Abstract
Aims/Introduction
Insulin has been associated with the risk of colorectal cancer (CRC). However, few studies have evaluated the association between insulin and colorectal adenoma. We investigated the relationship between fasting serum insulin levels or homeostasis model assessment of insulin resistance (HOMA‐IR) and colorectal adenoma.
Materials and Methods
We retrospectively enrolled 15,427 participants who underwent both fasting serum insulin measurement and colonoscopy for a routine health examination at Asan Medical Center from January 2007 to December 2008. Participants with a history of any cancer, previous colectomy or polypectomy, those taking antidiabetic medications, and inflammatory bowel disease, non‐specific colitis, non‐adenomatous polyps only or CRC on colonoscopic findings were excluded. Finally, 3,606 participants with histologically confirmed colorectal adenoma and 6,019 controls with no abnormal findings on colonoscopy were included. Participants were categorized into quartiles (Q) based on fasting serum insulin levels and HOMA‐IR.
Results
Fasting serum insulin and HOMA‐IR were significantly higher in participants with colorectal adenomas compared with controls. Multivariate regression analysis adjusting for age, sex, smoking habits, drinking habits and family history of CRC showed that participants with higher quartiles of fasting serum insulin levels (odd ratio [OR] 1.17 for 2nd Q, 1.19 for 3rd Q, and 1.42 for 4th Q, P < 0.05) or HOMA‐IR (OR 1.18 for 2nd Q and 1.45 for 4th Q, P < 0.05) showed significantly increased ORs of colorectal adenoma compared with the lowest quartiles.
Conclusions
These findings showed that increased serum insulin levels and insulin resistance were significantly associated with the presence of colorectal adenoma.
Keywords: Insulin, Insulin resistance, Colorectal adenoma
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide. Several risk factors for CRC are well known, such as high‐fat, low‐fiber intake, physical inactivity and a family history of CRC1. In addition, obesity and its related conditions, such as diabetes mellitus, insulin resistance, hyperinsulinemia and metabolic syndrome, have also been reported as risk factors for CRC4. Epidemiological and in vitro studies have supported the ‘insulin hypothesis’ of colorectal carcinogenesis7. In addition, several previous reports have shown a relationship between insulin or insulin resistance and CRC4.
Colorectal adenoma is considered a precursor of CRC through the adenoma–carcinoma sequence12. To prevent CRC, it is necessary to detect and treat colorectal adenoma13. Thus, it is important to identify the risk factors for colorectal adenoma.
Several studies have shown that obesity and metabolic syndrome or the components of metabolic syndrome are associated with colorectal adenoma14. However, the relationship between insulin or insulin resistance and colorectal adenoma has not been investigated in detail, and the results were controversial20. Some studies suggested that elevated serum insulin or homeostasis model assessment of insulin resistance (HOMA‐IR) were associated with colorectal adenoma20. In contrast, two Japanese studies showed that insulin levels were not related to colorectal adenoma22. Therefore, we carried out a study to assess the relationship between fasting serum insulin levels and colorectal adenoma in a population that underwent screening colonoscopy.
Materials and Methods
Study Population
We retrospectively analyzed 15,427 participants who underwent both serum insulin measurement and colonoscopy during routine health examinations at the Health Screening and Promotion Center of the Asan Medical Center (AMC, Seoul, Republic of Korea) from January 2007 to December 2008. Information on medication, previous medical or surgical diseases, family history of CRC in first‐degree relatives, and smoking and drinking habits were obtained from each participant using a standard questionnaire. Drinking habits were categorized as never and rarely or more than two times a week. Smoking habits were categorized as never, previous or current.
Subjects excluded were those with a history of cancer at other sites (n = 545), those taking antidiabetic medications (n = 934), those who underwent previous colectomy or polypectomy (n = 389) based on the questionnaire, those with incomplete studies (n = 127) and those with inflammatory bowel disease, non‐specific colitis (n = 320) or CRC (n = 39) based on colonoscopic findings. Therefore, after the exclusions criteria, 13,073 participants remained including 6,019 with normal findings and 7,054 with at least one or more colorectal polyp. In addition, 3,448 subjects with only hyperplastic or inflammatory polyps (n = 1,773), carcinoid (n = 29), or other non‐specific findings (n = 1,646) on pathology specimens were excluded. Finally, a total of 9,625 participants (3,606 subjects with histologically confirmed colorectal adenoma and 6,019 controls with no abnormal findings on colonoscopy) were evaluated in the present study (Figure 1). The study population was composed of 5,914 men (61.4%) and 3,711 women (38.6%) with a mean age of 50.4 ± 9.5 years.
Figure 1.
