Abstract
Introduction
Insulin degludec (IDeg) is an ultra‐long‐acting basal insulin with a consistent action profile of >42 h. This trial compared the efficacy and safety of IDeg with insulin glargine (IGlar) in insulin‐naïve Asian patients with type 2 diabetes.
Materials and Methods
In this multinational, 26‐week, open‐label, treat‐to‐target trial, 435 participants (202 females, 233 males; mean age 58.6 years; mean body mass index 25 kg/m2; mean glycated hemoglobin [HbA1c] 8.5%) were randomized (2:1) to IDeg or IGlar, each administered once daily with ≥1 oral antidiabetic drug(s) (OAD).
Results
After 26 weeks, HbA1c had decreased by 1.24 and 1.35% in the IDeg and IGlar groups, respectively (treatment difference [IDeg – IGlar] 0.11%, 95% confidence interval [CI] −0.03 to 0.24), confirming non‐inferiority. Rates of overall confirmed hypoglycemia were similar for IDeg and IGlar during the full trial period (3.0 vs 3.7 episodes/patient‐year of exposure [PYE]; rate ratio [RR] 0.82, 95% CI 0.60 to 1.11, P = 0.20), but significantly lower (by 37%) for IDeg during the maintenance period (from week 16 onward; RR 0.63, 95% CI 0.42 to 0.94, P = 0.02). No significant difference in the rate of nocturnal confirmed hypoglycemia was found between IDeg and IGlar in the full trial period (0.8 vs 1.2 episodes/PYE; RR 0.62, 95% CI 0.38 to 1.04, P = 0.07) or maintenance period (RR 0.52, 95% CI 0.27 to 1.00, P = 0.05). Adverse event rates were similar between treatments.
Conclusions
Initiating insulin therapy with IDeg in Asian patients with type 2 diabetes, inadequately controlled with OADs, provides similar improvements in long‐term glycemic control to IGlar, but at a significantly lower rate of overall confirmed hypoglycemia once stable glycemic control and insulin dosing are achieved. This trial was registered with www.clinicaltrials.gov (no. NCT01059799).
Keywords: Asian, Insulin degludec, Type 2 diabetes
Introduction
Approximately 36% (132 million) of the world's diabetes population is located in the Western Pacific region, where 8.0% of the adult population is currently estimated to have diabetes1. Compared with Caucasians, Asian patients with type 2 diabetes tend to be characterized more by impaired insulin secretion than increased insulin resistance2. Because of a progressive decline in pancreatic β‐cell function, most patients ultimately require insulin therapy alone, or in combination with other antidiabetic drugs (OADs), to achieve recommended levels of glycemic control5. Early initiation of insulin treatment is increasingly advocated to achieve glycemic targets6, with studies showing that intensive insulin therapy can counter β‐cell deterioration in patients newly diagnosed with type 2 diabetes8.
For many patients, insulin treatment is typically initiated through the use of intermediate‐ or long‐acting basal insulin. The long‐acting basal insulin analogs, insulin glargine (IGlar) and insulin detemir, mimic endogenous basal insulin action more closely than human insulin preparations, such as neutral protamine Hagedorn (NPH) insulin, and they are associated with lower rates of hypoglycemia9, a major barrier to the timely introduction and effective use of insulin10. However, despite these and other advances in diabetes management, many patients remain unable to meet the recommended levels of glycemic control11. Therefore, there continues to be a need for basal insulins with improved pharmacokinetic and pharmacodynamic properties that would allow more patients to reach and maintain glycemic targets at an even lower risk of hypoglycemia.
Insulin degludec (IDeg) is an ultra‐long‐acting basal insulin analog that is a full agonist at the insulin receptor, with a low affinity for the insulin‐like growth factor‐1 receptor (~2% of human insulin) and low mitogenic potency relative to human insulin15. On subcutaneous injection, IDeg forms a depot of soluble multi‐hexamers, resulting in a stable and consistent glucose‐lowering effect of >42 h at a steady state16. These properties are thought to contribute to the lower rates of hypoglycemia observed for IDeg compared with other basal insulin analogs20, and allow for the timing of a once‐daily injection to be varied from day to day, when required, without affecting glycemic control or risk of hypoglycemia23.
Here, we report the results of a phase 3, treat‐to‐target trial that compared the efficacy and safety of IDeg with IGlar, each given once daily, in insulin‐naïve Asian patients with type 2 diabetes inadequately controlled by OADs.
