Abstract
Optimal task performance requires anticipatory planning to select the most appropriate movement strategy. There is conflicting evidence for hemispheric specialisation of motor planning, with some suggesting left hemisphere dominance, claiming that children with right hemiplegic cerebral palsy (HCP) are therefore disproportionally affected. An alternative view is that there is a positive relationship between functional ability (rather than side of lesion) and motor planning skill. We aimed to compare children with right and left HCP on motor planning ability and to explore its relationship with functional manual ability. Participants were 76 children with HCP (40 left HCP; 30 female), aged 4-15y (Mean 9.09, SD 2.94). Motor planning was assessed using a measure of end-state comfort, which involved turning a hexagonal handle 180° without readjusting grasp. This is difficult, or in some cases impossible, to achieve unless an appropriate initial grasp is adopted. Children completed 24 turns (12 clockwise), which were video recorded for offline scoring. Functional manual ability was assessed with the ABILHAND-Kids questionnaire, completed by parents. Contrary to the existing literature, no differences were observed between right and left HCP. However, a significant interaction between direction of turn and side of hemiplegia indicated a preferential bias for turns in the medial direction, consistent with the “medial over lateral advantage”. There was no relationship between functional ability and motor planning. Therefore, motor planning may not be a priority for therapeutic intervention to improve functional ability in HCP.
Keywords: motor planning, hemiplegia, cerebral palsy, medial over lateral advantage, lateralisation
Introduction
Cerebral palsy can be defined as “a group of permanent disorders of the development of movement and posture, causing activity limitations that are attributed to non-progressive disturbances that occurred in the developing foetal or infant brain” (Rosenbaum et al. 2007). In hemiplegic cerebral palsy (HCP), the lesions lead to predominantly contralateral motor deficits. HCP is therefore traditionally thought of as a unilateral disorder of motor execution (Bax 1964), although often accompanied by sensory, perceptual and cognitive deficits (Rosenbaum et al. 2007). However, it has become increasingly apparent that some of the motor difficulties can be attributed to impaired planning (Steenbergen and Gordon 2006; Steenbergen et al. 2007). Specifically, anticipatory motor planning, in which movement strategy considers and adapts to the demands of the action’s goal (Janssen et al. 2011) has been studied in this context. A clear understanding of the nature of these planning difficulties is important to target them effectively with therapeutic interventions.
Motor planning assessments are generally based on the “end-state comfort effect”, i.e. that people will most often choose to terminate a movement in a comfortable position, even if it requires an awkward or uncomfortable initial posture (Rosenbaum et al. 1990). End-state comfort (ESC) may be prioritised because of efficiency (Rosenbaum et al. 1993), and because it allows for a greater range of potential movement for subsequent actions (Rosenbaum and Jorgensen 1992).
Although the earliest signs of anticipatory planning are detected in infancy (McCarty et al. 1999), motor planning skills mature gradually through childhood. This has most robustly been demonstrated by Jongbloed-Pereboom et al. (2013) in a sample of 351 children. The proportion of time ESC strategies were used increased between the ages of 3 and 10 years, but even at this age had neither plateaued nor reached ceiling. This suggests that ongoing development of motor planning occurs through adolescence. The study is in line with others which use appropriately complex tasks (van Swieten et al. 2010; Janssen and Steenbergen 2011), though studies using simple tasks have demonstrated ceiling effects by age 10 years or younger (Thibaut and Toussaint 2010; Knudsen et al. 2012). This indicates the importance of using an appropriately challenging task to achieve good discrimination between planning skills particularly when assessing older children.
Previous studies have suggested that the left hemisphere is specialised for motor planning (Janssen et al. 2011; Schluter et al. 1998). This is based on the theory that objective motor programs can be selected for each limb by the contralateral hemisphere, with a central system to integrate movements and monitor co-ordination. Because the majority of the population has a dominant right hand (Coren and Porac 1977) the left hemisphere is proposed as the planning centre (Sabate et al. 2004). An alternative account of motor planning proposes a more distributed system across the hemispheres. This is supported by evidence of distinct bilateral and contralateral activations in the precentral gyrus during movement, with the former thought to reflect planning and the latter execution (Baraldi et al. 1999).
Some studies supporting left hemisphere specialisation for motor planning have relied heavily on right-handed participants (Schluter et al. 1998; 2001), which may have led to bias. Janssen et al. (2009, 2011) have addressed this by comparing right- and left-handed participants on a bimanual task of forearm rotation. When the left hand ended in a horizontal position, participants tended not to employ ESC, in contrast to the right hand, suggesting left hemispheric dominance for motor planning. However, a dichotomous scoring system was used for ESC, determined by participant comfort ratings. Participants rated end-state postures on a scale of 1 (very uncomfortable) to 5 (very comfortable). Mean comfort ratings for all horizontal end postures were greater than 3- the midpoint on the scale likely to represent an equivocal state of (dis)comfort. Therefore, although one outcome was rated as more comfortable than the other, neither was reported as uncomfortable. For conditions in which the hand ended in a vertical position, where two end-states were clearly rated as uncomfortable and only one received a comfortable rating, no differences in performance were observed between hands. Therefore, these studies do not provide strong evidence of a hemispheric dominance for motor planning.
