Abstract
Aims/Introduction
The inverse association between soybean intake and type 2 diabetes mellitus has been reported. We investigated the effects of soybean product intake on the incidence of type 2 diabetes mellitus considering fasting and postload hyperglycemia.
Materials and Methods
The present 4‐year, cohort study included 1,738 men and 1,301 women, aged 30–69 years, without diabetes mellitus at baseline who underwent comprehensive medical check‐ups between April 2006 and March 2007 at Saku Central Hospital. Participants were stratified by sex and body mass index (BMI), and further classified into three groups based on soybean product intake: group 1 (0–1 time/week), group 2 (2–3 times/week) and group 3 (four or more times per week). Participants underwent annual standard 75‐g oral glucose tolerance testing during follow‐up periods until March 2011. Main outcomes were incidence of fasting hyperglycemia, postload hyperglycemia and type 2 diabetes mellitus.
Results
During 10,503 person‐years of follow up, 204 participants developed type 2 diabetes mellitus, including 61 who developed fasting hyperglycemia and 147 who developed postload hyperglycemia. Among men with a high BMI, group 3 had significantly lower risk for the incidence of type 2 diabetes mellitus, fasting hyperglycemia and postload hyperglycemia than group 1, and multivariable‐adjusted hazard ratios and 95% confidence intervals were 0.44 (0.22–0.89), 0.36 (0.15–0.96) and 0.40 (0.18–0.92), respectively. Similar results were not observed among men with low BMI or women.
Conclusions
Soybean product intake prevented fasting and postload hyperglycemia and type 2 diabetes mellitus in men with a high BMI. Further long‐term observation is necessary.
Keywords: Postload hyperglycemia, Soybean, Type 2 diabetes mellitus
Introduction
The prevalence of type 2 diabetes mellitus is increasing worldwide. Type 2 diabetes mellitus is a major risk factor for cardiovascular morbidity and mortality. It has been reported that among patients with coronary artery disease, approximately 35 and 15% have had diabetes or prediabetes, respectively1. In addition, individuals with high postload plasma glucose (PG) levels have a higher risk of cardiovascular and all‐cause mortality than individuals with high fasting plasma glucose (FPG) levels2. Therefore, it is important to prevent postload PG levels, as well as FPG levels, from rising.
Type 2 diabetes mellitus is strongly associated with lifestyle, and many modifiable risk factors have been reported. Dietary soy is beneficial to health because of a high polyunsaturated fat, fiber, vitamin and mineral content combined with a low saturated fat content6. A meta‐analysis showed that the substitution of soy protein for animal protein in the diet significantly lowered total cholesterol, low‐density lipoprotein (LDL) cholesterol and triglycerides without affecting high‐density lipoprotein (HDL) cholesterol7. Dietary soy also has the potential to decrease the incidence of type 2 diabetes mellitus. It has been reported that legume intake is associated with the modification of risk factors related to glucose intolerance8. Furthermore, the inverse association between soybean product intake and type 2 diabetes mellitus has been reported among women in two prospective studies9. However, the definition of type 2 diabetes mellitus in the two studies was based on self‐report. In addition, there has been no study investigating the effects of soybean product intake on the incidence of type 2 diabetes mellitus, and considering postload PG levels and FPG levels.
Therefore, we investigated the effects of soybean intake on postload PG levels, FPG levels and the incidence of type 2 diabetes mellitus. Specifically, the annual PG levels were determined in a Japanese population using a standard 75‐g oral glucose tolerance test (OGTT).
Materials and Methods
Participants
The Saku Study included community residents in the neighborhood around Saku Central Hospital (Nagano, Japan) who underwent annual comprehensive medical check‐ups for the prevention and early detection of various diseases, including type 2 diabetes mellitus, cardiovascular disease and cancer. The Saku Study attempted to elucidate the incidence of and risk factors for type 2 diabetes mellitus among the Japanese population11. The cohort consisted of 4,318 individuals, aged 30–69 years, who underwent a baseline comprehensive medical check‐up over 2 days and 1 night between April 2006 and March 2007 at Saku Central Hospital. Of these individuals, 3,726 did not have diabetes mellitus at baseline, based on four criteria: (i) no history of diabetes mellitus, as determined by interviews carried out by the physicians; (ii) FPG concentrations <7.0 mmol/L; (iii) 2‐h postload PG (2‐h PG) concentrations <11.1 mmol/L; and (iv) glycated hemoglobin (HbA1c) concentrations (National Glycohemoglobin Standardization Program [NGSP]) <6.5%. Of these individuals, 3,069 underwent at least one follow‐up examination by the end of March 2011; we excluded 30 participants with missing data at baseline and/or annual follow‐up examinations. Thus, a total of 3,039 participants (1,738 men and 1,301 women), aged 30–69 years, were eligible for our analysis. Of these 3,039 participants, 2,157 (71.0%) underwent comprehensive annual medical check‐ups for 4 years after the baseline examination.
The study protocol was in accordance with the Helsinki Declaration, and approved by the Ethical Committee of Saku Central Hospital.
