Abstract
Aims/Introduction
Postprandial hyperglycemia is a potent risk factor for cardiovascular disease. Serum glycated albumin (GA) has been reported to reflect postprandial blood glucose fluctuations. In the present study, we assessed the possible correlation of GA with the presence of carotid plaque to evaluate the potential clinical usefulness of GA for predicting atherosclerotic cardiovascular complications in patients with type 2 diabetes.
Materials and Methods
Patients with type 2 diabetes (n = 236) admitted to Nippon Medical School Hospital (Tokyo, Japan) for glycemic control (aged 19–86 years, 81 females and 155 males) were examined. Clinical measurements were taken on admission. The presence of carotid plaque was assessed by ultrasonography.
Results
In patients with carotid plaque (n = 154), GA (P = 0.023) was higher than those without carotid plaque (n = 82). In contrast, neither fasting plasma glucose (P = 0.48) nor glycated hemoglobin (P = 0.41) was significantly different between the groups. The results of logistic regression analysis showed that GA (age‐ and sex‐adjusted odds ratio [95% confidence interval], 1.05 [1.01–1.09]; P = 0.017) and glycated hemoglobin (1.17 [1.01–1.37]; P = 0.036) were significantly associated with the presence of carotid plaque.
Conclusions
The positive correlation of serum GA with the presence of carotid plaque in type 2 diabetes suggests that GA will serve as a useful clinical marker for predicting diabetic cardiovascular complications.
Keywords: Carotid plaque, Glycated albumin, Type 2 diabetes mellitus
Introduction
Glycated hemoglobin (HbA1c) is regarded as a gold standard for monitoring glycemic control. Most expert committees now recommend the use of HbA1c in the diagnosis of diabetes1. Although the overall usefulness of HbA1c is well accepted, epidemiological data suggest that HbA1c is not always versatile for predicting all types of diabetic complication; namely, whereas HbA1c is a good predictor of microvascular complications, it appears to be less so for macrovascular outcomes2. One interpretation is that the fluctuation of blood glucose contributes more to macrovascular diabetic complications than time‐averaged blood glucose concentration represented by HbA1c2. Postprandial acute glucose fluctuations are postulated to contribute to the pathogenesis of atherosclerotic cardiovascular complications through the induction of oxidative stress and consequent endothelial dysfunction3.
Whereas glycation of hemoglobin in erythrocytes (i.e., HbA1c) might not reflect blood glucose fluctuations, several reports have shown that glycation of serum albumin reflects the glycemic excursions6. The glycation of serum proteins has been long assessed by the measurement of fructosamine; however, the reduction reaction‐based colorimetric assay is influenced by protein concentration and other coexisting substances in serum8. For assessing serum protein glycation more accurately, glycated albumin (GA) assay has been developed. GA was originally analyzed by high‐performance liquid chromatography (HPLC), but it can currently be determined with automated clinical analyzers by a rapid and specific enzymatic method9. The measurement of GA is now available for monitoring glycemic control in patients with diabetes under public health insurance coverage in Japan. GA can be a potential index for predicting cardiovascular events, as it reflects blood glucose fluctuations better than HbA1c6. Postprandial hyperglycemia is epidemiologically a more potent risk factor for diabetic cardiovascular complications than fasting plasma glucose (FPG)10.
In the present study, we assessed the association of GA with the presence of carotid plaque, a surrogate marker for atherosclerotic disease, to evaluate the potential usefulness of the measurement of GA for predicting cardiovascular complications in type 2 diabetes.
Materials and Methods
Participants
Patients with type 2 diabetes who were admitted to Nippon Medical School Hospital (Tokyo, Japan) for glycemic control during 2005–2012 were enrolled in the present study (n = 236, aged 19–86 years, 81 females and 155 males). Exclusion criteria included diabetic nephropathy stage 3 or higher (urinary albumin exertion ≥300 mg/g·Cr [spot], or ≥300 mg/day; Joint Committee of the Japan Diabetes Society and the Japanese Society of Nephrology12), uncontrolled endocrine disease, steroid treatment, ketoacidosis, estimated primary hyperlipidemia, malignant disease, anemia (hemoglobin <10.0 g/dL) and other systemic disorders. The present study was approved by the Institutional Review Board, and all participants were enrolled after giving informed consent.