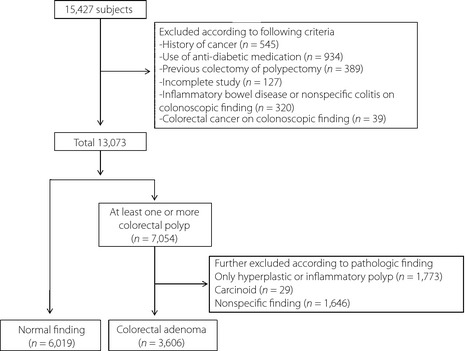
Study population selection.
All participants enrolled in the present study provided written informed consent. This study was approved by the institutional review board of the Asan Medical Center.
Measurements
Height and weight were measured by trained nurses with participants wearing light clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Waist circumference (cm) was measured at the midway point between the inferior margin of the last rib and the superior iliac crest in a horizontal plane.
Blood samples were obtained the morning after an overnight fast before colonoscopy. Plasma glucose was measured by the hexokinase method using an autoanalyzer (Toshiba, Tokyo, Japan). Fasting total cholesterol (TC), low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol and triglycerides were measured using the autoanalyzer (Toshiba). Serum insulin concentrations were obtained by immunoradiometric assay (TFB, Tokyo, Japan). The intra‐ and interassay coefficients of variation for insulin levels were 6.4 and 7.1%, respectively. The HOMA‐IR, an index of insulin resistance, was calculated as fasting plasma glucose (mg/dL) multiplied by fasting insulin (μIU/mL) divided by 40528.
Colonoscopic Examinations
After bowel preparation with 4 L of polyethylene glycol‐electrolyte oral lavage solution (Meditech Korea Pharma, Kyunggido, Korea), colonoscopy was carried out on each participant by one of eight experienced gastroenterologists using an EVIS‐260(B) colonoscope (Olympus, Tokyo, Japan), with the colon examined from the rectum to the cecum. All visualized lesions were biopsied and histologically assessed by experienced pathologists.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (median). Categorical variables were expressed as proportions (%). Variables that were not distributed normally, including lipids, insulin levels and HOMA‐IR, were log‐transformed before analysis. Student's t‐test was used to compare continuous variables between any two groups. The χ2‐test was used to compare proportions.
Fasting serum insulin levels and HOMA‐IR were categorized into quartiles (Q1–Q4) based on the levels of the whole study population (including both cases and controls). The ranges of each quartile of insulin and HOMA‐IR were <3.6, 3.6–5.3, 5.3–7.6, >7.6 μIU/mL and <0.83, 0.83–1.25, 1.25–1.89, >1.89, respectively. Demographic and biochemical characteristics of the study population sorted according to the quartiles of insulin or HOMA‐IR were compared using one‐way analysis of variance (anova) for continuous variables and the χ2‐test for categorical variables. The median values or adds ratios (ORs) for each quartile of insulin or HOMA‐IR were assessed for linear trends by P for trends. Multivariate analysis was carried out using logistic regression analysis adjusting for age (continuous), sex (categorical), alcohol intake (categorical: two categories of never or rarely and more than two times/week), smoking habit (categorical: two categories of never or past and current), first‐degree familial history of CRC (categorical: two categories of yes or no) and BMI (continuous). For each variable, the ORs, 95% confidence interval (CI) and P‐values were calculated. P‐values < 0.05 were considered statistically significant. Statistical analyses were carried out using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
The baseline characteristics of colorectal adenoma and control participants are summarized in Table 1. Compared with controls, participants with colorectal adenoma were significantly older and more likely to be men. Rates of current smoking and alcohol drinking were higher in participants with colorectal adenoma than in controls. However, the rate of family history of CRC did not differ significantly between the two groups. Participants with colorectal adenoma showed a higher mean BMI, waist circumference, TC, triglycerides, LDL cholesterol, fasting glucose, serum insulin, HOMA‐IR and lower HDL cholesterol compared with controls.
Table 1. Baseline clinical characteristics and laboratory findings of study participants.
