Summary
The electric light is one of the most important human inventions. Sleep and other daily rhythms in physiology and behavior however, evolved in the natural light-dark cycle[1] and electrical lighting is thought to have disrupted these rhythms. Yet how much the age of electrical lighting has altered the human circadian clock is unknown. Here we show that electrical lighting and the constructed environment is associated with reduced exposure to sunlight during the day, increased light exposure after sunset, and a delayed timing of the circadian clock as compared to a summer natural 14h40min:9h20min light-dark cycle camping. Furthermore, we find that after exposure to only natural light, the internal circadian clock synchronizes to solar time such that the beginning of the internal biological night occurs at sunset and the end of the internal biological night occurs before wake time just after sunrise. In addition, we find that later chronotypes show larger circadian advances when exposed to only natural light, making the timing of their internal clocks in relation to the light-dark cycle more similar to earlier chronotypes. These findings have important implications for understanding how modern light exposure patterns contribute to late sleep schedules and may disrupt sleep and circadian clocks.
Results and Discussion
The biological effects of light profoundly influence human physiology and behavior most notably permitting vision. Light also affects many non-image forming biological systems in humans and has been shown to increase physiological arousal and enhance cognition, disturb sleep, and permit the synthesis of vitamin D[2,3,4]. Light is also used medically to treat conditions such as jaundice, skin disorders and winter depression[2,5]. A key non-image forming response to light is entrainment of internal circadian clocks[2,6,7,8,9,10], which permits organisms to synchronize to environmental time allowing physiological functions to occur at optimal times of day[1]. Natural selection favored the human circadian clock system to promote energy intake and metabolism, physical activity, and cognition during the light portion of the day, and to promote sleep and related functions during darkness at night. Yet, the external lighting environment was dramatically altered in the 1930s when electrical power grids in North America and Europe provided electricity to power electrical lighting for the masses permitting humans to spend more time being active in indoor constructed environments. This ability to control our daily exposure to light with the flip of a switch has contributed to an increase in indoor activities and has expanded work and play hours far into the night. In the current study, we quantified how much electrical lighting and the associated reduction in exposure to sunlight in the constructed environment has altered the timing of the human circadian clock by comparing the effects of exposure to electrical plus natural light (referred to henceforth as electrical lighting-constructed environment) to that of exposure to only natural light.
Eight participants (two females) aged 30.3±8.5 years (mean ± standard deviation[SD]) completed a two-week long protocol during July in the Rocky Mountains of Colorado USA at latitude ~40°N and longitude between 105–106°W. We first examined the internal circadian timing of participants after one week of maintaining daily routines of work, school, social activities, self-selected sleep schedules, and exposure to the electrical lighting-constructed environment. This was compared to the effects of exposure to one week of outdoor camping in tents and exposure to only natural light (i.e., sunlight and camp fires; no flashlights, no personal electronic devices etc.) and self-selected sleep schedules (Fig. 1).
Figure 1. Experimental protocol from one participant.
