Abstract
Workers at the Mayak nuclear facility in the Russian Federation offer the only adequate human data for evaluating cancer risks from exposure to plutonium. Risks of mortality from cancers of the lung, liver and bone, the organs receiving the largest doses from plutonium, were evaluated in a cohort of 17,740 workers initially hired 1948–1972 using, for the first time, recently improved individual organ dose estimates. Excess relative risk (ERR) models were used to evaluate risks as functions of internal (plutonium) dose, external (primarily gamma) dose, gender, attained age and smoking. By December 31, 2003, 681 lung cancer deaths, 75 liver cancer deaths and 30 bone cancer deaths had occurred. Of these 786 deaths, 239 (30%) were attributed to plutonium exposure. Significant plutonium dose-response relationships (p < 0.001) were observed for all 3 endpoints, with lung and liver cancer risks reasonably described by linear functions. At attained age 60, the ERRs per Gy for lung cancer were 7.1 for males and 15 for females; the averaged-attained age ERRs for liver cancer were 2.6 and 29 for males and females, respectively; those for bone cancer were 0.76 and 3.4. This study is the first to present and compare dose-response analyses for cancers of all 3 organs. The unique Mayak cohort with its high exposures and well characterized doses has allowed quantification of the plutonium dose-response for lung, liver and bone cancer risks based on direct human data. These results will play an important role in plutonium risk assessment.
Keywords: lung cancer, liver cancer, bone cancer, plutonium, ionizing radiation, nuclear workers
Plutonium exposure has engendered considerable public interest, and is of concern because of occupational exposure in plutonium production, nuclear fuel reprocessing and clean-up operations and because of potential exposure to the general public from reactor accidents, nuclear wastes and space accidents. Human data on plutonium exposure are limited to epidemiologic studies of workers in the nuclear industry. Because of the small number of workers and the low levels of exposure, studies of workers in the United States and the United Kingdom have very limited potential for detecting and quantifying risks. During the early period of operations (1948–1958), workers at the Mayak nuclear facility in the Chelyabinsk region of the Russian Federation were exposed to inhaled plutonium at levels much higher than workers in other countries.1 Study of these workers provides a unique opportunity to evaluate the human health effects of plutonium exposure.
Because intact skin serves as an effective barrier to the alpha particles emitted by plutonium, plutonium is a biological hazard only if it is taken into the body. Experimental studies in dogs and rats, supplemented by autopsy studies in humans, have shown that inhalation is the route of exposure of greatest concern, that plutonium concentrates in the liver and skeleton and that the lung, liver and bone receive the largest doses from inhaled plutonium.2, 3 Plutonium is retained in the body with a long biological half-life, and can continue to deliver dose to adjacent tissues over a long period of time.
Because direct epidemiologic data are limited, quantitative estimates of cancer risks from exposure to plutonium have been inferred either from studies of persons exposed to other alpha-emitting radionuclides (e.g., radon and radium) or from studies of Japanese A-bomb survivors exposed primarily to gamma radiation.4 Because alpha particles have been demonstrated in experimental studies to cause more biological damage than gamma rays, it is necessary to apply a weighting factor to use the latter data.5 There is considerable uncertainty in whether these approaches are appropriate.
The Mayak cohort is the only study with reasonable statistical power for direct evaluation of the plutonium dose-response in humans and how it might be modified by gender and age. As a result of an extensive collaborative Russian and US dosimetry program, improved individual organ doses from both exposures to plutonium and to predominantly external gamma rays have recently become available for Mayak workers. Although previous analyses have linked lung, liver and bone cancer risks to plutonium exposure in Mayak workers,6–13 the current paper is the first to use these improved dose estimates to quantify risks including evaluation of the shape of the dose-response and modification of risk by gender, attained age, age at hire and time since exposure. It is also the first to analyze risks of lung, liver and bone cancer in parallel, thus allowing comparison of patterns of risks for the three cancer sites.
MATERIAL AND METHODS
This record-based epidemiological study required no contact with the cohort members. The project was reviewed and approved by the Institutional Review Boards of the Southern Urals Biophysics Institute and the Radiation Effects Research Foundation.
The study population and follow-up
The Mayak worker cohort and methods of follow-up have been described in detail elsewhere.1, 9, 14 The main plants of the Mayak nuclear facility, which began operations in 1948, include nuclear reactors, a radiochemical plant and a plutonium production facility, but only workers in the latter 2 facilities have potential for plutonium exposure. New doses have been estimated for 18,821 workers hired in the main plants 1948–1972 and vital status is known for 93% of these workers; the current analyses are based on 17,740 workers who were followed for at least 5 years (excluding 219 workers who died and 862 workers who were lost to follow-up in the first 5 years). By December 31, 2003, 8,839 of the 17,740 workers had died and cause of death was known for 8,407 (96%) of these deaths. About 25% of the cohort is female. Smoking data (used in lung cancer analyses) were obtained from medical records and were available for 89% of males and 84% of females with 75% of males and 4.2% of females reporting smoking.
Dosimetry
Analyses in this paper are based on recently improved individual annual external and internal doses to several organs of the body, including lung, liver and bone surfaces.15 Doses of external gamma-radiation were based primarily on film badge dosimeter readings with adjustments to correct for deficiencies in dosimeters (especially those used in early years) and to convert the originally recorded doses to organ doses.16 For the 15% of workers without film badge readings, external doses were reconstructed from detailed work histories.15 Repeat analyses excluding the 15% of workers with reconstructed doses did not substantially modify results.
