Abstract
Background:
This study was undertaken to investigate the expression of guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) in the cell lines and tissues in BPH and prostate cancer (PCa), as well as its associations with cancer invasion and prognosis in prostate carcinomas.
Methods:
Expression analysis of RasGRP3 was accomplished using immunohistochemical staining of PCa and BPH tissues. Pearson's χ2 test was used to analyze the association between RasGRP3 expression and specific clinical parameters. Survival and PSA relapse curves were evaluated using the Kaplan–Meier curves and log-rank tests, and the differences were assessed using the Cox regression methods. In addition, human PCa cell lines PC-3, DU145, LNCaP, PC3M-1E8, PC3M-2B4 and BPH-1 were examined for expression of RasGRP3 using western blot and quantitative polymerase chain reaction (Q-PCR) analysis. After PC-3 cells were transfected by small interfering RNA targeting RasGRP3, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and migratory assays were employed to determine the vitality and aggressive capability of tumor cell in vitro.
Results:
Expression of RasGRP3 was significantly correlated (P=0.038 and P=0.021) with Gleason score (⩽6 versus ⩾7) and T stage (T1–T2 versus T3–T4), respectively. PCa with RasGRP3-positive expression may increase the risk of PSA recurrence and decrease cancer-specific survival (P=0.0291 and P=0.0044). The expression of RasGRP3 was also associated with PSA recurrence and cancer-specific survival in univariate (P<0.001 and P<0.001) and multivariate analyses (P<0.001 and P=0.003). RasGRP3 mRNA and proteins were found to be positively expressed in PCa cell lines. There was higher expression of RasGRP3 in PC-3, DU145 and PC3M-1E8 than in LNCaP, PC3M-2B4 and BPH-1. Knockdown of RasGRP3 inhibited the proliferation, migration and invasion capabilities of PC-3 cells.
Conclusions:
These data suggested that elevated RasGRP3 expression may play a key role in the malignant progression of PCa, especially in invasion and metastasis, and may be a potential marker of biochemical recurrence.
Keywords: RasGRP3, recurrence, prognostic factor
Introduction
Prostate cancer (PCa) is one of the most common causes of death in men worldwide, causing >30 000 deaths annually in the United States alone.1 Because of the rapidly aging population and changing food consumption in China, more and more PCa cases have been reported in recent years.2 It is widely believed that PCa may be caused by abnormal overactivation of oncogenes or/and dysregulation of tumor-suppressor genes.3 So far, the PSA test is still the most widely used method for diagnosis of early-stage PCa. However, significant concerns exist regarding its specificity under certain conditions. Although numerous studies were carried out to perform variations of PSA tests (that is, changes in velocity, density, free versus bound and pro-isoforms) or discover novel biomarkers (that is, KLK2, EPCA, PCA3 and AMACR),4 very limited validated information is available for their uses in clinical settings.5 Having an accurate understanding of postoperation pathologic features and possible recurrence after treatments is important in selecting an effective and efficient method of treatment.
Guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) is a member of the RasGRP family and is able to promote guanine nucleotide exchange of Ha-Ras, R-Ras and Rap1.6 RasGRP3 is involved in a diverse range of important biological processes. For instance, RasGRP3 was initially reported to play important roles in regulation of human B cells, T cells and endothelial cells through multiple critical signaling pathways, such as PKC and Ras.7, 8 Later studies showed that its dysregulation is implicated in a number of human diseases, such as cancers.8, 9, 10 Overexpression of RasGRP3 is commonly associated with many malignancies, such as pre-B-cell leukemia, Burkitt's lymphoma and natural killer-like T-cell leukemia. The complementary DNA microarray data also revealed that RasGRP3 was significantly upregulated in metastatic PCa compared with normal prostate conditions.11, 12 Recently, Yang et al.13 also confirmed this upregulation of RasGRP3 mRNA in prostate tumors. In addition, they also reported that downregulation of endogenous RasGRP3 inhibited PCa cell proliferation, migration and induced apoptosis.13
In this study, we validated the hypothesis that RasGRP3 is a potentially valuable prognostic biomarker of PCa. The expression status of RasGRP3 in a series of human PCa cell lines and 169 prostate tissues has been tested to assess the relationship between RasGRP3 high expression and prognostic value in this cohort of patients. Furthermore, we attempted to explore its associated molecular mechanism in cell growth, migration and invasion of PCa cells using small interfering RNA (siRNA) in vitro.
Materials and methods
Patients and prostate specimens
A total of 117 prostatic carcinoma samples, consisting of 48 cases of TURP, 22 specimens of radical prostatectomy and 47 biopsies of the prostate, were collected from patients with an average age of 68.4 years (ages ranging from 52 to 83 years) at Tongji Hospital affiliated with Tongji Medical College at Huazhong University of Science and Technology (Wuhan, China). All patients underwent open or laparoscopic prostatectomies. Samples were collected from January 2005 to November 2009. The control group (BPH) comprised 52 age-matched patients with an average age of 67.5 years (ages ranging from 56 to 79 years), who were recruited during the same period. All slices were collected from areas of invasive adenocarcinoma and pathologically identified according to the hematoxylin and eosin staining. Three replicate tumor samples were taken from the same location for immunohistochemical staining. The tumor grade and clinical stage of these samples were assessed based on the 2002 TNM classification and Gleason system. Follow-up data were available for the case group, ranging from 7 to 93 months. This study was approved by the local ethical committee.