Studies of motor planning following a unilateral brain insult offer another way to examine hemispheric specialisation. Dean et al. (2012) found no differences between adults with stroke who had right and left hemisphere lesions, supporting the distributed view of motor planning. However, Steenbergen et al. (2004) and Craje et al. (2009) report disproportionate impairment among adolescents with right HCP. This is particularly surprising since one might expect cortical reorganisation and the development of compensatory planning strategies in this group (Williams et al. 2012; Carr et al. 1993) given the early nature of the damage. These are, to our knowledge, the only two studies of motor planning which directly compare adolescents with right and left HCP and both have relatively small sample sizes (n=11 and n=22 respectively). In addition, the tasks used by Craje et al. (2009) and Steenbergen et al. (2004) discriminate task outcome on the basis of relative states of end-comfort, as in Janssen et al. (2009, 2011). Because of the inherent difficulties with using relative comfort ratings, especially in small children, we used a task in which successful task completion relied upon effective planning.
Motor planning and motor imagery rely on internal simulation of movement and as a result, are thought to be functionally equivalent (Johnson 2000). Williams et al. (2012) showed that children with HCP are capable of performing simple motor imagery tasks. No differences were found between right- and left- hemiplegia groups but low functional ability was associated with poor motor imagery. The authors suggest that the role of function may be more significant than lesion side in motor imagery. Therefore, we hypothesised that there would also be a positive relationship between functional ability and motor planning in children with HCP.
One feature that has been demonstrated in motor imagery, but remains underexplored in motor planning, is the medial over lateral advantage (MOLA) (Funk and Brugger 2008). Medial hand rotatory movements (i.e. those towards the midline) can be completed faster and more easily than lateral movements (i.e. those away from the midline), as a result of intrinsic joint constraints, and this advantage is also reflected in mental rotation times during motor imagery (Parsons 1994, 1987). If this is also true for motor planning there are important implications for its assessment. Most tasks of motor planning, e.g. handle turning (Craje et al. 2010b), bar rotation (Thibaut and Toussaint 2010), grasping and placing a toy sword (Craje et al. 2010a), and turning over a cup (Knudsen et al. 2012) require medial and lateral hand movements so it is important to know if a MOLA exists. We hypothesised a medial advantage for motor planning: that children with right HCP would be better at planning clockwise turns and children with left HCP would be better at planning anticlockwise turns but that there would be no overall difference in motor planning ability between children with right and left HCP.
Our aims were threefold: to assess in children with HCP the effect of lesion side on motor planning, to explore the relationship between motor planning and functional ability and to investigate the MOLA.
Methods
Participants
Seventy-six children with HCP (40 left-sided, mean age 8.72 years, SD 3.05 years; 36 right-sided, mean age 9.5 years, SD 2.81 years) took part (Table 1). The children were taking part in either of two local studies of upper limb interventions in HCP; data were obtained from their baseline assessments. As all participants were under the age of 18, informed parental consent was obtained. Ethical approval was granted by the Newcastle & North Tyneside 2 Research Ethics Committee and the study adhered to the Declaration of Helsinki (1964).
Table 1. Participant information.