| Characteristics | Control (n = 6,019) | Adenoma (n = 3,606) | P |
|---|---|---|---|
| Age (years) | 48.7 ± 9.4 | 53.3 ± 8.8 | <0.05 |
| Sex, male (%) | 53.6 | 74.5 | <0.05 |
| Familial history of CRC (%) | 4.5 | 4.2 | NS |
| Current smoking (%) | 22.5 | 32.1 | <0.05 |
| Alcohol (%) | 38.3 | 49.5 | <0.05 |
| BMI (kg/m2) | 23.7 ± 2.9 | 24.6 ± 2.8 | <0.05 |
| Waist circumference (cm) | 81.6 ± 8.8 | 85.3 ± 8.4 | <0.05 |
| Male | 85.7 ± 7.4 | 87.4 ± 7.3 | <0.05 |
| Female | 76.8 ± 7.7 | 79.4 ± 8.3 | <0.05 |
| Total cholesterol (mg/dL) | 192.4 ± 33.4 | 195.5 ± 34.1 | <0.05 |
| Triglyceride (mg/dL) | 114.7 ± 73.7 | 131.7 ± 82.2 | <0.05 |
| HDL cholesterol (mg/dL) | 57.4 ± 14.3 | 54.7 ± 13.4 | <0.05 |
| LDL cholesterol (mg/dL) | 123.9 ± 29.7 | 127.4 ± 29.8 | <0.05 |
| Glucose (mg/dL) | 96.4 ± 13.2 | 99.6 ± 15.2 | <0.05 |
| Serum insulin (μIU/mL) | 5.9 ± 3.6 | 6.5 ± 4.0 | <0.05 |
| HOMA‐IR | 1.4 ± 1.1 | 1.6 ± 1.2 | <0.05 |
BMI, body mass index; CRC, colorectal cancer; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; NS, not significant.
The baseline clinical characteristics and laboratory findings according to quartiles of fasting serum insulin are shown in Table 2. Almost all clinical characteristics (except for mean age and rates of family history of CRC) and laboratory findings according to serum insulin quartiles were significantly different between groups.
Table 2. Clinical characteristics and laboratory findings of study participants according to insulin quartiles.
| Characteristics | Q1 (n = 2,269) | Q2 (n = 2,527) | Q3 (n = 2,412) | Q4 (n = 2,417) | P | P for trends |
|---|---|---|---|---|---|---|
| Insulin, μIU/mL (median) | <3.6 (2.49) | 3.6 –5.3 (4.39) | 5.3 –7.6 (6.30) | >7.6 (11.1) | <0.05 | |
| Age (years) | 50.1 ± 9.2 | 50.5 ± 9.1 | 50.1 ± 9.6 | 50.7 ± 9.9 | NS | NS |
| Sex, male (%) | 56.8 | 60.4 | 62.8 | 65.5 | <0.05 | |
| Familial history of CRC (%) | 4.9 | 4.2 | 4.4 | 4.3 | NS | |
| Current smoking (%) | 26.9 | 24.8 | 24.9 | 28 | <0.05 | |
| Alcohol (%) | 39.7 | 43 | 44.7 | 42.4 | <0.05 | |
| BMI (kg/m2) | 22.4 ± 2.4 | 23.5 ± 2.5 | 24.4 ± 2.7 | 25.8 ± 3.0 | <0.05 | <0.05 |
| Waist circumference (cm) | 78.2 ± 7.6 | 81.7 ± 8.0 | 84.0 ± 8.1 | 87.8 ± 8.5 | <0.05 | <0.05 |
| Male | 81.3 ± 6.7 | 85.2 ± 6.5 | 87.4 ± 6.4 | 90.8 ± 7.0 | <0.05 | <0.05 |
| Female | 74 ± 6.9 | 76.3 ± 7.2 | 78.1 ± 7.4 | 82.1 ± 8.3 | <0.05 | <0.05 |
| Total cholesterol (mg/dL) | 187.9 ± 32.9 | 193.1 ± 33.7 | 194.6 ± 32.9 | 198.2 ± 34.6 | <0.05 | <0.05 |
| Triglyceride (mg/dL) | 88 ± 48.3 | 108.2 ± 61.7 | 129.8 ± 72.7 | 156.7 ± 99.5 | <0.05 | <0.05 |
| HDL cholesterol (mg/dL) | 61.4 ± 14.4 | 57.9 ± 14.0 | 54.8 ± 13.6 | 51.8 ± 14.0 | <0.05 | <0.05 |
| LDL cholesterol (mg/dL) | 119.0 ± 29.0 | 125.0 ± 29.6 | 126.8 ± 29.6 | 129.7 ± 29.9 | <0.05 | <0.05 |
| Glucose (mg/dL) | 91.4 ± 12.0 | 96.1 ± 12.1 | 99.0 ± 13.2 | 103.5 ± 15.7 | <0.05 | <0.05 |
| HOMA‐IR | 0.6 ± 0.2 | 1.0 ± 0.2 | 1.5 ± 0.3 | 2.9 ± 1.2 | <0.05 | <0.05 |
BMI, body mass index; CRC, colorectal cancer; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; NS, not significant; Q, quartile.