Immunohistochemistry
In brief, tissue slices (4 μm) were deparaffinized and incubated in water with 0.3% H2O2 for 30 min to remove endogenous peroxidase activity. The sections were then immersed in 10 mM citrate buffer (pH=6) and heated in a microwave oven for 30 min. Sections were blocked with 5% normal goat serum in phosphate-buffered saline for 1 h and then incubated overnight with RasGRP3 antibody (Proteintech Group, Wuhan, China) at 4 °C. For the negative control, 5% normal goat serum was used to replace the primary antibody. Staining was detected using a standard avidin–biotin–peroxidase complex followed by Mayer's hematoxylin counterstain. Positive staining was defined as the brown oxidized 3,3'-Diaminobenzidine (DAB) in the given cellular compartments without background signal. The immunostaining scores (ISSs) of RasGRP3 were evaluated under a light microscope by three pathologists without patients' clinical information and were determined by multiplying the percentage of positive tumor cells and the average staining intensity. Proportion was scored on a scale of 0 (0–4%), 1 (5–24%), 2 (25–49%), 3 (50–74%) and 4 (75–100%). Intensity was scored on a scale of 0 (no stain), 1+ (weak stain), 2+ (medium stain) and 3+ (strong stain).
Cell culture and siRNA transfection
Human PCa cell lines LNCaP (ATCC#CRL-1740), DU-145 (ATCC#HTB-81) and PC-3 (ATCC#CRL-1435) were obtained from ATCC (Manassas, VA, USA) and BPH-1 was generously provided by Dr Weide Zhong (Guangzhou Medical University, Guangzhou, China). PC3M-1E8 (highly metastatic PC-3 variant) and PC3M-2B4 (nonmetastatic PC-3 variant) were obtained from Cancer Biology Research Center of Tongji Hospital (Wuhan, China), and were isolated and identified by Dr Jie Zheng (Beijing University, Beijing, China). Subclone 1E8 represented as the highly metastatic phenotype, whereas 2B4 was not metastatic after subcutaneous inoculation into nude mice.14, 15 Cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), penicillin (100 U ml−1) and streptomycin (100 μg ml−1; Invitrogen, Carlsbad, CA, USA) at 37 °C in a 5% CO2 incubator. Two RasGRP3-specific siRNAs and a nonspecific control siRNA were transfected into PC-3 cells with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. siRNA sequences were listed as following:
siRNA 774: 5′-GACCCAUUCUCAUCUUUCUUCAGAA-3′ and 5′-UUCUGAAGAAAGAUGAGAAUGGGUC-3′ siRNA 1260: 5′-CAUAAGGAAAUUAGUGGAGUCUGUA-3′ and 5′-UACAGACUCCACUAAUUUCCUUAUG-3′ Stealth siRNA Negative Control: 5′-CACCGCTGATAGCATTCGACGTCTATCAAGAGTAGACGTCGAATGCTATCAGC-3′.
mRNA expression and immunoblot analysis
Total RNA of human PCa cells was extracted with Trizol (Gibco-BRL, Paisley, Scotland) and reverse transcribed into complementary DNA by using oligo (DT) primers and M-MLV reverse transcriptase (ReverTra Ace, Toyobo, Osaka, Japan). The resultant complementary DNA was amplified with human RasGRP3 (NM_170672.2)-specific primers (sense 5′-TCAGCCTCATCGACATATCCA-3′ and antisense 5′-TCAGCCAATTCAATGGGCTCC-3′). Human 18S ribosomal RNA (GenBank: X03205.1) was amplified as internal control by using specific primers (sense: 5′-AACCCGTTGAACCCCATT-3′ and antisense: 5′-CCATCCAATCGGTAGTAGCG-3′). Real-time PCR was carried out on Applied Biosystems 7500 PCR systems by using the iQTM SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). Relative mRNA levels were determined using the 2−ΔΔCt interpretation and normalized to the 18S gene. For immunoblot analysis, total protein was isolated from PCa cells. Equal amounts of protein samples (60 μg) were subjected to a 10% SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked in 5% nonfat milk and then were incubated with primary antibody (anti-RasGRP3, 1:500; Proteintech Group) or β-actin (rabbit anti-β-actin, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight. The membranes were washed and incubated with specific peroxidase-conjugated secondary antibodies. Proteins were detected using the ECL system (Thermo Pierce, Rockford, IL, USA).
Cell vitality, invasion and migration assay
PC-3 cells were seeded into a 96-well plate at a density of 3000 cells per well and cultured for 72 h. Cell viability was determined by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer's protocol. Cell migration and invasion ability of PC-3 cells were measured by transwell assay. Then, 1 × 104 transfected cells were briefly plated in the upper chambers of the transwell (Costar, Cambridge, MA, USA) in fetal bovine serum-free medium and 10% fetal bovine serum-containing medium was deposited in the lower chambers. For cell invasion assays, transwell membranes were coated with matrigel (BD Biosciences, Franklin Lakes, NJ, USA) before plating cells. After 24 h, cell were removed from the upper chambers, whereas cells on the lower chambers were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Five independent fields for each well were counted and photographed by microscope.