Soybean Product Intake
Food intakes were assessed by a self‐administered questionnaire (see Appendix S1). Soybean products included soybean, tofu, koyadofu (freeze‐dried tofu), aburaage (deep‐fried tofu) and natto (fermented soybeans). Participants were asked about the frequency of soybean product intake. Participants described the frequency of intake by choosing from the following options: zero to one time/week, two to three times/week and four or more times/week. The standard portion size of soybean products per time was 100 g. Milk, egg, fish, meat, fruits, vegetables and grain intakes at baseline also were assessed by a self‐administered questionnaire. For grain intake only, participants were asked about the frequency of intake per day. A standard portion size was specified for each food.
Study Procedure
In the morning after an overnight fast (12 h), all participants underwent a standard 75‐g OGTT. Blood samples were obtained at 0 (fasting), 30, 60 and 120 min, with PG measured at all four times in the clinical laboratory of Saku Central Hospital. Blood glucose, HDL cholesterol and LDL cholesterol concentrations were measured by enzymatic methods. HbA1c concentrations were measured by high‐performance liquid chromatography. HbA1c (%) was estimated as a NGSP equivalent value (%) and calculated using the formula HbA1c (%) = HbA1c (Japan Diabetes Society; %) + 0.4%12.
Weight and height were measured in the morning during the fasting state. Body mass index (BMI) was calculated as the weight (kg) divided by the height squared (m2). Blood pressure was measured by trained nurses using an automatic sphygmomanometer, with the participant in a seated position after at least a 5‐min rest. Additionally, the examination included standard questionnaires regarding demographic characteristics, medical history, family history and health‐related habits. Smoking status was categorized as never‐smoker, ex‐smoker and current smoker. Alcohol consumption (ethanol) was categorized as 0, 1–139 or ≥140 g/week, and exercise was categorized into 0, 1–119 or ≥120 min/week.
Definition of Main Outcomes and Follow Up
We used the 1999 World Health Organization criteria to define type 2 diabetes mellitus14. Fasting hyperglycemia (FPG levels ≥7.0 mmol/L) and/or postload hyperglycemia (2‐h PG levels ≥11.1 mmol/L) were indicative of type 2 diabetes mellitus. In addition, receiving medical treatment for type 2 diabetes mellitus was included in the definition of type 2 diabetes mellitus. All 3,039 participants were followed up annually at Saku Central Hospital by comprehensive medical check‐ups over 2 days and 1 night, including the 75‐g OGTT, until they developed type 2 diabetes mellitus or until March 2011. Individuals not examined during follow up were censored on the date of their last examination.
Statistical Analysis
We stratified participants by sex and medians of BMI (23.6 kg/m2 in men and 22.0 kg/m2 in women) as follows: men with a low BMI, men with a high BMI, women with a low BMI and women with a high BMI. Additionally, based on their soybean product intake, participants were classified into three groups in each category of sex and BMI status: group 1 (0–1 time/week), group 2 (2–3 times/week) and group 3 (four or more times per week). Differences in baseline characteristics among these three groups were determined by using an analysis of variance for continuous data with a normal distribution, a Kruskal–Wallis H‐test for continuous data with a non‐normal distribution, and a chi‐squared‐test and Fisher's exact test for dichotomous data and categorical data.
Cox proportional hazards regression was used to estimate adjusted type 2 diabetes mellitus incidence hazard ratios (HRs) and 95% confidence intervals (CIs) for group 2 and group 3 compared with group 1. Furthermore, HRs and 95% CIs for fasting hyperglycemia (FPG levels ≥7.0 mmol/L) and postload hyperglycemia (2‐h PG levels ≥11.0 mmol/L) were estimated. Model 1 was adjusted by age, and model 2 was adjusted by age, BMI, smoking status, alcohol consumption, physical activity, a family history of diabetes mellitus, green vegetable intake and fruit intake. The assumptions required for proportional hazards were met, and these were assessed with graphs of log–log plots. In addition, incident rates of impaired glucose tolerance (2‐h PG ≥7.8 mmol/L) and impaired fasting glucose (FPG ≥6.1 mmol/L) among participants with normoglycaemia (2‐h PG <7.8 mmol/L and FPG <6.1 mmol/L) at baseline were calculated. All data were analyzed using SPSS statistical software (Version 19.0J; SPSS Japan Inc., Tokyo, Japan). All reported P‐values are two‐tailed; those <0.05 were considered statistically significant.
Results
Table 1 summarizes the baseline characteristics according to categories of soy product intake in men. In men with a low BMI, age and smoking status were significantly different among groups of different soy product intake. In men with a high BMI, alcohol consumption was significantly different among groups of different soy product intake. Table 2 summarizes the baseline characteristics according to categories of soy product intake in women. In women with a low BMI, age, HbA1c, smoking status and physical activity were significantly different among groups of different soy product intake. In women with a high BMI, age, alcohol consumption and physical activity were significantly different among groups of different soy product intake. Dietary intakes except for grain intake were significantly different among both sex and BMI groups.