| Participant | Age | Sex | Affected hand | Motor planning score |
ABILHAND-Kids Score (logits) | |
|---|---|---|---|---|---|---|
| Clockwise | Anticlockwise | |||||
| 1 | 4.0 | F | Right | 1.00 | 0.00 | a |
| 2 | 4.1 | F | Left | 0.85 | 1.00 | a |
| 3 | 4.2 | M | Left | 0.40 | 0.61 | a |
| 4 | 4.6 | F | Right | 0.92 | −1.00 | a |
| 5 | 4.7 | F | Right | 0.83 | 1.00 | a |
| 6 | 5.1 | M | Left | 0.07 | 0.89 | a |
| 7 | 5.2 | F | Left | 0.08 | 0.92 | a |
| 8 | 5.3 | M | Left | 0.64 | 0.83 | a |
| 9 | 5.3 | M | Left | 0.67 | 0.83 | a |
| 10 | 5.5 | F | Left | −0.14 | 1.00 | a |
| 11 | 5.5 | M | Left | −0.11 | 0.80 | a |
| 12 | 5.8 | M | Right | 0.77 | −0.27 | a |
| 13 | 6.1 | M | Right | 0.85 | 0.00 | 0.34 |
| 14 | 6.4 | M | Left | 0.75 | 0.75 | 0.32 |
| 15 | 6.5 | M | Left | 0.15 | 0.67 | 0.68 |
| 16 | 6.8 | M | Right | 0.30 | −0.18 | 1.03 |
| 17 | 6.9 | F | Right | 1.00 | −0.13 | 0.51 |
| 18 | 7.0 | M | Left | 0.83 | 0.67 | 6.09 |
| 19 | 7.1 | F | Right | 0.36 | 0.08 | 2.98 |
| 20 | 7.3 | M | Left | −0.18 | 0.64 | −1.35 |
| 21 | 7.3 | M | Right | 0.58 | −0.83 | 1.03 |
| 22 | 7.5 | F | Left | 1.00 | 1.00 | −1.08 |
| 23 | 7.6 | M | Left | 0.14 | 0.00 | 6.68 |
| 24 | 7.6 | M | Left | 0.38 | 0.40 | 1.38 |
| 25 | 7.6 | F | Left | 0.77 | 1.00 | 1.78 |
| 26 | 7.7 | F | Left | 0.55 | 1.00 | 1.91 |
| 27 | 7.8 | F | Left | 0.89 | 0.87 | 2.46 |
| 28 | 7.8 | M | Right | 1.00 | 0.08 | 1.65 |
| 29 | 7.8 | M | Left | −0.57 | 0.90 | 1.81 |
| 30 | 8.0 | F | Right | 0.70 | 0.79 | 3.50 |
| 31 | 8.1 | M | Right | 0.25 | −0.78 | 2.30 |
| 32 | 8.1 | M | Left | 1.00 | 0.67 | 3.70 |
| 33 | 8.1 | M | Left | 0.67 | 0.87 | 0.18 |
| 34 | 8.3 | M | Left | 0.17 | 0.73 | 6.68 |
| 35 | 8.3 | M | Right | 0.78 | 0.47 | −1.16 |
| 36 | 8.4 | M | Left | 1.00 | 0.92 | −0.12 |
| 37 | 8.5 | M | Left | 0.58 | 0.82 | 4.85 |
| 38 | 8.8 | F | Left | 0.40 | 0.75 | 2.17 |
| 39 | 9.0 | M | Right | 0.79 | −0.10 | 1.30 |
| 40 | 9.1 | M | Right | 0.33 | 0.00 | 0.85 |
| 41 | 9.2 | M | Left | 1.00 | 1.00 | 0.70 |
| 42 | 9.3 | F | Right | 0.92 | 1.00 | 2.89 |
| 43 | 9.3 | M | Right | 0.54 | 0.73 | 2.22 |
| 44 | 9.3 | F | Right | 0.57 | 0.20 | 1.38 |
| 45 | 9.5 | M | Left | 0.36 | 0.89 | 4.21 |
| 46 | 9.5 | M | Left | 0.67 | 1.00 | −0.10 |
| 47 | 9.6 | M | Right | 1.00 | 0.10 | 0.09 |
| 48 | 9.6 | F | Right | 0.50 | 0.64 | 1.57 |
| 49 | 9.7 | F | Right | 1.00 | 1.00 | 2.89 |
| 50 | 9.7 | F | Right | 0.85 | 0.90 | 1.15 |
| 51 | 9.7 | F | Left | 0.42 | 1.00 | −0.03 |
| 52 | 9.9 | F | Right | 1.00 | 1.00 | 2.33 |
| 53 | 9.9 | M | Left | 1.00 | 1.00 | 1.20 |
| 54 | 10.1 | M | Left | 0.31 | 0.73 | 2.82 |
| 55 | 10.3 | M | Left | 0.82 | 1.00 | 2.98 |
| 56 | 10.4 | F | Right | 0.83 | 0.75 | 4.31 |
| 57 | 10.8 | M | Right | 0.75 | 0.33 | 3.51 |
| 58 | 10.8 | F | Left | −0.25 | 0.57 | 1.14 |
| 59 | 11.1 | M | Right | 1.00 | 0.77 | 3.25 |
| 60 | 11.3 | M | Right | 1.00 | 1.00 | 1.68 |
| 61 | 11.7 | M | Right | 1.00 | 0.55 | 3.12 |
| 62 | 11.7 | F | Right | 1.00 | 0.83 | 3.46 |
| 63 | 11.9 | F | Left | 0.00 | 1.00 | 1.38 |
| 64 | 12.2 | F | Right | 1.00 | −0.25 | 1.68 |
| 65 | 12.8 | M | Right | 1.00 | 1.00 | 4.85 |
| 66 | 12.9 | F | Left | 0.77 | 0.30 | −0.50 |
| 67 | 13.0 | F | Left | −0.73 | 1.00 | 0.67 |
| 68 | 13.3 | F | Right | 1.00 | 0.18 | 2.63 |
| 69 | 13.7 | M | Right | 1.00 | 0.83 | 2.17 |
| 70 | 14.3 | M | Left | 0.92 | 1.00 | 4.37 |
| 71 | 14.3 | F | Right | 0.92 | 0.58 | 1.38 |
| 72 | 14.3 | M | Right | 1.00 | 0.17 | 2.17 |
| 73 | 14.5 | M | Right | 0.80 | 0.86 | 2.07 |
| 74 | 15.2 | M | Left | 0.33 | 0.83 | Data unavailable |
| 75 | 15.4 | M | Left | 0.54 | 1.00 | 0.15 |
| 76 | 15.7 | M | Left | 1.00 | 1.00 | 2.63 |
This assessment is validated for children aged 6-15 years.
