Abstract
Aims/Introduction
The goal of the study was to examine the effects of sitagliptin dose‐up or glimepiride dose‐up in Japanese patients with type 2 diabetes who were controlled inadequately by sitagliptin and glimepiride in combination.
Materials and Methods
A multicenter, prospective, randomized, open‐label study was carried out in 50 patients with type 2 diabetes treated with sitagliptin and low‐dose glimepiride. The patients were randomly assigned to receive the addition of 50 mg/day sitagliptin or 0.5 mg/day glimepiride. The primary end‐point was the percentage change in glycated hemoglobin (HbA1c).
Results
During a follow‐up period, the difference in the percentage changes in HbA1c between the two groups was not significant (P = 0.13). However, HbA1c was significantly decreased by glimepiride dose‐up (P < 0.01 vs baseline), but not by sitagliptin dose‐up (P = 0.74). Univariate linear regression analyses showed that the percentage change in HbA1c was significantly associated with the serum level of arachidonic acid (AA) in both groups.
Conclusions
There was no significant difference in the HbA1c‐lowering effects between the two groups. However, a significant HbA1c‐lowering effect from baseline of glimepiride dose‐up was found, and the AA level showed a negative correlation with the decrease in HbA1c in the sitagliptin dose‐up group, but a positive correlation in the glimepiride dose‐up group. These findings suggest that the AA level is associated with HbA1c reduction in response to dose‐up with these drugs in patients with type 2 diabetes in a combination therapy with sitagliptin and glimepiride. This trial was registered with UMIN (no. 000009544).
Keywords: Arachidonic acid, Glimepiride, Sitagliptin
Introduction
Type 2 diabetes is a chronic disease that usually requires treatment with multiple antihyperglycemic agents (AHAs) during the course of the disease. The American Diabetes Association/European Association for the Study of Diabetes algorithm for treating type 2 diabetes recommends metformin as initial monotherapy1. In contrast, insulin secretion capacity is genetically lower in Japanese subjects than in Caucasians. Therefore, sulfonylureas (SUs) are frequently used in treatment in approximately 60% of type 2 diabetes patients in Japan2. However, SUs often increase the risks of hypoglycemia and weight gain resulting in deterioration of glycemic control in the long term.
A dipeptidylpeptidase‐4 (DPP‐4) inhibitor is an AHA that prevents degradation of glucagon‐like peptide‐1 (GLP‐1) and gastric inhibitory polypeptide (GIP)3. Six different DPP‐4 inhibitors are available in Japan: sitagliptin, vildagliptin, alogliptin, linagliptin, teneligliptin and anagliptin. Among these agents, sitagliptin is the most widely used, because it was the first DPP‐4 inhibitor approved in Japan, and its efficacy and safety are proved in Japanese clinical practice4. Sitagliptin might be more effective for glycemic control in Japanese patients as compared with Caucasian patients5. Sitagliptin does not increase the incidence of hypoglycemia when used as monotherapy or with agents that do not cause hypoglycemia, such as metformin and thiazolidinediones, but might increase the incidence when used with agents that cause hypoglycemia8. The first report of severe hypoglycemia in a Japanese patient treated with sitagliptin combined with SUs was described in a case in which 50 mg/day sitagliptin was added to 6 mg/day glimepiride9. Since then, there have been more than 70 reported cases of severe hypoglycemia within a week after initiation of combination therapy with sitagliptin and SUs10. In most of these cases, a relatively high dose of SUs was administered to elderly patients who also had mild renal dysfunction. This led to establishment of a committee of diabetologists to examine the appropriate use of incretin‐based therapy. This committee recommended that the SU dose should be no more than 1.25 mg/day glibenclamide, 2.0 mg/day glimepiride or 40 mg/day gliclazide at the initiation of incretin‐based therapy11.