Clinical Measurements
All participants underwent a physical examination including height, bodyweight and blood pressure on the first morning of admission. Blood sample was taken after an overnight fast. FPG, HbA1c and serum GA were measured by the glucose oxidase method (ADAMS Glucose GA‐1170; Arkray, Kyoto, Japan), HPLC (ADAMS A1c HA‐8160; Arkray) and an enzymatic method using albumin‐specific proteinase and ketoamine oxidase (Lucica GA‐L; Asahi Kasei Pharma, Tokyo, Japan), respectively. HbA1c level was expressed as the percentage value of the National Glycohemoglobin Standardization Program according to the guideline of the Japan Diabetes Society13. GA was expressed as a percentage of total serum albumin. Serum total cholesterol, high‐density lipoprotein (HDL)‐cholesterol and triacylglycerol were measured enzymatically (Sekisui Medical, Tokyo, Japan). Low‐density lipoprotein cholesterol concentration was calculated by the Friedewald formula15. Smoking habit (current, past or never) and type 2 diabetes duration were assessed by interview.
Carotid Ultrasonography
Carotid artery status was examined with high‐resolution B‐mode ultrasound systems (SDU‐2000; Shimadzu, Kyoto, Japan; iU22 and EnVisor; Philips Healthcare, Andover, MA, USA; LOGIQ 7; GE Healthcare, Little Chalfont, England, UK) equipped with linear transducers with a frequency of 3–12 MHz as previously described16. Carotid plaque was defined as a focal intima‐media thickening ≥1.0 mm with marked protrusion into the lumen.
Statistical Analysis
Continuous variables are expressed as means ± standard deviation or median (interquartile range) for variables with normal or skewed distribution, respectively. Differences in clinical data between participants with and without carotid plaque were assessed by Student's t‐test, Mann–Whitney U‐test or χ2‐test as appropriate. Correlations between GA and other continuous variables were examined by Pearson's correlation analysis. A logistic regression model was applied to determine the odds ratio for the presence of carotid plaque. A P‐value of <0.05 was considered as significant. All analyses were carried out with jmp software (version 9.0; SAS Institute, Cary, NC, USA).
Results
Of 236 participants enrolled in the present study, 154 (65%) had carotid plaque. The clinical characteristics for each group are shown in Table 1. In the participants with carotid plaque, age was higher and the duration of type 2 diabetes was longer than in those without carotid plaque. Body mass index (BMI) was lower in the participants with carotid plaque compared with those without. In glycemic control indices, GA and GA‐to‐HbA1c ratio (GA/HbA1c) were higher in the participants with carotid plaque than in those without, whereas neither FPG nor HbA1c was significantly different between the two groups. With regard to diabetic complications, the participants with carotid plaque had a higher incidence of retinopathy or abnormal Achilles tendon reflex than those without. Regarding prehospital medication, a higher population of the participants with carotid plaque had been treated with an antihypertensive agent or antiplatelet agent than those without.
Table 1. Clinical characteristics of the participants.