Estimates of doses from plutonium (internal dose) are based on plutonium levels measured in urine and mathematical models of the behavior of plutonium in the body developed from measurements of plutonium alpha activity in urine and in body tissues at autopsy. In addition, workers' occupational histories, the physiochemical form of the plutonium aerosols, and whether or not workers smoked were taken into account.17, 18 Methods used to develop the recently improved estimates were comparable for all exposure periods. Routine urine monitoring did not begin until about 1970, and thus plutonium dose estimates are available for only 40% of workers potentially exposed to plutonium. To make it possible to use the full cohort for the purposes of evaluating baseline risks and the effects of external exposure, a categorical surrogate index of plutonium exposure was developed from occupational history data, including work locations, starting dates, measured body burden values and expert knowledge of working conditions at various times in the different facilities.14
Statistical methods
Lung and liver cancer analyses were based on cancers that were indicated as the underlying cause of death. Bone cancer analyses included, in addition, 3 deaths indicated as a contributing cause and 7 deaths with soft tissue cancer that occurred in tissue very close to the bone (2 angiosarcomas, 2 fibrosarcomas and 3 synovial sarcomas), similar to the approach in Koshurnikova et al.13
As in previous Mayak worker studies,9, 14 analyses were based on Poisson regression methods, and were implemented with the AMFIT module of the software package EPICURE.19 The follow-up period began 5 years after the date of employment in one of the main plants and ended on date of death, date lost to follow-up, or December 31, 2003, whichever occurred first. Separate person-year tables were created for lung, liver and bone cancer analyses. Analyses were based on dose received 5 or more years before the time at risk. Categories for lagged cumulative external and internal doses were a zero dose category and 14 other categories with boundaries of 0.2, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6 and 10 Gy. Additional categories with boundaries of 20 and 50 Gy were added for bone surface doses, and with boundaries of 0.05, 0.1, 0.3, and 0.4 Gy for low dose lung cancer analyses. For all analyses, unmonitored person-years were classified according to the surrogate. Because of indications that some workers were monitored for plutonium as a result of suspected diseases, person-years were classified as unmonitored until 2 years following the initial monitoring date.
Analyses were based on excess relative risk (ERR) models where the age-specific risk or hazard is given by
The logarithm of the baseline risk was modeled as a sex-specific linear-quadratic function of log(attained age), gender, birth cohort (4 categories with cut points of 1915, 1925 and 1935 for lung cancer; 2 categories with a cut point of 1930 for liver cancer, not included for bone cancer). For lung cancer, calendar period (1948–1972, 1973–2003) and sex-specific smoking status (nonsmoker, smoker and unknown smoking status) were also included. These models for the baseline risk were chosen after exploration of several alternative functions, including finer categories and continuous variables for birth cohort and calendar year, and stratification on age, gender, and birth cohort or age, gender and calendar period.
The total ERR was expressed as the sum of the excess risks for plutonium dose, external dose and plutonium surrogate categories for periods when plutonium dose could not be estimated. Most analyses were repeated with restriction to person-years where plutonium doses could be estimated with generally similar results.
The ERR for plutonium dose (ERRplu)was expressed as follows.
where dplu denotes plutonium dose, s is gender, a is attained age, axplu is the age at first plutonium dose and β, γi are parameters to be estimated; β is referred to as the ERR per Gy. In addition to the linear dose-response function in the above Equation, linear-quadratic and categorical functions were evaluated. Tests were conducted to indicate the need for each of the variables s, ln(a), and axplu. Final models were more selective as described in the Results section. Analogous expressions were used for the ERRs for external dose and the plutonium surrogate (with parameters ηj substituted for β dplu, where ηj indexes plutonium surrogate categories).
In all cases, parameter estimates were computed with maximum likelihood methods. Hypothesis tests and confidence intervals were based on likelihood ratio tests and direct evaluation of the profile likelihood. Two-sided p-values are used throughout and are referred to as significant if they are <0.05.
In addition to parameter estimates, we present estimates of the expected and excess cases, with the excess apportioned between plutonium and external exposures derived from the fitted models. These are calculated as described by Shilnikova et al.14
RESULTS
Of the 17,740 workers included in this study, plutonium doses could be estimated for 9,496 workers; 5,572 workers (59%) had positive plutonium doses, whereas 3,924 workers (41%) worked in the reactors with little potential for plutonium exposure (Table I). The mean plutonium doses to the lung, liver and bone surfaces were respectively 0.19, 0.27 and 0.98 Gy among workers with positive plutonium doses. The mean external doses to these organs were respectively 0.53, 0.53 and 0.57 Gy among all workers. An additional 8,244 workers had potential for plutonium exposure but were unmonitored. Of those with positive plutonium doses, 30% were female, 53% received their first such dose before 1959, 39% received their first dose before age 25, 52% worked in the radiochemical plant and 43% worked in the plutonium plant. In contrast to nuclear workers in other countries, females as well as male received substantial doses; in fact, mean plutonium doses were higher for females than males. Mean doses were highest for persons first exposed in the early calendar years, and were especially high for workers employed in the Main-1 department of the plutonium plant. There were 786 deaths from lung (681), liver (75) and bone (30) cancer.
TABLE I.
Number of Mayak Workers (Percent in Parenthses) by Plutonium Monitoring Status, Gender, Year of First Plutonium Dose, Age of First Plutonium Dose, External Dose And Plant
| All workers1 | No plutonium dose2 |
Positive plutonium dose and monitored3 |
Mean plutonium dose among those with positive doses(Gy)4 |
Pontential for plutonium dose, not monitored5 |
|||
|---|---|---|---|---|---|---|---|
| Lung | Liver | Bone surfaces | |||||
| Total | 17,740 (100) | 3,924 (100) | 5,572 (100) | 0.19 | 0.27 | 0.98 | 8,244 (100) |
| By gender | |||||||
| Males | 13,228(75) | 3,051 (78) | 3,874 (70) | 0.14 | 0.22 | 0.76 | 6,303 (76) |
| Females | 4,512 (25) | 873 (22) | 1,698 (30) | 0.29 | 0.38 | 1.47 | 1,941 (24) |
| By year of first plutonium dose6 | |||||||
| 1948–1953 | 7,553 (43) | 2,080 (53) | 1,872 (34) | 0.40 | 0.62 | 2.23 | 3,601 (44) |
| 1954–1958 | 3,612 (20) | 678 (17) | 1,092 (20) | 0.15 | 0.17 | 0.68 | 1,842 (22) |
| 1959–1963 | 3,579 (20) | 653 (17) | 1,133 (20) | 0.07 | 0.09 | 0.29 | 1,793 (22) |
| 1964–1972 | 2,996 (17) | 513 (13) | 1,475 (26) | 0.03 | 0.03 | 0.13 | 1,008 (12) |
| By age of first plutonium dose7 | |||||||
| 15–19 | 4,950 (28) | 933 (24) | 1,353 (24) | 0.09 | 0.15 | 0.56 | 2,664 (32) |
| 20–24 | 6,273 (35) | 1,590 (41) | 1,907 (34) | 0.22 | 0.37 | 1.32 | 2,776 (34) |
| 25–29 | 2,860 (16) | 707 (18) | 996 (18) | 0.22 | 0.29 | 1.07 | 1,157 (14) |
| 30+ | 3,657 (21) | 694 (18) | 1,316 (24) | 0.21 | 0.23 | 0.84 | 1,647 (20) |
| By external dose to the lung4 | |||||||
| 0.0Gy | 1,761 (10) | 248 (6.3) | 400 (7.2) | 0.06 | 0.07 | 0.27 | 1,113 (14) |
| <0.1 Gy | 4,750 (27) | 1,096 (28) | 1,340 (24) | 0.08 | 0.09 | 0.27 | 2,314 (28) |
| 0.1–1 Gy | 8,117 (46) | 2,336 (60) | 2,423 (43) | 0.26 | 0.33 | 1.14 | 3,358 (41) |
| 1+Gy | 3,112 (18) | 244 (6.2) | 1,409 (25) | 0.20 | 0.40 | 1.56 | 1,459 (18) |
| By plant8 | |||||||
| Reactor | 4,181 (24) | 3,923 (100) | 231 (4.1) | 0.03 | 0.04 | 0.13 | 279 (0.3) |
| Radiochemical | 7,365 (42) | 1 (0.0) | 2,921 (52) | 0.06 | 0.18 | 0.68 | 4,443 (54) |
| Pu-Auxilliary | 2,547 (14) | 0 | 834 (15) | 0.11 | 0.13 | 0.49 | 1,713 (21) |
| Pu-Main 2 | 1,503 (8.5) | 0 | 647 (12) | 0.13 | 0.10 | 0.29 | 856 (10) |
| Pu-Main 1 | 2,144 (12) | 0 | 939 (17) | 0.72 | 0.85 | 3.01 | 1,205 (15) |
Gy, gray. Mean plutonium doses to the lung, liver and bone surface by gender, year of first plutonium dose, age of first plutonium dose, external dose and plant.