Since this recommendation was established in Japan, the number of cases of severe hypoglycemia has decreased markedly, and it was reported that combination therapy with sitagliptin and a low dosage of SUs was safe and effective for glycemic control4. However, the effect of glimepiride dose‐up or sitagliptin dose‐up on glycemic control in patients with type 2 diabetes controlled inadequately by a combination therapy with sitagliptin and a low‐dose of glimepiride has not been determined. The standard initial dose of sitagliptin in Japan is 50 mg/day, and sitagliptin 50 mg/day dose‐up in patients who are poorly controlled with 50 mg/day sitagliptin is common in Japan, because the maximum recommended dosage of sitagliptin is 100 mg/day. The minimum dosage of glimepiride is 0.5 mg, and the maximum is 6 mg/day. Therefore, we carried out a prospective study to investigate the efficacy of the addition of 50 mg/day sitagliptin (maximum dose‐up) or 0.5 mg/day glimepiride (minimum dose‐up) for glycemic control in Japanese patients treated with a combination therapy.
Recently, Iwasaki et al.12 showed that alterations in the glycated hemoglobin (HbA1c) level on administration of DPP‐4 inhibitors as monotherapy are associated with estimated intake of fish, estimated intake of dietary n‐3 polyunsaturated fatty acids (PUFAs), and serum levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), but not with arachidonic acid (AA). Accordingly, the relationships between the percentage change in HbA1c and serum levels of PUFAs (AA, EPA, DHA and EPA/AA ratio) were also determined.
Materials and Methods
Participants and Study Design
The present study was carried out as a prospective, multicenter, randomized, open‐label, parallel‐group, intervention trial in 50 patients with type 2 diabetes who visited our medical institutions as outpatients. The study was designed in accordance with the principles stated in the Declaration of Helsinki. All participants gave written informed consent. The protocol was approved by the ethics committee of Kumamoto University (approval number 1469). This trial was registered with the University Hospital Medical Information Network (UMIN; no. 000009544).
Eligible patients were those with type 2 diabetes receiving treatment with sitagliptin (50 mg/day) and glimepiride (≤2.0 mg/day) with or without metformin at a stable dosage for at least 16 weeks, and aged ≥20 years with a HbA1c level ≥6.9% and no improvement in HbA1c within 12 weeks before dose‐up. Patients with type 1 diabetes, secondary diabetes, severe renal disease, severe hepatic disease, alcoholism, severe depression or a severe psychological condition, malignancy or abnormal hemoglobinemia were excluded. Patients who had received a blood transfusion within 4 months before the start of the study, and pregnant and nursing women were also excluded. Antidiabetic agents containing insulin, antihypertensive agents, statins or fibrates were not newly administered or their doses were not changed from 16 weeks before the start until the end of the study. The patients underwent baseline evaluation and were randomized 1:1 to receive 50 mg/day of sitagliptin dose‐up or 0.5 mg/day of glimepiride dose‐up using a computer‐generated allocation schedule. The patients were instructed to take sitagliptin or glimepiride each morning after breakfast, and were asked not to alter their lifestyle, including diet, exercise and habits, during the study.
The primary end‐point was the percentage change in HbA1c, calculated as: (post‐treatment value − baseline value) × 100/baseline value, at each assessment during the follow‐up period. The secondary end‐points included the percentage changes in body mass index (BMI), fasting plasma glucose (FPG) and high molecular weight (HMW) adiponectin at each assessment. The follow‐up period was 12 weeks. Fasting serum levels of AA, EPA and DHA were measured before dose‐up of sitagliptin or glimepiride. Hypoglycemia was defined as any symptomatic event with cognitive or adrenergic signs with or without confirmation of plasma glucose. Severe hypoglycemia was defined as any symptomatic event requiring assistance by another person.
Blood samples were obtained between 08.30 h and 09.30 h after overnight fasting. HbA1c was measured by high‐performance liquid chromatography. The values of HbA1c are presented as National Glycohemoglobin Standardization Program values (%) calculated by the formula HbA1c (%) = 1.02 × Japan Diabetes Society (%) + 0.25%, according to the recommendations of the Japan Diabetes Society13. Plasma insulin was measured by an electrochemiluminescence immunoassay (Eclusis insulin; Roche Diagnostics, Basel, Switzerland). Low‐density lipoprotein cholesterol levels were calculated using the formula of Friedewald et al.15. HMW adiponectin was measured by a latex turbidimetric immunoassay (Human adiponectin latex kit; Mitsubishi Chemical Medience Corporation, Tokyo, Japan). Serum AA, EPA and DHA were measured by gas chromatography by BML Inc. (Tokyo, Japan).
