Abstract
Type 2 diabetes is characterized by impaired insulin secretion from pancreatic β‐cells and/or reduced response of target tissues to insulin. Good glycemic control delays the development and slows the progression of micro‐ and macrovascular complications. Although there are numerous glucose‐lowering agents in clinical use, only approximately half of type 2 diabetic patients achieve glycemic control, and undesirable side‐effects often hamper treatment in those treated with the medications. There is a need for novel treatment options that can help overcome these difficulties. Sodium glucose cotransporter 2 (SGLT2) inhibitors have recently been developed as a novel potential therapeutic option for the treatment of type 2 diabetes. These drugs lower the plasma glucose concentration through inhibition of glucose reuptake in the kidney, independent of insulin secretion and insulin action, with a consequent lower risk of hypoglycemia. The data of clinical trials with monotherapy as well as combination therapy show that SGLT2 inhibitors have a blood glucose‐lowering effect and also reduce bodyweight. A follow‐up study shows long‐term efficacy and the durability of these effects. SGLT2 inhibitors have the potential to reverse glucose toxicity, and to improve insulin resistance, blood pressure and lipid profile. The available data suggest a good tolerability profile. However, clinicians should carefully prescribe these drugs in light of already reported and/or unexpected side‐effects. Further studies in larger numbers and longer‐term clinical use data are required to place these agents in standard treatment of type 2 diabetes.
Keywords: Novel antidiabetic agents, Renal glucose reabsorption, Sodium glucose cotransporter 2 inhibitors
Introduction
The increasing prevalence of diabetes now afflicts 382 million people worldwide, and this number is expected to rise to 592 million by 2,0351. The great majority (approximately 90%) of diabetes is type 2 diabetes2. Type 2 diabetes is characterized by impaired insulin secretion from pancreatic β‐cells and/or reduced response of target tissues to insulin (insulin resistance)3. Chronic hyperglycemia is associated with development of micro‐ and macrovascular complications, which contribute to mortality and morbidity5. Good glycemic control is desired to prevent the development and slow the progression of these complications7.
At present, there are numerous oral and injectable agents in clinical use11. Oral antihyperglycemic agents include biguanides, sulfonylureas, α‐glucosidase inhibitors, dipeptidyl peptidase‐4 (DPP4) inhibitors, meglitinides and thiazolidinediones. Injectable antihyperglycemic agents include incretin‐related agents, such as liraglitide and exenatide, and various insulins. Most of these agents are initially effective, but fail in the long term to maintain normoglycemia as monotherapy, resulting in a requirement for multiple antihyperglycemic therapies12. Despite the variety of treatment options, just half of type 2 diabetes patients achieve glycemic control with hemoglobin A1c lower than 7.0%13. In addition, undesirable side‐effects often hamper treatment with these medications. For example, insulin and insulin secretagogues, such as sulfonylureas, are associated with hypoglycemia and weight gain, thiazolidinediones are associated with weight gain and edema, metformin can cause gastrointestinal effects, and rarely, lactic acidosis. Hence, there is a need for novel treatment options that that can help overcome these difficulties.
Inhibitors of sodium glucose cotransporter 2 (SGLT2) have recently been developed as a novel potential therapeutic option for the treatment of type 2 diabetes14. SGLT2 inhibitors lower the plasma glucose concentration by inhibition of glucose reuptake in the kidney, without weight gain. As the mechanism of action of SGLT2 inhibitors is independent of insulin secretion and insulin action, they lower the plasma glucose concentration with lower risk of hypoglycemia. In the present article, we review the role of SGLT2 in glucose homeostasis, the development of SGLT2 inhibitors, findings from clinical trials of SGLT2 inhibitors, and the potentials and safety concerns in the treatment of type 2 diabetes.
Role of the Kidney in Glucose Homeostasis
The kidneys play an important role in glucose homeostasis, primarily through reabsorption of filtered glucose, glucose production (gluconeogenesis likely in the liver) and consumption16. The kidneys normally filter approximately 180 g of serum glucose in the glomeruli, which is then reabsorbed completely in the proximal tubules; urine is thus normally negative for glucose15. However, as plasma glucose concentrations approach approximately 180 mg/dL and cross over a threshold, glucose appears in the urine18. Glucose reabsorption at the renal tubules is mediated by two groups of transporters. These include glucose transporters (GLUTs) and sodium‐glucose cotransporters (SGLTs; Figure 1). GLUTs are facilitative or passive transporters that transport glucose along the concentration gradient. SGLTs are a large family of membrane proteins that transport glucose across the brush border membrane of the intestinal epithelium and proximal renal tubules using the electro‐chemical sodium gradient as the source of energy generated by Na+/K+ adenosine triphosphatase16. SGLT1 is a high‐affinity, low‐capacity transporter and is expressed mainly in the small intestine, where it plays a role in the absorption of glucose and galactose, as well as in the proximal tubules in the kidney. SGLT2 is a low‐affinity, high‐capacity transporter and is expressed particularly in proximal tubules in the kidney14. The proximal tubules in the kidney are divided to three segments (S1, S2 and S3) anatomically. SGLT2 is located in S1 and accounts for 90% of the glucose reabsorbed from the kidneys; SGLT1 is located in S2 and S3, and accounts for the remaining 10% (Figure 1).
Figure 1.
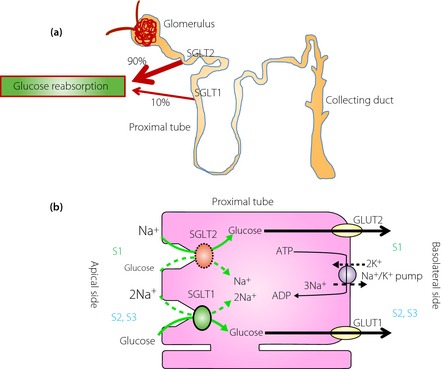
Renal glucose handling in a non‐diabetic individual. (a) Glucose reabsorption in the kidney. (b) Glucose reabsorption through sodium glucose cotransporter (SGLT)1 and SGLT2 in the proximal renal tubular cell. ADP, adenosine diphosphate; ATP, adenosine triphosphate; GLUT, glucose transporter; S1, segment1; S2, segment 2; S3, segment 3.