Statistical analysis
Pearson's χ2 test was used to analyze the association between RasGRP3 expression and specific clinical parameters, including T classification, N classification, surgical margin status, Gleason score and pretreatment PSA. Survival and PSA recurrence curves were evaluated using the Kaplan–Meier method, and the differences were assessed using the log-rank test. All clinical parameters were analyzed with a univariate Cox proportional hazards model; variables were considered significantly at a level of P<0.1. These significant variables were analyzed stepwise into multivariate Cox proportional hazards models. Related data were analyzed with t-test between two groups or analysis of variance among three groups. All statistical analyses were performed using the Statistical Package for the Social Sciences, Version 13.0 (SPSS, Chicago, IL, USA). P<0.05 was considered significant.
Results
RasGRP3 expression in PCa tissues and its correlation with clinical and predictive parameters
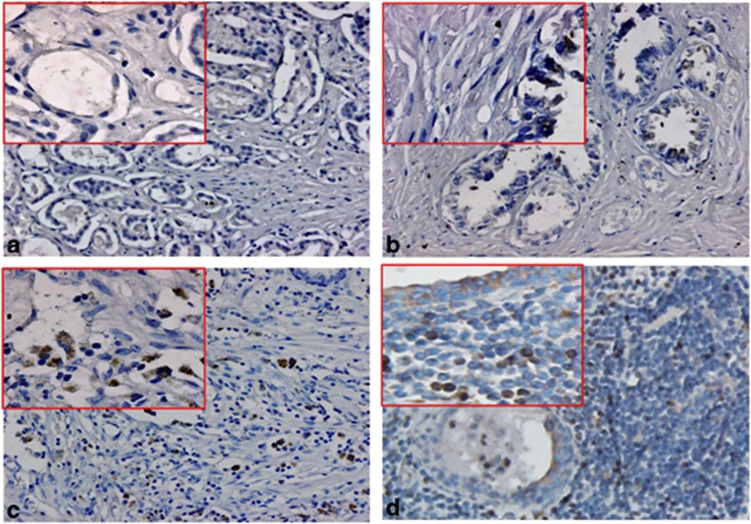
To investigate RasGRP3 protein expression levels in prostate tissues, 117 PCa patient samples with distinct clinical characteristics and outcomes (Tables 1 and 2) and 52 age-matched control samples (BPH) were evaluated by RasGRP3-specific immunohistochemistry staining. As shown in Figure 1, the staining results showed that 53 out of 117 PCa tissues (45.2%) were classified as RasGRP3 positive, with various staining intensities. In sharp contrast, RasGRP3 negative was extremely dominantly observed in 49 out of 52 (94.2%) benign prostate tissues, except 3 samples with ambiguous immunostaining. The RasGRP3 protein was mainly observed in the cytoplasms and membranes of PCa cells with occasional nuclear staining. This protein is one of those responsible for maintaining the balance of Ras-GTP levels. RasGRP3-mediated Ras activation and other diverse signaling may be important for survival of cancer cells from specific drugs.
Table 1. Prostate cancer patient characteristics.
| Number of patients | 117 |
|---|---|
| Age (years) | 52–83 |
| Mean (s.d.) | 68.4±4.7 |
| Serum PSA (ng ml−1) | 10–584 |
| Average (s.d.) | 46±76.4 |
| Follow-up period (month) | 7–93 |
| Mean (s.d.) | 48.0±25.1 |
| Outcome | |
| Alive (%) | 44 (46.2) |
| Cancer death (%) | 43 (36.8) |
| Other cause of death (%) | 20 (17.1) |
| Gleason score | |
| ⩽6 (%) | 52 (44.4) |
| ⩾7 (%) | 65 (55.6) |
| T classification | |
| T1–T2 (%) | 89 (76.1) |
| T3–T4 (%) | 28 (23.9) |
| N classification | |
| N0 (%) | 78 (66.7) |
| N+ (%) | 39 (33.3) |
Figure 1.
Immunohistochemical staining of guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) protein in prostate tissues. (a) RasGRP3 staining was negative in benign prostatic epithelia. (b, c, d) Representative positive staining of RasGRP3 in low-risk, intermediate-risk and high-risk prostate cancer. Low magnification is × 20; high magnification (inset) is × 40.
In addition, the association between RasGRP3 protein and clinical and prognostic parameters (T stage, N stage, Gleason score, pretreatment PSA level) was also analyzed. As shown in Table 2, immunohistochemical analysis revealed a positive correlation between RasGRP3 expression and Gleason score. For example, 35 out of 65 patients (53.8%) with Gleason scores of ⩾7 were RasGRP3 positive, but only 18 out of 52 (34.6%) patients with Gleason scores of ⩽6 were RasGRP3 positive (P=0.038). In addition, RasGRP3 upregulation was also significantly correlated with T stage (P=0.021). However, pretreatment PSA levels, node metastasis and positive surgical margin were not correlated with higher levels of RasGRP3 expression (P=0.841, P=0.914 and P=0.858).