Differences in characteristics across HOMA‐IR quartiles were also statistically significant, except for the rates of family history of CRC and smoking habits (data not shown). The prevalence of colorectal adenoma showed an increasing tendency across the quartiles of fasting serum insulin (P < 0.05). The prevalence of colorectal adenoma was 33.0% (748/1,521) for quartile 1, 37.1% (938/1,589) for quartile 2, 37.1% (894/1,518) for quartile 3 and 42.4% (1,026/1,391) for quartile 4. The prevalence of colorectal adenoma across the quartiles of HOMA‐IR also showed increasing tendency (P < 0.05), and was 32.5% (784/1,631) for quartile 1, 37.4% (897/1,499) for quartile 2, 36.0% (868/1,540) for quartile 3, and 43.9% (1,057/1,349) for quartile 4.
The ORs and 95% CI for colorectal adenoma according to the quartiles of fasting serum insulin are shown in Table 3. In univariate analysis, participants with higher quartiles of fasting serum insulin levels (OR 1.20, 95% CI 1.07–1.35 for Q2; OR 1.20, 95% CI 1.06–1.35 for Q3 and OR 1.50, 95% CI 1.33–1.69 for Q4, P < 0.05) showed significantly increased ORs of colorectal adenoma. After adjusting for age, sex, smoking habits, drinking habits and family history of CRC in a multivariate logistic regression analysis, participants with higher quartiles of fasting serum insulin levels (OR 1.17, 95% CI 1.03–1.34 for Q2, OR 1.19, 95% CI 1.04–1.36 for Q3 and OR 1.42, 95% CI 1.25–1.62 for Q4, P < 0.05) still showed significantly increased ORs of colorectal adenoma. Additional adjustment for BMI showed statistical significance only in the highest quartile (OR 1.17, 95% CI 1.01–1.35 for Q4, P < 0.05).
Table 3. Odds ratios of colorectal adenoma according to quartiles of fasting serum insulin.
| Insulin, μIU/mL (median) | OR (95% CI) | P for trends | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| <3.6 (2.49) | 3.6 –5.3 (4.39) | 5.3 –7.6 (6.30) | >7.6 (11.1) | ||
| Univariate | 1.0 (ref) | 1.20 (1.07–1.35)a | 1.20 (1.06 –1.35)a | 1.50 (1.33–1.69)a | <0.05 |
| Model 1 | 1.0 (ref) | 1.17 (1.03–1.34)a | 1.19 (1.04–1.36)a | 1.42 (1.25 –1.62)a | <0.05 |
| Model 2 | 1.0 (ref) | 1.10 (0.96 –1.26) | 1.06 (0.92–1.22) | 1.17 (1.01–1.35)a | NS (P = 0.07) |
P < 0.05. Model 1: Adjusted for age, sex, current smoking, alcohol drinking, family history of colorectal cancer. Model 2: Further adjusted for body mass index. CI, confidence interval; NS, not significant; OR, odds ratio; Q, quartile.
In addition, we analyzed the association between the quartiles of HOMA‐IR and the presence of colorectal adenoma (Table 4). In univariate analysis, participants with higher quartiles of HOMA‐IR (OR 1.25, 95% CI 1.11–1.40 for Q2, OR 1.17, 95% CI 1.04–1.32 for Q3 and OR 1.63, 95% CI 1.45–1.83 for Q4, P < 0.05) showed significantly increased ORs of colorectal adenoma. Multivariate logistic regression analysis after adjusting for confounding variables including age, sex, smoking habits, drinking habits and family history of CRC showed that the ORs of colorectal adenoma for the higher quartiles of HOMA‐IR were significantly increased (OR 1.18, 95% CI 1.04–1.35 for Q2 and OR 1.45, 95% CI 1.28–1.65 for Q4, P < 0.05) except for Q3. On additional adjustment for BMI, a significant positive association between HOMA‐IR and the presence of colorectal adenoma was observed only in the highest quartile of HOMA‐IR (OR 1.20, 95% CI 1.04–1.38 for Q4, P < 0.05).
Table 4. Odds ratios of colorectal adenoma according to quartiles of homeostasis model assessment of insulin resistance.
| HOMA‐IR (median) | OR (95% CI) | P for trends | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| <0.83 (0.57) | 0.83 –1.25 (1.05) | 1.25 –1.89 (1.54) | >1.89 (2.90) | ||
| Univariate | 1.0 (ref) | 1.25 (1.11–1.40)a | 1.17 (1.04–1.32)a | 1.63 (1.45–1.83)a | <0.05 |
| Model 1 | 1.0 (ref) | 1.18 (1.04–1.35)a | 1.10 (0.97–1.26) | 1.45 (1.28–1.65)a | <0.05 |
| Model 2 | 1.0 (ref) | 1.11 (0.97–1.27) | 0.99 (0.86–1.13) | 1.20 (1.04–1.38)a | NS (P = 0.07) |
P < 0.05. Model 1: Adjusted for age, sex, current smoking, alcohol drinking, family history of colorectal cancer. Model 2: Further adjusted for body mass index. CI, confidence interval; HOMA‐IR, homeostasis model assessment of insulin resistance; NS, not significant; OR, odds ratio; Q, quartile.