People with genetically inherited SGLT1 mutations show malabsorption, severe osmotic diarrhea and dehydration24. In contrast, people with genetically inherited SGLT2 mutations have familial renal glucosuria, but most cases are otherwise normal and healthy25. Interestingly, these people do not exhibit show regardless of virtually absent glucose reabsorption. In addition, it is reported that in individuals with type 2 diabetes, there is evidence that renal glucose reabsorption might be enhanced14. Therefore, even in the presence of hyperglycemia, the kidneys continue to reabsorb glucose, with the net effect of further promotion of hyperglycemia. These observations show that inhibition of SGLT2 might be a reasonable approach to the control of blood glucose levels in type 2 diabetes, and have led to the development of the SGLT2 inhibitors. Inhibition of glucose uptake by the kidneys appears to be a novel, unique and promising insulin‐independent approach to the treatment of type 2 diabetes.
Development of SGLT2 Inhibitors
Interest in SGLT2 inhibitors originated with the demonstration that phlorizin, originally isolated from the bark of apple trees in 1,835, in France27, non selectively inhibits SGLT1 and SGLT2, normalizes plasma glucose concentrations, and reverses insulin resistance in animal models of diabetes28. Phlorizin induces glucosuria by inhibiting SGLT in the kidneys27, and reduces serum glucose in diabetic animal models. Furthermore, phlorizin improves insulin resistance and β‐cell dysfunction by mitigating “glucose toxicity”, a deteriorating cycle in diabetes in which hyperglycemia itself can further compound the levels of insulin resistance and insulin deficiency. However, clinical development of phlorizin was stopped because of its poor absorption after oral administration and severe gastrointestinal side‐effects, such as diarrhea, induced by SGLT1 inhibition.
Efforts were made to develop new compounds to inhibit SGLT2 with high potency and selectivity. To overcome the limitations of phlorizin, a number of oral SGLT2 inhibitors, all derived from the basic structure of phlorizin, have been synthesized and are in clinical development for the treatment of type 2 diabetes (Table 1). These SGLT2 inhibitors have high potency and high selectivity against SGLT2 over SGLT1 (Table 2). Dapagliflozin was approved in Europe in 2012 and in the USA in 201429, canagliflozin was approved in the USA in 2013, ipraglifrozin has been approved in Japan in 2014, and these or several other SGLT2 inhibitors will soon be approved in Europe, the USA and other countries, such as Japan.
Table 1. List of sodium glucose cotransporter 2 inhibitors under clinical development.
| Drug | Company | Clinical stage |
|---|---|---|
| Canagliflozin | Mitsubishi Tanabe, Janssen | Approved in USA and EU, filed in Japan |
| Dapagliflozin | Bristol‐Myers, AstraZeneca | Approved in USA and EU, filed in Japan |
| Empagliflozin | Boehringer Ingelheim, Eli Lilly | Filed in USA, EU and Japan |
| Ipragliflozin | Astellas, Kotobuki | Approved in Japan |
| Luseogliflozin | Taisho | Filed in Japan |
| Tofogliflozin | Chugai, Kowa, Sanofi | Filed in Japan |
| Ertugliflozin | Merck, Pfizer | Phase III in USA,phase I in Japan |
| LX‐4211 | Lexicon | Phase II in USA |
EU, Europe.
Table 2. In vitro inhibitory concentration 50 values against human sodium glucose cotransporter 2 and sodium glucose cotransporter 1, and sodium glucose cotransporter 2 selectivity67.
| Drug | IC50 for human SGLT2 (nmol/L) | IC50 for human SGLT1 (nmol/L) | SGLT2 selectivity (fold) |
|---|---|---|---|
| Canagliflozin | 4.4 | 684 | 155 |
| Dapagliflozin | 1.12 | 1,391 | 1,242 |
| Empagliflozin | 3.1 | 8,300 | 2,680 |
| Ipragliflozin | 7.38 | 1,876 | 254 |
| Luseogliflozin | 2.26 | 3,990 | 1,770 |
| Tofogliflozin | 2.9 | 8,444 | 2,912 |
| Phrorizin | 34.6 | 210 | 6 |
Sodium glucose cotransporter (SGLT)2 selectivity was calculated by using the following formula: inhibitory concentration 50 (IC50) value for SGLT1/IC50 value for SGLT2.
In contrast, it has been reported that SGLT1‐deficient mice lose just ~3% of the filtered glucose into the urine, whereas SGLT2‐deficient mice lose ~60% of the filtered glucose into the urine, suggesting that wild‐type mice do not use the maximal transport capacity of SGLT1 under normoglycemic conditions30. In diabetic patients, the glucose concentration is overwhelming in early proximal tubules, and even more so in patients with an SGLT2‐specific inhibitor. In this condition, an SGLT1 transporter might be performing at full capacity, and therefore minimize the effects of the drug31. In this context, SGLT1 inhibition might have therapeutic potential. One mixed SGLT1 and SGLT2 inhibitor (LX‐4211) has been identified, and is currently in development33.
We next review the six representative types of SGLT2 inhibitor that offer the best available evidence in humans: dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, luseogliflozin and tofogliflozin.
Clinical Trials of SGLT2 Inhibitors
The data of clinical trials of these six agents with monotherapy for 16–24 weeks are shown in Table 3 and Figure 2. All types of SGLT2 inhibitors have a glucose‐lowering effect with monotherapy, and have an additional effect in reducing bodyweight. They lower the glycated hemoglobin (HbA1c) level by 0.58–1.03% from baseline. They are associated with clinically significant weight reductions by 2.2–3.4 kg, which have been attributed to glycosuria, with a loss of approximately 200–300 Kcal per day. Although several glucose‐lowering drugs exert a different effect in Caucasians and Asians because of differences of insulin secretory capacity and/or insulin sensitivity, the favorable effects of SGLT2 inhibitors are obtained to the same extent regardless of difference of race34. The reason might be derived from the unique mechanism of action of SGLT2 inhibitors, which act independently of insulin secretion and insulin sensitivity. Furthermore, because of this unique mechanism of action, SGLT2 inhibitors are effective in lowering HbA1c at all stages of diabetes, and can be used in combination with other glucose‐lowering agents including insulin36. In follow‐up clinical trials, the long‐term efficacy of SGLT2 inhibitors and their efficacy in combination therapy with other glucose‐lowering therapies became available.