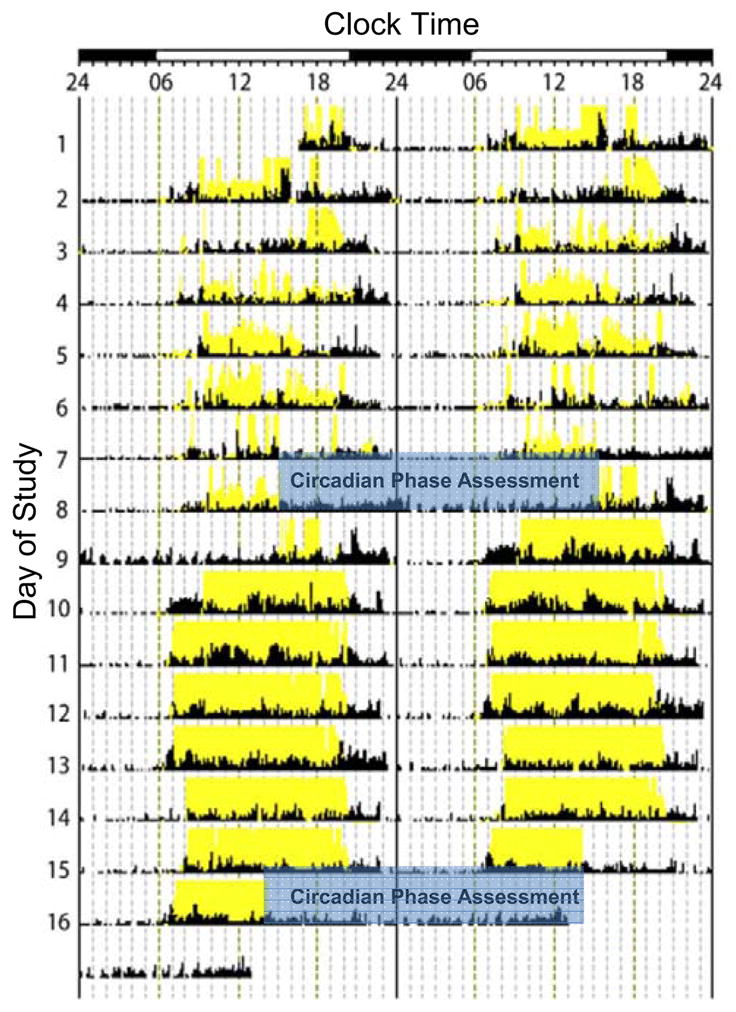
Data represent recordings from the Actiwatch-L recorder with activity denoted by black ticks and light exposure above ~1000 lux threshold denoted in yellow. Data are double plotted, with successive days plotted both next to and beneath each other and clock hour is indicated on the abscissa. Light-dark cycle (open and closed bars) on the top abscissa denotes approximate sunrise and sunset times. Sleep episodes are characterized by low activity levels. Exposure to electrical and natural lighting and sleep timing while the participant continued to live in their work-home-social constructed environment are shown on days 1–7. The initial assessment of internal circadian phase occurred on days 8–9 in the laboratory denoted by blue shading. After one night of sleep at home, exposure to the natural light-dark cycle while camping and associated sleep times occurred on days 10–16. On the last day of camping (day 16) subjects were driven directly to the laboratory for follow-up assessment of internal circadian phase on days 16–17. Conditions were sequential so that that sunrise and sunset times would be as similar as possible between conditions.
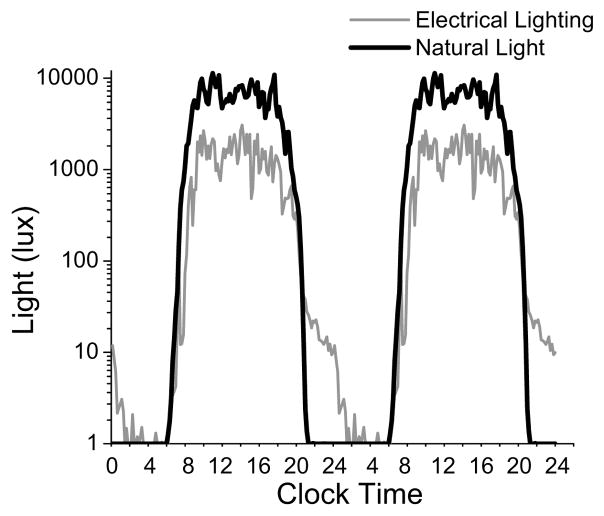
Participants were exposed to an average of 979±352 lux (±SD) during waking hours of the week of electrical lighting-constructed environment (Fig. 2). This illuminance level is greater than typically reported[11,12 (Supplemental information)] and likely reflects the outdoor lifestyle of participants and sunny climate of the mountain-desert region of Colorado. Nonetheless, participants’ average light exposure increased by more than fourfold (4487±552; p<0.0001, two tailed) during the week of natural lighting. We also examined light exposure in the first 2h after awakening, when the human circadian clock is most sensitive to the phase advancing effects of light[13,14] or to the compression of internal cycle length[15]. We found that during the first 2h awake, participants were exposed to a significantly higher average light level during natural (3074±1035 lux) versus electrical lighting-constructed environment (934±867; p<0.001, two tailed). In addition, the percentage and hours of the waking day spent above light thresholds of 50, 100, 550 and 1000 lux were all greater during natural lighting (Table 1). The only time of day when participants were exposed to more light during electrical versus natural lighting was between sunset and sleep start time (average 21±6 versus 8±2 lux, respectively; p=0.001, two tailed) when the human circadian clock is most sensitive to light-induced phase delays[13,14].