Materials and Methods
Study Design and Participants
The present phase 3, 26‐week, randomized, controlled, open‐label, multicenter, multinational, treat‐to‐target, non‐inferiority trial was carried out at 52 sites in six countries (Hong Kong, Japan, Malaysia, South Korea, Taiwan and Thailand) between February and December 2010. The trial protocol was approved by independent ethics committees or institutional review boards (with written informed consent obtained before patients entered the trial), and carried out according to the Declaration of Helsinki24 and Good Clinical Practice25.
Adults (aged ≥18 years; ≥20 years for Japan) diagnosed with type 2 diabetes for at least 6 months, with a body mass index (BMI) of ≤35 kg/m2, baseline glycated hemoglobin (HbA1c) 7.0–10.0% (both inclusive) and currently being treated with monotherapy or a combination of an insulin secretagogue (sulfonylurea or glinide) and metformin, with or without addition of α‐glucosidase inhibitors or a dipeptidyl peptidase‐4 (DPP‐4) inhibitor, with unchanged dosing for at least 3 months before the screening visit were eligible for enrolment in the trial. Patients were excluded if they were using glucagon‐like peptide‐1 receptor agonists (exenatide or liraglutide) or thiazolidinedione within 3 months of screening. Other exclusion criteria included impaired hepatic and renal function, severe hypertension, and cardiovascular disease within 6 months of the trial (stroke, decompensated heart failure categorized as New York Heart Association Class III or IV, myocardial infarction, unstable angina pectoris, or coronary arterial bypass graft or angioplasty).
Randomization and Masking
Patients were randomized in a 2:1 ratio (IDeg:IGlar) using an interactive voice/web system to receive IDeg (100 U/mL, 3 mL FlexPen®; Novo Nordisk, Bagsværd, Denmark) dosed once daily in the evening (start of main evening meal to bedtime) or IGlar (Lantus®; 100 U/mL, 3 mL SoloSTAR®; Sanofi, Paris, France) given according to approved local product labelling (once daily at any time during the day, but at the same time each day26). The trial was stratified according to two region levels: (i) Japan; and (ii) Asia without Japan (Hong Kong, South Korea, Malaysia, Taiwan and Thailand). Treatment group assignment was masked for individuals involved in titration surveillance, safety committee members and statistical/medical personnel.
Procedures
IDeg and IGlar were given subcutaneously. All participants continued their pre‐study OAD treatment without any change in dose or regimen, except for DPP‐4 inhibitors, which were to be discontinued. For both treatment groups, the recommended insulin starting dose was 10 U. On the basis of self‐measured plasma glucose (SMPG) concentrations before breakfast (mean value from three consecutive days), insulin doses were titrated individually once a week throughout the trial, aiming at a pre‐breakfast SMPG target of 3.9 to <5.0 mmol/L; Table S1).
Outcome Measures
The primary end‐point was a change from baseline in HbA1c concentration after 26 weeks of treatment. In this phase 3 program, HbA1c was measured using National Glycohemoglobin Standardization Program (NGSP) values regardless of where the trial was carried out. It was due to one of the regulatory requirements of the Food and Drug Administration, European Medicines Agency and Japanese Pharmaceuticals and Medical Devices Agency (PMDA) for insulin degludec global trials including the present study. Blood samples were sent to a few central laboratories listed below, and the unified and validated method was applied. HbA1c was measured using NGSP values, even in Japan where HbA1c was commonly measured using Japan Diabetes Society values at that time. Secondary efficacy end‐points included the proportion of patients achieving a HbA1c concentration of <7 and ≤6.5% (and proportion of patients achieving these targets in the absence of confirmed hypoglycemia the last 12 weeks of treatment), changes in laboratory‐measured fasting plasma glucose (FPG) and nine‐point SMPG profiles, within‐subject variability in self‐measured pre‐breakfast plasma glucose, and Health Related Quality of Life (HRQoL; assessed by Short‐Form 36 v.2 questionnaire). Safety assessments included adverse events, hypoglycemic episodes, injection‐site reactions, bodyweight, insulin dose, laboratory analyses (hematology, biochemistry and antibodies), physical examination, vital signs, fundoscopy and electrocardiogram. Confirmed hypoglycemia was defined as a measured plasma glucose value <3.1 mmol/L (regardless of symptoms) or if classified as severe (requiring assistance). Confirmed hypoglycemic episodes with an onset between 00:01 and 05:59 h (inclusive) were classified as nocturnal. Laboratory analyses were carried out by the following central laboratories: Quintiles East Asia Pte. Ltd (Singapore), Medca Japan Company Ltd. (Tenjin, Japan) and Quintiles Laboratories Japan (Tokyo, Japan). Antibodies specific to IDeg, and cross‐reacting between IDeg and human insulin were analyzed by Celerion (Fehraltorf, Switzerland) using a subtraction radioimmunoassay method27.