Table 5 showed the association between fasting insulin level and the presence of colorectal adenoma stratified by glucose levels. In both normoglycemic (<100 mg/dL) and hyperglycemic (≥100 mg/dL) groups, the participants with higher quartiles of fasting serum insulin levels (Q3 and Q4) showed significantly increased ORs of colorectal adenoma after adjusting for age, sex, current smoking, alcohol drinking and family history of CRC. After further adjustment for BMI, the highest quartile of fasting serum insulin levels (Q4) showed significantly increased OR of the colorectal adenoma only in the hyperglycemic group.
Table 5. Odds ratio of colorectal adenoma according to quartiles of fasting serum insulin stratified by fasting blood glucose.
| OR (95% CI) | P for trends | ||||
|---|---|---|---|---|---|
| FBS < 100 mg/dL | Q1 | Q2 | Q3 | Q4 | |
| Insulin, μIU/mL (median) | <3.3 (2.29) | 3.3–4.7 (4.03) | 4.7–6.6 (5.62) | >6.6 (9.57) | |
| Adenoma cases/non–cases | 516/1,130 | 529/1,025 | 520/954 | 557/985 | |
| Univariate | 1.0 (ref) | 1.13 (0.98 –1.31) | 1.19 (1.03 –1.39)a | 1.24 (1.07 –1.44)a | <0.05 |
| Model 1 | 1.0 (ref) | 1.14 (0.97 –1.34) | 1.22 (1.04 –1.44)a | 1.21 (1.03 –1.42)a | <0.05 |
| Model 2 | 1.0 (ref) | 1.06 (0.90 –1.25) | 1.09 (0.92 –1.30) | 0.99 (0.83 –1.18) | NS |
| FBS ≥ 100 mg/dL | Q1 | Q2 | Q3 | Q4 | P for trends |
|---|---|---|---|---|---|
| Insulin, μIU/mL (median) | <4.7 (3.53) | 4.7–6.5 (5.62) | 6.5–9.4 (7.84) | >9.4 (13.39) | |
| Adenoma cases/non‐cases | 360/509 | 340/499 | 372/493 | 412/424 | |
| Univariate | 1.0 (ref) | 0.96 (0.79 –1.17) | 1.07 (0.88 –1.29) | 1.37 (1.14 –1.66)a | <0.05 |
| Model 1 | 1.0 (ref) | 1.13 (0.91 –1.40) | 1.25 (1.01 –1.54)a | 1.70 (1.37 –2.10)a | <0.05 |
| Model 2 | 1.0 (ref) | 1.08 (0.87 –1.34) | 1.15 (0.93 –1.42) | 1.47 (1.17 –1.87)a | <0.05 |
P < 0.05. Model 1: Adjusted for age, sex, current smoking, alcohol drinking, family history of colorectal cancer. Model 2: Further adjusted for body mass index. CI, confidence interval; FBS, fasting blood sugar; NS, not significant; OR, odds ratio; Q, quartile; ref, reference.
In subgroup analysis according to BMI, the prevalence of colorectal adenoma tended to increase with increasing quartiles of insulin only in non‐obese group (BMI < 25 kg/m2; Table 6). The participants with the highest quartile of insulin in the non‐obese group showed a significantly increased OR of colorectal adenoma in both univariate and multivariate analysis.
Table 6. Odds ratios of colorectal adenoma according to quartiles of fasting serum insulin stratified by body mass index.
| OR (95% CI) | P for trend | ||||
|---|---|---|---|---|---|
| BMI < 25 kg/m2 | Q1 | Q2 | Q3 | Q4 | |
| Insulin, μIU/mL (median) | <3.2 (2.25) | 3.2 – 4.6 (3.92) | 4.6 – 6.5 (5.49) | >6.5 (9.19) | |
| Adenoma cases/non‐cases | 513/1,103 | 503/1,054 | 514/1,053 | 545/950 | |
| Univariate | 1.0 (ref) | 1.03 (0.88 –1.19) | 1.05 (0.91 –1.22) | 1.23 (1.06 –1.43)a | <0.05 |
| Model 1 | 1.0 (ref) | 1.08 (0.92 –1.28) | 1.11 (0.94 –1.31) | 1.26 (1.07 –1.49)a | <0.05 |
| BMI ≥ 25 kg/m2 | Q1 | Q2 | Q3 | Q4 | P for trend |
|---|---|---|---|---|---|
| Insulin, μIU/mL (median) | <4.9 (3.74) | 4.9 – 6.8 (5.89) | 6.8 – 9.7 (8.15) | >9.7 (13.89) | |
| Adenoma cases/non‐cases | 403/464 | 365/482 | 369/464 | 394/449 | |
| Univariate | 1.0 (ref) | 0.87 (0.72 –1.05) | 0.92 (0.76 –1.12) | 1.01 (0.84 –1.22) | NS |
| Model 1 | 1.0 (ref) | 0.95 (0.77 –1.17) | 1.01 (0.82 –1.24) | 1.13 (0.92 –1.39) | NS |
P < 0.05. Model 1: Adjusted for age, sex, current smoking, alcohol drinking, family history of colorectal cancer. CI, confidence interval; NS, not significant; OR, odds ratio; Q, quartile.