Table 1. Baseline characteristics according to groups categorized by intake of soybean products in men.
| Group 1 0 –1 time/week | Group 2 2 –3 times/week | Group 3 ≥4 times/week | P | |
|---|---|---|---|---|
| Men with low BMI, n (<23.6 kg/m2) | 71 | 304 | 487 | |
| Age (years) | 53.6 ± 9.3 | 55.2 ± 7.3 | 55.9 ± 8.1 | 0.042 |
| BMI (kg/m2) | 21.3 ± 1.7 | 21.7 ± 1.3 | 21.6 ± 1.5 | 0.080 |
| Systolic blood pressure (mmHg) | 116.8 ± 14.1 | 116.6 ± 16.1 | 117.6 ± 14.0 | 0.661 |
| HDL‐cholesterol (mmol/L) | 1.40 ± 0.33 | 1.46 ± 0.35 | 1.49 ± 0.37 | 0.100 |
| LDL‐cholesterol (mmol/L) | 3.11 ± 0.66 | 3.05 ± 0.76 | 3.08 ± 0.75 | 0.814 |
| Fasting PG (mmol/L) | 5.45 ± 0.4 | 5.48 ± 0.45 | 5.47 ± 0.48 | 0.860 |
| 2‐h PG (mmol/L) | 6.22 ± 1.26 | 6.55 ± 1.41 | 6.58 ± 1.40 | 0.126 |
| HbA1c (%) | 5.42 ± 0.41 | 5.49 ± 0.34 | 5.45 ± 0.32 | 0.156 |
| Smoking status, % (never, current, ex‐) | 26.8, 42.3, 31.0 | 26.3, 35.2, 38.5 | 29.2, 24.2, 46.6 | 0.001 |
| Alcohol consumption, % (0, 1 –139, ≥140 g/week) | 16.9, 49.3, 33.8 | 15.5, 43.1, 41.4 | 15.8, 44.8, 39.4 | 0.835 |
| Physical activity, % (0, 1 –119, ≥120 min/week) | 59.2, 22.5, 18.3 | 49.3, 31.9, 18.8 | 47.2, 32.2, 20.5 | 0.391 |
| Green vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 21.1, 46.5, 32.4 | 8.9, 41.4, 49.7 | 3.1, 20.3, 76.6 | <0.001 |
| Other vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 21.1, 36.6, 42.3 | 4.3, 37.2, 58.6 | 0.8, 15.6, 83.6 | <0.001 |
| Fruit intake, % (0 –1, 2 –3, ≥4 times/week) | 54.9, 25.4, 19.7 | 31.6, 36.8, 31.6 | 17.0, 32.0, 50.9 | <0.001 |
| Egg intake, % (0 –1, 2 –3, ≥4 times/week) | 28.2, 50.7, 21.1 | 12.8, 44.7, 42.4 | 10.1, 35.7, 54.2 | <0.001 |
| Milk intake, % (0 –1, 2 –3, ≥4 times/week) | 42.3, 19.7, 38.0 | 31.3, 22.7, 46.1 | 19.1, 16.8, 64.1 | <0.001 |
| Meat intake, % (0 –1, 2 –3, ≥4 times/week) | 26.8, 60.6, 12.7 | 16.4, 56.9, 26.6 | 13.1, 42.7, 44.1 | <0.001 |
| Fish intake, % (0 –1, 2 –3, ≥4 times/week) | 23.9, 53.5, 22.5 | 7.9, 53.0, 39.1 | 2.7, 24.2, 73.1 | <0.001 |
| Grain intake (times/day) | 3.5 ± 1.2 | 3.4 ± 1.0 | 3.6 ± 1.0 | 0.212 |
| Men with high BMI, n (≥23.6 kg/m2) | 62 | 317 | 497 | |
| Age (years) | 55.4 ± 6.8 | 55.1 ± 7.7 | 56.3 ± 8.2 | 0.098 |
| BMI (kg/m2) | 25.9 ± 1.7 | 25.9 ± 2.1 | 25.8 ± 2.0 | 0.814 |
| Systolic blood pressure (mmHg) | 124.5 ± 18.6 | 121.5 ± 14.4 | 122.8 ± 15.1 | 0.274 |
| HDL‐cholesterol (mmol/L) | 1.34 ± 0.38 | 1.28 ± 0.27 | 1.32 ± 0.29 | 0.122 |
| LDL‐cholesterol (mmol/L) | 3.20 ± 0.81 | 3.31 ± 0.77 | 3.24 ± 0.79 | 0.332 |
| Fasting PG (mmol/L) | 5.73 ± 0.46 | 5.66 ± 0.47 | 5.62 ± 0.45 | 0.127 |
| 2‐h PG (mmol/L) | 6.88 ± 1.61 | 6.83 ± 1.32 | 7.04 ± 1.36 | 0.098 |
| HbA1c (%) | 5.56 ± 0.34 | 5.56 ± 0.31 | 5.54 ± 0.34 | 0.707 |
| Smoking status (never, current, ex‐) | 16.1, 30.6, 53.2 | 24.0, 28.7, 47.3 | 26.6, 24.7, 48.7 | 0.342 |
| Alcohol consumption, % (0, 1 –139, ≥140 g/week) | 22.6, 30.6, 46.8 | 15.8, 45.1, 39.1 | 10.9, 48.7, 40.4 | 0.015 |
| Physical activity, % (0, 1–119, ≥120 min/week) | 53.2, 33.9, 12.9 | 52.1, 28.1, 19.9 | 44.3, 31.6, 24.1 | 0.093 |
| Green vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 33.9, 27.4, 38.7 | 6.6, 43.2, 50.2 | 2.2, 21.3, 76.5 | <0.001 |
| Other vegetable intake,% (0 –1, 2 –3, ≥4 times/week) | 12.9, 37.1, 50.0 | 2.8, 32.8, 64.4 | 0.4, 13.1, 86.5 | <0.001 |
| Fruit intake, % (0 –1, 2 –3, ≥4 times/week) | 54.8, 14.5, 30.6 | 33.8, 33.4, 32.8 | 18.1, 31.4, 50.5 | <0.001 |
| Egg intake, % (0 –1, 2 –3, ≥4 times/week) | 35.5, 32.3, 32.3 | 13.2, 45.7, 41.0 | 8.5, 30.8, 60.8 | <0.001 |