Table 2. RasGRP3 immunohistochemical staining results.
| Parameters | RasGRP3-positive group | RasGRP3-negative group | P-value |
|---|---|---|---|
| Patient (n) | 53 | 64 | |
| Serum PSA level (ng ml−1) | |||
| <10 | 25 | 29 | |
| ⩾10 | 28 | 35 | 0.841a |
| Gleason score | |||
| ⩽6 | 18 | 34 | |
| ⩾7 | 35 | 30 | 0.038a |
| T stage | |||
| T1–T2 | 35 | 54 | |
| T3–T4 | 18 | 10 | 0.021a |
| Lymph node | |||
| (−) | 37 | 51 | |
| (+) | 16 | 23 | 0.914a |
| Surgical margin status | |||
| (−) | 34 | 44 | |
| (+) | 19 | 20 | 0.858a |
Abbreviation: RasGRP3, guanyl nucleotide-releasing protein for Ras 3.
Calculated by Pearson's χ2 test.
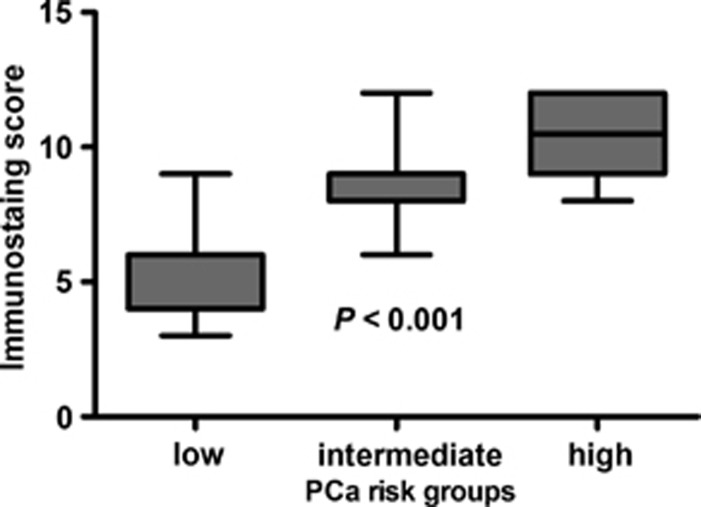
In order to have a better understanding of its potential predictive value in PCa progression, the relationship between RasGRP3 (ISS) and PCa risk was analyzed. PCa patients were stratified to low-, intermediate- and high-risk groups, according to National Comprehensive Cancer Network (NCCN) Guidelines.16 As shown in Figure 2, ISSs revealed significant difference in RasGRP3 levels among three PCa risk groups (P<0.001). More importantly, increased RasGRP3 protein expression was correlated with enhanced PCa risk. For instance, the average ISSs of low-, intermediate- and high-risk groups were 5.47±1.64, 8.30±1.34 and 10.44±1.62, respectively. Taken together, these observations illustrated that RasGRP3 is frequently upregulated in PCa with worse clinical and pathological parameters and has potential predictive value for evaluating increased PCa risk.
Figure 2.
Correlation of prostate cancer (PCa) risk and guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) protein expression. PCa risk groups were stratified by low-risk groups (n=15), intermediate-risk groups (n=20) and high-risk groups (n=18), according to the National Comprehensive Cancer Network Guidelines. Immunostaining score of RasGRP3 protein expression was determined by immunohistochemistry.
Correlation of RasGRP3 expression with PSA recurrence
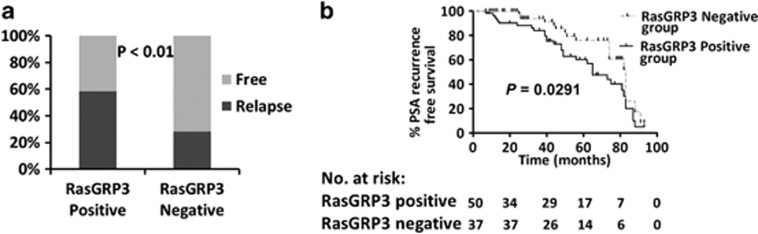
PSA recurrence reached a consensus according to the guidelines of the American Urological Association Localized Prostate Cancer Update Panel report, which is associated with accelerated PCa mortality.17 PSA recurrence-free survival (PRFS) was defined as the first time a PSA value of >0.2 ng ml−1 was recorded with a confirmatory value following radical prostatectomy.18 Of all the 117 PCa patients, 49 (41.9%) developed PSA recurrence. The PSA recurrence rate was 58.5% (31 out of 53) in the RasGRP3-positive group versus 28.1% (18 out of 64) in the RasGRP3-negative group (P<0.01; Figure 3a).We also found that PRFS rates at 3 years were 62.9 and 82.6% in the RasGRP3-positive group versus the RasGRP3-negative group. Abnormal RasGRP3 upregulation accelerated the progression of PSA recurrence (61.8% versus 73.8%, log-rank test, P=0.0291; Figure 3b). In particular, Table 3 showed the univariable and multivariable Cox regressions models predicting PRFS in the 117 patients. Univariable Cox regression analysis showed that Gleason score, T stage and RasgGRP3-positive expression were predictive of PRFS (P=0.026, P=0.004 and P<0.001). In multivariable analysis, only RasGRP3-positive expression was significantly associated with PRFS (P<0.001). Therefore, it is suggested that RasGRP3 expression is an independent risk factor for PSA recurrence.