Apparatus
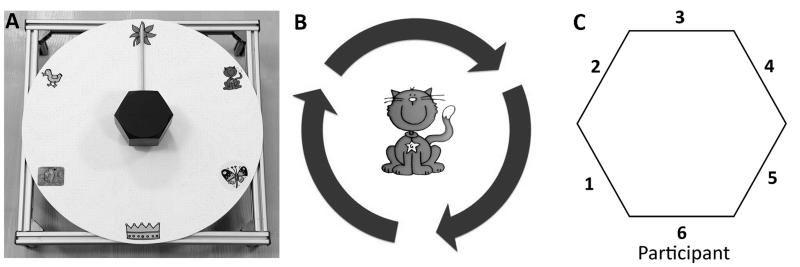
The apparatus (Figure 1A) was similar to that used by Mutsaarts et al. (2006), modified for use by young children. A plastic disk 40 cm in diameter was placed in front and sloping upwards in the direction away from the participant at an angle of 10°. The back of the disk was raised 11.5 cm from the table surface. Target pictures were placed at 0°, 60°, 120°, 180°, 240° and 300°. There were three interchangeable hexagonal handles, with diameters (as measured from graspable, opposing flat surfaces) 5 cm (for hand span <15 cm), 8 cm (for hand span 15-18 cm), and 11 cm (for hand span >18 cm), all 6 cm deep. Handles could rotate fully in both directions and had a wooden stick of length 14.7 cm attached to one side. A video camera was used to record participants’ hand position and movements. Stimuli were presented using Presentation software version 15.0 (Neurobehavioral Systems, Albany, NY, USA) on a 10” computer screen, positioned at a distance of 75 cm in front of the participant.
Fig. 1. Apparatus for motor planning test, adapted from Mutsaarts et al. (2006).
b Pictorial instructions for task (see text for explanation). c Diagram indicating possible thumb locations upon initial grasp
Procedure
ABILHAND-Kids questionnaire
The ABILHAND-Kids questionnaire (Arnould et al. 2004) is a measure of functional ability on a range of daily uni- and bimanual tasks, which has been validated for children with cerebral palsy aged 6-15 years. Parents completed the questionnaire, indicating whether their child could complete each task easily, with difficulty or not at all. Possible scores range from −6.75 (indicating poorest function) to 6.68 (indicating best function), and are not affected by age, sex or handedness.
Motor planning task
Participants were seated so that they could comfortably reach the handle without fully extending the forearm. A target picture was presented at the centre of the computer screen surrounded by moving arrows (Figure 1B). The task was completed with the non-paretic hand in order to remove confounding effects of motor execution difficulties (Craje et al. 2010a). Participants were instructed to turn the handle in the direction of the arrows so that the wooden stick (pointer) pointed to the target picture. The aim was to complete the turn in a single movement and not to adjust the initial grasp, if possible. This is a difficult, or in some circumstances impossible, task to complete comfortably for 180° turns, unless an appropriate initial grasp is selected. Using the same hand, participants clicked a button to indicate when the turn was complete. After 500 ms the next trial began. Trials were presented in a pseudorandom order. Following a practice session with 5 trials, participants performed 48 turns, half of which were 180° turns with an equal number in each direction. These 180° turns were critical trials which were analysed. Twelve 60° and twelve 120° turns were also completed in order to vary the start and end points of the pointer in the critical trials. These were not analysed as they could be completed comfortably without adjusting initial grasp. The optimal strategy for turns was not dependent on the start position of the pointer, as equivalent strategies were needed for critical turns in any one direction regardless of this. However, the approach of indicating the picture to which the pointer had to be turned was chosen in order to increase the accessibility of the test to young children, avoiding the need to describe the turn size in other ways.
