Abstract
Introduction
To evaluate the pharmacodynamics, pharmacokinetics, safety and tolerability of empagliflozin in Japanese patients with type 2 diabetes mellitus.
Materials and methods
In this 4‐week, multiple dose, randomized, parallel‐group, double‐blind, placebo‐controlled trial, patients (n = 100) were randomized to receive 1, 5, 10 or 25 mg of empagliflozin, or placebo once daily. Key end‐points were urinary glucose excretion (UGE), fasting plasma glucose (FPG) and eight‐point glucose profile.
Results
Data are presented for 1, 5, 10, 25 mg of empagliflozin and placebo groups, respectively. Adjusted mean changes from baseline to day 27 in UGE were 40.8, 77.1, 80.9, 93.0 and −2.1 g (P < 0.0001 for all empagliflozin groups vs placebo). Adjusted mean changes from baseline to day 28 in FPG were −1.56, −1.96, −2.31, −2.37 and −0.86 mmol/L (P < 0.01 for all empagliflozin groups vs placebo). Adjusted mean changes from baseline to day 27 in eight‐point glucose profile were −1.96, −2.21, −2.42, −2.54 and −0.97 mmol/L (P < 0.01 for all empagliflozin groups vs placebo). Empagliflozin reached peak plasma concentration 1.5–2 h after dosing. Mean steady state terminal elimination half‐lives ranged from 13.2 to 18.0 h. Of 100 patients, 25 experienced an adverse event, occurring more frequently for empagliflozin (29.1%) than placebo (9.5%); frequency was not dose related.
Conclusions
In Japanese patients with type 2 diabetes mellitus, empagliflozin at doses up to 25 mg once daily for 4 weeks was well tolerated and resulted in significant improvements in glycemic control compared with placebo. This trial was registered with ClinicalTrials.gov (no. NCT00885118).
Keywords: Diabetes, Empagliflozin, Sodium glucose cotransporter 2 inhibitor
Introduction
The global prevalence of diabetes was estimated at 366 million in 2011, and is increasing worldwide1. Japan was ranked among the top 10 countries in a global survey of the numbers of people aged between 20 and 79 years with diabetes, and the prevalence of the disease in the Japanese population increased by more than 4% between 2000 and 20102. Type 2 diabetes mellitus accounts for the majority of cases of diabetes in Japan4. Most patients with type 2 diabetes mellitus fail to attain and maintain glycemic targets with current therapies5. Barriers to achieving glycemic control include a progressive deterioration in β‐cell function, a part of the disease pathogenesis that reduces the efficacy of insulin‐dependent treatments over time6. Existing antidiabetic medications are also associated with side‐effects, such as weight gain, hypoglycemia and gastrointestinal effects, which limit their use8. There is a need for new antidiabetic drugs to overcome the shortcomings of current medications and improve treatment outcomes6.
The sodium glucose cotransporter 2 (SGLT2), located in the proximal tubule of the kidney, is estimated to facilitate approximately 90% of renal glucose reabsorption10. Empagliflozin, a potent selective inhibitor of SGLT211, is in development for the treatment of type 2 diabetes mellitus. Four weeks' treatment with multiple oral doses of empagliflozin up to 100 mg once daily in European patients with type 2 diabetes mellitus was well tolerated, and resulted in increases in urinary glucose excretion (UGE) and decreases in fasting plasma glucose (FPG) compared with placebo12. The present study evaluated the pharmacokinetics, pharmacodynamics, safety and tolerability of multiple doses of empagliflozin in Japanese patients with type 2 diabetes mellitus over 4 weeks.
Materials and Methods
Patients aged ≥20 to ≤70 years treated with diet and exercise alone or with one antidiabetic drug other than thiazolidinediones were eligible to enter the study. Other main inclusion criteria were glycated hemoglobin (HbA1c) ≥6.5% (≥48 mmol/mol) and ≤9.0% (≤75 mmol/mol) for patients on one antidiabetic drug or HbA1c ≥7.0% (≥53 mmol/mol) and ≤10.0% (≤86 mmol/mol) for drug naïve patients, and body mass index (BMI) ≥18.0 kg/m2 and ≤40.0 kg/m2. Exclusion criteria included fasting blood glucose levels >13.3 mmol/L; randomly determined blood glucose levels >22.2 mmol/L on two consecutive days during a wash‐out period (days −30 to −3); any clinically relevant concomitant diseases; or participation in another trial within 2 months before study drug administration. The trial (NCT00885118) was carried out in accordance with the Declaration of Helsinki (1996), the International Conference on Harmonization Good Clinical Practice (ICH‐GCP) guidelines and Japanese GCP regulations. The protocol was approved by the local ethics committee (institutional review board), and patients provided written consent before study commencement.