Statistical Analysis
Data are expressed as means ± standard deviation. Changes in clinical parameters from before to 12 weeks after sitagliptin or glimepiride dose‐up were evaluated by Wilcoxon signed‐rank test. Differences in clinical parameters between the two groups were evaluated by Mann–Whitney U‐test. To identify predictors of reductions of HbA1c after sitagliptin or glimepiride dose‐up, Spearman's correlation coefficient analysis was used to evaluate univariate correlations between improvement in HbA1c, and baseline levels of EPA, DHA, AA and EPA/AA ratio. P‐values <0.05 were considered to be statistically significant. Data analysis was carried out using SPSS version 11.5 for Windows (SPSS Inc., Chicago, IL, USA).
Results
There were no significant differences in baseline characteristics between the sitagliptin and glimepiride dose‐up groups (Table 1). All 50 participants completed the trial, and there were no clinically significant adverse events, except for hypoglycemia. Symptomatic hypoglycemia occurred in one patient in the glimepiride dose‐up group, but there was no severe hypoglycemic event in both groups. The final dosages of sitagliptin and glimepiride were 100 mg/day and 1.2 ± 0.6 mg/day in the sitagliptin dose‐up group, and 50 mg/day and 1.7 ± 0.5 mg/day in the glimepiride dose‐up group, respectively.
Table 1. Demographic and baseline characteristics of the participants.
| Characteristics | All | Sitagliptin dose‐up | Glimepiride dose‐up | P‐value* |
|---|---|---|---|---|
| n (Male/female) | 50 (31/19) | 25 (16/9) | 25 (15/10) | – |
| Age (years) | 63.1 ± 12.4 | 63.9 ± 10.3 | 62.4 ± 14.3 | 0.99 |
| BMI (kg/m2) | 25.1 ± 3.9 | 24.9 ± 4.1 | 25.3 ± 3.6 | 0.93 |
| SBP (mmHg) | 134 ± 15 | 132 ± 14 | 136 ± 16 | 0.45 |
| DBP (mmHg) | 78 ± 11 | 76 ± 13 | 80 ± 9 | 0.25 |
| BUN (mg/dL) | 15.6 ± 4.8 | 14.8 ± 4.6 | 16.4 ± 5.0 | 0.19 |
| Creatinine (mg/dL) | 0.77 ± 0.19 | 0.74 ± 0.18 | 0.81 ± 0.21 | 0.19 |
| HbA1c (%) | 7.4 ± 0.6 | 7.3 ± 0.5 | 7.5 ± 0.6 | 0.22 |
| TC (mg/dL) | 182 ± 27 | 185 ± 29 | 178 ± 23 | 0.21 |
| LDL‐C (mg/dL) | 102 ± 23 | 104 ± 27 | 99 ± 18 | 0.36 |
| HDL‐C (mg/dL) | 51 ± 13 | 55 ± 15 | 47 ± 10 | 0.05 |
| TG (mg/dL) | 143 ± 32 | 133 ± 71 | 157 ± 68 | 0.16 |
| Non‐HDL‐C (mg/dL) | 131 ± 28 | 131 ± 34 | 131 ± 22 | 0.92 |
| FPG (mg/dL) | 143 ± 32 | 142 ± 32 | 145 ± 33 | 0.68 |
| F‐IRI (μU/mL) | 8.1 ± 4.7 | 8.0 ± 4.6 | 8.3 ± 5.0 | 0.99 |
| HMW adiponectin (μg/mL) | 7.3 ± 3.5 | 7.0 ± 3.6 | 7.6 ± 3.5 | 0.54 |
| AA (μg/mL) | 196.9 ± 41.7 | 190.2 ± 31.6 | 203.3 ± 49.4 | 0.47 |
| EPA (μg/mL) | 82.6 ± 42.7 | 85.0 ± 33.5 | 80.3 ± 50.7 | 0.20 |
| DHA (μg/mL) | 154.3 ± 47.5 | 153.6 ± 42.5 | 155.0 ± 52.8 | 0.77 |
| EPA/AA ratio | 0.45 ± 0.27 | 0.46 ± 0.19 | 0.43 ± 0.33 | 0.16 |
| Dosage of glimepiride (mg/day) | 1.2 ± 0.5 | 1.2 ± 0.6 | 1.2 ± 0.5 | 0.99 |
| Metformin (n) | 25 | 14 | 11 | 0.40 |
| Dosage of metformin (mg/day) | 350 ± 388 | 400 ± 382 | 300 ± 395 | 0.24 |
AA, arachidonic acid; BMI, body mass index; DBP, diastolic blood pressure; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; F‐IRI, fasting immunoreactive insulin; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; HMW adiponectin, high molecular weight adiponectin; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride. Mean ± standard deviation values are shown. *P‐values for differences between the sitagliptin and glimepiride groups.
