. T Okamoto, A Sato, K Okuyama and JC Arjona Ferreira all declare that they are/were full‐time employees of MSD KK or Merck Sharp & Dohme Corp. at the time of the study, and might potentially own stock and/or hold stock options in the company. T Minamide is an employee of Ono Pharmaceutical Co. Ltd.
(J Diabetes Invest, doi: 10.1111/jdi.12116, 2013)
Portions of this research were presented at the American Diabetes Association 70th Scientific Sessions, 25–29, June 2010, Orlando, Florida, USA
References
- 1.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–865 [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 4.Cook MN, Girman CJ, Stein PP, et al Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med 2007; 24: 350–358 [DOI] [PubMed] [Google Scholar]
- 5.Herman GA, Stein PP, Thornberry NA, et al Dipeptidyl peptidase‐4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther 2007; 81: 761–767 [DOI] [PubMed] [Google Scholar]
- 6.Karasik A, Aschner P, Katzeff H, et al Sitagliptin, a DPP‐4 inhibitor for the treatment of patients with type 2 diabetes: a review of recent clinical trials. Curr Med Res Opin 2008; 24: 489–496 [DOI] [PubMed] [Google Scholar]
- 7.Herman GA, Bergman A, Stevens C, et al Effect of single oral doses of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006; 91: 4612–4619 [DOI] [PubMed] [Google Scholar]
- 8.Herman GA, Bergman A, Liu F, et al Pharmacokinetics and pharmacodynamic effects of the oral DPP‐4 inhibitor sitagliptin in middle‐aged obese subjects. J Clin Pharmacol 2006; 46: 876–886 [DOI] [PubMed] [Google Scholar]
- 9.Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007; 132: 2131–2157 [DOI] [PubMed] [Google Scholar]
- 10.Aschner P, Kipnes MS, Lunceford JK, et al Effect of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29: 2632–2637 [DOI] [PubMed] [Google Scholar]
- 11.Charbonnel B, Karasik A, Liu J, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638–2643 [DOI] [PubMed] [Google Scholar]
- 12.Hermansen K, Kipnes M, Luo E, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9: 733–745 [DOI] [PubMed] [Google Scholar]
- 13.Nonaka K, Kakikawa T, Sato A, et al Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79: 291–298 [DOI] [PubMed] [Google Scholar]
- 14.Raz I, Hanefeld M, Xu L, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49: 2564–2671 [DOI] [PubMed] [Google Scholar]
- 15.Raz I, Chen Y, Wu M, et al Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24: 537–550 [DOI] [PubMed] [Google Scholar]
- 16.Rosenstock J, Brazg R, Andryuk PJ, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clin Ther 2006; 28: 1556–1568 [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto Y, Taniguchi T, Nonaka K, et al Dose‐ranging efficacy of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J 2010; 57: 383–394 [DOI] [PubMed] [Google Scholar]
- 18.Kashiwagi A, Kadowaki T, Tajima N, et al Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Invest 2011; 2: 381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajima N, Kadowaki T, Odawara M, et al Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int 2011; 2: 32–44 [Google Scholar]
- 20.Goke B, Fuder H, Wieckhorst G, et al Voglibose (AO‐128) is an efficient alpha‐glucosidase inhibitor and mobilizes endogenous GLP‐1 reserve. Digestion 1995; 56: 493–501 [DOI] [PubMed] [Google Scholar]
- 21.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 23.Kosaka K, Hagura R, Kuzuya T, et al Insulin secretory response of diabetics during the period of improvement of glucose tolerance to normal range. Diabetologia 1974; 10: 775–782 [DOI] [PubMed] [Google Scholar]
- 24.Liang K, Zeger S. Longitudinal data analysis of continuous and discrete responses for pre‐post designs. Sankhya A 2000; 62: 134–148 [Google Scholar]
- 25.Iwamoto Y, Tajima N, Kadowaki T, et al Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double‐blind trial. Diabetes Obes Metab 2010; 12: 613–622 [DOI] [PubMed] [Google Scholar]
- 26.Odawara M, Kadowaki T, Tajima N, et al Long‐term safety, tolerability, and efficacy of the dipeptidyl peptidase‐4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetol Int 2011; 2: 94–105 [Google Scholar]
- 27.Barnett A, Allsworth J, Jameson K, et al A review of the effects of antihyperglycaemic agents on body weight: the potential of incretin targeted therapies. Curr Med Res Opin 2007; 23: 1493–1507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Time course of fasting plasma glucose (FPG).
Figure S2 | Time course of meal tolerance results in P/S group.
Figure S3 | Time course of meal tolerance results in S/S group.
Table S1 | Baseline patient characteristics of randomized patients.
Appendix S1 | MK‐0431/ONO5435, P104 Primary Investigators listing.