Table 3. Results of clinical trials with sodium glucose cotransporter 2 inhibitors39.
| Duration | Canagliflozin | Dapagliflozin | Empagliflozin | Ipragliflozin | Luseogliflozin | Tofogliflozin | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26W | 24W | 24W | 16W | 24W | 24W | ||||||||||||||
| Dose | PBO | 100 mg | 300 mg | PBO | 2.5 mg | 5 mg | 10 mg | PBO | 10 mg | 25 mg | PBO | 50 mg | PBO | 2.5 mg | PBO | 10 mg | 20 mg | 40 mg | |
| No. participants | n = 192 | n = 195 | n = 197 | n = 75 | n = 65 | n = 64 | n = 70 | n = 228 | n = 224 | n = 224 | n = 67 | n = 62 | n = 79 | n = 79 | n = 56 | n = 57 | n = 58 | n = 58 | |
| Race | Mainly in USA and Europe | Mainly in USA and Europe | Mainly in USA and Europe | Japanese | Japanese | Japanese | |||||||||||||
| HbA1c (%) | Mean ± SD baseline | 8.0 ± 1.0 | 8.1 ± 1.0 | 8.0 ± 1.0 | 7.84 ± 0.87 | 7.92 ± 0.90 | 7.86 ± 0.94 | 8.01 ± 0.96 | 7.91 | 7.87 | 7.86 | 8.25 | 8.4 | 8.17 | 8.14 | 8.41 | 8.45 | 8.35 | 8.37 |
| LS Mean ± SE change | 0.14 | −0.77 | −1.03 | −0.23 ± 0.10 | −0.58 ± 0.11 | −0.77 ± 0.11 | −0.89 ± 0.11 | 0.08 ± 0.05 | −0.66 ± 0.05 | −0.78 ± 0.05 | 0.48 | −0.76 | 0.13 | −0.63 | −0.03 | −0.8 | −1.02 | −0.87 | |
| Fasting plasma glucose (mg/dL) | Mean ± SD baseline | 165.6 ± 37.8 | 172.8 ± 43.2 | 172.8 ± 43.2 | 155.9 ± 42.1 | 164.1 ± 48.0 | 162.2 ± 45.0 | 166.6 ± 45.9 | – | – | – | – | – | 161.9 | 160.8 | 169.2 | 171 | 169.2 | 167.4 |
| LS Mean ± SE change | 9 | −27 | −34.2 | −4.1 ± 3.9 | −15.2 ± 4.2 | −24.1 ± 4.3 | −28.8 ± 4.0 | – | – | – | 5.9 | −39.9 | −0.8 | −28.3 | −8.56 | −31.868 | −35.899 | −32.327 | |
| Bodyweight (kg) | Mean ± SD baseline | 87.6 ± 19.5 | 85.8 ± 21.4 | 86.9 ± 20.5 | 88.8 ± 19.0 | 90.8 ± 22.8 | 87.6 ± 17.1 | 94.2 ± 18.7 | 78.23 | 78.35 | 77.8 | – | – | 66.7 | 70.2 | 71.2 | 67.26 | 68.06 | 68.72 |
| LS Mean ± SE change | −0.5 | −2.5 | −3.4 | −2.2 ± 0.4 | −3.3 ± 0.5 | −2.8 ± 0.5 | −3.2 ± 0.5 | −0.33 ± 0.17 | −2.26 ± 0.17 | −2.48 ± 0.17 | −0.89 | −2.36 | −0.9 | −2.7 | −0.36 | −2.23 | −2.85 | −2.97 | |
HbA1c, glycated hemoglobin; LS, least squares; PBO; placebo; SD, standard deviation; SE, standard error.
Figure 2.
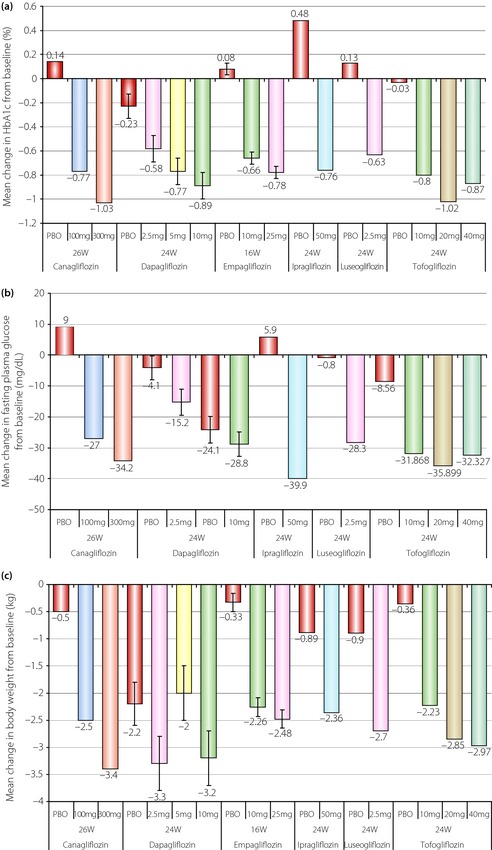
Results of trials with sodium glucose cotransporter 2 inhibitors. Changes in (a) glycated hemoglobin (HbA1c), (b) fasting plasma glucose and (c) bodyweight39. PBO, placebo.
Canagliflozin
Canagliflozin is the first SGLT2 inhibitor approved by the US Food and Drug Administration (FDA), and was available on the market in 201338. At week 26 of monotherapy, canagliflozin 100 mg and 300 mg reduced HbA1c vs placebo (−0.77, −1.03, +0.14%, respectively; P < 0.001)39. At week 52, canagliflozin 100 mg and 300 mg showed non‐inferiority, and canagliflozin 300 mg showed statistical superiority to sitagliptin in lowering HbA1c (−0.73, −0.88, −0.73%, respectively)40. Canagliflozin 100 and 300 mg reduced bodyweight vs placebo (week 26: −3.7, −4.2, −1.2%, respectively; P < 0.001) and sitagliptin (week 52: −3.8, −4.2, −1.3%, respectively; P < 0.001). Both canagliflozin doses reduced fasting blood glucose and systolic blood pressure vs placebo (week 26) and vs sitagliptin (week 52; P < 0.001). In the combination therapy, canagliflozin also improved glycemic control, reduced bodyweight and was generally well tolerated in type 2 diabetes patients on metformin plus sulphonylurea over 52 weeks41. HbA1c was significantly reduced with canagliflozin 100 and 300 mg vs placebo at week 26 (−0.85, −1.06, −0.13%; P < 0.001); these reductions were maintained at week 52 (−0.74, −0.96, 0.01%). Reductions in HbA1c with canagliflozin 100 mg (−0.82%) and 300 mg/day (−0.93%) were non‐inferior to those with glimepiride (titration of glimepiride ranged from a starting dose of 1 mg to a maximum dose of 6 or 8 mg; −0.81%) over the course of 52 weeks of treatment in patients on background metformin. Canagliflozin 300 mg/day was superior to glimepiride in reducing HbA1c, and both doses of canagliflozin were superior to glimepiride in reducing bodyweight (−3.7 kg with 100 mg/day, −4.0 kg with 300 mg/day vs +0.7 kg with glimepiride41. In the body composition substudy, patients had baseline characteristics and weight changes over 52 weeks that were generally similar to those reported in the main study. In the canagliflozin groups, roughly two‐thirds of the reduction in bodyweight was from fat mass, and one‐third was from lean body mass; the increase in bodyweight with glimepiride included both fat and lean body mass. Analysis of abdominal fat in the canagliflozin groups with computed tomography imaging showed a slightly greater reduction in visceral adipose tissue than that in subcutaneous adipose tissue. In the follow‐up study of the aforementioned study up to 2 years, HbA1c reductions were maintained with canagliflozin 100 and 300 mg, and glimepiride vs placebo at week 104 (−0.65, −0.746 and −0.55%), and both doses of canagliflozin were superior to glimepiride in reducing bodyweight (−4.1% with 100 mg/day, −4.2% with 300 mg/day vs +0.9% with glimepiride; Table 4)42.