| Milk intake, % (0 –1, 2 –3, ≥4 times/week) | 32.2, 25.8, 41.9 | 27.1, 22.7, 50.2 | 15.3, 20.5, 64.2 | <0.001 |
| Meat intake, % (0 –1, 2 –3, ≥4 times/week) | 45.2, 46.8, 8.1 | 17.0, 54.6, 28.4 | 14.5, 41.2, 44.3 | <0.001 |
| Fish intake, % (0 –1, 2 –3, ≥4 times/week) | 30.6, 48.4, 21.0 | 4.7, 49.2, 46.1 | 2.6, 26.0, 71.4 | <0.001 |
| Grain intake (times/day) | 3.4 ± 1.3 | 3.4 ± 1.0 | 3.5 ± 1.1 | 0.551 |
BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PG, plasma glucose.
Continuous data with a normal distribution were analyzed with the analysis of variance: mean ± standard deviation. Dichotomous and categorical data were analyzed with the chi‐squared‐test and Fisher's exact test: %.
Table 2. Baseline characteristics according to groups categorized by intake of soybean products in women.
| Group 1 0 –1 time/week | Group 2 2 –3 times/week | Group 3 ≥4 times/week | P | |
|---|---|---|---|---|
| Women with low BMI, n (<22.0 kg/m2) | 21 | 190 | 433 | |
| Age (years) | 51.3 ± 9.4 | 53.6 ± 7.8 | 56.1 ± 7.8 | <0.001 |
| BMI (kg/m2) | 20.1 ± 1.3 | 19.9 ± 1.4 | 20.0 ± 1.4 | 0.743 |
| Systolic blood pressure (mmHg) | 114.4 ± 19.9 | 114.8 ± 15.3 | 115.7 ± 15.8 | 0.775 |
| HDL‐cholesterol (mmol/L) | 1.62 ± 0.26 | 1.73 ± 0.36 | 1.74 ± 0.38 | 0.410 |
| LDL‐cholesterol (mmol/L) | 3.20 ± 0.84 | 3.09 ± 0.71 | 3.22 ± 0.69 | 0.091 |
| Fasting PG (mmol/L) | 5.21 ± 0.22 | 5.28 ± 0.42 | 5.29 ± 0.45 | 0.704 |
| 2‐h PG (mmol/L) | 6.30 ± 1.14 | 5.99 ± 1.31 | 6.00 ± 1.28 | 0.553 |
| HbA1c (%) | 5.40 ± 0.33 | 5.43 ± 0.38 | 5.51 ± 0.32 | 0.016 |
| Smoking status, % (never, current, ex‐) | 71.4, 19.0, 9.5 | 85.8, 9.5, 4.7 | 90.8, 4.4, 4.8 | 0.012 |
| Alcohol consumption, % (0, 1 –139, ≥140 g/week) | 57.1, 33.3, 9.5 | 56.3, 36.8, 6.8 | 56.1, 40.2, 3.7 | 0.370 |
| Physical activity, % (0, 1–119, ≥120 min/week) | 57.1, 38.1, 4.8 | 57.9, 26.3, 15.8 | 41.1, 33.7, 25.2 | 0.001 |
| Green vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 14.3, 47.6, 38.1 | 3.7, 33.7, 62.6 | 2.3, 12.2, 85.5 | <0.001 |
| Other vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 14.3, 42.9, 42.9 | 2.1, 26.3, 71.6 | 0.9, 6.7, 92.4 | <0.001 |
| Fruit intake, % (0 –1, 2 –3, ≥4 times/week) | 14.3, 38.1, 47.6 | 15.3, 27.9, 56.8 | 4.2, 20.1, 75.8 | <0.001 |
| Egg intake, % (0 –1, 2 –3, ≥4 times/week) | 19.0, 52.4, 28.6 | 14.2, 45.3, 40.5 | 9.5, 39.3, 51.3 | 0.033 |
| Milk intake, % (0 –1, 2 –3, ≥4 times/week) | 28.6, 19.0, 52.4 | 11.1, 26.8, 62.1 | 7.4, 16.4, 76.2 | <0.001 |
| Meat intake,% (0 –1, 2 –3, ≥4 times/week) | 42.9, 38.1, 19.0 | 15.3, 52.6, 32.1 | 13.4, 39.5, 47.1 | <0.001 |
| Fish intake,% (0 –1, 2 –3, ≥4 times/week) | 38.1, 28.6, 33.3 | 6.3, 45.8, 47.9 | 1.4, 25.2, 73.4 | <0.001 |
| Grain intake (times/day) | 3.0 ± 0.9 | 2.9 ± 0.7 | 2.9 ± 0.7 | 0.940 |
| Women with high BMI, n (≥22.0 kg/m2) | 19 | 182 | 456 | |
| Age (years) | 53.6 ± 8.5 | 54.5 ± 7.9 | 56.7 ± 7.2 | 0.001 |
| BMI (kg/m2) | 24.2 ± 2.3 | 24.8 ± 2.5 | 24.7 ± 2.5 | 0.618 |
| Systolic blood pressure (mmHg) | 117.0 ± 21.5 | 123.3 ± 17.0 | 123.3 ± 16.6 | 0.272 |
| HDL‐cholesterol (mmol/L) | 1.51 ± 0.41 | 1.55 ± 0.35 | 1.57 ± 0.34 | 0.675 |
| LDL‐cholesterol (mmol/L) | 3.36 ± 0.58 | 3.45 ± 0.71 | 3.46 ± 0.74 | 0.862 |
| Fasting PG (mmol/L) | 5.39 ± 0.35 | 5.49 ± 0.45 | 5.41 ± 0.43 | 0.122 |
| 2‐h PG (mmol/L) | 6.28 ± 1.32 | 6.65 ± 1.34 | 6.64 ± 1.35 | 0.504 |
| HbA1c (%) | 5.60 ± 0.29 | 5.60 ± 0.34 | 5.57 ± 0.31 | 0.605 |
| Smoking status,% (never, current, ex‐) | 94.7, 5.3, 0.0 | 89.0, 7.1, 3.8 | 93.9, 3.5, 2.6 | 0.253 |