Figure 3.
Correlation of PSA recurrence and guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) protein expression. (a) Probability of PSA recurrence. (b) Kaplan–Meier curves of PSA recurrence-free rate. Prostate cancer patients were stratified into a RasGRP3-positive group (n=53) and a RasGRP3-negative group (n=64).
Table 3. Association of clinical variables and immunostaining of RasGRP3 with PSA relapse-free survival based on Cox proportional hazards regression models.
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | Adjusted HR (95% CI) | Adjusted P-value | |
| Preoperative PSA | ||||
| <10 vs ⩾10 | 0.812 (0.462–1.427) | 0.469 | 1.099 (0.557–2.169) | 0.784 |
| Gleason score | ||||
| ⩽6 vs ⩾7 | 2.209 (1.099–4.441) | 0.026 | 1.266 (0.591–2.715) | 0.544 |
| T stage | ||||
| ⩽2 vs ⩾3 | 2.496 (1.334–4.668) | 0.004 | 1.71 (0.844–3.462) | 0.136 |
| Lymph node status | ||||
| Positive vs negative | 1.077 (0.595–1.948) | 0.807 | 0.617 (0.318–1.196) | 0.153 |
| Surgical margin status | ||||
| Positive vs negative | 1.357 (0.756–2.435) | 0.306 | 1.262 (0.629–2.529) | 0.513 |
| RasGRP3 expression | ||||
| Positive vs negative | 9.257 (4.314–19.864) | <0.001 | 9.464 (4.147–21.596) | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RasGRP3, guanyl nucleotide-releasing protein for Ras 3.
Correlation of RasGRP3 expression with patient survival
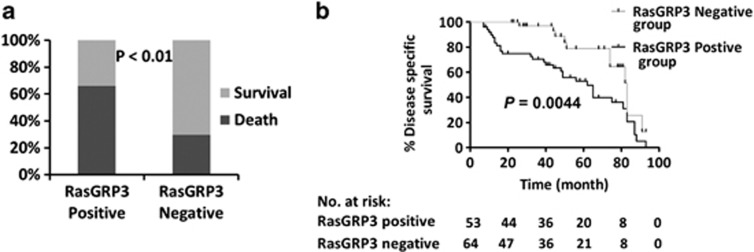
PCa patients who have received curative treatments, such as radical prostatectomies, have longer life expectancies, which are accompanied with obvious risks of mortality attributable to comorbidities. We chose death specifically from PCa as the experimental end point as it is the most definitive indication of the failure of curative treatment. As shown in Figure 4a, the prostate-specific death rate was significantly higher in the RasGRP3-positive group (66% 33 out of 50) than in the RasGRP3-negative group (29.8% 11 out of 37; P<0.01). We also found that PCa-specific survival rates at 3 years were 53.1% and 78.8% in the RasGRP3-positive group versus the RasGRP3-negative group, respectively. Compared with RasGRP3-positive patients, RasGRP3-negative patients had an apparent longer survival (55.0 versus 75.8, log-rank test, P=0.0044; Figure 4b). Exploratory univariable Cox regression analysis showed that Gleason score, T stage and RasgGRP3 positive expression were predictive of PCa-specific survival (P=0.045, P=0.022 and P<0.001). On multivariable analysis, only RasGRP3-positive expression was significantly associated with PCa-specific survival (P=0.003). Our data showed that RasGRP3 expression was an independent factor for cancer-specific survival (Table 4). Taken together, all of our immunohistochemistry results suggested that RasGRP3 may have a cancer-promoting function in PCa.
Figure 4.
Correlation of prostate-specific death and guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) protein expression. (a) Ratio and (b) speed of prostate cancer-specific death in the RasGRP3-positive group (n=50) and the RasGRP3-negative group (n=37).
Table 4. Association of clinical variables and RasGRP3 protein expression with cancer-specific survival based on Cox proportional hazards regression models.
| Variable |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | Adjusted HR (95% CI) | Adjusted P-value | |
| Preoperative PSA | ||||
| <10 vs ⩾10 | 0.927 (0.496–1.734) | 0.813 | 0.687 (0.254–1.859) | 0.459 |
| Gleason score | ||||
| ⩽6 vs ⩾7 | 2.222 (1.019–4.848) | 0.045 | 1.461 (0.675–3.162) | 0.336 |
| T stage | ||||
| ⩽2 vs ⩾3 | 2.260 (1.126–4.536) | 0.022 | 1.162 (0.452–2.992) | 0.755 |
| Lymph node status | ||||
| Positive vs negative | 1.328 (0.691–2.554) | 0.395 | 0.574 (0.263–1.254) | 0.164 |
| Surgical margin status | ||||
| Positive vs negative | 1.644 (0.873–3.097) | 0.124 | 1.807 (0.780–4.189) | 0.168 |
| RasGRP3 expression | ||||
| Positive vs negative | 7.798 (4.142–17.983) | <0.001 | 7.536 (4.405–16.015) | 0.003 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RasGRP3, guanyl nucleotide-releasing protein for Ras 3.