Statistical Analyses
The primary objective was to confirm non‐inferiority of IDeg to IGlar, both in combination with OAD(s), as assessed by change in HbA1c concentration from baseline after 26 weeks, with a non‐inferiority limit of 0.4% for the treatment difference29.
The sample size was determined by the primary objective with the assumption of a one‐sided t‐test at a significance level of 2.5%, a zero mean treatment difference and standard deviation of 1.1% for HbA1c. A total of 426 participants were to be randomized for at least 90% power after adjustment for a 15% dropout rate.
Statistical analyses of all efficacy end‐points, bodyweight, insulin dose and treatment comparisons of hypoglycemia included all randomized participants (full analysis set), following the intention‐to‐treat principle. Other safety end‐points were evaluated in participants exposed to treatment. Missing values were imputed using the last observation carried forward29.
Treatment differences in changes from baseline in HbA1c after 26 weeks were assessed using an analysis of variance (anova) model, with treatment, antidiabetic therapy at screening (monotherapy and combination therapy), sex and region as fixed factors, and age and baseline value as covariates. Non‐inferiority was confirmed if the upper limit of the 95% confidence interval (CI) for the treatment difference was 0.4% or less29. Details of sensitivity analyses of the primary end‐point are provided in Table S2. The proportion of patients attaining a HbA1c of <7.0 or ≤6.5% (and the proportion of patients achieving these targets without confirmed hypoglycemia in the last 12 weeks of treatment) was analyzed using a logistic regression model with treatment, antidiabetic therapy at screening, sex and region as fixed factors, and age and baseline HbA1c as covariates.
Treatment differences, such as change from baseline in laboratory‐measured FPG concentration, mean plasma glucose (based on the nine‐point SMPG profile), bodyweight, insulin dose (post‐hoc analysis) and HRQoL, were analyzed using an anova method similar to that used for the primary end‐point. The time to first achieve pre‐breakfast SMPG of 3.9 to <5 mmol/L was analyzed in a Cox proportional hazards model with treatment, antidiabetic therapy at screening, sex and region as fixed factors, and age as a covariate. Rate ratios of hypoglycemic episodes were estimated by use of a negative binomial regression model with treatment, antidiabetic therapy at screening, sex and region as fixed factors, and age as a covariate, for all treatment‐emergent episodes (predefined analysis). To establish the hypoglycemic profile after achievement of stable insulin dose and glycemic control for most participants, the model was also fitted in a post‐hoc analysis of episodes occurring in the maintenance period (from week 16 to week 26). The within‐subject variability in pre‐breakfast SMPG was estimated from a linear model with treatment, antidiabetic therapy at screening, sex and region as fixed factors, age as a covariate, and subject as random factor.
The overall type 1 error was controlled by using a hierarchical (fixed‐sequence) testing procedure for selected end‐points (Figure S1). All statistical analyses were run using sas version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
A total of 579 patients were screened for the trial, of which 144 failed the screening criteria. The remaining 435 patients were randomly assigned in a 2:1 ratio (IDeg:IGlar) to treatment; five patients in the IDeg group were withdrawn before receiving treatment. Overall, 89.3 and 93.2% of randomized patients completed the trial in the IDeg and IGlar groups, respectively (Figure 1). Baseline characteristics at randomization were comparable between treatment groups (Table S3). All participants were Asian (97.9% non‐Indian; 2.1% Indian), insulin‐naïve and treated with OADs at baseline, with most (~88%) taking at least two OADs.
Figure 1.
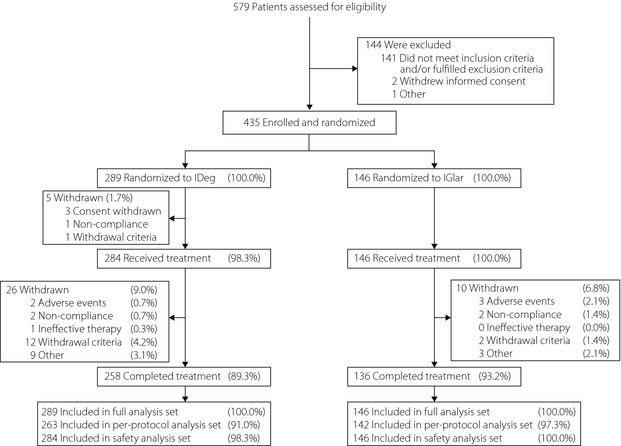
Trial flow diagram. Of the 12 patients in the insulin degludec (IDeg) group who were withdrawn due to ‘withdrawal criteria’, ten patients met withdrawal criterion #3 (‘Major protocol deviation having influence on efficacy or safety data as judged by the investigator’). Of the two remaining patients, one patient was withdrawn due to meeting withdrawal criterion #2 (‘Hypoglycemia during the treatment period posing a safety problem as judged by the investigator’), whereas the other met withdrawal criteria #2 and #3. Of the two patients in the insulin glargine (IGlar) group who were withdrawn due to withdrawal criteria, one patient met withdrawal criterion #3, whereas the other met withdrawal criterion #4 (‘Initiation or significant change of any systemic treatment which in the investigator's opinion could have interfered with glucose metabolism’). %, Proportion of randomized subjects.