In the present 4‐week, multiple dose, randomized, parallel‐group, double‐blind, placebo‐controlled trial, 100 patients were randomly assigned to receive 1, 5, 10 or 25 mg empagliflozin, or placebo, once daily. A meal tolerance test (MTT) was carried out on the mornings of days −1 and 28. The MTT used a standard drink (Calorie Mate®, 200 kcal; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) containing proteins (7.6 g), lipids (4.4 g), carbohydrate (31 g) and fiber (2.5 g), which was served 1 h after drug administration. A MTT was chosen rather than an oral glucose tolerance test, as it is considered a better option for quantitating the disease in patients who are known to have diabetes, and in order to avoid raising plasma glucose to extremely high levels.
Key end‐points were changes from baseline in UGE, FPG and eight‐point glucose profile.
Urinary glucose excretion was evaluated as the total amount of glucose in urine over 24 h on days −2, −1, 1, 27, 28, 29 and 30. FPG was measured on days −2, −1, 1, 2, 7, 14, 21, 26, 27, 28, 29 and 33; and eight‐point glucose profile was determined on days −2 to −1, 1–2, 7–8, 14–15, 21–22 and days 27–28. In addition, changes from baseline (day −1) to day 28 in HbA1c (National Glycohemoglobin Standardization Program values) were evaluated. HbA1c was measured at Covance (Asia) Pte. Ltd., Singapore. Pharmacokinetic end‐points were time from dosing to maximum concentration at steady state (tmax,ss), terminal half‐life of the analyte in plasma at steady state (t1/2,ss), area under the concentration‐time curve of the analyte in plasma over a uniform dosing interval τ at steady state (AUCτ,ss), maximum measured concentration of the analyte in plasma at steady state (Cmax,ss), apparent clearance of the analyte in plasma after extravascular administration at steady state (CL/F,ss), and fraction of the analyte excreted unchanged in urine at steady state from time interval 0–24 h (fe0–24,ss). Plasma sampling for pharmacokinetic measurements occurred on days 1, 2, 7, 14, 21, 26, 27, 28, 29, 30 and 31. Safety end‐points included adverse events (AEs; observed throughout the trial), vital signs (measured on days −1, 1, 2, 7, 14, 21, 26, 27, 28, 29 and 33), physical examinations (carried out at screening and any day between days 35 and 42) and routine clinical laboratory tests (measured on days −2, −1, 1, 2, 7, 14, 21, 27, 28 and 29).
Descriptive statistics were calculated for all end‐points. Analyses of key end‐points were carried out in an exploratory sense and were not adjusted for multiplicity. A two‐sided statistical testing strategy was used with α = 0.05 per standard conventions. For key end‐points and HbA1c, changes from baseline were compared by treatment using analysis of covariance (ancova). Dose proportionality (AUCτ,ss and Cmax,ss) was investigated using analysis of variance (anova).
Results
Of 100 patients randomized, three withdrew prematurely (because of AE, refusal to continue trial medication and poor compliance; Figure 1). A total of 84 patients were male, median age was 59.5 years (range 34–70 years), and median body mass index was 24.3 kg/m2 (range 18.0–39.1 kg/m2; Table 1).
Figure 1.
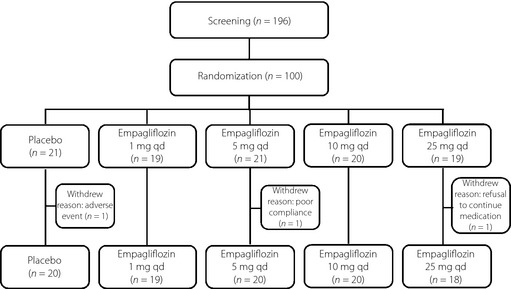
Disposition of patients. qd, Once daily.