Discussion
The results of the present study showed that fasting serum insulin levels were positively associated with the presence of colorectal adenoma. Furthermore, HOMA‐IR, an insulin resistance index, was also associated with the presence of colorectal adenoma. The association remained significant even after adjusting for risk factors of CRC, and further adjusting for BMI showed significance in the highest quartile level. These findings suggest a role for higher circulating insulin levels in the development of colorectal adenoma in the process of colorectal carcinogenesis. Many previous studies20 have tried to show an association between the risk of colorectal adenoma and insulin levels, but have yielded inconsistent results. The discrepancies in data between studies could in part result from differences in study design, selection of study population and the number of enrolled cases. Keku et al.26 showed that higher insulin levels were associated with increased colorectal adenoma (OR 2.2, 95% CI 1.1–4.2 compared with the lowest quartile). However, that study had a limitation in that the control group had a higher rate of familial history of CRC than the adenoma group. In the present study, the familial history of CRC was 4.3%. This rate was lower than those from Caucasian (7–26%)24 and Japanese (4.7–6.4%)20, but this was similar to other Korean studies (4.6–5%)19. In the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial24, insulin levels showed a positive association with colorectal adenoma (OR 2.1, 95% CI 1.3–3.6), especially with more advanced adenomas (OR 2.3, 95% CI 1.1–4.9 compared with the lowest quartile). However, 60% of the control participants in that study did not undergo complete colonoscopy. Flood et al.23 found that the highest quartile of insulin was associated with an approximately 50% increased recurrence risk of colorectal adenoma over 4 years in a multicentered randomized trial. However, that study had a small sample size. In a case–control study from Japan27, serum insulin levels were directly correlated with the presence of adenoma in the proximal colon, but the study population was composed of participants with lower gastrointestinal symptoms and positive screening results including positive findings at fecal occult blood or sigmoidoscopy. A recent study in Japan27 showed that multivariate adjusted OR of colorectal adenoma for the highest quartile compared with the lowest quartile of insulin levels was 3.45 (95% CI 1.73–6.87). However, the participants in that study were selected by a pit pattern using indigo carmine solution on the surface of polyps instead of histological confirmation. By contrast, in another study Japanese study22, insulin levels were positively associated with the development of early stage CRC, but showed no association with colorectal adenoma. That study had a serious limitation, because the control groups were not confirmed by colonoscopy. One prospective study found that higher levels of insulin were associated with colorectal adenoma, but this significance disappeared after multivariable adjustment for confounding variables, such as alcohol and smoking habits21. Nishii et al.25 reported that the prevalence of colon adenoma did not vary according to plasma insulin levels. The above‐mentioned studies were all composed of small populations of participants.
In the present study, we selected control participants with confirmed normal findings and who were free from other benign lesions. Participants of the adenoma groups had at least one or more adenoma confirmed by histopathology. Furthermore, we excluded participants who were taking antidiabetic medication, potentially interfering with circulating insulin levels. Therefore, we could select subjects with colorectal adenoma and controls exactly in comparison with other previous studies. The prevalence of colorectal adenoma in the present study was 23.3% in a total of 15,427 participants and 37.5% in the 9,625 participants after excluding 5,802 participants according to exclusion criteria. This is similar to the prevalence of colorectal adenoma (30.2%) by Multi‐Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management in Korea31.
We also showed that HOMA‐IR was associated with colorectal adenoma, and multivariate adjusted risk estimate achieved statistical significance. HOMA‐IR, an insulin resistance index, has also been evaluated as a potential risk factor for colorectal adenoma21. One study in Japanese men demonstrated that increased HOMA‐IR according to three categories, normal (<1.6), indeterminate (1.6–2.5) and insulin resistance (≥2.5), showed a significantly increased risk for colorectal adenoma after adjusting for waist circumference (OR 1.62–2.23, 95% CI 1.07–2.45)29. Ortiz et al.21 reported that the OR of the highest relative to the lowest HOMA‐IR group increased significantly by 2.11 in the Cancer and Energetics Colon Polyps Study. A case–control study in Koreans showed that increased HOMA‐IR was associated with the presence of colorectal adenoma (OR 1.99, 95% CI 1.35–2.92, compared with the lowest quintile)30. By contrast, Yamamoto et al.22 could not show any association between HOMA‐IR and colorectal adenoma in a small Japanese population study.