Table 4. Clinical data of long‐term efficacy using sodium glucose cotransporter 2 inhibitors.
| Canagliflozin | Dapagliflozin | Empagliflozin | ||||||
|---|---|---|---|---|---|---|---|---|
| Combination with metformin | Combination with metformin | Combination with insulin | ||||||
| 104 weeks | 4 years | 78 weeks | ||||||
| SU | 100 mg | 300 mg | SU | Dapagliflozin | PBO | 10 mg | 25 mg | |
| HbA1c change (%) | −0.55 | −0.65 | −0.74 | 0.2 | −0.1 | −0.02 | −0.48 | −0.64 |
| Bodyweight change (kg) | – | – | – | 1.12 | −3.95 | 0.7 | −2.2 | −2 |
| Bodyweight change (%) | 0.9 | −4.1 | −4.2 | – | – | – | – | – |
HbA1c, glycated hemoglobin; PBO; placebo, SU; sulfonylurea.
Dapagliflozin
Dapagliflozin was the first approved SGLT2 inhibitor, and there is much published clinical trial data. In 24‐week, placebo‐controlled, phase 3 trials, dapagliflozin (2.5, 5 and 10 mg once daily) used as monotherapy or as add‐on therapy to metformin43, glimepiride44, pioglitazone45 or insulin46 reduced HbA1c and fasting plasma glucose in patients with type 2 diabetes. A long‐term follow‐up trial also showed beneficial effects of dapagliflozin. Dapagliflozin added to metformin for 102 weeks resulted in sustained reductions in HbA1c, fasting blood glucose and weight without increased risk of hypoglycemia in patients with type 2 diabetes inadequately controlled by metformin alone47. At week 102, mean changes from baseline HbA1c (8.06%) were +0.02% for placebo compared with −0.48% (P = 0.0008), −0.58% (P < 0.0001) and −0.78% (P < 0.0001) for dapagliflozin 2.5–5 and 10 mg, respectively. In addition, all dapagliflozin groups had sustained reductions from baseline in fasting plasma glucose and bodyweight at 102 weeks, whereas increases were noted in placebo‐treated patients for both of these outcomes. Dapagliflozin was also compared with glipizide in patients whose hyperglycaemia was inadequately controlled by metformin48. After 1 year, a similar HbA1c reduction from baseline of −0.52% was seen with dapagliflozin (≤10 mg/day) and glipizide (≤20 mg/day). A decrease from baseline in bodyweight of −3.2 kg occurred with dapagliflozin, compared with a weight gain of 1.4 kg with glipizide. Dapagliflozin has the longest term follow‐up results among SGLT2 inhibitors. In a randomized, double‐blind trial of dapagliflozin (≤10 mg/day) vs glipizide (≤20 mg/day) as add‐on to metformin in type 2 diabetes, dapagliflozin was non‐inferior to glipizide in HbA1c change at 52 weeks (both −0.52%), and produced weight loss. The 4‐year data showed that the effect of therapy on HbA1c was attenuated over time in both groups, but dapagliflozin showed more persistent benefits vs glipizide (change from baseline of −0.1 vs +0.2%): treatment difference −0.30% (95% confidence interval (CI) −0.51 to −0.09; Table 4)49. Sustained and stable weight loss was observed with dapagliflozin vs weight gain with glipizide (−3.95 vs +1.12 kg): treatment difference −5.07 kg (95% CI −6.21 to −3.93). Mean systolic blood pressure was reduced with dapagliflozin, but not with glipizide (difference: −3.7 mmHg, 95% CI −5.9 to −1.4).
Empagliflozin
Empagliflozin (5–25 mg/day for 12 weeks) increased glucose excretion, and decreased fasting plasma glucose (−31.1 mg/dL at 25 mg vs an increase of 0.8 mg/dL with placebo), HbA1c (−0.63% at 25 mg vs an increase of 0.09%) and bodyweight (−2.0 kg at 2 mg vs −0.8 kg) in patients with type 2 diabetes50. In a randomized to double‐blind empagliflozin (10, 25 mg) or placebo add‐on to basal insulin for 78 weeks, empagliflozin significantly reduced HbA1c (empagliflozin 10 mg: −0.48%,empagliflozin 25 mg: −0.64%,placebo: −0.02%), bodyweight (empagliflozin 10 mg: −2.2 kg,empagliflozin 25 mg: −2.0 kg,placebo: +0.7 kg; Table 4). Furthermore, empagliflozin 10 mg significantly reduced systolic blood pressure (empagliflozin 4 mg: −4.1 mmHg, empagliflozin 25 mg: −2.4 mmHg,placebo: +0.1 mmHg)51. In a randomized, open‐label, 78‐week blinded study of empagliflozin (monotherapy at doses of 10 mg or 25 mg, and add‐on to metformin) with metformin, and sitagliptin as add‐on to metformin, changes from baseline in HbA1c at week 90 were −0.34 to −0.63% with empagliflozin, −0.56% with metformin, and −0.40% with sitagliptin. Changes in weight from baseline at week 90 were −2.2 to −4.0 kg with empagliflozin, −1.3 kg with metformin and −0.4 kg with sitagliptin52. Thus, long‐term empagliflozin treatment can provide sustained glycemic and weight control in patients with type 2 diabetes.