Data acquisition and analysis
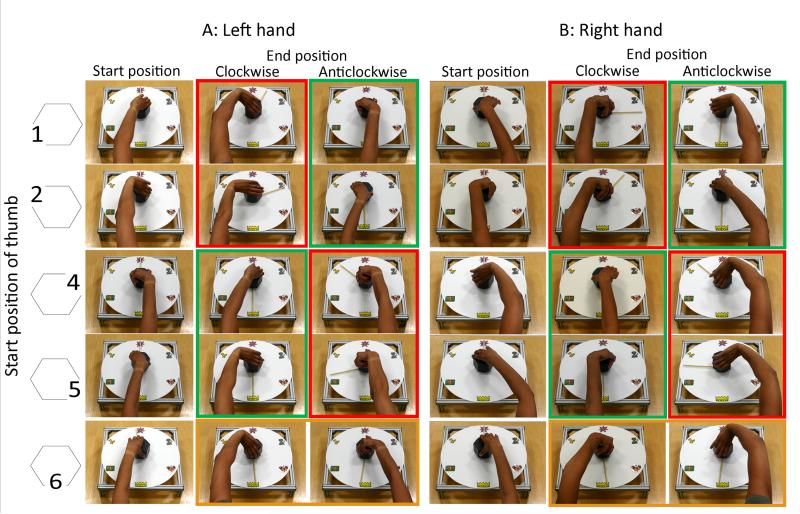
Data were extracted from video recordings. Initial grasp was coded according to position of the thumb (Figure 1C). A score of 1 (effective anticipatory planning), 0 (no evidence of anticipatory planning) or −1 (maladaptive planning strategy) was then assigned for each trial for each participant.
Effective anticipatory planning
In order to comfortably complete the task it was necessary to initially rotate the hand in the opposite direction of the anticipated turn (Figure 2). For clockwise turns this meant that the thumb was at position 4 or 5 in Figure 1C upon initial grasp, whichever hand was used. For anticlockwise turns the thumb was at positions 1 or 2 in Figure 1C upon initial grasp, whichever hand was used. This was considered effective anticipatory motor planning and each trial in which this strategy was used was given a score of 1 point. It was possible to complete tasks with the thumb at position 3 on Figure 1C, providing the hand was initially rotated in the appropriate direction. These trials also were given a score of 1 point but this was an unusual strategy, adopted by only five participants.
Fig. 2. Starting thumb positions (as numbered in Figure 1C) indicated with left hand (A) and right hand (B) and consequences for clockwise and anticlockwise 180° turns to move the pointer from the flower (top) to the crown (bottom).
Initial grasps with the thumb at position 1 or 2 were suitable for comfortable completion of anticlockwise turns but made clockwise turns impossible. Initial grasps with thumb at position 4 or 5 were suitable for comfortable completion of clockwise turns but made anticlockwise turns impossible. It was possible to complete turns in either direction with initial grasps with thumb at position 6 but these ended in uncomfortable, awkward postures. Position 3 is not shown due to infrequency of use. Green frames indicate effective planning strategies. Red frames indicate maladaptive planning strategies. Orange frame indicates no evidence of planning strategy
No evidence of anticipatory planning
If the hand was not rotated before turning the handle (thumb at position 6 in Figure 1C) it was possible to complete the task but the end position of the hand was very uncomfortable and unstable. In light of the end-state comfort effect, this was not considered effective planning. A score of 0 was applied to these trials given that there was no evidence of either successful or maladaptive planning.
Maladaptive planning strategy
If the hand was initially rotated in the same direction as the anticipated turn, the task was impossible to complete (Figure 2). Clockwise turns starting with the thumb at positions 1 or 2 and anticlockwise turns with the thumb at positions 4 or 5 in Figure 1C were therefore given a negative score by subtracting 1 point per trial.
A composite motor planning score was then calculated for 180° turns for each participant using the following formula:
This gave a possible range of −1 to +1, where −1 indicated maladaptive planning for all turns, +1 indicated that all turns were effectively planned and 0 showed no evidence of planning.
A two-way mixed analysis of covariance (ANCOVA) was undertaken with between-group variable side of hemiplegia (right vs. left), and within-group variable direction of turn (clockwise vs. anticlockwise). Age was included as a covariate to account for developmental changes associated with motor planning. Post hoc paired t-tests, with Bonferroni adjusted alpha, were used to explore the observed interaction.
Raw ABILHAND-Kids scores were converted to a linear measure by Rasch analysis using the online scoring tool (Arnould et al. 2004). To study the relationship between motor planning and functional ability, a Pearson product-moment correlation was performed on converted ABILHAND-Kids scores and motor planning scores. A subgroup of 64 children (32 left-sided, mean age 9.64 years, SD 2.68 years; 32 right-sided, mean age 10.09 years, SD 2.37 years) was included in this analysis, omitting those under age 6 years (for whom the ABILHAND test has not been validated) and one child age 15 years for whom ABILHAND-Kids baseline data was unavailable.
Results
Independent t-tests confirmed that right and left hemiplegia groups did not differ in terms of age t (74) = −1.17, p = 0.25, or functional manual ability t (61) = −0.24, p = 0.81. Age was significantly related to motor planning score F (1, 73) = 7.33, p = 0.01, ηp2 = 0.09.