There was no significant difference in the percentage change in HbA1c between the two groups (sitagliptin −0.3 ± 4.8% vs glimepiride −3.0 ± 5.3%, P = 0.13; Figure 1). However, HbA1c significantly decreased from 7.5 ± 0.6% at baseline to 7.3 ± 0.7% after 12 weeks in the glimepiride dose‐up group (P < 0.01). In contrast, HbA1c in the sitagliptin dose‐up group had no significant change from baseline (7.3 ± 0.5%) to after 12 weeks (7.3 ± 0.7%).
Figure 1.
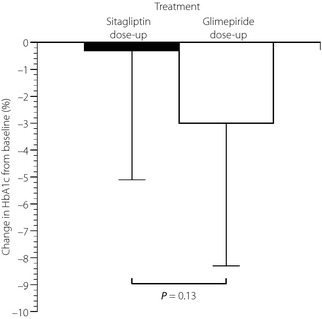
Percentage change in glycated hemoglobin (HbA1c) from baseline with 50 mg/day of sitagliptin dose‐up (black bar) and 0.5 mg/day of glimepiride dose‐up (white bar) during follow up. *P < 0.05 vs baseline.
For the secondary end‐points, there were no significant differences between the groups in percentage change in BMI (sitagliptin 0.1 ± 1.7% vs glimepiride 0.7 ± 2.1%, P = 0.32), FPG (3.5 ± 18.6% vs 2.4 ± 19.9%, P = 0.41) and HMW adiponectin (8.7 ± 18.9% vs 8.2 ± 17.1%, P = 0.62). However, in the glimepiride dose‐up group, HMW adiponectin increased significantly from 7.6 ± 3.5 to 8.1 ± 3.4 μU/mL after 12 weeks (P < 0.05). In the sitagliptin dose‐up group, high‐density lipoprotein cholesterol (HDL‐C) increased significantly from 55 ± 15 to 58 ± 18 mg/dL after 12 weeks (P < 0.05; Table 2).
Table 2. Comparison of clinical parameters at baseline and at 12 weeks after additional administration of sitagiptin or glimepride.
| Sitagliptin dose‐up (n = 25) | Glimepiride dose‐up (n = 25) | P‐value** | |||||
|---|---|---|---|---|---|---|---|
| Before | 12 weeks after | P‐value* | Before | 12 weeks after | P‐value* | ||
| HbA1c (%) | 7.3 ± 0.5 | 7.3 ± 0.7 | 0.74 | 7.5 ± 0.6 | 7.3 ± 0.7 | <0.01 | 0.74 |
| BMI (kg/m2) | 24.9 ± 4.1 | 25.0 ± 4.2 | 0.81 | 25.3 ± 3.7 | 25.5 ± 3.7 | 0.05 | 0.69 |
| TC (mg/dL) | 185 ± 29 | 195 ± 28 | 0.07 | 178 ± 23 | 185 ± 39 | 0.48 | 0.17 |
| LDL‐C (mg/dL) | 104 ± 27 | 108 ± 30 | 0.49 | 99 ± 18 | 105 ± 31 | 0.66 | 0.57 |
| HDL‐C (mg/dL) | 55 ± 15 | 58 ± 18 | 0.01 | 47 ± 10 | 49 ± 11 | 0.18 | 0.05 |
| TG (mg/dL) | 133 ± 71 | 142 ± 73 | 0.63 | 157 ± 68 | 155 ± 104 | 0.27 | 0.85 |
| Non‐HDL‐C (mg/dL) | 131 ± 34 | 136 ± 35 | 0.24 | 131 ± 22 | 136 ± 36 | 0.70 | 0.87 |
| FPG (mg/dL) | 142 ± 32 | 144 ± 30 | 0.31 | 145 ± 33 | 147 ± 41 | 0.70 | 0.97 |
| F‐IRI (μU/mL) | 8.0 ± 4.6 | 7.7 ± 4.1 | 0.69 | 8.3 ± 5.0 | 7.2 ± 4.7 | 0.14 | 0.46 |
| HMW adiponectin (μg/mL) | 7.0 ± 3.6 | 7.5 ± 4.3 | 0.10 | 7.6 ± 3.5 | 8.1 ± 3.4 | 0.03 | 0.47 |
BMI, body mass index; FPG, fasting plasma glucose; F‐IRI, fasting immunoreactive insulin; HDL‐C, high‐density lipoprotein cholesterol; HMW adiponectin, high molecular weight adiponectin; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride. Mean ± standard deviation values are shown. *P‐values for differences between data before and after 12 weeks of treatment. **P‐values for differences between the sitagliptin and glimepiride groups after 12 weeks of treatment.