| Alcohol consumption, % (0, 1 –139, ≥140 g/week) | 47.4, 52.6, 0.0 | 68.1, 26.4, 5.5 | 58.6, 36.8, 4.6 | 0.045 |
| Physical activity, % (0, 1 –119, ≥120 min/week) | 73.7, 21.1, 5.3 | 58.8, 26.9, 14.3 | 42.5, 35.5, 21.9 | 0.001 |
| Green vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 15.8, 47.4, 36.8 | 2.2, 33.0, 64.8 | 0.7, 11.8, 87.5 | <0.001 |
| Other vegetable intake, % (0 –1, 2 –3, ≥4 times/week) | 5.3, 36.8, 57.9 | 0.5, 22.0, 77.5 | 0.0, 6.4, 93.6 | <0.001 |
| Fruit intake, % (0 –1, 2 –3, ≥4 times/week) | 21.1, 26.3, 52.6 | 14.8, 36.3, 48.9 | 8.1, 19.7, 72.1 | <0.001 |
| Egg intake,% (0 –1, 2 –3, ≥4 times/week) | 31.6, 47.4, 21.1 | 12.6, 49.5, 37.9 | 10.5, 33.8, 55.7 | <0.001 |
| Milk intake, % (0 –1, 2 –3, ≥4 times/week) | 31.6, 26.3, 42.1 | 15.9, 25.8, 58.2 | 10.7, 20.0, 69.3 | 0.005 |
| Meat intake, % (0 –1, 2 –3, ≥4 times/week) | 21.1, 68.4, 10.5 | 18.1, 50.5, 31.3 | 13.6, 39.5, 46.9 | <0.001 |
| Fish intake, % (0 –1, 2 –3, ≥4 times/week) | 21.1, 52.6, 26.3 | 8.8, 41.8, 49.5 | 2.9, 17.3, 79.8 | <0.001 |
| Grain intake (times/day) | 2.9 ± 0.5 | 2.9 ± 0.8 | 2.9 ± 0.7 | 0.973 |
BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; PG, plasma glucose.
Continuous data with a normal distribution were analyzed with the analysis of variance: mean ± standard deviation. Dichotomous and categorical data were analyzed with the chi‐squared‐test and Fisher's exact test: %.
The median follow up was 4.0 years (total person‐years: 10,503), during which 204 individuals developed type 2 diabetes mellitus, including 26 defined as having type 2 diabetes mellitus by receiving medical treatment for this disease. Of those, 61 participants developed fasting hyperglycemia and 147 participants developed postload hyperglycemia. Because the youngest of these individuals was 40‐years‐old at baseline, all incident cases were assumed to be type 2 diabetes mellitus. Table 3 shows the multivariable‐adjusted HRs and 95% CIs for the incidence of type 2 diabetes mellitus, fasting hyperglycemia and postload hyperglycemia according to groups categorized by soybean product intake in men. Among men with a high BMI, men of group 3 had a significantly lower risk for incidences of type 2 diabetes mellitus, fasting hyperglycemia and postload hyperglycemia than men of group 1, and the multivariable‐adjusted HRs and 95% CIs were 0.44 (0.22–0.89), 0.36 (0.15–0.96) and 0.40 (0.18–0.92), respectively. In addition, there were significant linear decreases in the multivariable‐adjusted HRs for the incidence of type 2 diabetes mellitus and postload hyperglycemia (P for trend = 0.014 and 0.032, respectively). Figure 1 shows FPG and 2‐h PG levels at the follow‐up end among men who were diagnosed type 2 diabetes mellitus by 75‐g OGTT. Of the men who developed type 2 diabetes mellitus among the men with high BMI, the number of men whose HbA1c increased to more than 6.5% was eight (group 1: 0, group 2: 4, group 3: 4). When high BMI was defined as BMI ≥25.0 kg/m2, the number of men with high BMI were 514 (group 1: 37, group 2: 189, group 3: 288) and the multivariable‐adjusted HRs and 95% CIs for type 2 diabetes mellitus, fasting hyperglycemia and postload hyperglycemia among group 3 in men with high BMI were 0.35 (0.15–0.81), 0.32 (0.09–1.11) and 0.31 (0.12–0.83), respectively. These significant associations between soybean product intake and incidence of type 2 diabetes mellitus were observed after further adjustment for fish intakes.