Effect of lesion side on motor planning
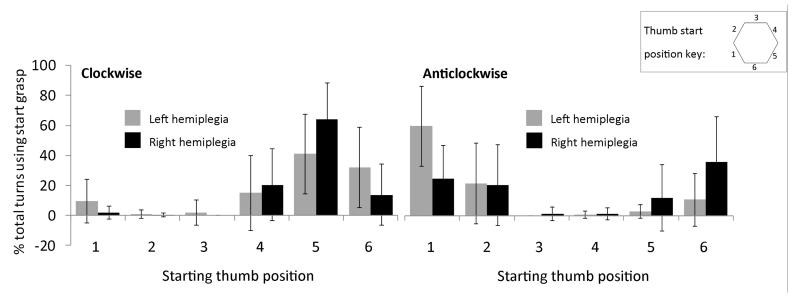
Controlling for age, no significant difference in motor planning score was found between the right and left HCP groups F (1, 73) = 1.65, p = 0.20, ηp2 = 0.02. The distribution of initial grasps is shown in Figure 3.
Fig. 3. Distribution of start grasps used for clockwise (left) and anticlockwise (right) turns in right and left hemiplegia.
Error bars indicate ±1SD
Relationship between motor planning and functional manual ability
No relationship was found between functional manual ability (ABILHAND-Kids score) and motor planning score r (62) = 0.10, p = 0.45.
Medial over lateral advantage
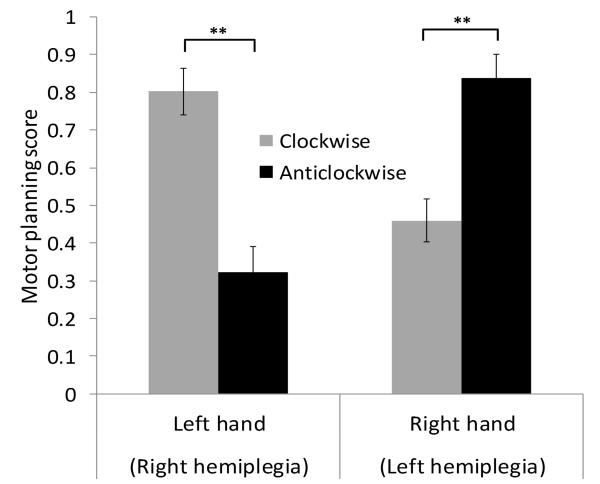
While there was no main effect of turn direction on motor planning scores F (1, 73) = 2.62, p = 0.11, ηp2 = 0.04, a significant interaction between turn direction and side of hemiplegia was observed F (1, 73) = 56.21, p < 0.001, ηp2= 0.44 (Figure 4). Post-hoc paired t-tests confirmed a MOLA, in which the right HCP group were significantly better at planning clockwise (Mean score = 0.80, SE = 0.06) than anticlockwise turns (Mean score = 0.32, SE = 0.07), t (35) = 5.34, p < 0.001, r = 0.67. The left HCP group showed higher planning scores for anticlockwise turns (Mean score = 0.84, SE = 0.06) over clockwise turns (Mean score = 0.46, SE = 0.06), t (39) = −4.96, p < 0.001, r = 0.62.
Fig. 4. Mean motor planning scores for clockwise (black bars) and anticlockwise (grey bars) 180° turns in children with left and right HCP.
Error bars indicate ±1SE
Discussion
Our paper represents to our knowledge the largest study of motor planning in HCP. We have shown that, contrary to previous published findings, there is no difference in motor planning performance between children with left and right HCP with our task. We also found that motor planning is unrelated to functional ability in activities of daily living. Of note, we have demonstrated for the first time a strong MOLA in motor planning.
In contrast to Steenbergen et al. (2004) and Craje et al. (2009), our results do not indicate a significant difference in motor planning between right and left HCP. Craje et al. (2009) categorised participants according to their adopted strategy, e.g. tendency to start in pronated position, and found different distributions of planning style in right and left HCP groups. However, comparing their group data in terms of ESC, as in the current study, no difference is observed. Steenbergen et al. (2004) also concluded that in specific contexts, particularly when the task lacked ecological validity, adolescents with right HCP were more impaired at motor planning than those with left HCP. However, this study had very small groups of 5-6 participants each, making it difficult to draw reliable conclusions. Additionally, end-states prescribed as uncomfortable were relatively less comfortable than the alternative but are unlikely have been sufficiently uncomfortable to elicit appropriate motor planning behaviour. These factors may account for the apparent contradictory results in the current study. Our findings are in keeping with the notions of a distributed system of motor planning across hemispheres and/or cortical reorganisation following damage in early life (Williams et al. 2012; Carr et al. 1993). Based on Williams et al. (2012) we hypothesised that, similar to motor imaging, motor planning may be related to functional ability; however, this was not supported by our results. The ABILHAND-Kids questionnaire (Arnould et al. 2004) was used as a measure of functional ability. This is a useful assessment to gain insight into the child’s ability to perform a range of daily tasks. A possible explanation for the lack of relationship with motor planning is that this questionnaire focuses on whether the child can achieve a goal but not on the manner in which this is done. Children may easily complete an activity in a step-wise manner which does not rely on anticipatory planning. Perhaps an alternative assessment such as the Assisting Hand Assessment (Krumlinde-Sundholm et al. 2007), which measures unprompted use of the paretic hand during bimanual activities, would provide further insight, and we intend to investigate this.