Correlations of AA, EPA, DHA and EPA/AA ratio with the percentage change in HbA1c determined by linear regression analysis are shown in Table 3. Simple linear regression analysis showed that baseline AA and the percentage change in HbA1c were negatively correlated in the sitagliptin dose‐up group (r = −0.497, P < 0.05), but positively correlated in the glimepiride dose‐up group (r = 0.531, P < 0.01; Figure 2a). To further investigate the impact of AA on the efficacy of the two treatments, the participants in each group were divided into two subgroups by average level of AA (196.9 μg/mL), and percentage change in HbA1c in these subgroups was analyzed. In patients with AA ≤196.9 μg/mL, the percentage change in HbA1c in the glimepiride dose‐up group (−5.2 ± 5.6%) significantly decreased more than that in the sitagliptin dose‐up group (0.3 ± 4.6%; P < 0.01). In contrast, in patients with AA >196.9 μg/mL, the percentage change in HbA1c in the sitagliptin dose‐up group (−3.9 ± 3.1%) significantly decreased more than that in the glimepiride dose‐up group (0.13 ± 2.9%, P < 0.01; Figure 2b).
Table 3. Correlations of arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid and eicosapentaenoic acid to arachidonic acid ratio with the percentage change in glycated hemoglobin determined by linear regression analysis.
| r | P‐value | |
|---|---|---|
| Sitagliptin dose‐up | ||
| AA | −0.497 | 0.016 |
| EPA | 0.059 | 0.778 |
| DHA | −0.171 | 0.414 |
| EPA/AA ratio | 0.225 | 0.280 |
| Glimepiride dose‐up | ||
| AA | 0.531 | 0.008 |
| EPA | −0.066 | 0.756 |
| DHA | −0.023 | 0.912 |
| EPA/AA ratio | 0.011 | 0.959 |
AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Figure 2.
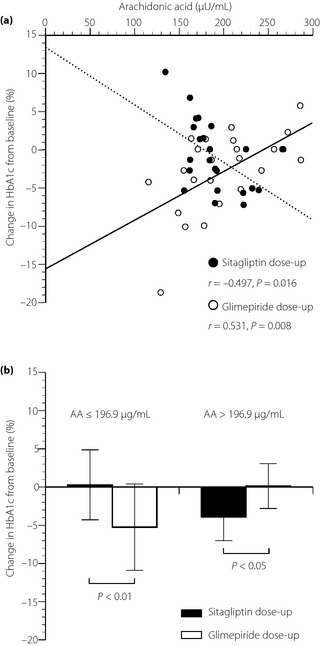
(a) Correlations between the percentage changes in glycated hemoglobin (HbA1c) and serum levels of arachidonic acid (AA) at baseline with 50 mg/day of sitagliptin dose‐up (closed circles, dotted line) and 0.5 mg/day of glimepiride dose‐up (open circles, solid line). (b) Comparison of the percentage changes in glycated hemoglobin (HbA1c) with between 50 mg/day of sitagliptin dose‐up (black bars) and 0.5 mg/day of glimepiride dose‐up (white bars), when the patients in each group were divided into two subgroups by average level of AA.