| Variable | All participants | Carotid plaque | P‐value* | |
|---|---|---|---|---|
| (n = 236) | + (n = 154) | – (n = 82) | ||
| Age (years) | 56 ± 13 | 60 ± 11 | 50 ± 14 | <0.0001 |
| Sex (female/male) | 81/155 | 52/102 | 29/53 | 0.81 |
| Duration of type 2 diabetes (years) | 5 [0–11] | 6 [1–15] | 3 [0–10] | 0.0069 |
| Systolic blood pressure (mmHg) | 127 ± 15 | 128 ± 15 | 125 ± 15 | 0.073 |
| Diastolic blood pressure (mmHg) | 75 ± 11 | 75 ± 10 | 74 ± 11 | 0.58 |
| BMI (kg/m2) | 25.4 ± 4.9 | 24.8 ± 4.5 | 26.4 ± 5.6 | 0.014 |
| Smoking habit, current or past (n [%]) | 148 [63] | 102 [66] | 46 [56] | 0.13 |
| Fasting plasma glucose (mmol/L) | 9.79 ± 2.99 | 9.89 ± 3.04 | 9.60 ± 2.89 | 0.48 |
| HbA1c (%) | 10.3 ± 2.1 | 10.4 ± 2.1 | 10.1 ± 1.9 | 0.41 |
| GA (%) | 28.5 ± 8.4 | 29.4 ± 8.9 | 26.8 ± 7.0 | 0.023 |
| GA/HbA1c | 2.74 ± 0.45 | 2.80 ± 0.45 | 2.63 ± 0.43 | 0.0037 |
| Total cholesterol (mmol/L) | 5.31 ± 1.06 | 5.28 ± 1.06 | 5.37 ± 1.07 | 0.55 |
| HDL‐cholesterol (mmol/L) | 1.27 ± 0.35 | 1.26 ± 0.32 | 1.27 ± 0.41 | 0.89 |
| LDL‐cholesterol (mmol/L)* | 3.26 ± 0.89 | 3.26 ± 0.89 | 3.25 ± 0.88 | 0.93 |
| Non HDL‐cholesterol (mmol/L) | 4.05 ± 1.05 | 4.02 ± 1.04 | 4.10 ± 1.08 | 0.58 |
| Triacylglycerols (mmol/L) | 1.72 ± 0.94 | 1.68 ± 0.88 | 1.81 ± 1.05 | 0.31 |
| Retinopathy (n [%]) | 49 [21] | 41 [27] | 8 [10] | 0.0014 |
| Albuminuria, >30 mg/mg·Cr (n [%]) | 36 [15] | 26 [17] | 10 [12] | 0.33 |
| Abnormal Achilles tendon reflex (n [%]) | 98 [42] | 75 [49] | 23 [28] | 0.0019 |
| Prehospital medication | ||||
| Insulin (n [%]) | 29 [12] | 23 [15] | 6 [7] | 0.078 |
| Oral hypoglycemic agent (n [%]) | 120 [51] | 81 [53] | 39 [48] | 0.46 |
| Statin (n [%]) | 47 [20] | 36 [23] | 11 [13] | 0.061 |
| Antihypertensive agent (n [%]) | 75 [32] | 57 [37] | 18 [22] | 0.016 |
| Antiplatelet agent (n [%]) | 26 [11] | 23 [15] | 3 [4] | 0.0043 |
Continuous variables are expressed as means ± SD or median [interquartile range]. *For differences between the subjects with (+) and without (–) carotid plaque. †As low‐density lipoprotein (LDL)‐cholesterol was calculated by the Friedewald formula, three participants (one in + and two in –) with hypertriacylglycerolemia (≥4.5 mmol/L) were excluded from the statistical analysis of LDL‐cholesterol. BMI, body mass index; GA, glycated albumin; GA/HbA1c, glycated albumin‐to‐glycated hemoglobin ratio; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein.
Table 2 shows Pearson's correlation coefficients between GA and other continuous variables. GA was positively correlated with FPG, HbA1c and HDL‐cholesterol, whereas inversely with systolic blood pressure, diastolic blood pressure and BMI.
