Summary
The identification and characterization of age-related degenerative changes is a critical goal because it can elucidate mechanisms of aging biology and contribute to understanding interventions that promote longevity. Here we document a novel, age-related degenerative change in C. elegans hermaphrodites, an important model system for the genetic analysis of longevity. Matricidal hatching—intra-uterine hatching of progeny that causes maternal death—displayed an age-related increase in frequency and affected ∼70% of mated, wild-type hermaphrodites. The timing and incidence of matricidal hatching were largely independent of the levels of early and total progeny production and the duration of male exposure. Thus, matricidal hatching appears to reflect intrinsic age-related degeneration of the egg-laying system rather than use-dependent damage accumulation. Consistent with this model, mutations that extend longevity by causing dietary restriction significantly delayed matricidal hatching, indicating age-related degeneration of the egg-laying system is controlled by nutrient availability. To identify the underlying tissue defect, we analyzed serotonin signaling that triggers vulval muscle contractions. Mated hermaphrodites displayed an age-related decline in the ability to lay eggs in response to exogenous serotonin, indicating that vulval muscles and/or a further downstream function that is necessary for egg-laying degenerate in an age-related manner. By characterizing a new, age-related degenerative event displayed by C. elegans hermaphrodites, these studies contribute to understanding a frequent cause of death in mated hermaphrodites and establish a model of age-related reproductive complications that may be relevant to the birthing process in other animals such as humans.
Introduction
Reproductive aging of human females results in a progressive decline in reproductive success culminating in reproductive cessation at menopause (Hartge 2009). Prior to menopause, age-related degeneration of the reproductive system increases the frequency of a wide variety of pregnancy-related complications that threaten the life of the mother and fetus. For example, women over 40 years of age are twice as likely to have emergency cesarean sections due to complications in the birthing process than women under 35 years of age (Cleary-Goldman et al. 2005).
To elucidate the causes of age-related degeneration of the reproductive system in animals, we used the nematode C. elegans as a model. Reproductive aging in mated C. elegans hermaphrodites is characterized by a progressive, age-related decline of reproductive function that begins on about day 5 of adulthood, and reproduction ceases after 10-14 days of adulthood (Hughes et al. 2007; Andux & Ellis 2008; Mendenhall et al. 2011). C. elegans eggs are fertilized internally when a mature oocyte is ovulated and passed into the spermatheca where it is fertilized by either self-sperm generated during the L4 larval stage or male sperm deposited after copulation (Greenstein 2005). In young adult hermaphrodites, fertilized eggs develop in the hermaphrodite for ∼2.5 hours, are laid into the environment, and hatch after ∼10 hours of further development (Schafer 2005). However, if a fertilized egg is not laid within ∼12.5 hours, then it will hatch internally and the larva will begin feeding, which kills the hermaphrodite. This event is called matricidal hatching, or endotokia matricida, and is defined as intra-uterine hatching and larval development leading to the destruction of the female by the larvae (Luc et al. 1979). This form of cannibalism is common among Rhabditida nematodes (Johnigk & Ehlers 1999; Luong et al. 1999) and may enhance larval viability and promote dauer formation in nutrient-deficient conditions (Chen & Caswell-Chen 2004). The phenomenon is not restricted to nematodes; for example, Adactylidium mites display matricidal hatching (Kirkwood & Cremer 1982).
Matricidal hatching in C. elegans, colloquially known as the bag-of-worms phenotype, has been extensively characterized as a result of genetic screens for mutant animals defective in egg laying. These studies demonstrated that formation of the vulva, the vulval muscles, and the HSN neurons that innervate the vulval muscles are essential for efficient egg-laying, since mutations that impair these systems cause egg retention and matricidal hatching (Trent et al. 1983; Ferguson & Horvitz 1985; Weinshenker et al. 1995). Furthermore, reproductive hermaphrodites exposed to nutrient deprivation stop laying eggs and experience matricidal hatching (McCulloch & Gems 2003; Chen & Caswell-Chen 2004).
Here we focus on a third cause of matricidal hatching that has not been previously characterized: age-related degeneration of the egg-laying system. We demonstrate that matricidal hatching, hereafter termed MH, displays an age-related increase in frequency and occurs primarily in mated hermaphrodites that reproduce late in life. The timing and incidence of MH was largely independent of the level of early and total progeny production and the duration of exposure to males, suggesting it is not caused by use-dependent damage accumulation and may reflect intrinsic age-related degeneration of the egg-laying system. Consistent with this model, mutations that cause dietary restriction delayed MH, indicating that nutritional status influences the age-related degeneration of the egg-laying system. To dissect this age-related decline, we showed that hermaphrodites displayed age-related loss of serotonin-mediated egg-laying, suggesting that vulval muscles or a further downstream function declines in an age-related fashion. The age-related degeneration of the C. elegans reproductive system may be relevant to other animals and provide a model system that can elucidate human complications associated with giving birth.