Interventions to improve motor planning through bimanual training (Janssen and Steenbergen 2011) and motor imagery (Craje et al. 2010b) have been proposed for HCP. While previous studies suggest that this would primarily benefit children with right HCP (Craje et al. 2009), our results show that children with both right and left HCP could benefit. However, the lack of relationship between motor planning and functional ability suggest that motor planning may not play a key role in effective completion of many daily activities. Functional success has been identified as a meaningful goal for parents (Wiart et al. 2010) and should be a priority in therapeutic intervention.
We showed a strong MOLA for motor planning, reflecting upper limb biomechanical constraints, which is seen also in motor imagery and execution (Parsons, 1994, 1987). Children with left HCP showed a preference for anticlockwise turns and the opposite preference was observed in children with right HCP. This has not previously been demonstrated in motor planning and highlights an important methodological issue in the need to ensure tasks assessing end-state comfort, which commonly require forearm rotation, are balanced with respect to medial and lateral rotation.
We have shown that motor planning is not lateralised in children with hemiplegia, as right and left HCP groups performed to an equal level. Furthermore we have highlighted the importance of balancing tasks appropriately in light of the MOLA in motor planning. Finally, we suggest that interventions to improve motor planning may not be a priority in therapeutic plans to improve functional outcomes for activities of daily living for children with HCP.
Acknowledgements
Apparatus construction: Vincent Willey. Data collection: Erin Baker, Hannah Preston. Thanks to all participants and clinicians who assisted with participant identification and referral, in particular Dr Jill Kisler. Funders: Newcastle University, WellChild Trust.
Abbreviations
- HCP
Hemiplegic cerebral palsy
- MOLA
medial over lateral advantage
- ESC
end-state comfort
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology. 2004;63(6):1045–1052. doi: 10.1212/01.wnl.0000138423.77640.37. [DOI] [PubMed] [Google Scholar]
- Baraldi P, Porro CA, Serafini M, Pagnoni G, Murari C, Corazza R, Nichelli P. Bilateral representation of sequential finger movements in human cortical areas. Neurosci Lett. 1999;269(2):95–98. doi: 10.1016/s0304-3940(99)00433-4. [DOI] [PubMed] [Google Scholar]
- Bax MC. Terminology and Classification of Cerebral Palsy. Dev Med Child Neurol. 1964;6:295–297. doi: 10.1111/j.1469-8749.1964.tb10791.x. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(Pt 5):1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C. Fifty centuries of right-handedness: the historical record. Science. 1977;198(4317):631–632. doi: 10.1126/science.335510. [DOI] [PubMed] [Google Scholar]
- Craje C, Aarts P, Nijhuis-van der Sanden M, Steenbergen B. Action planning in typically and atypically developing children (unilateral cerebral palsy) Research in developmental disabilities. 2010a;31(5):1039–1046. doi: 10.1016/j.ridd.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Craje C, van der Kamp J, Steenbergen B. Visual information for action planning in left and right congenital hemiparesis. Brain Res. 2009;1261:54–64. doi: 10.1016/j.brainres.2008.12.074. [DOI] [PubMed] [Google Scholar]
- Craje C, van Elk M, Beeren M, van Schie HT, Bekkering H, Steenbergen B. Compromised motor planning and Motor Imagery in right Hemiparetic Cerebral Palsy. Research in developmental disabilities. 2010b;31(6):1313–1322. doi: 10.1016/j.ridd.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Dean PJ, Seiss E, Sterr A. Motor planning in chronic upper-limb hemiparesis: evidence from movement-related potentials. Plos One. 2012;7(10):e44558. doi: 10.1371/journal.pone.0044558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk M, Brugger P. Mental rotation of congenitally absent hands. Journal of the International Neuropsychological Society : JINS. 2008;14(1):81–89. doi: 10.1017/S1355617708080041. [DOI] [PubMed] [Google Scholar]
- Janssen L, Beuting M, Meulenbroek R, Steenbergen B. Combined effects of planning and execution constraints on bimanual task performance. Exp Brain Res. 2009;192(1):61–73. doi: 10.1007/s00221-008-1554-y. [DOI] [PubMed] [Google Scholar]