Table 2. Pearson's correlation coefficients between glycated albumin and other continuous variables.
| Variable | r | P‐value |
|---|---|---|
| Age | 0.0077 | 0.91 |
| Duration of type 2 diabetes | −0.041 | 0.53 |
| Systolic blood pressure | −0.19 | 0.0028 |
| Diastolic blood pressure | −0.13 | 0.046 |
| BMI | −0.39 | <0.0001 |
| Fasting plasma glucose | 0.71 | <0.0001 |
| HbA1c | 0.83 | <0.0001 |
| Total cholesterol | 0.11 | 0.10 |
| HDL‐cholesterol | 0.15 | 0.022 |
| LDL‐cholesterola | 0.083 | 0.20 |
| Non HDL‐cholesterol | 0.056 | 0.39 |
| Triacylglycerols | −0.044 | 0.50 |
Three participants were excluded from the statistical analysis of low‐density lipoprotein (LDL)‐cholesterol (see the footnote to Table 1). BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein.
Table 3 shows the results of logistic regression analysis for the presence of carotid plaque. Age, duration of type 2 diabetes, GA and GA/HbA1c were positively associated with the presence of carotid plaque in unadjusted univariate analysis, whereas BMI was inversely associated with that. After the analysis was adjusted for age and sex, GA and HbA1c were significant predictors of the carotid plaque presence. In contrast, no significant association was found between GA/HbA1c and the presence of carotid plaque after the adjustment. To address the discrepancies between these glycemic control indices (GA, HbA1c and GA/HbA1c) and the carotid plaque presence with or without the adjustment, we analyzed the correlations between these glycemic control indices and age. Whereas no correlation was found between GA and age (Figure 1a), HbA1c was inversely correlated with age (Figure 1b). As a consequence, GA/HbA1c was positively correlated with age (Figure 1c).
Table 3. Odds ratios of variables for the presence of plaque.
| Variable | Unadjusted | Age‐ and sex‐adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Age (per 1 year) | 1.07 (1.04–1.09) | <0.0001 | ||
| Sex (male)* | 1.07 (0.61–1.88) | 0.81 | ||
| Duration of type 2 diabetes (per 1 year) | 1.05 (1.01–1.09) | 0.0050 | 1.01 (0.98–1.05) | 0.51 |
| BMI (per 1 kg/m2) | 0.93 (0.88–0.99) | 0.015 | 0.98 (0.92–1.04) | 0.49 |
| Smoking habit (current or past)† | 1.54 (0.89–2.66) | 0.13 | 1.80 (0.97–3.37) | 0.062 |
| Fasting plasma glucose (per 1 mmol/L) | 1.03 (0.94–1.13) | 0.48 | 1.06 (0.96–1.17) | 0.24 |
| HbA1c (per 1%) | 1.06 (0.93–1.21) | 0.41 | 1.17 (1.01–1.37) | 0.036 |
| GA (per 1%) | 1.04 (1.01–1.08) | 0.020 | 1.05 (1.01–1.09) | 0.017 |
| GA/HbA1c (per 1) | 2.57 (1.36–5.05) | 0.0032 | 1.74 (0.87–3.60) | 0.12 |
| Total cholesterol (per 1 mmol/L) | 0.93 (0.72–1.19) | 0.55 | 1.00 (0.76–1.32) | 0.98 |
| HDL‐cholesterol (per 1 mmol/L) | 0.95 (0.45–2.05) | 0.89 | 0.42 (0.17–1.01) | 0.052 |
| Non HDL‐cholesterol (per 1 mmol/L) | 0.93 (0.72–1.20) | 0.58 | 1.09 (0.82–1.44) | 0.56 |
| LDL‐cholesterol (per 1 mmol/L)‡ | 1.01 (0.75–1.38) | 0.93 | 1.08 (0.78–1.52) | 0.63 |
| Triacylglycerols (per 1 mmol/L) | 0.87 (0.65–1.15) | 0.31 | 1.15 (0.85–1.59) | 0.37 |
BMI, body mass index; CI, confidence interval; GA, glycated albumin; GA/HbA1c, glycated albumin‐to‐glycated hemoglobin ratio; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; OR, odds ratio. *Female as reference. †Never as reference. ‡Three participants were excluded from the statistical analysis of low‐density lipoprotein (LDL)‐cholesterol (see the footnote to Table 1).
Figure 1.