RasGRP3 expression levels and siRNA-based RasGRP3 knockdown in PCa cells
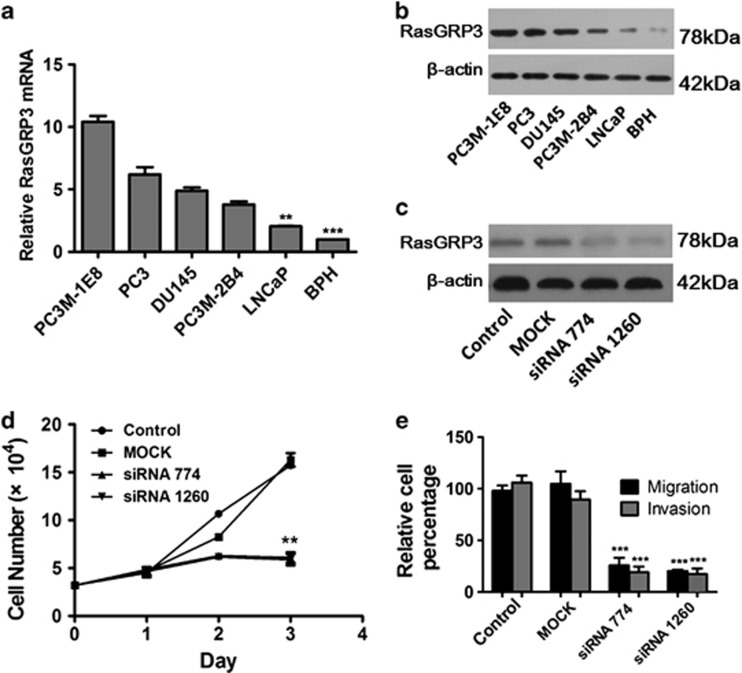
RasGRP3 mRNA and protein expression levels of five prostate cell lines were evaluated using real-time-PCR and western blots, respectively. Both RasGRP3 mRNA and protein in androgen-independent cell lines DU145, PC3 and its variant cell lines (PC3M-1E8 and PC3M-2B4) were obviously higher than those in androgen-dependent cell line LNCaP (Figures 5a and b), which is consistent with previous reports.13 To explore the function of RasGRP3 in PCa cells, we decided to knock down endogenous RasGRP3expression by RasGRP3-specific siRNA 774 and siRNA 1260. As shown in Figure 5c, both siRNAs were able to effectively inhibit RasGRP3 expression in PC-3 cells. Compared with mock and control siRNA-transfected cells, PC-3 cells transfected with siRNAs exhibited significantly decreased cell proliferation (P<0.01) as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Figure 5d). Upregulation in metastatic prostate tissue suggests that RasGRP3 may also have a role in cell migration and invasion. Therefore, we also evaluated cell migration and invasion abilities of PC-3 cells. As shown in Figure 5e, transwell assays indicated that downregulation of RasGRP3 by siRNA was significantly associated with inhibited cell migration and invasion in PC-3 cells (P<0.001). Taken together, these in vitro results further confirm the putative oncogene role of RasGRP3 by promoting cell proliferation, migration and invasion of PCa cells.
Figure 5.
(a) Quantitative polymerase chain reaction (Q-PCR) analysis of guanyl nucleotide-releasing protein for Ras 3 (RasGRP3) in prostate cancer (PCa) cell lines represents the mean±s.d.; Q-PCR analysis of RasGRP3 mRNA was normalized to 18S. (b) Western blot analysis of RasGRP3 in PCa cell lines. (c) Downregulation of the RasGRP3 protein in PC-3 cells treated with small interfering RNA (siRNA). (d) Proliferation of PC-3 cells was inhibited after downregulation of the RasGRP3. (e) Comparisons of PC-3 migration and invasion abilities after RasGRP3 siRNA transfection. **P<0.01, ***P<0.001.
Discussion
Localized PCa was found to have high cure rates and excellent long-term survival rates with standard approaches.19 However, patients with high-risk, locally advanced, metastatic PCa face life-threatening conditions that cannot be treated using any currently available approaches. RasGRP3 is here proposed as a candidate tumor biomarker to evaluate the accuracy of early diagnosis and prognosis of PCa.