Table 1. Baseline demographics and characteristics.
| Empagliflozin | Placebo (n = 21) | Total (n = 100) | ||||
|---|---|---|---|---|---|---|
| 1 mg (n = 19) | 5 mg (n = 21) | 10 mg (n = 20) | 25 mg (n = 19) | |||
| Men, n (%) | 16 (84.2) | 20 (95.2) | 17 (85.0) | 15 (78.9) | 16 (76.2) | 84 (84.0) |
| Median (range) age (years) | 58.0 (43–70) | 54.0 (34–67) | 57.5 (42–70) | 61.0 (42–70) | 59.0 (38–70) | 59.5 (34–70) |
| Median (range) bodyweight (kg) | 63.1 (45.0–93.4) | 74.8 (51.7–90.7) | 68.7 (43.4–98.0) | 62.5 (48.0–89.3) | 71.9 (47.6–98.3) | 68.0 (43.4–98.3) |
| Median (range) waist circumference (cm) | 84.8 (69.0–104.5) | 89.2 (77.8–102.2) | 87.0† (73.7–115.0) | 87.0 (75.5–100.3) | 91.0 (75.0–112.8) | 87.5 (69.0–115.0) |
| Median (range) BMI (kg/m2) | 22.4 (19.1–32.6) | 25.1 (21.2–39.1) | 24.0 (19.2–31.5) | 24.7 (18.0–30.0) | 26.4 (20.1–34.6) | 24.3 (18.0–39.1) |
| Mean (SD) UGE (g) | ||||||
| Day −1 | 12.6 (12.0) | 7.6 (11.6) | 10.5 (12.4) | 9.2 (14.6) | 5.8 (8.7) | 9.1 (11.9) |
| Day −2‡ | 17.9 (15.4) | 8.4 (8.0) | 15.6 (12.6) | 7.6 (8.6) | 9.1 (13.5) | 11.6 (12.4) |
| Mean (SD) eight‐point glucose, mmol/L (Day −2) | 11.9 (2.0) | 10.2 (1.7) | 11.2 (1.7) | 10.6 (1.6) | 10.3 (1.5) | 10.8 (1.8) |
| Mean (SD) FPG, mmol/L (Day −1) | 9.5 (1.8) | 8.6 (1.4) | 8.9 (1.7) | 8.9 (1.3) | 8.8 (1.3) | 8.9 (1.5) |
| Mean (SD) HbA1c, % (Day −1) | 8.5 (0.9) | 7.8 (0.7) | 8.2 (0.7) | 8.0 (0.8) | 8.0 (0.7) | 8.0 (0.8) |
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; SD, standard deviation; UGE, urinary glucose excretion. †n = 19. ‡n = 18, 19 and 18 for empagliflozin 1, 10 and 25 mg, respectively.
Adjusted mean changes from baseline to day 27 in UGE increased with empagliflozin dose (40.8, 77.1, 80.9, 93.0 and −2.1 g for the 1, 5, 10, 25 mg empagliflozin and placebo groups, respectively; P < 0.0001 vs placebo, for all; Figure 2a). Adjusted mean changes from baseline to day 28 in FPG also increased with dose (−1.56, −1.96, −2.31, −2.37 and −0.86 mmol/L for the 1, 5, 10, 25 mg empagliflozin and placebo groups, respectively; P < 0.01 vs placebo, for all; Figure 2b). Adjusted mean changes from baseline to day 27 in eight‐point glucose profile decreased dose‐dependently in all empagliflozin groups (−1.96, −2.21, −2.42, −2.54 and −0.97 mmol/L for the 1, 5, 10, 25 mg empagliflozin and placebo groups, respectively; P < 0.01 vs placebo; Figure 2c). Adjusted mean changes from baseline to day 28 in HbA1c were −0.66% in the empagliflozin 1 mg group, −0.72% in the empagliflozin 5 mg group, −0.85% in the empagliflozin 10 mg group, −0.82% in the empagliflozin 25 mg group and −0.42% in the placebo group (Figure 2d). Differences compared with placebo were significant in all empagliflozin groups except the 1 mg group (Figure 2d).
Figure 2.