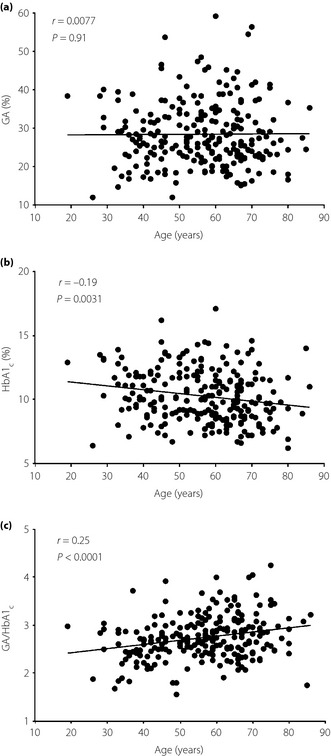
Correlations of (a) glycated albumin (GA), (b) glycated hemoglobin (HbA1c) and (c) GA‐to‐HbA1c ratio (GA/HbA1c) with age in the participants. Pearson's correlation coefficient is shown in each panel.
Discussion
HbA1c and GA basically provide similar clinical information on recent glycemic control. However, owing to the shorter half‐life of serum albumin than erythrocyte hemoglobin, GA reflects shorter‐term blood glucose concentration (over 2–3 weeks) compared with HbA1c, which reflects that over 2–3 months17. GA should therefore be a more suitable index of glycemic control than HbA1c in cases where rapid changes might occur in blood glucose concentration; for example, when starting or changing diabetes treatments6. GA is also proposed as a useful measure of glycemic control in populations for whom HbA1c might not reflect glycemic status accurately; that is, anemics, those with hemoglobinopathies or neonates19. More intriguingly, GA has been reported to reflect blood glucose fluctuations7. A recent report clearly showed that GA, but not HbA1c, was positively associated with glycemic excursions, which were assessed with 48‐h continuous glucose monitoring20.
Because GA has been reported to reflect blood glucose fluctuations and individuals with greater postprandial glycemic excursions are more likely to develop cardiovascular disease10, GA might serve as a useful marker for predicting diabetic cardiovascular complications. A recent study actually reported that GA, but not HbA1c, was associated with the increasing degree of coronary stenosis in type 2 diabetes21. The higher GA level in subjects with carotid plaque among the present participants (Table 1) also supports the clinical usefulness of GA for evaluating the risk of cardiovascular complications.
The positive correlations of GA with other glycemic indices (FPG and HbA1c) suggest that GA increases with the deterioration of overall glycemic control (Table 2). Furthermore, GA was associated with the presence of carotid plaque, and the association remained significant after the logistic regression model was adjusted for age and sex (Table 3). As a recent meta‐analysis of prospective cohorts22 and an additional relevant report23 showed, there is no doubt that higher HbA1c level is associated with the risk of cardiovascular events in the general population without diabetes. However, in subjects with type 2 diabetes, epidemiological evidence indicates a weaker association of HbA1c with macrovascular outcomes than that with microvascular complications24. Ishizaka et al.25 actually demonstrated that HbA1c level was strongly associated with the presence of carotid plaque in normal subjects (normal FPG and normal glucose tolerance); however, the association was not observed in subjects with diabetes or prediabetes. Similar to that report, no significant difference was found in HbA1c between the subjects with and without carotid plaque in the present study (Table 1). As patients with poor glycemic control were enrolled in the present study, their higher HbA1c levels within a narrow range might be one reason for the lack of association. However, age‐ and sex‐adjusted logistic regression analysis showed the association between HbA1c and carotid plaque prevalence (Table 3).