RasGRP3 is one of the four known members of the RasGRP3 family of RasGEFs.6 Aside from the characterized catalytic domains related to CDC25, which are the Ras exchange factors of Saccharomyces cerevisiae, RasGRP has an atypical pair of EF (two helixes, E and F) hands that bind the calcium and diacylglycerol-binding domain. Research has shown that RasGRP3 is regulated by binding tumor-promoting phorbol esters. This serves as a PKC-independent pathway, inducing activation of Ras. For instance, Ras signaling increases in autocrine and paracrine growth factor loops, and epidermal growth factor, transforming growth factor-α, keratinocyte growth factor, basic fibroblast growth factor and insulin-like growth factor 1 and their cognate ligands have all been found to be overexpressed in advanced PCa.20 Moreover, Ras was an intermediate of the downstream signaling driving progression to androgen-independent PCa.21 The findings of previously published studies clearly implicated that Ras signaling was correlated with progression of PCa.22, 23 RasGRP3 and other guaninenucleotide exchange factors function as molecular switches and promote the exchange of guanosine diphosphate to guanosine triphosphate binding of the Ras family G proteins.24 RasGRP family members are involved in cancer development: Suzuki et al.25 identified four Ras genes by retroviral tagging, and RasGRP3 was upregulated during tumor angiogenesis and regulated by vascular endothelial growth factor.8 RasGRP1 showed a novel role in skin carcinogenesis.26 Increased RasGRP1 levels reduced p38 kinase activity in resistance to methyl ethyl ketone inhibitors.27 Yang et al.13 revealed RasGRP3 expression in the PCa cell lines as well as PCa tissues; its expression played an influential role on the proliferation and tumorigenesis of the PCa cell lines and contributed to resistance to chemotherapeutic drugs in the PCa cell lines.
Our findings support that upregulation of RasGRP3 may participate in the carcinogenesis of PCa. First, human PCa cells (PC-3, DU145, LNCaP, PC3M-1E8, PC3M-2B4), each of which exhibits distinct features with respect to PCa status, were taken to adequately represent the clinical development of PCa. We have found RasGRP3 to be overexpressed in all PCa cell lines, and high levels of RasGRP3 expression were observed in highly malignant and highly metastatic characteristics of human PCa cells (PC-3, DU145, PC3M-1E8). Less expression was observed in weakly malignant and nonmetastatic cell lines (LNCaP and PC3M-2B4). Second, siRNA is transformed by double-stranded RNA or synthesized artificially during the process of posttranscriptional gene silencing. In our study, siRNA of RasGRP3 was transfected into PC-3 cells, and results showed that knockdown of RasGRP3 inhibited cell growth, migration and invasion. Third, more RasGRP3 expression was detected in PCa tissues than in BPH tissues, and increased ISS of RasGRP3 was associated with a higher NCCN PCa risk group. Kaplan–Meier and Cox regression proportional analysis showed RasGRP3 expression to be a significant factor in cancer-specific survival and PSA recurrence. This test may be suitable for use in clinical settings.
Mutations in Ras have been reported for PCa, although they show considerable frequency in Western populations.28 These mutations can lead to the activated, oncogenic form of Ras, Ras-GTP. Ras-GTP can specifically bind to the catalytic subunit of phospatidylinositol 3-kinase (PI3K). In human leukocyte-derived cells expressing RasGRP3 facilitated a RasGRP/Ras/PI3K signaling axis, the following PI3K pathway has been confirmed in prostate carcinogenesis and important in castration-resistant PCa.29, 30, 31 Voeller et al.32 confirmed that transfection of wild-type Ras is sufficient to cause increased anchorage-independent growth in the androgen-dependent LNCaP PCa cell line.33 Overexpression of wtRas can cause normal prostatic cell lines to become metastatic.34 Epithelial-to-mesenchymal transition (EMT) is a critical mechanism for the initiation of PCa metastasis, and ectopic expression of oncogenic H-Ras is involved in the changes in adhesion and metastasis. Increased RhoA, Rac1 and Cdc42 expression in colon adenocarcinoma cells mediated cell migration, and Ras-specific guaninenucleotide exchange factors were confirmed to reduce Ras-dependent insulin-like growth factor 1-induced migration and invasion of human bladder cancer cells.35, 36, 37 As an activator of Ras, increased levels of RasGRP3 may contribute to PCa cell proliferation, migration and invasion through activation of Ras-related signaling. EMT is a physiologic process by which epithelial cells gain migratory and invasive capabilities convert into mesenchymal cells. It has been confirmed that EMT is involved in the progression of PCa, regulated by several signaling pathways, such as E-cadherin, transforming growth factor-β, growth factors, Wnt, Notch and so on.38 Loss of phosphatase and tensin homolog is considered as mediation event in EMT, accompanied by Ras activation.39 Further research into the molecular mechanism behind this is underway, and a paradigm for anti-RasGRP3 targeting drug development may be established.
Conclusion
To the best of our knowledge, this is the first thorough analysis of RasGRP3 with respect to the development of PCa. Our findings suggest a role for RasGRP3 in the progression of PCa that is of certain prognostic significance, as well as suggest a more complex relationship within the Ras signaling pathway. More research is needed to address these regulatory possibilities.
Acknowledgments
The project was supported by the National Natural Science Foundation of China (No. 81173608 and No. 81101944), the Natural Science Foundation of Hubei Province (2011CDB202) and the Seed Foundation of HUST (No. 2011JC038).