Employed in the main plants in the period 1948–1972 and followed for at least 5 years.
Worked only in the reactors and were not monitored for plutonium 2 or more years before end of follow-up.
Primarily radiochemical and plutonium plant workers but includes 231 workers who worked only in the reactors.
Cumulative dose up to 5-years before the end of follow-up.
Worked in the radiochemical or plutonium plants and were not monitored for plutonium 2 or more years before end of follow-up.
Year of hire for those with no plutonium dose or not monitored for plutonium dose.
Age at hire for those with no plutonium dose or not monitored for plutonium dose.
Classified by the “most dangerous” (in the order given) plant prior to 1973.
Employed in radiochemical or plutonium plants 1973 or later and not monitored for plutonium.
Among workers whose plutonium doses could be estimated, 354 workers died of lung cancer, 40 of liver cancer and 11 of bone cancer (Table II). Increases in risk with increasing dose were observed for both lung and liver cancer. Elevated risks for bone cancer were observed only in the 10+ Gy category. All 3 endpoints exhibited significant dose-response relationships (p < 0.001).
TABLE II.
Number of Person-Years, Deaths, And Relative Risks (With 95% CI) For Lung, Liver, And Bone Cancer Mortality By Categories of Plutonium Dose to the Cancer Site1
| Plutonium organ dose (Gy) |
Lung cancer | Liver cancer | Bone cancer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Person-years | Lung cancers |
Relative risk (95% CI) |
Person-years | Liver cancers |
Relative risk (95% CI) |
Person-years | Bone cancers |
Relative risk (95% CI) |
|
| 02 | 144,023 | 139 | 1.0 | 144,022 | 14 | 1.0 | 144,023 | 5 | 1.0 |
| >0–0.1 | 67,980 | 111 | 0.98 (<1–1.3) | 59,754 | 9 | 1.033 (<1–1.8) | 34,209 | 1 | |
| 0.1–0.2 | 6,928 | 16 | 1.4 (<1–2.4) | 10,319 | 0 | 14,406 | 0 | ||
| 0.2–0.3 | 2,864 | 14 | 3.3 (1.7–5.8) | 4,306 | 1 | 1.54 (<1–3.2) | 7,725 | 0 | 0.96 (<1–4.3) |
| 0.3–0.5 | 2,472 | 14 | 4.5 (2.4–7.7) | 3,656 | 1 | 8,599 | 2 | ||
| 0.5–1.0 | 1,972 | 15 | 6.4 (3.5–11) | 3,078 | 0 | 8,643 | 0 | ||
| 1.0–2.0 | 1,129 | 16 | 15 (8.1–25) | 1,831 | 2 | 4.05 (1.2–13) | 5,171 | 0 | 0.07 (0.0–8.7) |
| 2.0–3.0 | 450 | 8 | 18 (8.3–35) | 599 | 1 | 1,839 | 0 | ||
| 3.0–5.0 | 342 | 7 | 17 (7.1–35) | 518 | 3 | 16 (3.3–58) | 1,674 | 0 | |
| 5.0–10.0 | 273 | 6 | 27 (10–58) | 400 | 7 | 43 (12–134) | 1,163 | 0 | 0.0 (0.0–61) |
| 10.0+ | 183 | 8 | 186 (69–466) | 133 | 2 | 36 (4.5–196) | 1,164 | 3 | 82 (17–338) |
| Total | 228,616 | 354 | 228,616 | 40 | 228,616 | 11 | |||
Gy, gray; CI, confidence interval.
Includes only person-years and cancers for which plutonium doses can be estimated.
Referent group.
For dose category >0–0.2 Gy.
For dose category 0.2–1.0 Gy.
For dose category 1.0–3.0 Gy.
For dose category >0–1.0 Gy.
For dose category 1.0–5.0 Gy.
For lung cancer, baseline rates for male smokers were 9.4 (95% CI: 6.2–15) times than those for nonsmokers; for males with unknown smoking status, this relative risk was 4.7 (95% CI: 2.7–8.3). For females, these relative risks were respectively 4.7 (95% CI = 2.1–9.1) and 1.4 (95% CI = 0.6–2.6). The lower relative risks for females are likely due to females having smoked lower amounts of tobacco than males. The baseline risk for nonsmoking males was about 1.5 (95% CI = 0.8–2.6) times that for nonsmoking females.