Figure 2. Light exposure.
Average light exposure (lux) plotted on a log scale during the week of exposure to electrical lighting in the constructed environment and exposure to the natural light-dark cycle while camping. Data are double plotted so that light levels across midnight (24h local clock time) can be more easily observed. For reference, one lux is equivalent to the light exposure received by the eye when gazing at a candle 1m away, moonlight is ~0.1 lux, typical indoor lighting is ~200 lux, sunrise or sunset is ~10,000 lux and looking at a bright blue midday sky is >100,000 lux.
Table 1.
Average exposure of participants to light intensities during waking hours for light levels with characterized circadian properties in accordance with intensity and phase response curves to light[13,14,16] are provided. Exposure to above 1,000 lux is commonly used to represent exposure to outdoor light (Supplemental information). Findings from laboratory phase shifting studies that exclude natural light show that exposure to 550 lux induces a saturating phase delay shift when comparing a range of light intensities from 3 to ~9,100 lux[16]; exposure to 100 lux induces half of the maximal circadian phase delay achieved by exposure to ~9,100 lux, whereas exposure to 50 lux or less produces smaller phase delay shifts[16]. Table 1 shows that exposure to electrical lighting and constructed indoor work-home-social environments is associated with reduced exposure to sunlight as compared to only natural light while camping.
| Light exposure above circadian derived thresholds | Exposure to electrical plus natural light in the work-home-social constructed environment (±SD) | Exposure to only natural light while camping (±SD) | p value two tailed |
|---|---|---|---|
| Percent of waking day spent above | |||
| 1000 lux | 23 ± 6 | 71 ± 2 | < 0.0000001 |
| 550 lux | 31 ± 10 | 76 ± 2 | < 0.00001 |
| 100 lux | 62 ± 14 | 82 ± 1 | 0.005 |
| 50 lux | 70 ± 13 | 84 ± 1 | 0.023 |
| Hours of waking day spent above | |||
| 1000 lux | 3.8 ± 1.0 | 11.4 ± 0.2 | < 0.0000001 |
| 550 lux | 5.1 ± 1.5 | 12.2 ± 0.3 | < 0.00001 |
| 100 lux | 10.1 ± 2.2 | 13.2 ± 0.2 | 0.005 |
| 50 lux | 11.4 ± 2.0 | 13.4 ± 0.3 | 0.023 |
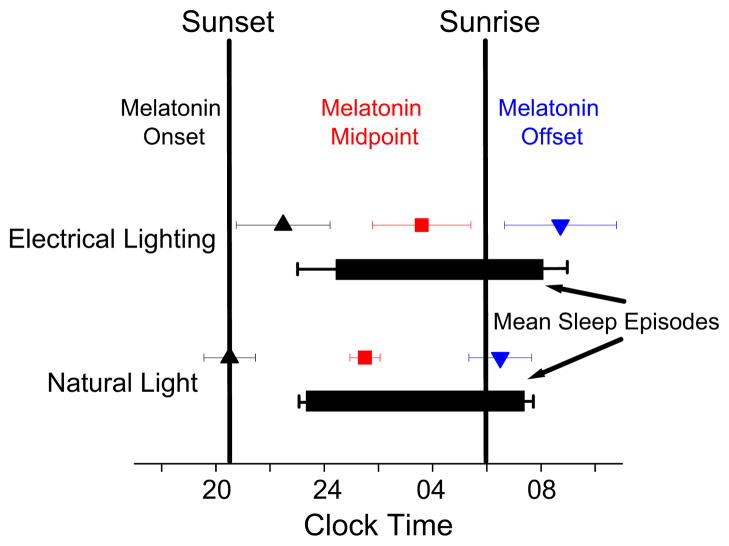
Following the week of exposure to the electrical lighting-constructed environment, characteristic circadian and sleep patterns for adults were observed. Specifically, average melatonin onset occurred ~2h prior to sleep start time (Fig. 3 and Supplemental information Fig. S1A), sleep start time was around 12:30 AM, melatonin midpoint occurred in the second half of the solar night, and melatonin offset occurred after wake time around 8:00 AM[19,20,21]. Following the week of exposure to natural lighting, all measured markers of internal circadian time were ~2h earlier (Fig 3., all p<0.01; Bonferroni correction required p<0.0167, one tailed) and remarkably, melatonin onset occurred on average near sunset, the melatonin midpoint occurred in the middle of the solar night, and melatonin offset occurred before wake time just after sunrise. Sleep timing was related to circadian timing after electrical lighting-constructed conditions and less so after exposure to only natural light (Supplemental information Table S1 and Fig. S2). The advance in timing of melatonin onset was significantly larger than changes in sleep start and wake times which changed by ~1.2h (Fig. 3, both p<0.02; Bonferroni correction required p<0.025, two