Table 3. Multivariable adjusted hazard ratios for incidence of diabetes according to groups categorized by intake of soybean products.
| Group 1 0 –1 time/week | Group 2 2 –3 times/week | Group 3 ≥4 times/week | P for trend | |
|---|---|---|---|---|
| Men with low BMI, n (<23.6 kg/m2) | 71 | 304 | 487 | |
| Incidence of type 2 diabetes mellitus | ||||
| Case | 2 | 23 | 28 | |
| Incidence rate/1000 person‐years | 8.3 | 21.8 | 16.9 | |
| HRs (95% CIs) | ||||
| Model 1 | 1.00 | 2.55 (0.60–10.81) | 1.79 (0.43–7.52) | 0.816 |
| Model 2 | 1.00 | 2.49 (0.58–10.67) | 1.84 (0.42–8.02) | 0.952 |
| Incidence of fasting hyperglycemia | ||||
| Case | 1 | 3 | 8 | |
| Incidence rate/1000 person‐years | 4.1 | 2.8 | 4.7 | |
| HRs (95% CIs) | ||||
| Model 1 | 1.00 | 0.67 (0.07–6.48) | 1.01 (0.13–8.09) | 0.721 |
| Model 2 | 1.00 | 0.74 (0.08–7.29) | 1.17 (0.13–10.52) | 0.656 |
| Incidence of postload hyperglycemia | ||||
| Case | 2 | 20 | 22 | |
| Incidence rate/1000 person‐years | 8.3 | 18.9 | 13.0 | |
| HRs (95% CIs) | ||||
| Model 1 | 1.00 | 2.21 (0.52–9.44) | 1.39 (0.33–5.93) | 0.543 |
| Model 2 | 1.00 | 2.17 (0.50–9.39) | 1.48 (0.33–6.54) | 0.729 |
| Men with high BMI, n (≥23.6 kg/m2) | 62 | 317 | 497 | |
| Incidence of type 2 diabetes mellitus | ||||
| Case | 11 | 38 | 44 | |
| Incidence rate/1000 person‐years | 53.9 | 35.2 | 25.5 | |
| HRs (95% CIs) | ||||
| Model 1 | 1.00 | 0.66 (0.34–1.29) | 0.45 (0.23–0.88) | 0.011 |
| Model 2 | 1.00 | 0.65 (0.33–1.29) | 0.44 (0.22–0.89) | 0.014 |
| Incidence of fasting hyperglycemia | ||||
| Case | 6 | 14 | 18 | |
| Incidence rate/1000 person‐years | 28.6 | 12.6 | 10.3 | |
| HRs (95% CIs) | ||||
| Model 1 | 1.00 | 0.44 (0.17–1.15) | 0.35 (0.14–0.89) | 0.060 |
| Model 2 | 1.00 | 0.46 (0.17–1.21) | 0.36 (0.15–0.96) | 0.075 |
| Incidence of postload hyperglycemia | ||||
| Case | 8 | 24 | 29 | |
| Incidence rate/1000 person‐years | 38.3 | 21.8 | 16.6 | |
| HRs (95% CIs) | ||||
| Model 1 | 1.00 | 0.57 (0.26–1.28) | 0.41 (0.19–0.90) | 0.030 |
| Model 2 | 1.00 | 0.57 (0.25–1.28) | 0.40 (0.18–0.92) | 0.032 |
HR, hazard ratio; CI, confidence interval; FPG, fasting plasma glucose; PG, plasma glucose.
Model 1 adjusted by age. Model 2 adjusted by age, body mass index (BMI), alcohol consumption, smoking status, physical activity, family history of diabetes, green vegetable intake and fruit intake.
Figure 1.
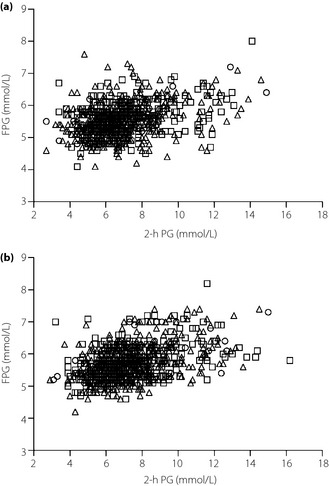
Fasting plasma glucose (FPG) levels and 2‐h plasma glucose (PG) levels at the end of follow up among men. (a) Men with low body mass index (BMI). (b) Men with high BMI. The circles show group 1, the squares show group 2 and the triangles show group 3.