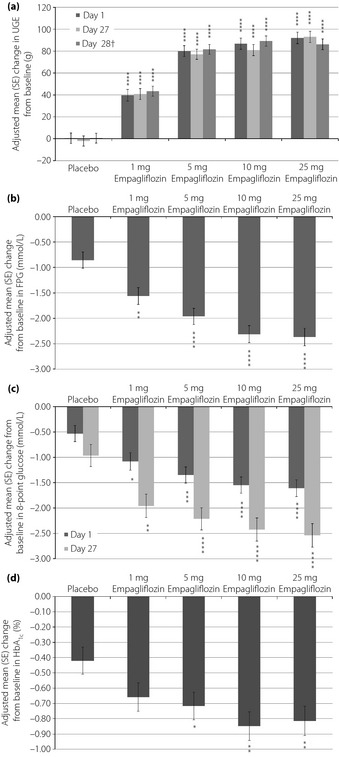
Adjusted mean (standard error [SE]) changes from baseline in (a) cumulative urinary glucose excretion (UGE) after first (day 1) and multiple (days 27 and 28†) drug administration, (b) fasting plasma glucose (FPG) after multiple drug administration (day 28), (c) eight‐point glucose profile after first (day 1) and multiple drug administration (day 27) and (d) glycated hemoglobin (HbA1c) after multiple drug administration (day 28). †A meal tolerance test was carried out on day 28. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs placebo.
A total of 25 (25.0%) patients experienced at least one AE (Table 2). The frequency of AEs was not dose‐dependent, and all AEs were mild or moderate in intensity. The most frequently reported AEs in the empagliflozin groups were constipation and nasopharyngitis, each experienced by four patients (Table 2). One patient in the 1 mg group reported a serious AE (transient ischemic attack), which was not regarded as related to the study drug. One patient on placebo reported hyperglycemia that led to premature discontinuation. No deaths occurred. Symptomatic hypoglycemia, judged as mild by the investigator, occurred in one patient in each of the 1 mg (cold sweat and facial pallor; blood glucose level within the 3.0–3.9 mmol/L range) and 10 mg (finger shivering and cold sweat; blood glucose level >3.9 mmol/L) groups after the MTT on day 28. There was one case of cystitis (25 mg group), one case of nocturia (10 mg group) and one case of pollakiuria (25 mg group). No genital tract infections were reported. No clinically relevant findings were noted in laboratory tests, vital signs, physical findings or any other safety‐related observations.
Table 2. Summary of adverse events.
| No. participants with AEs, n (%) | Empagliflozin | Placebo (n = 21) | |||
|---|---|---|---|---|---|
| 1 mg (n = 19) | 5 mg (n = 21) | 10 mg (n = 20) | 25 mg (n = 19) | ||
| Any AE | 6 (31.6) | 4 (19.0) | 6 (30.0) | 7 (36.8) | 2 (9.5) |
| Most frequently reported AEs by preferred term† | |||||
| Nasopharyngitis | 2 (10.5) | 1 (4.8) | 0 (0.0) | 1 (5.3) | 0 (0.0) |
| Constipation | 0 (0.0) | 1 (4.8) | 2 (10.0) | 1 (5.3) | 1 (4.8) |
| Upper abdominal pain | 0 (0.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) | 0 (0.0) |
| Drug‐related AEs‡ | 2 (10.5) | 0 (0.0) | 4 (20.0) | 3 (15.8) | 1 (4.8) |
†Reported by ≥10% of subjects in ≥1 group. ‡Investigator defined. AEs, adverse events.
Increases in empagliflozin exposure (AUCτ,ss and Cmax,ss) were dose proportional. Peak plasma concentrations of empagliflozin were reached after a median of 1.5 h, and declined with a mean (% coefficient of variation [% CV]) t1/2 of 13.2 h (45.3 h) to 18.0 h (40.7 h) on day 28. The mean (%CV) fraction of empagliflozin excreted in urine from the time interval 0–24 h ranged from 21.4% (24.7%) to 22.3% (25.7%).
Discussion
In Japanese patients with type 2 diabetes mellitus, treatment with empagliflozin at doses up to 25 mg once daily for 4 weeks increased UGE and resulted in improvements in glycemic control. Exposure to empagliflozin showed linearity over the whole dose range. These findings are in line with observations from other empagliflozin studies12.
Compared with the European cohort12, Japanese patients with type 2 diabetes mellitus showed slightly higher Cmax,ss and AUCτ,ss (1.4‐ to 1.5‐fold). Higher exposure in Japanese patients was considered to be associated with their lower bodyweight (median of 68 kg for Japanese patients compared with 90 kg for Caucasian patients) and BMI (median 24 kg/m2 for Japanese patients compared with 29 kg/m2 for Caucasian patients), therefore these differences were not considered clinically relevant. Changes in FPG after multiple oral doses of 10 mg and 25 mg empagliflozin were similar between European patients (−2.43 and −1.90 mmol/L, respectively) and Japanese patients (−2.31 and −2.37 mmol/L, respectively).