Ipragliflozin
In a 24‐week trial in patients with type 2 diabetes inadequately controlled with metformin alone, ipragliflozin (50 mg/day) decreased HbA1c by −0.87% vs an increase of 0.38% with placebo (P < 0.001). Bodyweight was also reduced with ipragliflozin (−2.3 kg vs −0.6 kg)53. The beneficial effects of ipragliflozin (50–100 mg/day) on HbA1c (−0.51%) and bodyweight (−3.41 kg) were sustained for up to 52 weeks in Japanese patients with type 2 diabetes54.
Luseogliflozin
Clinical trials of luseogliflozin as monotherapy or add‐on therapy to five types of oral antidiabetic drugs for 52 weeks in Japanese patients with type 2 diabetes mellitus showed that luseogliflozin improves glycemic control and reduces bodyweight. Changes in HbA1c after 52 weeks were monotherapy: −0.50%,add‐on to glimepiride: −0.63%,add‐on to metformin: −0.61%,add‐on to DPP4 inhibitors: −0.52%,add‐on to pioglitazone: −0.60%,add‐on to glinide: −0.59%,and add‐on to α‐glucosidase inhibitor: −0.68%.Changes in bodyweight after 52 weeks were monotherapy: −2.7 kg,add‐on to glimepiride: −2.2 kg,add‐on to metformin: −2.9 kg,add‐on to DPP4 inhibitors: −2.0 kg,add‐on to pioglitazone: −2.3 kg,add‐on to glinide: −2.9 kg and add‐on to α‐glucosidase inhibitor: −2.8 kg.Furthermore, luseogliflozin decreased blood pressure and showed trends toward improvement in plasma lipids (triglyceride and high‐density lipoprotein (HDL) cholesterol) at week 52 compared with baseline55.
Tofogliflozin
Clinical trials of tofogliflozin as monotherapy or add‐on therapy to oral antidiabetic drugs for 52 weeks in Japanese patients with type 2 diabetes mellitus showed that tofogliflozin improved glycemic control and reduced bodyweight. With monotherapy, change in HbA1c from baseline was −0.7% (both 20 and 40 mg), and change in bodyweight was –3.1 kg (20 mg) and –3.4 kg (40 mg). With combination therapy, change in HbA1c from baseline was –0.8% (20 mg) and –0.9% (40 mg), and change in bodyweight was −2.5 kg (20 mg) and −3.0 kg (40 mg). Furthermore, reduced systolic and diastolic blood pressure, improved homeostasis model assessment of insulin resistance, increased serum adiponectin and HDL cholesterol levels were secondarily observed58.
Safety of SGLT2 Inhibitors
Urinary Tract and Genital Infections
One of the major safety concerns of SGLT2 inhibition is that by their very nature, the drugs cause glucose elevation in the urine that can lead to urinary tract and genital infections, electrolyte imbalances, and increased urinary frequency. For example, frequency in urinary infections and genital infections reported in clinical trials were 2.8% (dapaglifrogin 5 mg), 7.2% (canagliflozin 100 mg)39. In the meta‐analysis, urinary tract infections were more common among patients treated with SGLT2 inhibitors than among those receiving placebo (odds ratio [OR] 1.34, 95% CI 1.03–1.74]; I2 = 0%)59. We also found an increased incidence of genital tract infections with SGLT2 inhibitors compared with placebo (OR 3.50, 95% CI 2.46–4.99; I2 = 0%) and active comparators (OR 5.06, 95% CI 3.44–7.45; I2 = 0%). Females were more prone to infection than males.
Hypoglycemia
Because their mechanism of action is not dependent on insulin secretion, SGLT2 inhibitors are less likely to cause hypoglycemia, an adverse effect of some antihyperglycemics60. The incidence of hypoglycemia was low in most treatment groups, except among patients receiving a sulfonylurea or insulin as allocated treatment or background therapy59.
Cancer Risk
An increased incidence of bladder and breast cancer was identified in the dapagliflozin trials. Among 5,478 patients who received dapagliflozin, there were nine cases of bladder cancer compared with one case in 3,136 patient controls60. There were nine cases of breast cancer in 2,223 patients receiving dapagliflozin therapy compared with one case in 1,053 controls61. In 2011, a FDA Advisory Committee voted against approval of dapagliflozin because of concerns about increased risk for bladder and breast cancer61, and the FDA requested additional clinical trial data to determine the risk‐to‐benefit ratio of this therapy. Further data analysis has been ongoing to determine the potential increased risk of cancer with dapagliflozin therapy. In January 2014, the FDA approved dapaglifozin to improve glycemic control, along with diet and exercise, in adults with type 2 diabetes29. A pooled analysis of nine trials with approximately 8,000 person‐years of exposure did not show any difference in incidence of bladder cancer between canagliflozin (5 of 6,648 patients) and control (4 of 3,640 patients) groups59. Similarly, the incidence of breast cancer did not differ between canagliflozin (12 of 2,827 patients) and comparators (6 of 1,501 patients). However, clinicians must be cautious of the incidence of cancer risk in long‐term clinical use of SGLT2 inhibitors, and long‐term follow‐up data and cumulative data on the association of cancer incidence and SGLT2 inhibitors are required.
Cardiovascular Outcomes
The meta‐analysis of cardiovascular outcomes for dapagliflozin, which was based on 14 trials (n = 6,300), yielded an OR of 0.73 (95% CI 0.46–1.16; I2 = 0%) compared with control59. In a pooled analysis of two dapagliflozin trials in patients with established cardiovascular disease63, the hazard ratio for the composite cardiovascular end‐point (cardiovascular death, myocardial infarction, stroke and hospitalization for unstable angina) was 1.07 (95% CI 0.64–1.72) vs placebo65. Canagliflozin was not associated with an increased risk for the composite cardiovascular outcome compared with placebo or active comparator on the basis of data from 10 trials that included a total of 10,474 patients. In the FDA report66, the HR for non‐fatal stroke was higher in patients receiving canagliflozin (6,876 patient‐years) than in the control groups (3,470 patient‐years; HR 1.46, 95% CI 0.83–2.58). In addition, an imbalance in the incidence of cardiovascular events observed during the first 30 days of the dedicated cardiovascular trial66 between canagliflozin (13/2,886 patients) and a placebo (1/1,441 patients) resulted in a HR of 6.50 (95% CI 0.85–49.66), possibly as a result of volume depletion after canagliflozin initiation. This imbalance was not evident after 30 days. Data on cardiovascular outcomes and death were inconclusive. The numerical imbalance in non‐fatal stroke events among patients treated with canagliflozin requires clarification and confirmation. Follow‐up trials of cardiovascular outcomes in clinical use of several SGLT2 inhibitors including a canagliflozin are ongoing60.