After 26 weeks of treatment, the observed mean HbA1c concentration was similar for IDeg (7.2%) and IGlar (7.1%), as were mean decreases from baseline (−1.24 and −1.35%, respectively; Figure 2a). The estimated mean treatment difference (ETD) between IDeg and IGlar was 0.11% [95% CI −0.03 to 0.24], showing that IDeg was non‐inferior to IGlar in lowering HbA1c. The results of the primary analysis were supported by a per‐protocol analysis and additional sensitivity analyses (Table S2).
Figure 2.
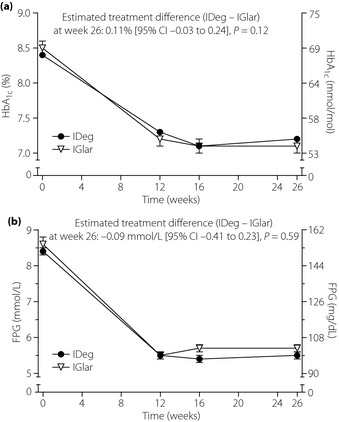
Mean (a) glycated hemoglobin (HbA1c) and (b) fasting plasma glucose (FPG) over time. Data are observed mean values for all randomized participants (last observation carried forward is used for each post‐baseline time‐point). Error bars show standard error of the mean. IDeg, insulin degludec; IGlar insulin glargine.
No statistically significant difference was found between IDeg and IGlar with respect to the proportion of patients achieving an end‐of‐trial HbA1c concentration of <7.0% (40.8% vs 48.6% of patients; P = 0.10) or ≤6.5% (18.0% vs 24.7% of patients; P = 0.08). Likewise, no statistically significant difference was found between IDeg and IGlar in terms of the proportion of patients who attained a HbA1c of <7.0% (29.1% vs 31.5%; P = 0.63) or ≤6.5% (11.6% vs 18.2%; P = 0.06) in the absence of confirmed hypoglycemia in the last 12 weeks of treatment.
At end‐of‐trial, mean FPG levels (laboratory measured) were similar for IDeg (5.5 mmol/L) and IGlar (5.7 mmol/L; Figure 2b); mean levels were reduced from baseline by 2.88 and 2.97 mmol/L, respectively (ETD; IDeg–IGlar: −0.09 mmol/L [95% CI −0.41 to 0.23], P = 0.59). The estimated within‐subject day‐to‐day variation (CV%) in pre‐breakfast SMPG levels (measured during week 26 of treatment) was significantly lower for IDeg than IGlar (16.3 vs 18.2%; treatment ratio [IDeg/IGlar]: 0.89 [95% CI 0.80 to 0.99], P = 0.013). Both treatment groups had improvements from baseline in mean nine‐point SMPG profile (Figure S2). After 26 weeks of treatment, the estimated overall mean of the nine‐point SMPG profile (defined as the area under the profile divided by measurement time) was 8.1 and 7.8 mmol/L for IDeg and IGlar, respectively (ETD [IDeg – IGlar]: 0.24 mmol/L [95% CI −0.11 to 0.59], P = 0.18). No statistically significant differences were observed between IDeg and IGlar with respect to any of the categories/domains of the SF‐36 v2 HRQoL questionnaire (data not shown).
Insulin doses were adjusted during the trial to achieve the specified pre‐breakfast SMPG target of 3.9 to <5.0 mmol/L; the median time to first achieve this target was similar for IDeg (5 weeks) and IGlar (7 weeks; estimated hazard ratio [IDeg/IGlar] 1.19 [95% CI 0.96 to 1.48], P = 0.11). Mean doses did not differ between groups at the initiation of treatment (both groups: 9 U; 0.14 U/kg), but by end‐of‐trial they were significantly lower (by 20%) for IDeg (19 U; 0.28 U/kg) than IGlar (24 U; 0.35 U/kg; mean ratio: 0.80 [95% CI 0.71 to 0.90], P = 0.0004). See Figure S3 for mean insulin dose over time.