Even though we excluded patients with anemia (hemoglobin <10.0 g/dL) and renal failure (stage 3 or higher), HbA1c showed a significant inverse correlation with age in the present participants (Figure 1b). Several reports suggested that HbA1c can be affected by age26 and ethnic group28. Recently, Khoo et al.30 reported a discrepancy between the oral glucose tolerance test (OGTT) and HbA1c to diagnose diabetes in elderly populations. They suggested a higher false negative rate when diagnosing diabetes by HbA1c alone as compared with by OGTT. These results suggest that HbA1c alone is not enough to evaluate glycemic control, especially in elderly patients with type 2 diabetes. Conversely, as GA was not affected by age (Figure 1a), the measurement of GA might have clinical value for evaluating glycemic control more accurately, and for predicting atherosclerotic cardiovascular outcomes, especially in patients with type 2 diabetes.
As GA reflects blood glucose fluctuations better than HbA1c6, GA/HbA1c might reflect the postprandial glycemic response and could be useful for predicting diabetic complications31. Most recently, possible clinical use of both GA and GA/HbA1c for predicting the presence of carotid atherosclerosis32 and the progression of intima‐media thickness33 were also reported in outpatients with type 2 diabetes. However, in the present study, the association between GA/HbA1c and the presence of carotid atherosclerosis was not significant after the adjustment for age and sex. Similarly, no significant correlation was found between GA/HbA1c and intima‐media thickness after the adjustment (data not shown). One of the possible reasons for the differences from the previous reports32 is the age distribution. Relative to the participants in those reports (aged 40–70 years and 53–68 years in the reports of Moon et al.32 and Song et al.33, respectively), the present participants had a wider age distribution (19–86 years). As HbA1c was inversely correlated with age (Figure 1b), GA/HbA1c (the index defined by reciprocal value of HbA1c) showed a positive correlation with age in the present study (Figure 1c). These results suggest that age‐related differences among the glycemic control indices should be taken into account for clinical use of GA/HbA1c as a surrogate marker of diabetic complications.
In the present study, BMI was inversely correlated with GA (Table 2). Although the reasons remain unknown, several reports also showed that BMI was inversely correlated with GA34. Further investigation into the causes of the relationship between GA and BMI might provide new insights into the interpretation of glycemic control indices in the research and clinical practice of diabetes and its complications.
In conclusion, the present results showed that GA and GA/HbA1c were higher in subjects with carotid plaque among patients with type 2 diabetes. Logistic regression analysis showed the positive association of GA and HbA1c with the presence of carotid plaque when the models were adjusted for age and sex. Because this was a cross‐sectional study, the present data provide just a snapshot of each patient at admission; this is a limitation of the present study. As atherosclerotic lesion formation is a longitudinal event, the longstanding history of vascular conditions should be taken into account. Nevertheless, the present cross‐sectional data should warrant further prospective investigation to evaluate the clinical usefulness of GA for predicting cardiovascular outcomes in patients with type 2 diabetes.
Acknowledgements
The authors declare that there is no duality of interest associated with this manuscript.
(J Diabetes Invest, doi: 10.1111/jdi.12085, 2013)
References
- 1.Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: response to the International Expert Committee. Diabetes Care 2009; 32: 159–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen RM, Smith EP. Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care 2008; 11: 512–517 [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Esposito K, Piconi L, et al Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57: 1349–1354 [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1–7 [DOI] [PubMed] [Google Scholar]
- 5.Monnier L, Mas E, Ginet C, et al Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1687 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi S, Uchino H, Shimizu T, et al Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short‐term changes in glycemic control. Endocr J 2007; 54: 139–144 [DOI] [PubMed] [Google Scholar]