Others
A higher risk of hypotension with SGLT2 inhibitors was induced than with other antidiabetic medications (OR 2.68, 95% CI 1.14–6.29)59. In patients with moderate renal impairment, the incidence of renal‐related adverse events resulting in renal impairment induced by osmotic diuresis and volume depletion was increased with dapagliflozin and canagliflozin compared with the placebo. Regarding liver‐related adverse events, slight imbalances among patients treated with dapagliflozin or canagliflozin and control groups were probably not associated with the study drug.
Clinical Potential of SGLT2 Inhibitors
The data from all of the clinical trials clearly show that SGLT2 inhibitors have the favorable effects of lowering blood glucose levels as well as reducing bodyweight. The energy deficit resulting from excretion of calories into the urine induces weight loss or has a weight‐neutral effect. A follow‐up study of these trials showed the long‐term efficacy and durability of these effects; several SGLT2 inhibitors, such as dapagliflozin, are superior to sulfonylureas in terms of changes in HbA1c and bodyweight loss49. In addition to the blood glucose‐lowering effect and the reducing effect on bodyweight, SGLT2 inhibitors have a potential in amelioration of metabolic and cardiovascular risk factors, blood pressure, lipid profile (HDL cholesterol), adiponectin, and liver dysfunction induced by fatty liver.
SGLT2 inhibitors might also have a preventive effect on the progression of diabetes by ameliorating β‐cell dysfunction as well as insulin resistance. Although improvement of β‐cell function was found first in animal models67, there have been some reports of improved β‐cell function in clinical studies39. These effects might be derived from indirect effects induced by the attenuation of glucotoxity, as SGLT2 inhibitors do not directly influence insulin secretion. SGLT2 inhibitors thus have long durability of good glycemic control regardless of β‐cell conditions. In contrast to other current antidiabetic agents that directly influence insulin secretion, inhibition of SGLT2 represents a particularly appealing approach to diabetes treatment because of its novel mechanism of action. The mechanism of action also suggests that SGLT2 inhibitors have the potential to be used in combination with other oral antidiabetic agents as well as insulin to exert additive or synergic effects on lowering glucose levels in type 2 diabetes. In fact, clinical data on combination therapy has shown favorable effects, with efficacy in lowering HbA1c and reduction of bodyweight not inferior to that in use in monotherapy.
The available data suggest a good tolerability profile. However, SGLT2 inhibitors have no experience of long clinical use. Clinicians should carefully prescribe these drugs in light of already reported and/or unexpected side‐effects. In particular, increasing risks of repeated urinary tract infections and genital infections should be kept in mind. SGLT2 inhibitors might cause hypovolemia as a result of their diuretic effect; the relevant side‐effects, such as stroke, should be of concern, especially during a hot season. In patients with moderate renal impairment, the use of dapagliflozin or high doses of canagliflozin was associated with increased incidence of renal‐related adverse events. In addition, in lean, elderly patients, there might be a risk for sarcopenia as a result of bodyweight loss. Furthermore, a potential for an increase in hypoglycemia after combination therapy, especially with sulfonylureas, has also been noted74.
Conclusion
Inhibition of the SGLT2 glucose transporter is a new therapeutic approach for the treatment of type 2 diabetes. Clinical trials of the SGLT2 inhibitors have shown therapeutic benefits in attaining better glycemic control and reducing bodyweight in type 2 diabetes patients. Some of these are now, or will soon be, in clinical use. Although the available data suggest a good tolerability profile, clinicians should carefully prescribe these drugs in light of already reported and/or unexpected side‐effects. Further studies in large numbers and long‐term clinical use data are required to delineate efficacy and safety, and to place these agents in the standard treatment of type 2 diabetes.
Acknowledgements
Nobuya Inagaki has received research grants, consultancy fees and honoraria for lectures from Astellas Pharma Inc., Taisho Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited., GlaxoSmithKline plc, Daiichi Sankyo Company, Limited., MSD, Sanofi, Novartis Pharma, Dainippon Sumitomo Pharma Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Eli Lilly Japan K.K., Shiratori Pharmaceutical Co., Ltd., Roche Diagnostics, JT, Nippon Boehringer Ingelheim Co ., Ltd., Ono Pharmaceutical Co. Ltd., AstraZeneca PLC, Kowa Company, Ltd., and Japan Diabetes Foundation. Yoshihito Fujita declares no conflict of interest.
J Diabetes Invest 2014; 5: 265–275
References
- 1.International Diabetes Federation . IDF Diabetes Atlas, 6th edn International Brussels, Brussels, Belgium, Belgium, International Diabetes Federation, 2013 [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet 2011; 378: 31–40 [DOI] [PubMed] [Google Scholar]
- 3.Prentki M, Nolan CJ. Islet cell failure in type 2 diabetes. J Clin Invest 2006; 116: 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Rahilly S. Human obesity and insulin resistance: lessons from experiments of nature. Biochem Soc Trans 2007; 35: 33–36 [DOI] [PubMed] [Google Scholar]
- 5.Pirola L, Balcerczyk A, Okabe J, et al Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol 2010; 6: 665–675 [DOI] [PubMed] [Google Scholar]
- 6.L'Abbate A. Large and micro coronary vascular involvement in diabetes. Pharmacol Rep 2005; 57(Suppl.): 3–9 [PubMed] [Google Scholar]
- 7.UKPDS 34 . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–865 [PubMed] [Google Scholar]
- 8.UKPDS 33 . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853 [PubMed] [Google Scholar]
- 9.Ohkubo Y, Kishikawa H, Araki E, et al Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association . Standards of medical care in diabetes–2014. Diabetes Care 2014; 37(Suppl 1): S14–S80 [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE, Haffner SM, Heise MA, et al Glycemic durability of rosiglitazone, metformin or glyburide monotherapy. N Engl J Med 2007; 356: 1387–1388 [DOI] [PubMed] [Google Scholar]
- 13.Ong KL, Cheung BM, Wong LY, et al Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18: 222–229 [DOI] [PubMed] [Google Scholar]
- 14.Rahmoune H, Thompson PW, Ward JM, et al Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes 2005; 54: 3427–3434 [DOI] [PubMed] [Google Scholar]