Insulin has long been considered a primary mediator of CRC risk in obese populations. Insulin itself has direct growth‐promoting effects and mitogenic activity. Insulin also activates insulin‐like growth factor (IGF)‐1, which plays a role in cell proliferation and apoptosis32. Additionally, insulin stimulates growth of normal colonic and carcinoma cells in vitro33. The mitogenic properties of insulin might be mediated through IGF‐1 receptors and increased bioactive IGF‐134. An in vivo study showed that circulating insulin, at levels seen in insulin resistance, increased proliferation of normal colorectal epithelial cells in a dose‐dependent manner, which implies that hyperinsulinemia could be the main risk factor for colorectal neoplasm36. Epidemiological and clinical studies have shown the association of CRC with circulating insulin4, and those results have supported the insulin hypothesis in colorectal carcinogenesis. The present study showed that insulin and HOMA‐IR were associated with the presence of colorectal adenoma. These results suggest that insulin and insulin resistance are involved in the early stages of colorectal carcinogenesis.
We also analyzed the association between fasting insulin level and the presence of colorectal adenoma stratified by glucose or BMI. The higher quartiles of insulin level were associated with the presence of colorectal adenoma in both normoglycemic and hyperglycemic groups (model 1 in Table 5). After stratification by BMI, the significant association was also observed in the non‐obese group (Table 6). These results suggest that increased fasting insulin levels were still significantly associated with the presence of colorectal adenoma after controlling the confounding effects of glucose levels or adiposity. However, the association weakened in the normoglycemic group after controlling BMI (model 2 in Table 5), and the association was not observed in the obese group (Table 6). These results might be as a result of the complex interaction among serum insulin and glucose levels and BMI, especially in the obese group.
The present study had several limitations. We analyzed one‐time measures of serum insulin, and single measurements of serum insulin levels might not accurately reflect levels over time. Second, data on other residual confounders, such as physical activity, dietary factors and detailed past history of smoking, were not available. Third, neither size nor number of colorectal adenomas was taken into account in the present study. In addition, we cannot determine causality from our findings, because it was a cross‐sectional study. Nevertheless, the present study had certain strengths, such as the inclusion of a large number of participants and the inclusion of histopathologically confirmed colorectal adenoma in all study participants. In addition, for the control group, we used an apparently healthy population that had undergone a screening health check‐up, and such populations approximately reflect the general population. Finally, the collected data were rigorously standardized and of high quality.
In conclusion, the present study showed that increased serum insulin level and insulin resistance were significantly associated with the presence of colorectal adenoma. These results suggest that insulin and insulin resistance might contribute to the development of colorectal adenoma. Further prospective studies are required to clarify the role of insulin in the development of colorectal adenoma.
Acknowledgements
The authors have no conflict of interest.
J Diabetes Invest 2014; 5: 297–304
References
- 1.Fung T, Hu FB, Fuchs C, et al Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med 2003; 163: 309–314 [DOI] [PubMed] [Google Scholar]
- 2.Keku TO, Millikan RC, Martin C, et al Family history of colon cancer: what does it mean and how is it useful? Am J Prev Med 2003; 24: 170 –176 [DOI] [PubMed] [Google Scholar]
- 3.White E, Jacobs EJ, Daling JR. Physical activity in relation to colon cancer in middle‐aged men and women. Am J Epidemiol 1996; 144: 42–50 [DOI] [PubMed] [Google Scholar]
- 4.Schoen RE, Tangen CM, Kuller LH, et al Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999; 91: 1147–1154 [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, et al Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet 2008; 371: 569–578 [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007; 86: s836–842 [DOI] [PubMed] [Google Scholar]
- 7.Koohestani N, Tran TT, Lee W, et al Insulin resistance and promotion of aberrant crypt foci in the colons of rats on a high‐fat diet. Nutr Cancer 1997; 29: 69–76 [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995; 6: 164–179 [DOI] [PubMed] [Google Scholar]
- 9.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev 1996; 5: 1013–1015 [PubMed] [Google Scholar]