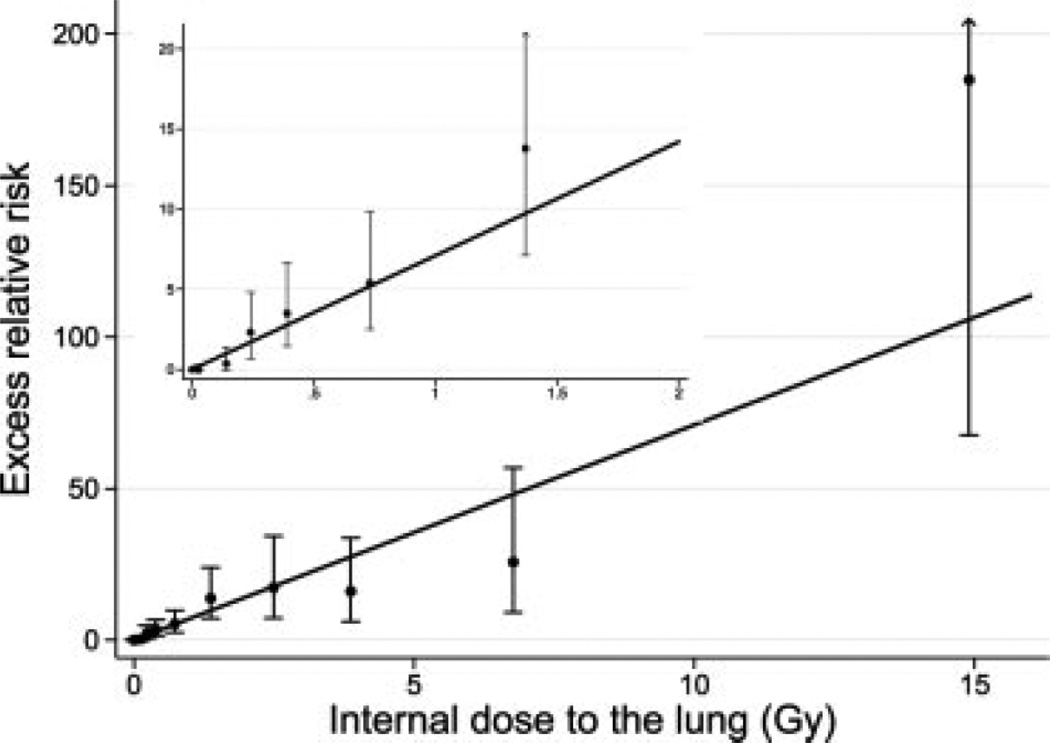
The lung cancer ERR per Gy for females was 2.1 (95% CI = 1.0–4.3) times that for males (Table III). The ERR declined with both attained age (p = 0.002) and age at first plutonium dose (p = 0.025) when the 2 variables were evaluated separately. With both variables in the model, attained age remained statistically significant (p = 0.006) while age at first plutonium dose was of borderline statistical significance (p = 0.079). Lung cancer risks were well described by a linear dose response (Fig. 1), and a linear-quadratic function did not significantly improve the fit over a linear one (p > 0.5), A significant dose-response was observed when analyses were restricted to doses less than 0.3 Gy (p = 0.007), but not when restricted to doses less than 0.2 Gy (p = 0.47). Repeat analyses without adjustment for smoking yielded similar results (ERR per Gy for males at attained age 60 = 6.5; 95% CI = 4.4–9.2) although, without the smoking adjustment, there was little evidence of a decline in the ERR with increasing age at first plutonium dose (p = 0.30).
TABLE III.
Excess Relative Risk (Err) Per Gy for Plutonium Dose by Gender, Attained Age and Age at First Plutonium Dose
| Lung | Liver | Bone | ||||
|---|---|---|---|---|---|---|
| No. of deaths1 |
ERR per Gy (95% CI) |
No. of deaths1 |
ERR per Gy (95% CI) |
No. of deaths1 |
ERR per Gy (95% CI) |
|
| BY gender2 | ||||||
| Males | 191 | 7.1 (4.9–10) | 14 | 2.6 (0.7–6.9) | 4 | 0.76 (<0–5.2) |
| Females | 24 | 15 (7.6–29) | 12 | 29 (9.8–95) | 2 | 3.4 (0.4–20) |
| P for difference | 0.047 | <0.001 | 0.26 | |||
| By attained age3 | ||||||
| <55 years | 33 | 10.7 (5.4–20) | 6 | 4.4 (0.8–16) | 5 | 9.2 (2.0–34) |
| 55–64 years | 79 | 6.8 (4.2–10) | 9 | 1.8 (0.3–6.3) | 0 | |
| 65–74 years | 83 | 3.7 (1.9–6.3) | 8 | 1.8 (0.3–6.7) | 1 | −0.015 (<0–1.3) |
| 75 + years | 20 | 4.1 (0.9–10) | 3 | 7.5 (1.0–34) | 0 | |
| p trend4 | 0.002 | >0.5 | 0.011 | |||
| By age at first plutonium dose3 | ||||||
| <20 years | 25 | 10.7 (4.5–20) | 4 | 6.0 (0.6–28) | 2 | 5.7 (0.3–28) |
| 20–24 years | 95 | 8.0 (5.1–12) | 12 | 2.6 (0.6–7.8) | 4 | |
| 20–30 years | 42 | 4.9 (2.3–8.9) | 3 | 0.5 (<0–4.2) | 0 | 1.16 (0.1–5.3) |
| 30 + years | 53 | 3.4 (1.8–5.9) | 7 | 2.9 (0.6–9.3) | 0 | |
| p trend4 | 0.025 | 0.41 | 0.22 | |||
Gy = gray; CI = confidence interval.
Number of deaths with positive plutonium doses.
For lung cancer, ERR estimates are for attained age 60 (based on a model that included attained age). For liver and bone cancers, ERR estimates are for all attained ages.
For lung and liver cancers, ERR estimates are for males (based on a model that included gender). For bone cancer, ERR estimates are for both genders.
Based on trend over 5-year categories.
For attained age category 55+ years.
For age category 20+ years.
FIGURE 1.
Excess relative risk (with 95% CI) of lung cancer by categories of plutonium dose to the lung. Shown for males at age 60. Estimated linear function also shown.