In addition, incident rates of impaired glucose tolerance and impaired fasting glucose among a subcohort of men with normoglycaemia at baseline are shown in Table 4. Incident rates in group 3 were lower than that in group 1 among men with high BMI. The number of incident cases in women was too small to calculate the adjusted HRs, especially in group 1: 26 cases (group 1: 0, group 2: 11, group 3: 15) among women with a low BMI and 32 cases (group 1: 1, group 2: 7, group 3: 24) among women with a high BMI.
Table 4. Incident rates of impaired glucose tolerance and impaired fasting glucose according to groups categorized by intake of soybean products among a subcohort of men with normoglycemia at baseline.
| Group 1 0 –1 time/week | Group 2 2 –3 times/week | Group 3 ≥4 times/week | |
|---|---|---|---|
| Case (incident rates/1000 person‐years) | |||
| Men with low BMI, n (<23.6 kg/m2) | 57 | 213 | 341 |
| Impaired glucose tolerance | 12 (67.4) | 53 (77.5) | 86 (78.2) |
| Impaired fasting glucose | 6 (32.6) | 34 (47.8) | 33 (28.4) |
| Men with high BMI, n (≥23.6 kg/m2) | 46 | 219 | 346 |
| Impaired glucose tolerance | 19 (159.7) | 75 (113.6) | 129 (125.4) |
| Impaired fasting glucose | 9 (63.4) | 38 (53.4) | 63 (55.3) |
BMI, body mass index.
Impaired glucose tolerance was defined as 2 h plasma glucose ≥7.8 mmol/L. Impaired fasting glucose was defined as fasting plasma glucose ≥6.1 mmol/L.
Discussion
This is the first report of a prospective cohort study investigating the association between soybean product intake and incidence of type 2 diabetes mellitus considering fasting and postload hyperglycemia. The main findings showed that soybean product intake prevented the increase of 2‐h PG, as well as FPG, in men with a high BMI. The findings are very important with regard to the prevention of cardiovascular disease, as well as type 2 diabetes mellitus.
The effect of soybean product intake on type 2 diabetes mellitus might be accounted for by the dietary fiber content and the glycemic index (GI) of soybean products. A previous study involving rats reported that long‐term dietary fiber intake significantly improved the area under the curve of plasma glucose concentration over time, and lowered FPG and HbA1c levels15. In addition, it has been reported that ingesting a low‐glycemic load meal containing dietary fiber at breakfast significantly improves the breakfast postload glycemic response in adults with type 2 diabetes mellitus16. Soybean products include substantial concentrations of dietary fiber. Although rice, which is a major grain in Japan, has a high GI, the reduction of the GI occurred whether soybean products were taken together, before or after rice intake17. These effects of soybean products might play an important role in the prevention of increasing 2‐h PG levels. In contrast, a previous cross‐sectional study reported that dietary fiber intake was not associated with impaired glucose tolerance or fasting glucose18. The FPG, 2‐h PG and HbA1c levels at baseline were not associated with soybean product intake in the present study (Table 1). Therefore, the effects of soybean product intake might appear in the long term.
The effects of soybean product intake on type 2 diabetes mellitus were not observed in men with a low BMI. Obesity is a major risk factor for the incidence of type 2 diabetes mellitus. FPG, 2‐h PG and HbA1c levels were better at baseline in men with a low BMI than in men with a high BMI. Therefore, men with a low BMI were at a lower risk for incidence of type 2 diabetes mellitus than men with a high BMI. To confirm the effects of soybean product intake on type 2 diabetes mellitus in men with a low BMI, long‐term observation might be necessary.
Participants with a high intake of soybean products also consumed other healthy foods, such as vegetables or fruits. It has been reported that dietary patterns including higher intake of vegetables, fruits and soybean products were inversely associated with the risk of incidence of type 2 diabetes mellitus19. Because we adjusted for vegetable and fruit intake in estimating the risk of type 2 diabetes mellitus, soybean product intake could be effective for type 2 diabetes mellitus alone. However, we cannot completely eliminate the effects of the intake of other healthy foods.
The strengths of the present study include the large, community‐based cohort and extensive data on the diagnosis of type 2 diabetes mellitus based on OGTT using the current definition of the disease14. Furthermore, the 12‐h overnight fast before OGTT was managed by being hospitalized from the day before, and the type 2 diabetes mellitus patients were screened every year. However, the study had several limitations. First, a non‐validated questionnaire for food intake was used in the present study so that elderly participants could answer easily. Some misclassification of exposure to soybean product intake was inevitable. Second, an issue regarding dietary assessment is that total energy and nutrient intake determined from the questionnaire were not calculated and validated. Participants with a high intake of soybean products probably consumed more energy than those with a low intake of soybean products; high‐energy intake usually increases the risk of type 2 diabetes mellitus, and the lack of adjustment for energy intake might cause an erroneous positive association. However, energy adjustment only strengthens, rather than diminishes, the inverse association between soybean product intake and the incidence of type 2 diabetes mellitus, which is the major finding of the present study. Third, the number of cases in some groups was small. The statistical power of group 3 in men with high BMI for the incidence of type 2 diabetes mellitus was approximately 75%. Further studies with large sample size are required. Finally, the estimated risks might be underestimated because of the regression dilution bias given that the question about soybean product intake was asked only once21.