Discussion
In the present study, no significant difference in the HbA1c‐lowering effects between 50 mg/day of sitagliptin dose‐up and 0.5 mg/day of glimepiride dose‐up was found in Japanese patients with type 2 diabetes controlled inadequately by a combination of sitagliptin and glimepiride. However, it showed that the dose‐up of glimepiride significantly decreased HbA1c in Japanese patients with type 2 diabetes controlled inadequately with both sitagliptin and glimepiride, and that, under these conditions, the percentage change in HbA1c by the addition of sitagliptin or glimepiride was negatively or positively correlated significantly with the serum level of AA, respectively.
Sitagliptin is administered as a single 100‐mg tablet in non‐Japanese patients16. In contrast, the usual dose of sitagliptin in Japan is 50 mg/day in patients with normal to mildly impaired renal function, because HbA1c, FPG and 2‐h postprandial plasma glucose do not differ significantly in 50, 100, and 200 mg/day sitagliptin monotherapy in Japanese patients20. Furthermore, the present study showed that the addition of 50 mg/day of sitagliptin did not significantly improve HbA1c levels in the participants treated with 50 mg/day of sitagliptin. These findings suggest that 50 mg/day of sitagliptin dose‐up might not be effective enough to lower HbA1c in patients with type 2 diabetes who are controlled inadequately by combination therapy with sitagliptin and glimepiride.
In the present study, a significant improvement of HDL‐C occurred by the addition of sitagliptin without the decrease in triglyceride (Table 2). The effect of sitagliptin on HDL‐C in subjects with type 2 diabetes is still controversial. Bergenstal et al.21 and Charbonnel et al.22 found that 100 mg/day sitagliptin significantly increased HDL‐C. However, a significant change in HDL‐C has not been found in Japanese patients receiving 50 mg/day sitagliptin monotherapy for type 2 diabetes23.
Interestingly, AA had a negative correlation with the decrease in HbA1c in the sitagliptin dose‐up group, but a positive correlation in the glimepiride dose‐up group. The exact reason for these findings is not clear, but one possible explanation is as follows. AA potentiates insulin secretion in β‐cells24, but prostaglandin E2 (PGE2), an AA metabolite, reduces insulin secretion through the inhibition of cyclic adenosine monophosphate (cAMP)‐accumulation and insulin sensitivity32. Furthermore, Ehses et al.28 reported that GIP stimulated AA release from clonal β‐cells through stimulation of cAMP signaling. It is possible that an AA level might have increased after administration of sitagliptin in patients who are highly responsive to GIP. Therefore, a higher AA level before dose‐up might have resulted in a larger decrease in HbA1c in the sitagliptin dose‐up group, because an increase in GIP induced by sitagliptin dose‐up counteracted the inhibition of cAMP‐accumulation by PGE2. In contrast, Qi et al.39 reported that AA elevated the intracellular Ca2+ concentration in a dose‐dependent manner, and that glimepiride reduced the intracellular Ca2+ elevation induced by AA in platelets. That report suggests that insulin secretion induced by glimepiride might be varied by the AA level. Therefore, it is speculated that the inhibitory effect of glimepiride on insulin secretion potentiated by AA might be lower in patients with lower AA treated with both sitagliptin and glimepiride. Thus, glimepiride reduced HbA1c more effectively in patients with lower AA. Clarification of this mechanism is important for understanding of the relationship between the decrease of HbA1c and the serum AA level. This will require further appropriate clinical and basic studies in vivo and in vitro.
Interestingly, in the range of reference values of AA (112.7~237.9 μg/mL), the percentage change in HbA1c in the glimepiride dose‐up group (−3.9 ± 5.4%) significantly decreased more than that in the sitagliptin dose‐up group (−0.6 ± 4.7%; P < 0.01). Therefore, from the viewpoint of HbA1c reduction, glimepiride dose‐up is more reasonable than sitagliptin dose‐up in most Japanese patients with type 2 diabetes controlled inadequately by sitagliptin and glimepiride in combination. However, when AA level is above the reference range, sitagliptin dose‐up is more effective than glimepiride dose‐up, because decreases in HbA1c were positively correlated with AA levels in patients receiving glimepiride dose‐up. These findings suggest that the serum AA level might provide the important information for selecting sitagliptin or glimepiride dose‐up in patients with type 2 diabetes receiving both sitagliptin and glimepiride treatment.