tailed). Weekly sleep duration (6.7±0.9h electrical versus 6.8h±0.7h natural lighting; p=0.268, twotailed) and sleep efficiency (87.6±5.6% electrical versus 87.0±4.6% natural lighting; p=0.49, two-tailed) did not significantly change indicating that a change in sleep amount or efficiency did not contribute to the current findings. We did find however, that the circadian advance in melatonin onset was significantly correlated with the change in sleep timing (Pearson correlation: DLMO25% versus sleep start time r=0.85, p<0.005; DLMO25% versus wake time r=0.87, p<0.005; DLMOff25% versus sleep start time r=0.48, p=0.11; non-significant trend for DLMOff25% versus wake time r=0.77, p<0.0133; Bonferroni correction required p<0.0125; two-tailed).
Figure 3. Circadian and sleep timing.
Timing of the average melatonin onset (black upward triangles), melatonin midpoint (red squares), and melatonin offset (blue downward triangles) after a week of exposure to electrical lighting in the constructed environment versus exposure to the natural light-dark cycle while camping. Average sunrise and sunset times are provided for the ~two week study. Average sunrise time occurred 11 minutes later and sunset 4 minutes earlier during the week of camping as compared to the week of electrical lighting. Sleep start and wake times are presented as average times during each week. Error bars represent ± standard deviations. We did not observe a change in the duration of the melatonin rhythm defined by the time between melatonin onset and offset (p=0.66, two tailed). Exposure to longer dark episodes, as occur naturally at latitudes away from the equator during winter are likely necessary to expand the duration of the melatonin rhythm[17,18]. Larger differences in circadian timing during exposures to the electrical lighting-constructed environment versus natural light than that observed in our midsummer study may also be expected during winter.
Our findings demonstrate a fundamental physiological principle of human circadian timing—internal biological time under natural light-dark conditions tightly synchronizes to environmental time and in this regard, humans are comparable to other animals[22,23,24]. Our findings also speak to a paradox in our understanding of the neurobiology of brain arousal. Specifically, the circadian low point in brain arousal, as defined by cognitive performance level or physiological markers of sleepiness, occurs approximately two hours after habitual wake time[25,26] near to the timing of the melatonin offset. This paradox, that we are most sleepy from a circadian perspective after habitual wake time, may be a consequence of the change in the circadian timing of wake time in the electrical lighting-constructed environment (Fig. 3). After exposure to natural light, we found the timing of the circadian clock to be ~2h earlier and melatonin offset to occur more than 50 minutes prior to wake time suggesting that if human circadian and sleep timing was in synchrony with the natural light-dark cycle, the circadian low point in brain arousal would move to before the end of the sleep episode making it easier to awaken in the morning. Related, the earlier timing of the melatonin onset after exposure to natural light would promote earlier bedtimes, whereas the later evening timing of melatonin onset and light exposure in the electrical light-constructed environment would promote brain arousal and could contribute to later bedtimes and disturbed sleep.