Small and similar increases in mean bodyweight were observed from baseline to week 26 for IDeg (1.3 kg) and IGlar (1.4 kg; IDeg – IGlar: −0.17 kg [95% CI −0.59 to 0.26], P = 0.44).
At least one episode of confirmed hypoglycemia (PG <3.1 mmol/L or severe) was reported for 50 and 53% of participants in the IDeg and IGlar groups, respectively (Table S4). One episode of severe hypoglycemia was reported during the trial (for a patient in the IGlar group). The overall rate of confirmed hypoglycemia did not differ significantly between IDeg and IGlar (3.0 vs 3.7 episodes/patient‐year of exposure [PYE]; rate ratio [RR] IDeg/IGlar 0.82 [95% CI 0.60 to 1.11], P = 0.20; Figure 3a). Similarly, no statistically significant difference was found between treatments with respect to rates of nocturnal confirmed hypoglycemia for the full trial period (0.8 vs 1.2 episodes/PYE; RR IDeg/IGlar 0.62 [95% CI 0.38 to 1.04] P = 0.07; Figure 3b). However, for the maintenance period of the trial (from week 16 to end‐of‐trial, when insulin doses and glycemic indicators appeared to have stabilized for most patients), the rate of overall confirmed hypoglycemia was significantly lower (by 37%) with IDeg (RR 0.63 [95% CI 0.42 to 0.94], P = 0.0242). A lower mean rate of nocturnal confirmed hypoglycemia was also observed for IDeg relative to IGlar in the maintenance period, but this did not reach statistical significance (RR 0.52 [95% CI 0.27 to 1.00] P = 0.05).
Figure 3.
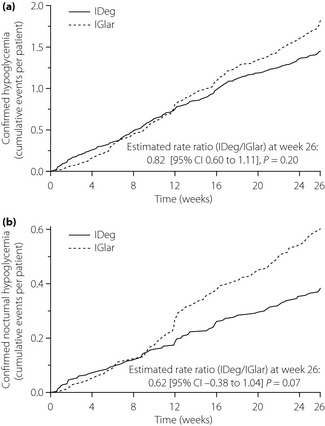
Cumulative number of (a) confirmed hypoglycemic episodes and (b) confirmed nocturnal hypoglycemic episodes. IDeg, insulin degludec; IGlar insulin glargine.
In total, 59 and 65% of patients in the IDeg and IGlar groups, respectively, reported at least one adverse event (Table S5), of which the majority (~99%) were mild or moderate in severity. No apparent treatment group‐specific patterns or clustering were observed; the most frequently reported adverse events in both groups were nasopharyngitis, upper respiratory tract infection and diabetic retinopathy (Table S6). Rates of serious adverse events were similar between groups (both: 0.1 events/PYE). Two serious adverse events were considered possibly related to the trial product by the investigator: a severe case of hypoglycemia in the IGlar group (from which the patient fully recovered) and a death from drowning in the IDeg group. No other fatal adverse events were reported. Few patients reported injection‐site reactions with IDeg (1.8% of patients) or IGlar (2.1% of patients); all reactions were mild in severity.
Levels of IDeg‐ and IGlar‐specific antibodies remained close to zero throughout the trial. Mean levels of antibodies cross‐reacting between IDeg and human insulin were low at baseline, and were maintained at the same level throughout the trial. In the IGlar group, the mean level of antibodies cross‐reacting between IGlar and human insulin was low at baseline, and increased marginally with a peak level at week 12.
No clinically relevant differences were noted between treatments in physical examination findings, vital signs, standard laboratory analyses (hematology and biochemistry), fundoscopic exam or electrocardiogram.
Discussion
Due to evidence that Asian and Caucasian patients with type 2 diabetes have differences in pathophysiology2 and lifestyle background, it is important to evaluate the efficacy and safety of IDeg – a new ultra‐long‐acting insulin analog – in both these patient populations. Accordingly, the present 26‐week, treat‐to‐target trial was carried out in insulin‐naïve Asian patients with type 2 diabetes inadequately controlled with OAD therapy as part of the global clinical development program for IDeg, as well as to fulfil PMDA requirements for regulatory approval. Once‐daily administration of IDeg was compared with IGlar, a basal insulin analog that is commonly used when initiating insulin therapy.
As would be expected from the treat‐to‐target trial design, similar improvements in HbA1c were observed with IDeg and IGlar; the mean reduction in HbA1c of ~1.3% was of similar magnitude to two other phase 3 trials of IDeg in insulin‐naïve (predominantly Caucasian) patients with type 2 diabetes that used an identical treatment algorithm30. Mean laboratory‐measured FPG and nine‐point SMPG profiles were also closely matched between treatment groups at study end, and comparable proportions of patients achieved HbA1c targets of <7.0 and ≤6.5%.