The frequency of AEs was higher in the empagliflozin groups than the placebo group. In the empagliflozin groups, the most frequently reported type of AE was a gastrointestinal disorder. The number of patients with urinary tract infections was low, and no patients reported genital tract infections. Longer‐term data from larger studies will provide further information on the risk of urinary tract infections and genital tract infections with SGLT2 inhibitors. Mild symptomatic hypoglycemia occurred in one patient in each of the 1 and 10 mg empagliflozin groups after the MTT on day 28. Both patients recovered without treatment within the day. In the corresponding European study, mild hypoglycemia was reported in two patients (6.7%) treated with 100 mg empagliflozin and one patient (6.3%) treated with placebo after the oral glucose tolerance test (OGTT) held on that day12. These hypoglycemic events might be related to the prolonged period of fasting required for the MTT and OGTT.
In conclusion, treatment with empagliflozin at doses up to 25 mg once daily for 4 weeks in Japanese patients with type 2 diabetes mellitus was well tolerated and resulted in significant improvements in glycemic control. The efficacy and safety of longer‐term treatment with empagliflozin will be evaluated in a large, international phase III clinical program.
Acknowledgments
K Koiwai, A Taniguchi, A Sarashina, L Seman and HJ Woerle are employees of Boehringer Ingelheim. S Kanada was Coordinating Investigator of the study. The study was funded by Boehringer Ingelheim. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Keni Lee and Elizabeth Ng of Fleishman‐Hillard Group Ltd during the preparation of this manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
(J Diabetes Invest, doi: 10.1111/jdi.12110, 2013)
Glycated hemoglobin values are expressed as National Glycohemoglobin Standardization Program values
References
- 1.Whiting DR, Guariguata L, Weil C, et al IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94: 311–321 [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14 [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, et al Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053 [DOI] [PubMed] [Google Scholar]
- 4.Neville SE, Boye KS, Montgomery WS, et al Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25: 705–716 [DOI] [PubMed] [Google Scholar]
- 5.Esposito K, Chiodini P, Bellastella G, et al Proportion of patients at HbA1c target <7% with 8 classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab 2012; 14: 228–233 [DOI] [PubMed] [Google Scholar]
- 6.Cefalu WT. Evolving treatment strategies for the management of type 2 diabetes. Am J Med Sci 2012; 343: 21–26 [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA. Overview of newer agents: where treatment is going. Am J Med 2010; 123: S38–S48 [DOI] [PubMed] [Google Scholar]
- 8.Cobble ME, Peters AL. Clinical practice in type 2 diabetes: after metformin and lifestyle, then what? J Fam Pract 2009; 58: S7–S14 [PubMed] [Google Scholar]
- 9.Pollack MF, Purayidathil FW, Bolge SC, et al Patient‐reported tolerability issues with oral antidiabetic agents: associations with adherence; treatment satisfaction and health‐related quality of life. Diabetes Res Clin Pract 2010; 87: 204–210 [DOI] [PubMed] [Google Scholar]
- 10.Bakris GL, Fonseca VA, Sharma K, et al Renal sodium‐glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75: 1272–1277 [DOI] [PubMed] [Google Scholar]
- 11.Grempler R, Thomas L, Eckhardt M, et al Empagliflozin, a novel selective sodium glucose cotransporter‐2 (SGLT‐2) inhibitor: characterisation and comparison with other SGLT‐2 inhibitors. Diabetes Obes Metab 2012; 14: 83–90 [DOI] [PubMed] [Google Scholar]
- 12.Heise T, Seewaldt‐Becker E, Macha S, et al Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks' treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013; 15: 613–621doi: 10.1111/dom.12073 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Seman L, Macha S, Nehmiz G, et al Empagliflozin (BI 10773), a potent and selective SGLT‐2 inhibitor, induces dose‐dependent glucosuria in healthy subjects. Clinical Pharm in Drug Dev 2013; 2: 152–161doi 10.1002/cpdd.16 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Sarashina A, Koiwai K, Seman LJ, et al Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter‐2 (SGLT‐2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet 2012????: ???–???? [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