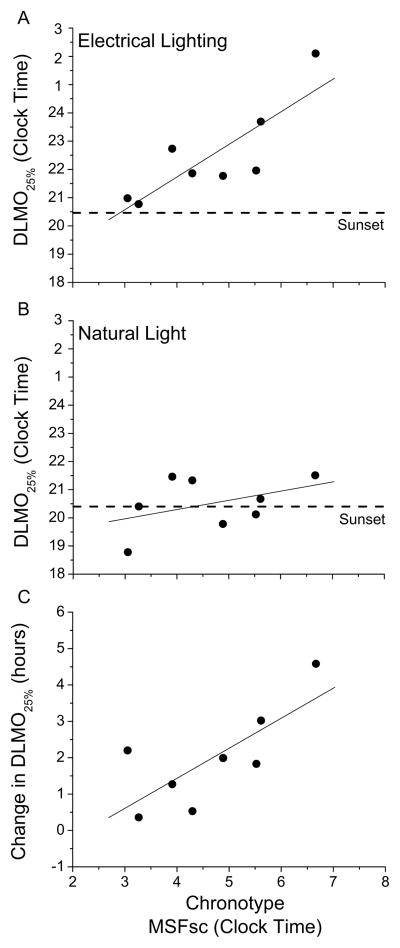
We also observed that exposure to natural light reduced individual differences in the timing of the melatonin rhythm and sleep (Fig. 3), and reduced individual differences in the timing between melatonin onset and sunset (Figs. 3, 4A, 4B) resulting in larger circadian advances for those with later chronotypes (Fig. 4C). Thus, the clocks of more evening types appear to become even later than earlier chronotypes when exposed to less sunlight and electrical lighting in the constructed environment, which may exacerbate sleep and circadian problems such as delayed sleep phase[30] and social jetlag[29]. Social behaviors, removal of electrical lighting, and increased physical activity (Supplemental information Fig. S3) during camping may also have contributed to the earlier sleep timing.
Figure 4. Association between chronotype and circadian timing and the change in circadian timing.
Chronotypes derived from the Morningness-Eveningness Questionnaire (MEQ)[27] and the Munich Chronotype Questionnaire (MCTQ)[28,29] were significantly correlated (r=−0.96, p<0.001, two-tailed). Associations between chronotype scores and circadian phase were similar regardless of the chronotype measure used, therefore we present data from the MCTQ using the timing of midsleep on free days corrected (MSFsc)[28]. After exposure to electrical lighting,. we found a strong association between chronotype and the timing of the DLMO25% (Panel A; r=0.83, p=0.01, two-tailed). After exposure to natural light however, no significant association between chronotype and circadian timing was observed (Panel B; r=0.42, p=0.28, two-tailed) likely due to a reduction in variance of the clock hour of the DLMO25% in the stronger zeitgeber of the natural light-dark cycle. Chronotype was significantly correlated with the change in DLMO25%, (Panel C; r=0.75, p=0.031, two-tailed) such that participants with later chronotypes, denoted by later clock times of the MSFsc, showed larger advances in the timing of their DLMO25%. On average, for every hour of MSFsc chronotype under electrical lighting conditions, DLMO25% moved 0.83 hours earlier after exposure to natural light. Symbols represent individual subjects and positive values indicate advances in the DLMO25% in hours. The solid line represents a linear fit of the data and the dashed line represents sunset.