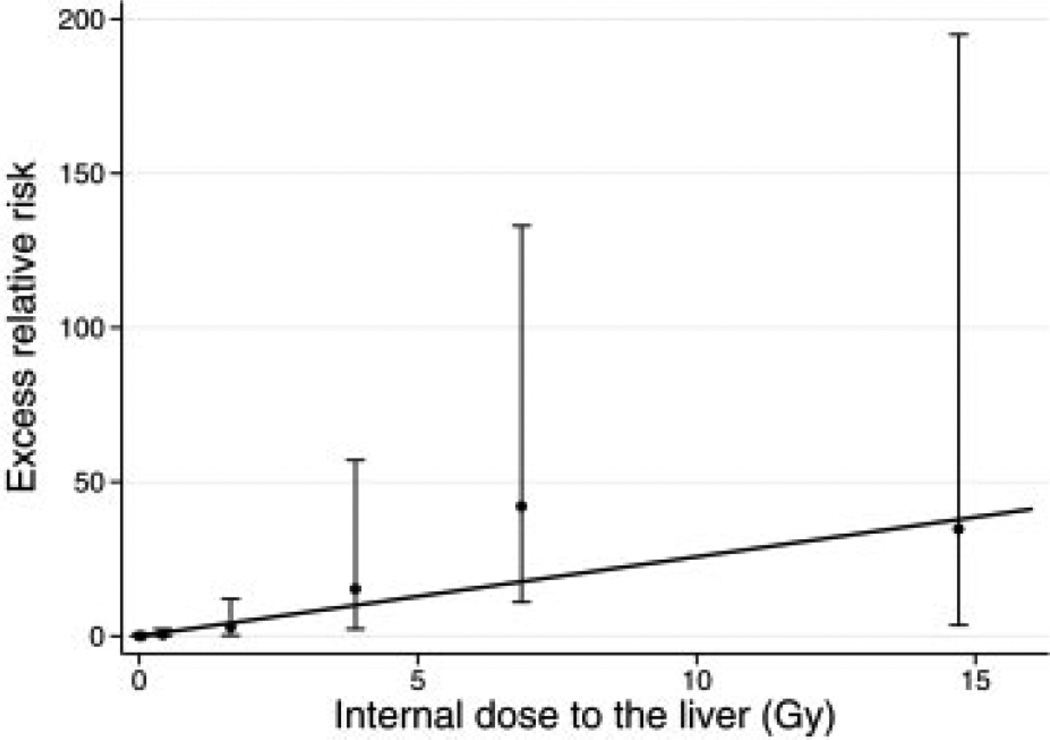
For liver cancer, baseline rates for males were 4.3 (95% CI = 1.8–12) times those for females. The ERR per Gy for females was 11.2 (95% CI = 2.7–59) times that for males, but did not depend on attained age (p > 0.5) or age at first plutonium dose (p = 0.41). Liver cancer risks were described reasonably well by a linear dose response (Fig. 2), and a linear-quadratic function did not significantly improve the fit over a linear one (p = 0.30). A significant dose-response was observed when analyses were restricted to doses less than 3.5 Gy (p = 0.020), but not when restricted to doses less than 3 Gy (p = 0.098).
FIGURE 2.
Excess relative risk (with 95% CI) of liver cancer by categories of plutonium dose to the liver. Shown for males (all ages). Estimated linear function also shown.
For bone cancer, baseline rates for males were 1.5 (95% CI: 0.41–6.9) times those for females. The evidence for a bone cancer dose-response rests on 3 deaths with doses exceeding 10 Gy: 1 male death with a dose of 18 Gy and 2 female deaths with doses of 31 and 69 Gy. The ERR per Gy did not differ significantly by gender (p = 0.26) and was 1.6 (95% CI: 0.3–6.8) for the combined sexes. The ERR declined with attained age (p = 0.011); in fact, evidence for dose-response was found only for attained ages under 55. The ERR was not significantly modified by age at first plutonium dose. There was no evidence (p > 0.5) of dose-response when analyses were restricted to doses less than 10 Gy (ERR per Gy = −0.10, 95%CI = <0–2.0). In a model with attained age included as a modifying variable, a linear-quadratic model improved the fit over a linear one but this improvement was not statistically significant (p = 0.10); a pure quadratic model fitted the data as well as the linear-quadratic model (p > 0.5).
There was no evidence that the ERR per Gy for lung, liver or bone cancer varied significantly among the 4 categories for first year of plutonium dose (1948–1953, 1954–1958, 1959–1963, 1964–1972). However, the lower doses and younger ages observed in the later periods limited the statistical power to detect differences. For lung cancer, significant dose response-relationships were found for all but the latest period; for liver cancer, significant dose-response relationships were found for only the first 2 periods; whereas for bone cancer, a significant dose response was found only for the 1948–1953 period.
Among workers whose plutonium doses could not be estimated, RR for categories of the plutonium surrogate were estimated (data not shown). For lung cancer, the RR increased consistently with increasing surrogate category. The RRs for the highest category, which consisted of those who worked in the most dangerous department of the plutonium plant in the period 1948–1953, were 3.7 (95% CI = 2.4–5.4; 37 deaths) for males and 18 (95% CI = 8.3–35; 15 deaths) for females. For liver and bone cancers, elevated risks that approached statistical significance were observed only in the highest category. For liver cancer, the RRs were 3.2 (95% CI = 0.3–8.7; 4 deaths) for males and 30 (95% CI = 9.4–105; 7 deaths) for females, while for bone cancer the RRs were 9.6 (95% CI = 1.3–47; 2 deaths) for males and 31 (95% CI = 7.0–136; 4 deaths) for females.
The ERR per Gy for external dose was 0.19 (95% CI = 0.05–0.39; p = 0.006) for lung cancer, 0.21 (95% CI = <0–1.0; p = 0.34) for liver cancer and 0.35 (95% CI = <0–4.4; p > 0.5) for bone cancer with no evidence of statistically significant modification by gender, attained age or age at first external dose for any of the endpoints. The ERR per Gy for lung cancer remained significantly elevated when analyses were restricted to data where plutonium doses could be estimated (ERR/Gy = 0.21; 95% CI: 0.02–0.49; p = 0.026).
Table IV shows the numbers of excess deaths attributable to plutonium and external dose. For lung and liver cancer, these are based on the linear models shown at the top of Table III. For bone cancer, they are based on a pure quadratic function with modification by attained age. Overall, 239 (30%) of the 786 lung, liver and bone cancers could be attributed to plutonium exposure, whereas about 68 (8.6%) such deaths could be attributed to external exposure. Of the 239 plutonium excess deaths, 112 occurred in workers with positive plutonium doses, whereas 127 occurred in workers whose plutonium doses could not be estimated. Lung cancer contributed the largest numbers of plutonium excess deaths (199), but the percentage of deaths attributed to plutonium exposure was highest for bone cancer (47%).
TABLE IV.