- 10.Trevisan M, Liu J, Muti P, et al Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev 2001; 10: 937–941 [PubMed] [Google Scholar]
- 11.Limburg PJ, Stolzenberg‐Solomon RZ, Vierkant RA, et al Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin Gastroenterol Hepatol 2006; 4: 1514–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelstein B, Fearon ER, Hamilton SR, et al Genetic alterations during colorectal‐tumor development. N Engl J Med 1988; 319: 525–532 [DOI] [PubMed] [Google Scholar]
- 13.Winawer SJ, Zauber AG, O'Brien MJ, et al Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328: 901–906 [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Colditz GA, Stampfer MJ, et al Physical activity, obesity, and risk of colorectal adenoma in women (United States). Cancer Causes Control 1996; 7: 253–263 [DOI] [PubMed] [Google Scholar]
- 15.Wise LA, Rosenberg L, Palmer JR, et al Anthropometric risk factors for colorectal polyps in African‐American women. Obesity (Silver Spring) 2008; 16: 859–868 [DOI] [PubMed] [Google Scholar]
- 16.Hu NC, Chen JD, Lin YM, et al Stepwise relationship between components of metabolic syndrome and risk of colorectal adenoma in a Taiwanese population receiving screening colonoscopy. J Formos Med Assoc 2011; 110: 100–108 [DOI] [PubMed] [Google Scholar]
- 17.Liu CS, Hsu HS, Li CI, et al Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol 2010; 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SE, Shim KN, Jung SA, et al An association between obesity and the prevalence of colonic adenoma according to age and gender. J Gastroenterol 2007; 42: 616–623 [DOI] [PubMed] [Google Scholar]
- 19.Oh TH, Byeon JS, Myung SJ, et al Visceral obesity as a risk factor for colorectal neoplasm. J Gastroenterol Hepatol 2008; 23: 411–417 [DOI] [PubMed] [Google Scholar]
- 20.Sasaki Y, Takeda H, Sato T, et al Serum Interleukin‐6, insulin, and HOMA‐IR in male individuals with colorectal adenoma. Clin Cancer Res 2012; 18: 392–399 [DOI] [PubMed] [Google Scholar]
- 21.Ortiz AP, Thompson CL, Chak A, et al Insulin resistance, central obesity, and risk of colorectal adenomas. Cancer 2012; 118: 1774–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Nakagawa T, Matsushita Y, et al Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care 2010; 33: 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flood A, Mai V, Pfeiffer R, et al Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology 2007; 133: 1423–1429 [DOI] [PubMed] [Google Scholar]
- 24.Schoen RE, Weissfeld JL, Kuller LH, et al Insulin‐like growth factor‐I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology 2005; 129: 464–475 [DOI] [PubMed] [Google Scholar]
- 25.Nishii T, Kono S, Abe H, et al Glucose intolerance, plasma insulin levels, and colon adenomas in Japanese men. Jpn J Cancer Res 2001; 92: 836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keku TO, Lund PK, Galanko J, et al Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 2005; 14: 2076–2081 [DOI] [PubMed] [Google Scholar]
- 27.Yoshida I, Suzuki A, Vallee M, et al Serum insulin levels and the prevalence of adenomatous and hyperplastic polyps in the proximal colon. Clin Gastroenterol Hepatol 2006; 4: 1225–1231 [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Takeda H, Sasaki Y, et al Increased homeostasis model assessment‐insulin resistance is a risk factor for colorectal adenoma in Japanese males. Tohoku J Exp Med 2011; 223: 297–303 [DOI] [PubMed] [Google Scholar]
- 30.Kang HW, Kim D, Kim HJ, et al Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross‐sectional, case‐control study. Am J Gastroenterol 2010; 105: 178–187 [DOI] [PubMed] [Google Scholar]
- 31.Lee BI, Hong SP, Kim S‐E, et al Korean Guidelines for Colorectal Cancer Screening and Polyp Detection. Intest Res 2012; 10: 67–88 [Google Scholar]
- 32.Renehan AG, Zwahlen M, Minder C, et al Insulin‐like growth factor (IGF)‐I, IGF binding protein‐3, and cancer risk: systematic review and meta‐regression analysis. Lancet 2004; 363: 1346 –1353 [DOI] [PubMed] [Google Scholar]
- 33.Watkins LF, Lewis LR, Levine AE. Characterization of the synergistic effect of insulin and transferrin and the regulation of their receptors on a human colon carcinoma cell line. Int J Cancer 1990; 45: 372–375 [DOI] [PubMed] [Google Scholar]
- 34.Bjork J, Nilsson J, Hultcrantz R, et al Growth‐regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non‐transformed intestinal epithelial cell line IEC‐6 and the colon cancer cell line HT 29. Scand J Gastroenterol 1993; 28: 879–884 [DOI] [PubMed] [Google Scholar]
- 35.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin‐like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res 1989; 80: 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran TT, Naigamwalla D, Oprescu AI, et al Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology 2006; 147: 1830–1837 [DOI] [PubMed] [Google Scholar]