- 15.Bakris GL, Fonseca VA, Sharma K, et al Renal sodium‐glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75: 1272–1277 [DOI] [PubMed] [Google Scholar]
- 16.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91: 733–794 [DOI] [PubMed] [Google Scholar]
- 17.Abdul‐Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes. Endocr Pract 2008; 6: 782–790 [DOI] [PubMed] [Google Scholar]
- 18.Ferrannini E. Learning from glycosuria. Diabetes 2011; 60: 695–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hediger MA, Kanai Y, You G, et al Mammalian ion‐coupled solute transporters. J Physiol 1995; 482: 7S–17S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Williams S, Ho S, et al Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 2010; 1: 57–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallon V, Platt KA, Cunard R, et al SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011; 22: 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch 2004; 447: 510–518 [DOI] [PubMed] [Google Scholar]
- 23.Wright EM. Renal Na–glucose cotransporters. Am J Renal Physiol 2001; 280: F10–F18 [DOI] [PubMed] [Google Scholar]
- 24.Martín MG, Turk E, Lostao MP, et al Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose‐galactosemalabsorption. Nat Genet 1996; 12: 216–220 [DOI] [PubMed] [Google Scholar]
- 25.van den Heuvel LP, Assink K, Willemsen M, et al Autosomal recessive renal glucosuria attributable to a mutation in the sodium glucose cotransporter (SGLT2). Hum Genet 2002; 111: 544–547 [DOI] [PubMed] [Google Scholar]
- 26.Calado J, Santer R, Rueff J. Effect of kidney disease on glucose handling (including genetic defects). Kidney Int Suppl 2011; 79: S7–S13 [DOI] [PubMed] [Google Scholar]
- 27.Ehrenkranz JR, Lewis NG, Kahn CR, et al Phlorizin: a review. Diabetes Metab Res Rev 2005; 21: 31–38 [DOI] [PubMed] [Google Scholar]
- 28.Rossetti L, Smith D, Shulman GI, et al Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987; 79: 1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA, FDA NEWS RELEASE . Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm380829.htm‐
- 30.Gorboulev V, Schürmann A, Vallon V, et al Na(+)‐D‐glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose‐dependent incretin secretion. Diabetes 2012; 6: 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada N, Inagaki N. Role of sodium‐glucose transporters in glucose uptake of the intestine and kidney. J Diabetes Invest 2012; 3: 352–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdul‐Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30‐50% of filtered glucose load in humans. Diabetes 2013; 10: 3324–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrowicz B, Freiman J, Brown PM, et al LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo‐controlled trial. Clin Pharmacol Ther 2012; 92: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66(Suppl 1): S37–S43 [DOI] [PubMed] [Google Scholar]
- 35.Inagaki N, Kondo K, Yoshinari T, et al Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, 12‐week study. Diabetes Obes Metab 2013; 15: 1136–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Feng Y, List J, et al Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 2010; 12: 510–516 [DOI] [PubMed] [Google Scholar]
- 37.Clar C, Gill JA, Court R, et al Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012; 2: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietrich E, Powell J, Taylor JR. Canagliflozin: a novel treatment option for type 2 diabetes. Drug Des Devel Ther 2013; 22: 1399–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenlöf K, Cefalu WT, Kim KA, et al Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15: 372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavalle‐González FJ, Januszewicz A, Davidson J, et al Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013; 56: 2582–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cefalu WT, Leiter LA, Yoon KH, et al Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013; 382: 941–950 [DOI] [PubMed] [Google Scholar]
- 42.Cefalu WT, Leiter LA, Yoon KH, et al Canagliflozin demonstrates durable glycemic improvements over 104 weeks versus glimepiride in subjects with type 2 diabetes mellitus on metformin. Diabetes 2013; 62(Suppl 1A): LB18 [Google Scholar]
- 43.Bailey CJ, Gross JL, Pieters A, et al Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 2223–2233 [DOI] [PubMed] [Google Scholar]
- 44.Strojek K, Yoon KH, Hruba V, et al Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2011; 13: 928–938 [DOI] [PubMed] [Google Scholar]
- 45.Rosenstock J, Vico M, Wei L, et al Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012; 35: 1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilding JPH, Woo V, Soler NG, et al Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin. Ann Intern Med 2012; 156: 405–415 [DOI] [PubMed] [Google Scholar]
- 47.Bailey CJ, Gross JL, Hennicken D, et al Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med 2013; 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nauck MA, Del Prato S, Meier JJ, et al Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care 2011; 34: 2015–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prato SD, et al Durability of dapagliflozin vs. glipizide as add‐on therapies in T2DM inadequately controlled on metformin: 4‐year data. Diabetes 2013; 62(Suppl 1A): LB17 [Google Scholar]
- 50.Ferrannini E, Seman LJ, Seewaldt‐Becker E, et al The potent and highly selective sodium‐glucose co‐transporter (SGLT‐2) inhibitor BI10773 is safe and efficacious as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2010; 53: S351 [Google Scholar]
- 51.Rosenstock J, Jelaska A, Wang F, et al Empagliflozin as add‐on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin‐treated type 2 diabetes. Diabetes 2013; 62: A285 [Google Scholar]
- 52.Ferrannini E, Berk A, Hantel S, et al Long‐term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active‐controlled, parallel‐group, randomized, 78‐week open‐label extension study in patients with type 2 diabetes. Diabetes Care 2013; 36: 4015–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goto K, Kashiwagi A, Kazuta K, et al Ipragliflozin reduces A1C and body weight in type 2 diabetes patients who have inadequate glycemic control on metformin alone: ILLUMINATE study. Diabetes 2012; 61: A269 [Google Scholar]
- 54.Kawano H, Kashiwagi A, Kazuta K, et al Long‐term safety, tolerability and efficacy of ipragliflozin in Japanese patients with type 2 diabetes mellitus: IGNITE. Diabetes 2012; 61(Suppl 1): A610 [Google Scholar]