In conclusion, the present findings suggest that soybean product intake prevented fasting and postload hyperglycemia in men with a high BMI. There was a dose–response relationship between soybean product intake and these outcomes. Although obesity is a major risk factor of type 2 diabetes mellitus, dietary habits could lower the risk of postload hyperglycemia, as well as fasting hyperglycemia. Furthermore, long‐term observation is necessary to confirm the effects of soybean product intake on fasting and postload hyperglycemia in men with a low BMI.
Supplementary Material
Appendix S1| Food frequency questionnaire in the present study.
Acknowledgements
All authors were involved in the writing of the manuscript and approved the final version of this article. This work was supported in part by grants from the Ministry of Health, Labour and Welfare, Japan, and grants from the Fuji Foundation for Protein Research. We sincerely thank all researchers and co‐medical staff of Saku Central Hospital for their excellent medical examinations and follow‐up surveys. There is no conflict of interest in all the authors listed.
(J Diabetes Invest, doi: 10.1111/jdi.12100, 2013)
References
- 1.Bartnik M, Ryden L, Ferrari R, et al Euro Heart Survey Investigators. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J 2004; 25: 1880–1890 [DOI] [PubMed] [Google Scholar]
- 2.Barr EL, Zimmet PZ, Welborn TA, et al Risk of cardiovascular and all‐cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study. Circulation 2007; 116: 151–157 [DOI] [PubMed] [Google Scholar]
- 3.DECODE Study Group the European Diabetes Epidemiology Group . Glucose tolerance and cardiovascular mortality: comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med 2001; 161: 397–405 [DOI] [PubMed] [Google Scholar]
- 4.Oizumi T, Daimon M, Jimbu Y, et al Impaired glucose tolerance is a risk factor for stroke in a Japanese sample–the Funagata study. Metabolism 2008; 57: 333–338 [DOI] [PubMed] [Google Scholar]
- 5.Tominaga M, Eguchi H, Manaka H, et al Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999; 22: 920–924 [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Lichtenstein A, Van Horn L, et al Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006; 113: 1034–1044 [DOI] [PubMed] [Google Scholar]
- 7.Anderson JW, Johnstone BM, Cook‐Newell ME. Meta‐analysis of the effects of soy protein intake on serum lipids. N Engl J Med 1995; 333: 276–282 [DOI] [PubMed] [Google Scholar]
- 8.Feskens EJ, Bowles CH, Kromhout D. Carbohydrate intake and body mass index in relation to the risk of glucose intolerance in an elderly population. Am J Clin Nutr 1991; 54: 136–140 [DOI] [PubMed] [Google Scholar]
- 9.Villegas R, Gao YT, Yang G, et al Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr 2008; 87: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanri A, Mizoue T, Takahashi Y, et al Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J Nutr 2010; 140: 580–586 [DOI] [PubMed] [Google Scholar]
- 11.Morimoto A, Tatsumi Y, Deura K, et al Impact of cigarette smoking on impaired insulin secretion and insulin resistance in Japanese men: the Saku Study. J Diabetes Invest 2012; doi: 10.1111/jdi.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . Report of a WHO Consultation: Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Part 1. Diagnosis and Classification of Diabetes Mellitus. Department of Noncommunicable Disease Surveillance, Geneva, Switzerland, 1999 [Google Scholar]
- 15.Li J, Kaneko T, Qin LQ, et al Long‐term effects of high dietary fiber intake on glucose tolerance and lipid metabolism in GK rats: comparison among barley, rice, and cornstarch. Metabolism 2003; 52: 1206–1210 [DOI] [PubMed] [Google Scholar]
- 16.Clark CA, Gardiner J, McBurney MI, et al Effects of breakfast meal composition on second meal metabolic responses in adults with Type 2 diabetes mellitus. Eur J Clin Nutr 2006; 60: 1122–1129 [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama M, Tang AC, Wakaki Y, et al Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr 2003; 57: 743–752 [DOI] [PubMed] [Google Scholar]
- 18.Sartorelli DS, Freire RD, Ferreira SR, et al Japanese‐Brazilian Diabetes Study Group. Dietary fiber and glucose tolerance in Japanese Brazilians. Diabetes Care 2005; 28: 2240–2242 [DOI] [PubMed] [Google Scholar]
- 19.Morimoto A, Ohno Y, Tatsumi Y, et al Effects of healthy dietary pattern and other lifestyle factors on incidence of diabetes in a rural Japanese population. Asia Pac J Clin Nutr 2012; 21: 601–608 [PubMed] [Google Scholar]
- 20.Odegaard AO, Koh WP, Butler LM, et al Dietary patterns and incident type 2 diabetes in chinese men and women: the singapore chinese health study. Diabetes Care 2011; 34: 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMahon S, Peto R, Cutler J, et al Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335: 765–774 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1| Food frequency questionnaire in the present study.