- 7.Yoshiuchi K, Matsuhisa M, Katakami N, et al Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008; 55: 503–507 [DOI] [PubMed] [Google Scholar]
- 8.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987; 33: 2153–2163 [PubMed] [Google Scholar]
- 9.Kohzuma T, Koga M. Lucica GA‐L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther 2010; 14: 49–51 [DOI] [PubMed] [Google Scholar]
- 10.Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia 2001; 44: 2107–2114 [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Hanefeld M, Leiter L, et al Postprandial glucose regulation and diabetic complications. Arch Intern Med 2004; 164: 2090–2095 [DOI] [PubMed] [Google Scholar]
- 12.Inomata S, Haneda M, Moriya T, et al Revised criteria for the early diagnosis of diabetic nephropathy. J Jpn Diabetes Soc 2005; 48: 757–759 (Japanese). [PubMed] [Google Scholar]
- 13.Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502 [PubMed] [Google Scholar]
- 16.Tanimura K, Nakajima Y, Nagao M, et al Association of serum apolipoprotein B48 level with the presence of carotid plaque in type 2 diabetes mellitus. Diabetes Res Clin Pract 2008; 81: 338–344 [DOI] [PubMed] [Google Scholar]
- 17.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995; 18: 440–447 [DOI] [PubMed] [Google Scholar]
- 18.Koga M, Murai J, Saito H, et al Prediction of near‐future glycated hemoglobin levels using glycated albumin levels before and after treatment for diabetes. J Diabetes Invest 2011; 2: 304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010; 57: 751–762 [DOI] [PubMed] [Google Scholar]
- 20.Suwa T, Ohta A, Matsui T, et al Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J 2010; 57: 135–140 [DOI] [PubMed] [Google Scholar]
- 21.Pu LJ, Lu L, Shen WF, et al Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J 2007; 71: 1067–1073 [DOI] [PubMed] [Google Scholar]
- 22.Santos‐Oliveira R, Purdy C, da Silva MP, et al Haemoglobin A1c levels and subsequent cardiovascular disease in persons without diabetes: a meta‐analysis of prospective cohorts. Diabetologia 2011; 54: 1327–1334 [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Steffes MW, Zhu H, et al Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stratton IM, Adler AI, Neil HA, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizaka N, Ishizaka Y, Takahashi E, et al Association between insulin resistance and carotid arteriosclerosis in subjects with normal fasting glucose and normal glucose tolerance. Arterioscler Thromb Vasc Biol 2003; 23: 295–301 [DOI] [PubMed] [Google Scholar]
- 26.Lipska KJ, De Rekeneire N, Van Ness PH, et al Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. J Clin Endocrinol Metab 2010; 95: 5289–5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TT, Pin FJ, Tan E, et al HbA1c may not be a sensitive determinant of diabetic status in the elderly. Diabetes Res Clin Pract 2011; 92: 31–33 [DOI] [PubMed] [Google Scholar]
- 28.Christensen DL, Witte DR, Kaduka L, et al Moving to an A1C‐based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 2010; 33: 580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract 2010; 87: 415–421 [DOI] [PubMed] [Google Scholar]
- 30.Khoo J, Tay TL, Foo JP, et al Sensitivity of A1C to diagnose diabetes is decreased in high‐risk older Southeast Asians. J Diabetes Complications 2012; 26: 99–101 [DOI] [PubMed] [Google Scholar]
- 31.Imai T, Oikawa Y, Shimada A. Improved monitoring of the hyperglycemic state in type 1 diabetes patients by use of the glycoalbumin/HbA1c ratio. Rev Diabet Stud 2007; 4: 44–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon JH, Chae MK, Kim KJ, et al Decreased endothelial progenitor cells and increased serum glycated albumin are independently correlated with plaque‐forming carotid artery atherosclerosis in type 2 diabetes patients without documented ischemic disease. Circ J 2012; 76: 2273–2279 [DOI] [PubMed] [Google Scholar]
- 33.Song SO, Kim KJ, Lee B‐W, et al Serum glycated albumin predicts the progression of carotid arterial atherosclerosis. Atherosclerosis 2012; 225: 450–455 [DOI] [PubMed] [Google Scholar]
- 34.Koga M, Matsumoto S, Saito H, et al Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J 2006; 53: 387–391 [DOI] [PubMed] [Google Scholar]
- 35.Koga M, Otsuki M, Matsumoto S, et al Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta 2007; 378: 48–52 [DOI] [PubMed] [Google Scholar]
- 36.Selvin E, Francis LM, Ballantyne CM, et al Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011; 34: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]