- Janssen L, Meulenbroek RG, Steenbergen B. Behavioral evidence for left-hemisphere specialization of motor planning. Exp Brain Res. 2011;209(1):65–72. doi: 10.1007/s00221-010-2519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L, Steenbergen B. Typical and atypical (cerebral palsy) development of unimanual and bimanual grasp planning. Res Dev Disabil. 2011;32(3):963–971. doi: 10.1016/j.ridd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Johnson SH. Thinking ahead: the case for motor imagery in prospective judgements of prehension. Cognition. 2000;74(1):33–70. doi: 10.1016/s0010-0277(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Jongbloed-Pereboom M, Nijhuis-van der Sanden MW, Saraber-Schiphorst N, Craje C, Steenbergen B. Anticipatory action planning increases from 3 to 10 years of age in typically developing children. J Exp Child Psychol. 2013;114(2):295–305. doi: 10.1016/j.jecp.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Knudsen B, Henning A, Wunsch K, Weigelt M, Aschersleben G. The end-state comfort effect in 3- to 8-year-old children in two object manipulation tasks. Frontiers in psychology. 2012;3:445. doi: 10.3389/fpsyg.2012.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The assisting hand assessment: current evidence of validity, reliability, and responsiveness to change. Developmental Medicine and Child Neurology. 2007;49(4):259–264. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- McCarty ME, Clifton RK, Collard RR. Problem solving in infancy: the emergence of an action plan. Dev Psychol. 1999;35(4):1091–1101. doi: 10.1037//0012-1649.35.4.1091. [DOI] [PubMed] [Google Scholar]
- Mutsaarts M, Steenbergen B, Bekkering H. Anticipatory planning deficits and task context effects in hemiparetic cerebral palsy. Exp Brain Res. 2006;172(2):151–162. doi: 10.1007/s00221-005-0327-0. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Imagined spatial transformations of one’s hands and feet. Cognitive psychology. 1987;19(2):178–241. doi: 10.1016/0010-0285(87)90011-9. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. Journal of experimental psychology Human perception and performance. 1994;20(4):709–730. doi: 10.1037//0096-1523.20.4.709. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D, Marchak F, Barnes HJ, Vaughan J, Slotta J, Jorgensen MJ. Constraints for Action Selection: Overhand Versus Underhand Grips. In: Jeannerod M, editor. Attention and performance XIII: Motor representation and control. Lawrence Erlbaum Associates; Hove and London: 1990. pp. 321–342. [Google Scholar]
- Rosenbaum DA, Engelbrecht SE, Bushe MM, Loukopoulos LD. A Model for Reaching Control. Acta Psychol. 1993;82(1-3):237–250. doi: 10.1016/0001-6918(93)90014-i. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Jorgensen MJ. Planning Macroscopic Aspects of Manual Control. Hum Movement Sci. 1992;11(1-2):61–69. [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- Sabate M, Gonzalez B, Rodriguez M. Brain lateralization of motor imagery: motor planning asymmetry as a cause of movement lateralization. Neuropsychologia. 2004;42(8):1041–1049. doi: 10.1016/j.neuropsychologia.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39(2):105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121(Pt 5):785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Gordon AM. Activity limitation in hemiplegic cerebral palsy: evidence for disorders in motor planning. Developmental Medicine and Child Neurology. 2006;48(9):780–783. doi: 10.1017/S0012162206001666. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Meulenbroek RGJ, Rosenbaum DA. Constraints on grip selection in hemiparetic cerebral palsy: effects of lesional side, end-point accuracy, and context. Brain Res Cogn Brain Res. 2004;19(2):145–159. doi: 10.1016/j.cogbrainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Verrel J, Gordon AM. Motor planning in congenital hemiplegia. Disabil Rehabil. 2007;29(1):13–23. doi: 10.1080/09638280600947591. [DOI] [PubMed] [Google Scholar]
- Thibaut JP, Toussaint L. Developing motor planning over ages. Journal of experimental child psychology. 2010;105(1-2):116–129. doi: 10.1016/j.jecp.2009.10.003. [DOI] [PubMed] [Google Scholar]
- van Swieten LM, van Bergen E, Williams JH, Wilson AD, Plumb MS, Kent SW, Mon-Williams MA. A test of motor (not executive) planning in developmental coordination disorder and autism. J Exp Psychol Hum Percept Perform. 2010;36(2):493–499. doi: 10.1037/a0017177. [DOI] [PubMed] [Google Scholar]
- Wiart L, Ray L, Darrah J, Magill-Evans J. Parents’ perspectives on occupational therapy and physical therapy goals for children with cerebral palsy. Disability and Rehabilitation. 2010;32(3):248–258. doi: 10.3109/09638280903095890. [DOI] [PubMed] [Google Scholar]
- Williams J, Anderson V, Reid SM, Reddihough DS. Motor imagery of the unaffected hand in children with spastic hemiplegia. Developmental neuropsychology. 2012;37(1):84–97. doi: 10.1080/87565641.2011.560697. [DOI] [PubMed] [Google Scholar]