- 55.Seino Y, Sasaki T, Fukatsu A, et al Luseogliflozin, a SGLT2 inhibitor, improves glycaemic control and reduces body weight as monotherapy up to 52 weeks in Japanese patients with type 2 diabetes mellitus. Diabetologia 2013; 56(Suppl 1): S384 [Google Scholar]
- 56.Inagaki N, Seino Y, Sasaki T, et al Luseogliflozin, a selective SGLT2 inhibitor, added on to glimepiride for 52 weeks improves glycaemic control with no major hypoglycaemia in Japanese type 2 diabetes patients. Diabetologia 2013; 56(Suppl 1): S82 [Google Scholar]
- 57.Haneda M, Seino Y, Sasaki T, et al Luseogliflozin, a SGLT2 inhibitor, as add‐on therapy to 5 types of oral antidiabetic drugs improves glycaemic control and reduces body weight in Japanese patients with type 2 diabetes mellitus. Diabetologia 2013; 56(Suppl 1): S384 [Google Scholar]
- 58.Tanizawa Y, Araki E, Tobe K, et al Efficacy and safety of tofogliflozin administered for 52 weeks as monotherapy or combined with other oral hypoglycaemic agents in Japanese patients with type 2 diabetes. Diabetologia 2013; 56(Suppl 1): S82–S83 [Google Scholar]
- 59.Vasilakou D, Karagiannis T, Athanasiadou E, et al Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med 2013; 159: 262–274 [DOI] [PubMed] [Google Scholar]
- 60.Riser TaylorS, Harris KB. The clinical efficacy and safety of sodium glucose cotransporter‐2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy 2013; 33: 984–999 [DOI] [PubMed] [Google Scholar]
- 61.Food and Drug Administration . FDA Briefing Document NDA 202293. Dapagliflozin 5 and 10 mg. 2011. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262994.pdf
- 62.Burki TK. FDA rejects novel diabetes drug over safety fears. Lancet 2012; 379: 507. [DOI] [PubMed] [Google Scholar]
- 63.Cefalu WT, Leiter LA, Debruin TW, et al Dapagliflozin treatment for type 2 diabetes mellitus patients with comorbid cardiovascular disease and hypertension. Diabetes 2012; 61(Suppl 1): A271 [Google Scholar]
- 64.Leiter LA, Cefalu WT, Debruin TW, et al Efficacy and safety of dapagliflozin for type 2 diabetes mellitus patients with a history of cardiovascular disease. Diabetes 2012; 61(Suppl 1): A287 [Google Scholar]
- 65.European Medicines Agency . Assessment Report: Forxiga (Dapagliflozin). Procedure no. EMEA/H/C/002322. London: European Medicines Agency; 2012. Accessed at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002322/WC500136024.pdf [Google Scholar]
- 66.Matthews DR, Fulcher G, Perkovic V, et al Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co‐transporter 2 (SGLT2), added‐on to insulin therapy +/− oral agents in type 2 diabetes. Diabetologia 2012; 55(Suppl 1): S314–S315 [Google Scholar]
- 67.Han S, Hagan DL, Taylor JR, et al Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 2008; 57: 1723–1729 [DOI] [PubMed] [Google Scholar]
- 68.Oku A, Ueta K, Arakawa K, et al T‐1095, an inhibitor of renal Na+‐glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 1999; 48: 1794–1800 [DOI] [PubMed] [Google Scholar]
- 69.Oku A, Ueta K, Arakawa K, et al Correction of hyperglycemia and insulin sensitivity by T‐1095, an inhibitor of renal Na+‐glucose cotransporters, in streptozotocin‐induced diabetic rats. Jpn J Pharmacol 2000; 84: 351–354 [DOI] [PubMed] [Google Scholar]
- 70.Luippold G, Klein T, Mark M, et al Empagliflozin, a novel potent and selective SGLT‐2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin‐induced diabetic rats, a model of type 1 diabetes mellitus. Diabetes Obes Metab 2012; 14: 601–607 [DOI] [PubMed] [Google Scholar]
- 71.Suzuki M, Honda K, Fukazawa M, et al Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Pharmacol Exp Ther 2012; 341: 692–701 [DOI] [PubMed] [Google Scholar]
- 72.Arakawa K, Ishihara T, Oku A, et al Improved diabetic syndrome in C57BL/KsJ‐db/db mice by oral administration of the Na+‐glucose cotransporter inhibitor T‐1095. Br J Pharmacol 2001; 132: 578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang Y, Arakawa K, Ueta K, et al Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS ONE 2012; 7: e30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicolle LE, Capuano G, Ways K, et al Effect of canagliflozin, a sodium‐glucose cotransporter 2(SGLT2) inhibitors, on bacteria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12‐week, phase 2 study. Curr Med Res Opin 2012; 28: 1167–1171 [DOI] [PubMed] [Google Scholar]
- 75.Kim Y, Babu AR. Clinical potential of sodium‐glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012; 5: 313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tahrani AA, Bailey CJ, Del Prato S, et al Management of type 2 diabetes: new and future developments in treatment. Lancet 2011; 378: 182–197 [DOI] [PubMed] [Google Scholar]
- 77.Tahara A, Kurosaki E, Yokono M, et al Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol 2012; 385: 423–436 [DOI] [PubMed] [Google Scholar]
- 78.Grempler R, Thomas L, Eckhardt M, et al Empagliflozin, a novel selective sodium glucose cotransporter‐2 (SGLT‐2) inhibitor: characterisation and comparison with other SGLT‐2 inhibitors. Diabetes Obes Metab 2012; 14: 83–90 [DOI] [PubMed] [Google Scholar]
- 79.Ohtake Y, Sato T, Kobayashi T, et al Discovery of tofogliflozin, a novel C‐Arylglucoside with an O‐spiroketal ring system, as a highly selective sodium glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 2012; 55: 7828–7840 [DOI] [PubMed] [Google Scholar]
- 80.Kakinuma H, Oi T, Hashimoto‐Tsuchiya Y, et al (1S)‐1,5‐anhydro‐1‐[5‐(4‐ethoxybenzyl)‐2‐methoxy‐4‐methylphenyl]‐1‐thio‐d‐glucito l (TS‐071) is a potent, selective sodium‐dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem 2010; 53: 3247–3261 [DOI] [PubMed] [Google Scholar]
- 81.Kurosaki E, Ogasawara H. Ipragliflozin and other sodium‐glucose cotransporter‐2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther 2013; 139: 51–59 [DOI] [PubMed] [Google Scholar]
- 82.Ferrannini E, Ramos SJ, Salsali A, et al Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise. Diabetes Care 2010; 33: 2217–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roden M, Weng J, Eilbracht J, et al Empagliflozin monotherapy improves glucose control in drug‐naïve patients with type 2 diabetes. Diabetes 2013; 62(Suppl 1): A280 [Google Scholar]
- 84.Kashiwagi A, Takinami Y, Kazuta K, et al Efficacy of Ipragliflozin, a new SGLT2 inhibitor, in the treatment of type 2 diabetes. J Jpn Diabetes Soc 2012; 55(Suppl 1): S‐276 (Japanese). [Google Scholar]
- 85.Araki E, Kaku K, Watada H, et al Verification of the efficacy and safety of tofogliflozin, a novel SGLT2 inhibitor, in Japanese patients with type 2 diabetes mellitus: results from a phase 2/3 clinical study. Diabetologia 2013; 56(Suppl 1): S371 [Google Scholar]