Observed and Predicted Deaths from Lung, and Bone Cancer, and Estimated Excess Deaths Associated with Plutonium And External Exposure
| Observed | Predicted by model |
Background1 | Excess | ||
|---|---|---|---|---|---|
| Plutonium exposure | External exposure | ||||
| Lung2 | |||||
| Plutonium alpha dose | |||||
| Estimated as zero3 | 139 | 124.3 | 114.0 (92) | 0.0 (0.0) | 10.3 (8.3) |
| Estimated as positive4 | 215 | 222.2 | 107.9 (49) | 91.9 (41) | 22.3 (10) |
| Could not be estimated5 | 327 | 334.5 | 201.9 (60) | 106.8 (32) | 25.8 (7.7) |
| Total | 681 | 681 | 423.9 (62) | 198.7 (29) | 58.4 (8.6) |
| Liver6 | |||||
| Plutonium alpha dose | |||||
| Estimated as zero3 | 14 | 12.5 | 11.5 (92) | 0.0 (0.0) | 1.1 (8.5) |
| Estimated as positive4 | 26 | 28.2 | 8.9 (32) | 17.2 (61) | 2.1 (7.4) |
| Could not be estimated5 | 35 | 34.3 | 21.9 (64) | 9.3 (27) | 3.1 (9.0) |
| Total | 75 | 75 | 42.3 (56) | 26.5 (35) | 6.3 (8.3) |
| Bone7 | |||||
| Plutonium alpha dose | |||||
| Estimated as zero3 | 5 | 3.8 | 3.2 (83) | 0.0 (0.0) | 0.6 (17) |
| Estimated as positive4 | 6 | 6.7 | 2.8 (42) | 3.0 (44) | 0.9 (14) |
| Could not be estimated5 | 19 | 19.6 | 7.0 (36) | 11.1 (56) | 1.5 (7.6) |
| Total | 30 | 30 | 13.0 (43) | 14.0 (47) | 3.0 (10) |
| Lung, liver and bone | |||||
| Plutonium alpha dose | |||||
| Estimated as zero3 | 158 | 140.7 | 128.6 (91) | 0.0 (0.0) | 12.0 (8.5) |
| Estimated as positive4 | 247 | 257.0 | 119.6 (47) | 112.1 (44) | 25.4 (9.9) |
| Could not be estimated5 | 381 | 388.3 | 230.9 (60) | 127.1 (33) | 30.4 (7.8) |
| Total | 786 | 786 | 479.1 (61) | 239.2 (30) | 67.7 (8.6) |
Percentage are given in parentheses.
Deaths that would have occurred in the absence of external or plutonium radiation exposure.
Estimated from linear model with modification by gender and attained age.
Primarily persons who worked only in reactor or auxiliary plants.
Primarily person who worked in the radiochemical or plutonium plant and were monitored for plutonium.
Worked in radiochemical or plutonium plant and not monitored for plutonium.
Estimated from linear model with modification by gender.
Estimated from pure quadratic model with modification by attained age.
DISCUSSION
The Mayak worker study provides the only direct evidence that internal plutonium exposure increases cancer risks in humans. On the basis of individual improved plutonium organ dose estimates, lung and liver cancer risks increased in a dose-dependent fashion with the dose-response relationships reasonably described by linear functions. Bone cancer risk also showed a significant dose-response, but elevated risks were observed only for those with plutonium doses exceeding 10 Gy. For all 3 endpoints, the ERR per Gy for plutonium dose was higher for females than males; for lung and liver cancer, this finding may reflect higher baseline risks for males because of their greater smoking and alcohol consumption. For lung and bone cancer, the ERR declined with attained age, and for lung cancer, it also declined with age at first plutonium dose. In addition to the dose-response relationships, those who worked in the most dangerous department of the plutonium plant prior to 1954 but were not monitored for plutonium exposure exhibited significant excess risks for all 3 endpoints with higher relative risks for females than males.
For lung cancer, the use of improved dose estimates and adjustment for smoking strengthens our previous results.9 The estimated ERR per Gy for plutonium dose at attained age 60 in this paper was slightly higher for males (7.1; 95% CI = 4.9–10.1 when compared to 4.7; 95% CI = 3.3–6.7 reported earlier) and slightly lower for females (15.1; 95% CI = 7.6–21 when compared to 19; 95% CI = 9.5–39). Modification by attained age was also similar to earlier results. Unlike our previous paper, we found evidence that the ERR per Gy declined with age at first plutonium dose, a difference that appears to come about because of the adjustment for smoking. In contrast to previous findings, a significant dose-response for external dose was found even when analyses were restricted to data where plutonium doses could be estimated, thus making it less likely that this relationship is the result of confounding by plutonium exposure. Other evaluations of lung cancer risks in Mayak workers6–8, 10 were based on preliminary dose estimates, less extensive follow-up, and, in most cases, did not include females or investigate modification of risk by gender and attained age. For radiation protection purposes, estimates of the relative biological effectiveness of plutonium dose relative to external gamma dose are of interest, and such estimates will be given attention in future papers.
Earlier evaluation of liver11 and bone13 cancer mortality risks in Mayak workers established associations with plutonium exposure, but dose-response relationships were not evaluated. A case control study of liver cancer risks in Mayak workers was recently conducted and included 44 morphologically confirmed deaths from liver cancer and 111 matched controls.12 The study linked liver cancer risk to plutonium exposure and alcohol consumption, but did not use the improved dose estimates and did not evaluate alternative dose-response functions or modification of the dose response by gender and age.
Studies of other plutonium workers3, 20–25 provide little evidence of exposure-related lung cancer risk although plutonium exposure-response relationships were suggested in selected subgroups.24, 25 There was a single death from liver cancer in a plutonium worker at Hanford and a single death from bone cancer in a plutonium worker at Los Alamos, but no additional deaths from these cancers in either US or UK plutonium workers. The low doses these workers received undoubtedly limit the statistical power of these studies. For example, the mean lung dose among plutonium exposed workers in the UK was about 0.01 Gy23 compared to 0.19 Gy in monitored Mayak workers. In the case-control study of workers at the Rocky Flats plant,25 only about 5% of subjects (8 cases and 40 controls) had estimated internal lung doses (including dose from plutonium and other radionuclides) exceeding 0.047 Gy. The maximum body burden among all US workers was 3.2 kBq22 (organ dose estimates are not available); more than 400 Mayak workers had plutonium body burdens that exceeded this.