Multiple photoreceptors in the retina send environmental lighting information via intrinsically photosensitive retinal ganglion cells directly to the master circadian clock located in the suprachiasmatic nucleus (SCN) of the mammalian brain[31,32,33,34]. This light information is processed by the SCN resulting in photic entrainment to the light-dark cycle. In the current study, we did not determine how many days of exposure to the natural light-dark cycle are needed to entrain the circadian clock to solar time, but such knowledge could aid in developing multicomponent treatments for circadian sleep disorders. For example, exposure to the natural light-dark cycle may help to obtain a desired earlier timing of the circadian clock and sleep for patients with delayed sleep phase. In addition, increased exposure to sunlight combined with a fixed earlier sleep schedule[35] may help adolescents and young adults who need to awaken early for school and work to maintain earlier sleep and wake timings[36,37]. We have previously shown that exposure to only electrical lighting in the laboratory[9] or to sunlight and electrical lighting in the laboratory under conditions of insufficient sleep[38] delays the circadian clock even later than electrical lighting in the home, work, school and social constructed environment. That suggests electrical lighting can result in even later sleep timing than observed here.
As our study was conducted in a small number of adult participants under midsummer sunlight conditions in Colorado, U.S.A., additional research is necessary to address study limitations by testing a larger number of subjects of different ages and cultures, and by examining effects of different latitudes and season to more fully explore the influence of natural light on human circadian physiology. More research designed to explore the contribution, effects and potential benefits of increased exposure to natural or simulated daytime sunlight and reduced exposure to electrical light in the constructed environment on human circadian physiology is also warranted.
In summary, our findings clearly demonstrate that reduced exposure to sunlight and the widespread use of electrical lighting in the constructed environment has altered human circadian physiology leading to a major change in the timing of our sleep and wakefulness. Natural sunlight is a stronger environmental zeitgeber or time cue for the internal circadian clock than is electrical lighting in the constructed environment. Our findings show that individual differences in the timing of internal circadian clocksare more likely to be expressed under the weaker environmental time cue of constructed environments, reduced exposure to sunlight and exposure to electrical lighting. Exposure to only sunlight while living outdoors reduced individual differences in circadian timing as predicted by entrainment theory for circadian clocks in the presence of strong zeitgebers[39]. Increased exposure to sunlight may help to reduce the physiological, cognitive and health consequences of circadian disruption.
Experimental Procedures
Chronotype was determined once using the morningness-eveningness questionnaire (MEQ)[27] and the Munich Chronotype Questionnaire (MCTQ)[28,29] for each participant’s habitual diurnal preference/behavior. According to the MEQ, one subject was definitely an evening type, one a moderate evening type, four were intermediate types, and two were moderate morning types. Wrist activity monitors, with photodiodes that measure lux, were used to determine average weekly activity levels, sleep start time, wake time, sleep duration, sleep efficiency—percent time spent asleep during the sleep episode using the medium sensitivity threshold—and light exposure levels for each participant (Actiwatch-L, Minimitter Respironics, Bend, OR; Supplemental information Methods and Fig. S3). Sunrise and sunset times were obtained from the United States National Oceanic and Atmospheric Association. After each lighting condition, we assessed internal circadian time of participants in the laboratory under dim lighting, ~1.9 lux (~0.6Watts/m2) in the angle of gaze, and seated or supine posture conditions to control for factors that influence markers of the circadian clock. Napping was permitted overnight between saliva samples to reduce influences of sleep deprivation on subsequent behavior the next day. Circadian timing was determined by the timing of the dim light salivary onset, midpoint and offset of the circadian melatonin rhythm (LDN Melatonin Direct RIA; Rocky Mountain Diagnostics, Colorado Springs, CO) as these measures are the most commonly used and precise markers of the circadian clock in humans[40]. Moreover, melatonin onset and offset represent the beginning and end of the internal biological night, respectively[17,18,41]. To control for individual differences in melatonin amplitude, melatonin onset (DLMO25%) and melatonin offset (DLMOff25%) were defined as the linear interpolated point in time at which melatonin levels reached 25% of the fitted peak-to-trough amplitude of the 3-harmonic of each individual’s data [9,10]. The melatonin midpoint (MP25%) was calculated as the midpoint between onset and offset. Unless noted, data were analyzed with mixed-effects ANOVAs using condition as a fixed factor and participant as a random factor. One tailed tests were used for directional hypotheses and Bonferroni corrections were used for multiple comparisons. Data are presented as mean ± standard deviation.