Iwasaki et al.12 found that the reduction of HbA1c by administration of DPP‐4 inhibitors as monotherapy significantly correlated with baseline levels of EPA and DHA, but not with that of AA, by the simple linear regression analysis, through unknown mechanisms. The background of the patients in the present study was different from that in the report by Iwasaki et al., but the associations of alterations in HbA1c level with serum levels of polyunsaturated fatty acids in both studies is of interest. Clarification of the relationship between change of HbA1c and serum levels of AA, EPA, DHA, and EPA/AA ratio will require multivariate analysis in a large number of patients in a future study.
In conclusion, there was no significant difference in the HbA1c‐lowering effects between 50 mg/day sitagliptin dose‐up and 0.5 mg/day glimepiride dose‐up. However, a significant HbA1c reduction from baseline was observed in the glimepiride dose‐up group. The serum AA level is associated with HbA1c reduction in response to dose‐up with these drugs in patients with type 2 diabetes receiving combination therapy with sitagliptin and glimepiride.
Acknowledgements
The authors report no potential conflicts of interest relevant to this article.
J Diabetes Invest 2014; 5: 320–326
References
- 1.Nathan DM, Buse JB, Davidson MB, et al Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai K, Matoba K, Hirao K, et al Present status of sulfonylurea treatment for type 2 diabetes in Japan: second report of a cross‐sectional survey of 15,652 patients. Endocr J 2010; 57: 499–507 [DOI] [PubMed] [Google Scholar]
- 3.Tahrani AA, Bailey CJ, Del Prato S, et al Management of type 2 diabetes: new and future developments in treatment. Lancet 2011; 378: 182–197 [DOI] [PubMed] [Google Scholar]
- 4.Harashima SI, Ogura M, Tanaka D, et al Sitagliptin add‐on to low dosage sulphonylureas: efficacy and safety of combination therapy on glycaemic control and insulin secretion capacity in type 2 diabetes. Int J Clin Pract 2012; 66: 465–476 [DOI] [PubMed] [Google Scholar]
- 5.Nonaka K, Kakikawa T, Sato A, et al Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79: 291–298 [DOI] [PubMed] [Google Scholar]
- 6.Tajima N, Kadowaki T, Odawara M, et al Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int 2011; 2: 32–44 [Google Scholar]
- 7.Hermansen K, Kipnes M, Luo E, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9: 733–745 [DOI] [PubMed] [Google Scholar]
- 8.Vilsbøll T, Rosenstock J, Yki‐Järvinen, et al Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 167–177 [DOI] [PubMed] [Google Scholar]
- 9.Iwakura T, Fujimoto K, Tahara Y, et al A case of severe hypoglycemia induced by sitagliptin added to ongoing glimepiride therapy in patients with type 2 diabetes. J Jpn Diabetes Soc 2010; 53: 505–508 [Google Scholar]
- 10.Sitagliptin post‐marketing surveillance study. MSD KK, Ono Pharmaceutical Co. Ltd., August 2010 (Japanese). [Google Scholar]
- 11.Inagaki N, Iwakura T, Iwamoto Y, et al Committee on appropriate use of incretin‐based therapy. http://www.nittokyo.or.jp/kinkyu_incretin100408m.html
- 12.Iwasaki M, Hoshian F, Tsuji T, et al Predicting efficacy of dipeptidyl peptidase‐4 inhibitors in patients with type 2 diabetes: association of glycated hemoglobin reduction with serum eicosapentaenoic acid and docosahexaenoic acid levels. J Diabetes Invest 2012; 3: 464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502 [PubMed] [Google Scholar]
- 16.Scott R, Wu M, Sanchez M, et al Efficacy and tolerability of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61: 171–180 [DOI] [PubMed] [Google Scholar]
- 17.Hanefeld M, Herman GA, Wu M, et al Sitagliptin Study 014 Investigators. Once‐daily sitagliptin, a dipeptidyl peptidase‐4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007; 23: 1329–1339 [DOI] [PubMed] [Google Scholar]
- 18.Aschner P, Kipnes MS, Lunceford JK, et al Effect of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29: 2632–2637 [DOI] [PubMed] [Google Scholar]