Lung, liver and bone cancer risks have been linked with exposure to alpha-emitters other than plutonium. Clear exposure-response relationships have been demonstrated for lung cancer in numerous studies of underground miners exposed to radon,26, 27 and for bone cancer in patients who received injections of radium28 and radium dial painters.29 Excess liver cancer has been observed in several studies of thorotrast-exposed patients.2, 30 In addition, lung and liver cancer risks have been clearly linked with external exposure in studies of A-bomb survivors31 while bone cancer has been linked with high dose external exposure in childhood cancer survivor studies.30 Finally, experimental studies in dogs and rats exposed to plutonium provide strong evidence of dose-related risks of lung, liver and bone cancers.2, 32
A major reason for studying Mayak workers is to allow estimation of risks from plutonium exposure in other populations, especially in persons exposed at low doses, a task that requires information on the dose-response relationship and also on modification of risk by gender and age. For lung cancer, the Mayak data as well as data on other alpha emitters such as radon26, 27 support the use of linear extrapolation, and modifying effects are reasonably well quantified. For bone cancer and, to a lesser extent, liver cancer, sparse data limit conclusions that can be drawn. Although liver cancer risks could reasonably be described by linear functions, there was no direct evidence of risk at doses below about 3 Gy. For bone cancer, there were only 6 deaths with positive plutonium doses and only 5 with doses of 0. Although this was sufficient to establish a dose-response, it was not possible to reliably evaluate the shape of the dose-response or the modifying effects of gender and age. For all three cancer endpoints, the lack of plutonium monitoring data for a large proportion of workers potentially exposed to plutonium clearly reduces the power of analyses.
Although there is a clear evidence of dose-response relations for lung, liver and bone cancer mortality, our quantitative estimates are subject to potential bias from several sources. First, data on life-style factors and other occupational exposures were limited. Although lung cancer analyses in this paper were adjusted for smoking, data on smoking were limited to a mostly self-reported “Yes/No” assessment and were not available for 12% of the workers. Nevertheless, a clear gradient in the relative risks for the three smoking categories (no/unknown/yes) was observed in both males and females. Furthermore, the smoking adjustment did not greatly modify lung cancer results suggesting that smoking is not an important confounder in this study. Alcohol consumption is an important risk factor for liver cancer and likely contributes strongly to the gender difference in baseline risks. In addition, some workers may have received other occupational exposures either at Mayak or before employment at Mayak. However, these exposures would be confounders only if they were related to plutonium dose.
Another potential source of bias relates to the reliability of health endpoint data. Vital status was known for 93% of the cohort, and cause of death was known for 96% of deceased workers. In a small study comparing cause of death from autopsy data with that from death certificates, confirmation rates and detection rates for lung, liver and bone cancers ranged from 81% to 100%.1 These findings compare favorably with a study of deaths in Japanese atomic bomb survivors, where the confirmation and detection rates for all neoplasms combined were 91% and 76% respectively, with substantially lower rates for liver cancer (35 and 55% respectively).33 Nevertheless, some cancers indicated as the cause of death, especially liver cancers, could represent metastasis from primary cancers of other organs. This misclassification could lead to underestimation of the ERR.
Analyses in this paper were based on improved plutonium and external organ dose estimates. Nevertheless, estimating internal organ doses from plutonium and their pattern over time is subject to many uncertainties including imprecision in urine measurements, uncertainties in when plutonium exposure occurred and the form of the plutonium, uncertainties in the biokinetic models and parameter values used to estimate deposition and clearance in organs of the body, and the fact that models can only approximate the behavior of plutonium in a given individual. Current estimates use different models for smokers and nonsmokers, but could not take account of detailed smoking histories, and smoking data were not available for all workers. The Mayak data provide the first direct estimates of cancer risks from plutonium. It is expected that future plutonium risk assessments will make strong use of these data along with data from other sources including data on persons exposed to other alpha-emitters.
REFERNCE
- 1.Koshurnikova NA, Shilnikova NS, Okatenko PV, Kreslov VV, Bolotnikova MG, Sokolnikov ME, Khokhryakov VF, Suslova KG, Vassilenko EK, Romanov SA. Characteristics of the cohort of workers at the Mayak nuclear complex. Radiat Res. 1999;152:352–363. [PubMed] [Google Scholar]
- 2.National Research Council. Committee on the Biological Effects of Ionizing Radiation, Health Effects of Radon and Other Internally Deposited Alpha Emitters (BEIR IV) Washington, DC: National Academy Press; 1988. p. 602. [Google Scholar]
- 3.National Council on Radiation Protection and Measurements. NCRP Report No. 131. Bethesda, MD: National Council of Radiation Protection and Measurements; 2001. Scientific Basis for Evaluating the Risks to Populations From Space Applications of Plutonium; p. 280. [Google Scholar]
- 4.Grogan HA, Sinclair WK, Voilleque PG. Risks of fatal cancer from inhalation of 239,240Plutonium by humans: a combined four-method approach with uncertainty evaluation. Health Phys. 2001;80:447–461. doi: 10.1097/00004032-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 5.International Commission on Radiological Protection (ICRP) 1990 Recommendations of the International Commission on Radiological Protection. Oxford: Pergamon Press; 1991. p. 201. ICRP Publication; 60. [Google Scholar]
- 6.Koshurnikova NA, Bolotnikova MG, Ilyin LA, Keirim-Markus IB, Menshikh ZS, Okatenko PV, Romanov SA, Tsvetkov VI, Shilnikova NS. Lung cancer risk due to exposure to incorporated plutonium. Radiat Res. 1998;149:366–371. [PubMed] [Google Scholar]