Supplementary Material
Highlights.
Internal biological time tightly synchronizes to a midsummer natural light-dark cycle
Electric lighting and reduced exposure to sunlight delays circadian timing in humans
Exposure to only natural light reduces individual differences in circadian timing
Circadian clocks of evening chronotypes are later when exposed to electrical lighting
Acknowledgments
Supported in part by NIH RO1 HL081761. The authors wish to thank MK McHill, RR Markwald, and GK Wright for their assistance with the study as well as R Kram and MK LeBourgeois for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 2.Wurtman RJ. The effects of light on the human body. Sci Am. 1975;233:69–77. [PubMed] [Google Scholar]
- 3.Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50:583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 4.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal NE, Sack DA, Carpenter CJ, Parry BL, Mendelson WB, Wehr TA. Antidepressant effects of light in seasonal affective disorder. Am J Psychiatry. 1985;142:163–170. doi: 10.1176/ajp.142.2.163. [DOI] [PubMed] [Google Scholar]
- 6.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents-IV. Entrainment: Pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 7.Wever RA. The circadian system of man: Results of experiments under temporal isolation. New York: Springer; 1979. [Google Scholar]
- 8.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 9.Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-hour pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci USA. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47:921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 12.Thorne HC, Jones KH, Peters SP, Archer SN, Dijk DJ. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009;26:854–866. doi: 10.1080/07420520903044315. [DOI] [PubMed] [Google Scholar]
- 13.Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhythms. 2005;28:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 14.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590:3035–3045. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roenneberg T, Hut R, Daan S, Merrow M. Entrainment concepts revisited. J Biol Rhythms. 2010;25:329–339. doi: 10.1177/0748730410379082. [DOI] [PubMed] [Google Scholar]
- 16.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehr TA, Giesen HA, Moul DE, Turner EH, Schwartz PJ. Suppression of men’s responses to seasonal changes in day length by modern artificial lighting. Am J Physiol. 1995;269:R173–R178. doi: 10.1152/ajpregu.1995.269.1.R173. [DOI] [PubMed] [Google Scholar]
- 18.Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (Photoperiod) J Clin Endocrinol Metab. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- 19.Wright KP, Jr, Gronfier C, Duffy JE, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395:191–195. doi: 10.1016/j.neulet.2005.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol Int. 2009;26:474–493. doi: 10.1080/07420520902821077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daan S, Aschoff J. Circadian rhythms of locomotor activity in captive birds and mammals: Their variations with season and latitude. Oecologia. 1975;18:269–316. doi: 10.1007/BF00345851. [DOI] [PubMed] [Google Scholar]
- 23.Nelson RJ, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol. 1981;69A:145–148. [Google Scholar]
- 24.Roenneberg T, Foster RG. Twilight times: Light and the circadian system, Photochem. Photobiol. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 25.Czeisler CA, Dijk DJ, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day. In: Ogilvie RD, Harsh HR, editors. Sleep Onset: Normal and Abnormal Processes. Washington DC: American Psychological Association; 1994. pp. 89–110. [Google Scholar]
- 26.Wright KP, Lowry CA, LeBourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 28.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV. American Academy of Sleep Medicine. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm: An American Academy of Sleep Medicine Review. Sleep. 2007;30:1480–1497. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos Trans R Soc Lond B Biol Sci. 2009;364:2849–2865. doi: 10.1098/rstb.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–692. doi: 10.1016/j.sleep.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol. 2007;17:R44–R45. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro Endocrinol Lett. 2010;31:92–96. [PMC free article] [PubMed] [Google Scholar]
- 38.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roenneberg T, Daan S, Merrow M. The art of entrainment. J Biol Rhythms. 2003;18:183–194. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- 40.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 41.Wehr TA, Aeschbach D, Duncan WC., Jr Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 2001;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.