Because similar degrees of glycemic control were achieved with IDeg and IGlar, valid comparisons could be made between treatment groups with respect to the frequency and severity of hypoglycemia29. No statistically significant difference in overall or nocturnal confirmed hypoglycemia was found between IDeg and IGlar. However, it is noteworthy that the numerically lower rates of hypoglycemia associated with IDeg are consistent with findings from two other trials (26 and 52 weeks' duration) that compared IDeg with IGlar in insulin‐naïve (predominantly Caucasian) patients with type 2 diabetes30 where a significantly lower rate of nocturnal hypoglycemia was found for IDeg after 52 weeks of treatment30. Indeed, in a pre‐planned meta‐analysis using pooled, individual patient‐level data from all three phase 3 trials comparing IDeg with IGlar in insulin‐naïve patients with type 2 diabetes, IDeg was found to have significantly lower rates of both overall (by 17%) and nocturnal confirmed hypoglycemia (by 36%)32. Differences between treatments in observed rates of overall and nocturnal confirmed hypoglycemia were of a similar magnitude in the present study; based on the increasing difference in rates of hypoglycemia (in particular nocturnal hypoglycemia) as the trial progressed, it is possible that the lower rates observed for IDeg might have reached statistical significance if the trial had continued beyond 26 weeks.
Hypoglycemia and fear of hypoglycemia can result in a reluctance to use insulin therapy and optimize glycemic control33, with cases reported of patients deliberately maintaining blood glucose levels above the recommended targets to avoid hypoglycemia34. Although unwelcome at any time, hypoglycemia (especially severe hypoglycemia) is a particular concern at night, because a patient might be less able to get assistance or, if asleep, be unaware of symptoms that would have prompted corrective action. In addition to potential adverse clinical consequences, nocturnal hypoglycemia has also been shown to negatively affect the patient's well‐being and work productivity the next day35.
The consistently lower rates of hypoglycemia observed for IDeg across these and other trials21 in the development program is likely attributable to IDeg having a more consistent pharmacokinetic profile throughout the 24 h after once‐daily dosing compared with IGlar (where 60% of insulin exposure occurs in the first 12 h post‐dosing)38. The reduced day‐to‐day and hour‐to‐hour pharmacodynamic variability in insulin action observed with IDeg relative to IGlar19 is also thought to contribute to the lower rates of hypoglycemia observed for IDeg, especially once insulin doses have stabilized. In this regard, it was notable that IDeg was associated with significantly lower within‐subject day‐to‐day variation in pre‐breakfast SMPG levels (measured at end‐of‐trial), as well as a significantly lower rate of overall confirmed hypoglycemia (by 37%) compared with IGlar in the maintenance phase of the current study (from week 16 to end‐of‐trial), when the majority of patients had reached stable glycemic control and insulin doses. Similar findings were also obtained from the meta‐analysis of phase 3 trials in insulin‐naïve patients with type 2 diabetes, where, compared with the full trial period, differences in hypoglycemia rates between IDeg and IGlar were even greater during the maintenance phase32.
By the end of the trial, patients in the IGlar group were using ~20% higher doses compared with those treated with IDeg. This could suggest that higher doses of IGlar are required to achieve sufficient 24‐h coverage when used once daily, with higher doses contributing to the higher rate of hypoglycemia associated with IGlar. It was of note that end‐of‐trials doses (U/kg) of IDeg and IGlar were ~50% lower than observed in other phase 3 trials of IDeg in insulin‐naïve (predominantly Caucasian) patients with type 2 diabetes30.
Severe hypoglycemia was reported for one patient in this trial and is rare in insulin‐naïve patients with type 2 diabetes. However, it is of interest that a significant, 86% lower rate of severe hypoglycemia was found for IDeg vs IGlar in the meta‐analysis of all phase 3 trials in this patient population32.
Apart from the lower rates of hypoglycemia observed with IDeg, the present study found no differences between treatment groups with respect to standard safety assessments.
A possible limitation of the present trial was the open‐label design; a blinded, double‐dummy design was not possible because appropriate placebo‐containing injection devices were not available. Consequently, it cannot be ruled out that the open‐label design might have influenced efforts by patients and investigators to attain the target blood glucose level, and possibly influenced the reporting of hypoglycemia, adverse events and patient‐reported outcomes. As in any open‐label trial, there could have been greater caution in adjusting doses of the new drug (IDeg). To minimize potential reporting bias for hypoglycemia, we used confirmed hypoglycemia (PG <3.1 mmol/L or severe episodes requiring assistance) instead of hypoglycemic symptoms to compare rates between treatment groups.