- 7.Tokarskaya ZB, Scott BR, Zhutova GV, Okladnikova ND, Belyaeva ZD, Khokhryakov VF, Schollnberger H, Vasilenko EK. Interaction of radiation and smoking in lung cancer induction among workers at the Mayak Nuclear Enterprise. Health Phys. 2002;83:833–846. doi: 10.1097/00004032-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kreisheimer M, Sokolnikov ME, Koshurnikova NA, Khokhryakov VF, Romanov SA, Shilnikova NS, Okatenko PV, Nekolla EA, Kellerer AM. Lung cancer mortality among nuclear workers of the Mayak facilities in the former Soviet-Union. An updated analysis considering smoking as the main confounding factor. Radiat Environ Biophys. 2003;42:129–135. doi: 10.1007/s00411-003-0198-3. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert ES, Koshurnikova NA, Sokolnikov ME, Shilnikova NS, Preston DL, Ron E, Okatenko PV, Khokhryakov VF, Vasilenko EK, Miller S, Eckerman K, Romanov SA. Lung cancer in Mayak workers. Radiat Res. 2004;162:505–516. doi: 10.1667/rr3259. [DOI] [PubMed] [Google Scholar]
- 10.Jacob V, Jacob P, Meckbach R, Romanov SA, Vasilenko EK. Lung cancer in Mayak workers: interaction of smoking and plutonium exposure. Radiat Eniron Biophy. 2005;44:119–129. doi: 10.1007/s00411-005-0012-5. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert ES, Koshurnikova NA, Sokolnikov M, Khokhryakov VF, Miller S, Preston DL, Romanov SA, Shilnikova NS, Suslova KG, Vostrotin VV. Liver cancers in Mayak workers. Radiat Res. 2000;154:246–252. doi: 10.1667/0033-7587(2000)154[0246:lcimw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Tokarskaya ZB, Zhuntova GV, Scott BR, Khokhryakov VF, Belyaeva ZD, Vasilendo EK, Syrchikov VA. Influence of alpha and gamma radiations and non-radiation risk factors on the incidence of malignant liver tumors among Mayak PA workers. Health Phys. 2006;91:296–310. doi: 10.1097/01.HP.0000215840.24538.8b. [DOI] [PubMed] [Google Scholar]
- 13.Koshurnikova NA, Gilbert ES, Sokolnikov M, Khokhryakov VF, Miller S, Preston DL, Romanov SA, Shilnikova NS, Suslova KG, Vostrotin VV. Bone cancers in Mayak workers. Radiat Res. 2000;154:237–245. doi: 10.1667/0033-7587(2000)154[0237:bcimw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, Kreslov VV, Koshurnikova NA. Cancer mortality risk among workers at the Mayak Nuclear. Radiat Res. 2003;159:787–798. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Vasilenko EK, Khokhryakov VF, Miller SC, Fix JJ, Eckerman K, Choe DO, Gorelov M, Khokhryakov VV, Knyasev V, Krahenbuhl MP, Scherpelz RI, Smetanin M, et al. Mayak worker dosimetry study: an overview. Health Phys. 2007;93:190–206. doi: 10.1097/01.HP.0000266071.43137.0e. [DOI] [PubMed] [Google Scholar]
- 16.Choe DO, Shelkey BN, Wilde JL, Walk HA, Slaughter DA. Calculated organ doses for Mayak production association central hall using ICRP and MCNP. Health Phys. 2003;84:317–321. doi: 10.1097/00004032-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Leggett RW, Eckerman KF, Khokhryakov VF, Suslova KG, Krahenbuhl MP, Miller SC. Mayak worker study: an improved biokinetic model for reconstructing doses from internally deposited plutonium. Radiat Res. 2005;164:111–122. doi: 10.1667/rr3371. [DOI] [PubMed] [Google Scholar]
- 18.Khokhryakkov VF, Sluslova KG, Vostrotin VV, Romanov SA, Eckerman KF, Krahenbuhl MP, Miller SC. Adaptation of the ICRP publication 66 respiratory tract model to data on plutonium biokinetics for Mayak workers. Health Phyr. 2005;88:125–132. doi: 10.1097/01.hp.0000144575.37546.9d. [DOI] [PubMed] [Google Scholar]
- 19.Preston DL, Lubin JH, Pierce DA. EPICURE user’s guide. Seattle, WA: HiroSoft International Corporation; 1993. [Google Scholar]
- 20.Wilkinson GS, Tietjen GL, Wiggs LD, Galke WA, Acquavella JF, Reyes M, Voelz GL, Waxweiler RJ. Mortality among plutonium and other radiation workers at a plutonium weapons facility. Am J Epidemiol. 1987;125:231–250. doi: 10.1093/oxfordjournals.aje.a114523. [DOI] [PubMed] [Google Scholar]
- 21.Wiggs LD, Johnson ER, Cox-DeVore CA, Voelz GL. Mortality through 1990 among white male workers at the Los Alamos National Laboratory: considering exposures to plutonium and external ionizing radiation. Health Phy. 1994;67:577–588. doi: 10.1097/00004032-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Voelz GL, Lawrence JNP, Johnson ER. Fifty years of plutonium exposure to the Manhattan Project plutonium workers: an update. Health Phys. 1997;73:611–619. doi: 10.1097/00004032-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Omar RZ, Barber JA, Smith PJ. Cancer mortality and morbidity among plutonium workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer. 1998;79:1288–1301. doi: 10.1038/sj.bjc.6690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wing S, Richardson D, Wolf S, Mihlan G. Plutonium-related work and cause-specific mortality at the United Sates Department of Energy Hanford Site. Am J Ind Med. 2004;45:153–164. doi: 10.1002/ajim.10332. [DOI] [PubMed] [Google Scholar]
- 25.Brown SC, Schonbeck MF, McClure D, Baron AE, Navidi WC, Byers T, Ruttenber AJ. Lung cancer and internal lung doses among plutonium workers at the Rocky Flats Plant: a case-control study. Am J Epidemiol. 2004;160:163–172. doi: 10.1093/aje/kwh192. [DOI] [PubMed] [Google Scholar]
- 26.Lubin JH, Boice JD, Jr, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA, Morrison HI, Radford EP, Samet JM. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J NCI. 1995;87:817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council. Committee on the Biological Effects of Exposure to Radon, Health Effects of Exposure to Radon (BEIR VI) Washington (DC): National Academy Press; 1999. p. 500. [Google Scholar]
- 28.Nekolla EA, Kreisheimer M, Kellerer AM, Kuse-Isingschulte M, Gossner W, Spiess H. Induction of malignant bone tumors in radium- 224 patients: risk estimates based on the improved dosimetry. Radiat Res. 2000;153:93–103. doi: 10.1667/0033-7587(2000)153[0093:iombti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Carnes BA, Groer PG, Kotek TJ. Radium dial workers: issues concerning dose response and modeling. Radiat Res. 1997;147:707–714. [PubMed] [Google Scholar]
- 30.United National Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Sources and Effects of Ionizing Radiation. Volume II: Effects. New York: United Nations; 2000. Report to the General Assembly, with Scientific Annexes; p. 566. [Google Scholar]
- 31.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert ES, Griffith WC, Boecker BB, Dagle GE, Guilmette RA, Hahn FF, Muggenburg BA, Park JF, Watson CR. Statistical modeling of carcinogenic risks in dogs that inhaled 238PuO2. Radiat Res. 1998;150:66–82. [PubMed] [Google Scholar]
- 33.Ron E, Carter R, Jablon S, Mabuchi K. Agreement between death certificate and autopsy diagnoses among atomic bomb survivors. Epidemiology. 1994;5:48–56. doi: 10.1097/00001648-199401000-00009. [DOI] [PubMed] [Google Scholar]