The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Gu F. Changing constituents of genitourinary cancer in recent 50 years in Beijing. Chin Med J (Engl) 2003;116:1391–1393. [PubMed] [Google Scholar]
- Foster CS, Cornford P, Forsyth L, Djamgoz MB, Ke Y. The cellular and molecular basis of prostate cancer. Bju Int. 1999;83:171–194. doi: 10.1046/j.1464-410x.1999.00954.x. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Scherr DS, Gupta A, Bianco FJ, Karakiewicz PI, Zeltser IS, et al. Emerging biomarkers for prostate cancer diagnosis, staging, and prognosis. Arch Esp Urol. 2011;64:681–694. [PubMed] [Google Scholar]
- DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood. 2005;105:3648–3654. doi: 10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Anderson AL, Hidaka M, Swetenburg RL, Patterson C, Stanford WL, et al. A vascular gene trap screen defines RasGRP3 as an angiogenesis-regulated gene required for the endothelial response to phorbol esters. Mol Cell Biol. 2004;24:10515–10528. doi: 10.1128/MCB.24.24.10515-10528.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- Yang D, Kedei N, Li L, Tao J, Velasquez JF, Michalowski AM, et al. RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res. 2010;70:7905–7917. doi: 10.1158/0008-5472.CAN-09-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zheng J, Fang W, You J, Wang J, Cui X, et al. [Isolation and characterization of human prostate cancer cell subclones with different metastatic potential] Zhonghua Bing Li Xue Za Zhi. 1999;28:361–364. [PubMed] [Google Scholar]
- Yang W, Luo D, Wang S, Wang R, Chen R, Liu Y, et al. TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clin Cancer Res. 2008;14:5494–5502. doi: 10.1158/1078-0432.CCR-08-0233. [DOI] [PubMed] [Google Scholar]
- Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999;17:1983–1987. doi: 10.1200/JCO.1999.17.7.1983. [DOI] [PubMed] [Google Scholar]
- Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D'Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- Culig Z, Klocker H, Bartsch G, Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer. 2002;9:155–170. doi: 10.1677/erc.0.0090155. [DOI] [PubMed] [Google Scholar]
- Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, et al. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am J Pathol. 2003;162:655–663. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;442:42–44. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63:1981–1989. [PubMed] [Google Scholar]
- Shou C, Farnsworth CL, Neel BG, Feig LA. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992;358:351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shen H, Akagi K, Morse HC, Malley JD, Naiman DQ, et al. New genes involved in cancer identified by retroviral tagging. Nat Genet. 2002;32:166–174. doi: 10.1038/ng949. [DOI] [PubMed] [Google Scholar]
- Oki-Idouchi CE, Lorenzo PS. Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Res. 2007;67:276–280. doi: 10.1158/0008-5472.CAN-06-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauchle JO, Kim D, Le DT, Akagi K, Crone M, Krisman K, et al. Response and resistance to MEK inhibition in leukaemias initiated by hyperactive Ras. Nature. 2009;461:411–414. doi: 10.1038/nature08279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BS, Epstein JI, Isaacs WB. ras gene mutations in human prostate cancer. Cancer Res. 1990;50:6830–6832. [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Poon HY, Stone JC. Functional links between diacylglycerol and phosphatidylinositol signaling systems in human leukocyte-derived cell lines. Biochem Biophys Res Commun. 2009;390:1395–1401. doi: 10.1016/j.bbrc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Shen MM, Abate-Shen C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 2007;67:6535–6538. doi: 10.1158/0008-5472.CAN-07-1271. [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Wilding G, Gelmann EP. v-rasH expression confers hormone-independent in vitro growth to LNCaP prostate carcinoma cells. Mol Endocrinol. 1991;5:209–216. doi: 10.1210/mend-5-2-209. [DOI] [PubMed] [Google Scholar]
- Carey AM, Pramanik R, Nicholson LJ, Dew TK, Martin FL, Muir GH, et al. Ras-MEK-ERK signaling cascade regulates androgen receptor element-inducible gene transcription and DNA synthesis in prostate cancer cells. Int J Cancer. 2007;121:520–527. doi: 10.1002/ijc.22715. [DOI] [PubMed] [Google Scholar]
- Partin AW, Isaacs JT, Treiger B, Coffey DS. Early cell motility changes associated with an increase in metastatic ability in rat prostatic cancer cells transfected with the v-Harvey-ras oncogene. Cancer Res. 1988;48:6050–6053. [PubMed] [Google Scholar]
- Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- Makrodouli E, Oikonomou E, Koc M, Andera L, Sasazuki T, Shirasawa S, et al. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Mol Cancer. 2011;10:118. doi: 10.1186/1476-4598-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco E, Metalli D, Spinelli M, Manzoni R, Samalikova M, Grandori R, et al. Novel RasGRF1-derived Tat-fused peptides inhibiting Ras-dependent proliferation and migration in mouse and human cancer cells. Biotechnol Adv. 2012;30:233–243. doi: 10.1016/j.biotechadv.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle. Nat Rev Urol. 2011;8:428–439. doi: 10.1038/nrurol.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]