These possible limitations notwithstanding, the findings from the present and other studies in insulin‐naïve patients with type 2 diabetes show the potential of IDeg to further improve basal insulin treatment at a lower risk of hypoglycemia and, with longer‐term treatment, possibly bring more patients to recommended levels of glycemic control at a lower risk of hypoglycemia.
In summary, the present study showed that initiating insulin therapy with IDeg in Asian patients with type 2 diabetes, inadequately controlled with OADs, provides effective glycemic control with a significantly lower rate of overall confirmed hypoglycemia once stable glycemic control and insulin dosing are achieved. IDeg might therefore represent a more suitable treatment option for this patient population.
Supplementary Material
Table S1 | Titration algorithm.
Table S2 | Sensitivity analyses of the primary end‐point.
Table S3 | Baseline characteristics of randomized population.
Table S4 | Summary of hypoglycemic episodes.
Table S5 | Summary of adverse events.
Table S6 | Adverse events occurring with a frequency of ≥5%.
Figure S1 | Hierarchical testing scheme.
Figure S2 | Mean nine‐point SMPG profiles at baseline and week 26.
Figure S3 | Mean insulin dose over time.
Acknowledgements
The trial was sponsored by Novo Nordisk (Bagsværd, Denmark). The patients, trial investigators and trial staff are thanked for their participation. All authors approved the final version of the manuscript. The authors thank Paul G Drake, PhD (Novo Nordisk), for assistance with manuscript preparation and Daria Renshaw (Watermeadow Medical; sponsored by Novo Nordisk) for assisting with the submission. Yukiko Onishi, Yasuhiko Iwamoto, Soon Jib Yoo and Sungwoo Park have no conflicts of interest to declare; Per Clauson and Søren C Tamer are employees of Novo Nordisk, and own stock in the company.
(J Diabetes Invest, doi: 10.1111/jdi.12102, 2013)
References
- 1.International Diabetes Federation . Diabetes Atlas. 5 edn Brussels, 2012. update. [Google Scholar]
- 2.Chen KW, Boyko EJ, Bergstrom RW, et al Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5‐Year follow‐up of initially nondiabetic Japanese‐American men. Diabetes Care 1995; 18: 747–753 [DOI] [PubMed] [Google Scholar]
- 3.Fukushima M, Usami M, Ikeda M, et al Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835 [DOI] [PubMed] [Google Scholar]
- 4.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66(Suppl 1): S37–S43 [DOI] [PubMed] [Google Scholar]
- 5.The Japan Diabetes Society . Evidence‐Based Practice Guideline for the Treatment of Diabetes in Japan. Nankodo Co,. Ltd, Bunkyo‐ku, Tokyo, Japan, 2010. (Japanese). [Google Scholar]
- 6.Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577–1596 [DOI] [PubMed] [Google Scholar]
- 7.Rodbard HW, Jellinger PS, Davidson JA, et al Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15: 540–559 [DOI] [PubMed] [Google Scholar]
- 8.Wajchenberg BL. Beta‐cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28: 187–218 [DOI] [PubMed] [Google Scholar]
- 9.Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care 2009; 32: S253–S259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002; 45: 937–948 [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Li C, Little RR, et al Trends in A1C concentrations among U.S. adults with diagnosed diabetes from 1999 to 2004. Diabetes Care 2008; 31: 102–104 [DOI] [PubMed] [Google Scholar]
- 12.Hoerger TJ, Segel JE, Gregg EW, et al Is glycemic control improving in U.S. adults? Diabetes Care 2008; 31: 81–86 [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick ES, Das AK, Orskov C, et al Good glycaemic control: an international perspective on bridging the gap between theory and practice in type 2 diabetes. Curr Med Res Opin 2008; 24: 2651–2661 [DOI] [PubMed] [Google Scholar]
- 14.Kanatsuka A, Kawai K, Hirao K, Japan Diabetes Clinical Data Management Study Group . Survey of drug therapy in type 2 diabetes mellitus (V). Combined treatment with insulin plus an oral hypoglycemic agents; clinical aspects and glycemic control (JDDM 19). J Japan Diab Soc 2010; 53: 737–744 (Japanese). [Google Scholar]
- 15.Nishimura E, Sørensen A, Hansen BF, et al Insulin degludec is a new generation ultra‐long acting basal insulin designed to maintain full metabolic effect while minimizing mitogenic potential. Diabetes 2010; 59(Suppl 1): A375 [Google Scholar]
- 16.Jonassen I, Havelund S, Hoeg‐Jensen T, et al Design of the novel protraction mechanism of insulin degludec, an ultra‐long‐acting basal insulin. Pharm Res 2012; 29: 2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzhals P, Heise T, Strauss HM, et al Multi‐hexamer formation is the underlying basis for the ultra‐long glucose‐lowering effect of insulin degludec. Diabetologia 2011; 54(Suppl 1): S426 [Google Scholar]