- 19.Raz I, Hanefeld M, Xu L, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49: 2564–2571 [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto Y, Taniguchi T, Nonaka K, et al Dose‐ranging efficacy of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J 2010; 57: 383–394 [DOI] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Wysham C, Macconell L, et al Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION‐2): a randomised trial. Lancet 2010; 376: 431–439 [DOI] [PubMed] [Google Scholar]
- 22.Charbonnel B, Karasik A, Liu J, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638–2643 [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto Y, Tajima N, Kadowaki T, et al Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double‐blind trial. Diabetes Obes Metab 2010; 12: 613–622 [DOI] [PubMed] [Google Scholar]
- 24.Ramanadham S, Gross R, Turk J. Arachidonic acid induces an increase in the cytosolic calcium concentration in single pancreatic islet beta cells. Biochem Biophys Res Commun 1992; 184: 647–653 [DOI] [PubMed] [Google Scholar]
- 25.Band AM, Jones PM, Howell SL. Arachidonic acid‐induced insulin secretion from rat islets of Langerhans. J Mol Endocrinol 1992; 8: 95–101 [DOI] [PubMed] [Google Scholar]
- 26.Turk J, Gross RW, Ramanadham S. Amplification of insulin secretion by lipid messengers. Diabetes 1993; 42: 367–374 [DOI] [PubMed] [Google Scholar]
- 27.Simonsson E, Ahrén B. Phospholipase A2 and its potential regulation of islet function. Int J Pancreatol 2000; 27: 1–11 [DOI] [PubMed] [Google Scholar]
- 28.Ehses JA, Lee SS, Pederson RA, et al A new pathway for glucose‐dependent insulinotropic polypeptide (GIP) receptor signaling: evidence for the involvement of phospholipase A2 in GIP‐stimulated insulin secretion. J Biol Chem 2001; 276: 23667–23673 [DOI] [PubMed] [Google Scholar]
- 29.Cohen G, Riahi Y, Shamni O, et al Role of lipid peroxidation and PPAR‐δ in amplifying glucose‐stimulated insulin secretion. Diabetes 2011; 60: 2830–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho YS, Kim CH, Kim KY, et al Protective effects of arachidonic acid against palmitic acid‐mediated lipotoxicity in HIT‐T15 cells. Mol Cell Biochem 2012; 364: 19–28 [DOI] [PubMed] [Google Scholar]
- 31.Yeung‐Yam‐Wah V, Lee AK, Tse A. Arachidonic acid mobilizes Ca2+ from the endoplasmic reticulum and an acidic store in rat pancreatic β cells. Cell Calcium 2012; 51: 140–148 [DOI] [PubMed] [Google Scholar]
- 32.Robertson RP, Gavareski DJ, Porte D Jr, et al Inhibition of in vivo insulin secretion by prostaglandin E1. J Clin Invest 1974; 54: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson RP. Arachidonic acid metabolite regulation of insulin secretion. Diabetes Metab Rev 1986; 2: 261–296 [DOI] [PubMed] [Google Scholar]
- 34.Seaquist ER, Walseth TF, Nelson DM, et al Pertussis toxin‐sensitive G protein mediation of PGE2 inhibition of cAMP metabolism and phasic glucose‐induced insulin secretion in HIT cells. Diabetes 1989; 38: 1439–1445 [DOI] [PubMed] [Google Scholar]
- 35.Dachicourt N, Serradas P, Giroix MH, et al Decreased glucose‐induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. Am J Physiol 1996; 271: E725–E732 [DOI] [PubMed] [Google Scholar]
- 36.Persaud SJ, Muller D, Belin VD, et al The role of arachidonic acid and its metabolites in insulin secretion from human islets of Langerhans. Diabetes 2007; 56: 197–203 [DOI] [PubMed] [Google Scholar]
- 37.Luo P, Wang MH. Eicosanoids, β‐cell function, and diabetes. Prostaglandins Other Lipid Mediat 2011; 95: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parazzoli S, Harmon JS, Vallerie SN, et al Cyclooxygenase‐2, not microsomal prostaglandin E synthase‐1, is the mechanism for interleukin‐1β‐induced prostaglandin E2 production and inhibition of insulin secretion in pancreatic islets. J Biol Chem 2012; 287: 32246–32253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi R, Ozaki Y, Satoh K, et al Sulphonylurea agents inhibit platelet aggregation and [Ca2+]i elevation induced by arachidonic acid. Biochem Pharmacol 1995; 49: 1735–1739 [DOI] [PubMed] [Google Scholar]