- 18.Heise T, Nosek L, Bottcher SG, et al Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metab 2012; 14: 944–950 [DOI] [PubMed] [Google Scholar]
- 19.Heise T, Hermanski L, Nosek L, et al Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab 2012; 14: 859–864 [DOI] [PubMed] [Google Scholar]
- 20.Birkeland KI, Home PD, Wendisch U, et al Insulin Degludec in Type 1 Diabetes: a randomized controlled trial of a new‐generation ultra‐long‐acting insulin compared with insulin glargine. Diabetes Care 2011; 34: 661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garber AJ, King AB, Del Prato S, et al Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal‐Bolus Type 2): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1498–1507 [DOI] [PubMed] [Google Scholar]
- 22.Heller S, Buse J, Fisher M, et al Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal‐Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1489–1497 [DOI] [PubMed] [Google Scholar]
- 23.Meneghini L, Atkin SL, Gough SCL, et al The efficacy and safety of insulin degludec given in variable once‐daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: A 26‐week, randomized, open‐label, parallel‐group, treat‐to‐target trial in people with type 2 diabetes. Diabetes Care 2013; 36: 858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Medical Association . Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc 2009; 107: 403–405 [PubMed] [Google Scholar]
- 25.International Conference on Harmonisation (ICH) . ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J Postgrad Med 2001; 47: 199–203 [PubMed] [Google Scholar]
- 26.Lantus: European Public Assessmnt Report – Annex I: Summary of Product Characteristics. Available at: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000284/WC500036082.pdf (accessed 16 December 2010).
- 27.Lindholm A, Jensen LB, Home PD, et al Immune responses to insulin aspart and biphasic insulin aspart in people with type 1 and type 2 diabetes. Diabetes Care 2002; 25: 876–882 [DOI] [PubMed] [Google Scholar]
- 28.Mire‐Sluis AR, Barrett YC, Devanarayan V, et al Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods 2004; 289: 1–16 [DOI] [PubMed] [Google Scholar]
- 29.Food and Drug Administration . Food and Drug Administration, Code of Federal Regulations, Guidance for Industry, Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention. Draft Guidance. Available at: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm071624.pdf (accessed 16 December 2010).
- 30.Zinman B, Philis‐Tsimikas A, Cariou B, et al Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes. A 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergenstal R, Bhargava A, Jain R, et al 200 U/mL Insulin degludec improves glycemic control similar to insulin glargine with a low risk of hypoglycemia in insulin‐naïve people with type 2 diabetes. American Association of Clinical Endocrinologists 21st Annual Scientific Meeting and Clinical Congress2012; A26
- 32.Ratner R, Gough S, Mathieu C, et al Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakar S, Yitzhaki G, Rosenberg R, et al Transition to insulin in Type 2 diabetes: family physicians' misconception of patients' fears contributes to existing barriers. J Diabetes Complications 2007; 21: 220–226 [DOI] [PubMed] [Google Scholar]
- 34.Wild D, von Maltzahn R, Brohan E, et al A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 2007; 68: 10–15 [DOI] [PubMed] [Google Scholar]
- 35.King P, Kong MF, Parkin H, et al Well‐being, cerebral function, and physical fatigue after nocturnal hypoglycemia in IDDM. Diabetes Care 1998; 21: 341–345 [DOI] [PubMed] [Google Scholar]
- 36.Brod M, Christensen T, Thomsen TL, et al The impact of non‐severe hypoglycemic events on work productivity and diabetes management. Value Health 2011; 14: 665–671 [DOI] [PubMed] [Google Scholar]
- 37.Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev 2008; 24: 87–92 [DOI] [PubMed] [Google Scholar]
- 38.Heise T, Hövelmann U, Nosek L, et al Insulin degludec: two‐fold longer half‐life and a more consistent pharmacokinetic profile than insulin glargine. Diabetologia 2011; 54(Suppl 1): S425 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Titration algorithm.
Table S2 | Sensitivity analyses of the primary end‐point.
Table S3 | Baseline characteristics of randomized population.
Table S4 | Summary of hypoglycemic episodes.
Table S5 | Summary of adverse events.
Table S6 | Adverse events occurring with a frequency of ≥5%.
Figure S1 | Hierarchical testing scheme.
Figure S2 | Mean nine‐point SMPG profiles at baseline and week 26.
Figure S3 | Mean insulin dose over time.