Results
Age-related matricidal hatching is a major cause of mortality for mated hermaphrodites
MH can be scored accurately with a dissecting microscope by observing internally hatched progeny (Movie S1). A hermaphrodite that ceased reproduction without internally hatched progeny and died a senescent death was scored as escaping MH. We compared the incidence and timing of MH for wild-type (WT) hermaphrodites never exposed to males (self-fertile) and hermaphrodites exposed to 3 WT males for 24 hours on the first day of adulthood (mated). This protocol minimizes male exposure while allowing adequate sperm transfer, so that mated hermaphrodites typically generate cross-progeny for the entire reproductive period (Hughes et al. 2007). By monitoring progeny production, we identified hermaphrodites that reverted to self-progeny production and excluded them from the analysis.
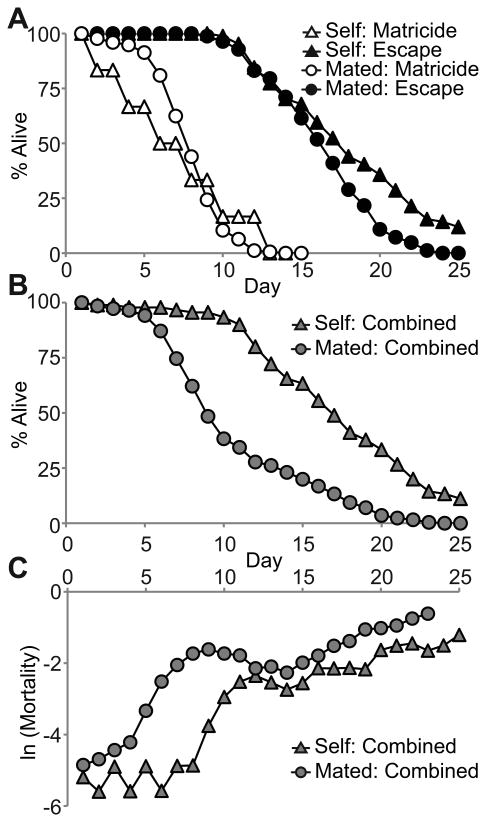
For self-fertile WT hermaphrodites, 6% died by MH, and these animals exhibited a mean life span of 6.2±4.0 days (Fig. 1A, Table 1). The 94% of animals that escaped displayed a significantly longer mean life span of 17.3±5.3 days. Mated hermaphrodites generate more progeny for an extended period compared to self-fertile hermaphrodites (Ward & Carrel 1979). For mated WT hermaphrodites, 68% died by MH, consistent with the finding that MH occurs frequently in mated hermaphrodites (Luo et al. 2009). MH occurred primarily on days 6-10, and these animals displayed a mean life span of 7.2±2.2 days (Fig. 1A, Table 1). The 32% of animals that escaped exhibited a significantly longer mean life span of 15.5±3.4 days (Fig. 1A, Table 1). Thus, the incidence of MH is significantly higher in mated compared to self-fertile hermaphrodites, whereas the timing of MH is similar (p=0.27). For animals that escaped MH, self-fertile hermaphrodites had a slightly longer mean life span than mated hermaphrodites, but this difference was not statistically significant (p=0.08).
Figure 1.
Matricidal hatching is the predominant cause of mortality in mated WT hermaphrodites. A) Life span curves of self-fertile (Self) and mated (Mated) WT hermaphrodites that died by MH (Matricide) or that escaped MH (Escape). B) Life span curves and C) Natural log of age-specific mortality for the combined population of hermaphrodites that died by MH and escaped MH. See Table 1 for summary statistics and N values.
Table 1. Life spans of WT hermaphrodites that died by matricidal hatching or escaped matricidal hatching.
| %† | Life Span (Days)‡ | N§ | ||
|---|---|---|---|---|
| Mated | Matricidal Hatching | 68 | 7.2±2.2* | 175 |
| Escape | 32 | 15.5±3.4 | 82 | |
| Combined | 100 | 9.8±4.7* | 257 | |
|
| ||||
| Self | Matricidal Hatching | 6 | 6.2±4.0* | 6 |
| Escape | 94 | 17.3±5.3 | 91 | |
| Combined | 100 | 16.7±5.9 | 97 | |
Hermaphrodites were either self-fertile (Self) or mated to WT males on day 1 of adulthood (Mated).
The percentage of animals that died by MH, escaped MH, or the combined population.
Mean life span ± standard deviation.
The number of animals.
p<0.001 compared to animals that escaped matricidal hatching.
The life span of mated and self-fertile hermaphrodites that escaped MH was not significantly different (p=0.08).
To examine the entire populations, we analyzed the life span of the combined group of animals that died by MH or escaped. The combined mated population exhibited a mean life span of 9.8±4.7 days that was significantly shorter than the life span of animals that escaped (Fig. 1B, Table 1). The combined self-fertile population exhibited a mean life span of 16.7±5.9 days that was not significantly different than the life span of animals that escaped (Fig. 1B, Table 1). Thus, MH in the mated WT hermaphrodite population is a frequent event that significantly shortens the mean life span, whereas MH in the self-fertile WT hermaphrodite population is a rare event that only slightly reduces the mean life span.
To quantify the age-specific mortality rate in mated and self-fertile populations, we calculated the natural log of the mortality rate. For the population of mated animals, the death rate was low for the first 4 days, began to rise substantially on day 5, and reached an initial peak at day 9 (Fig. 1C). The population of self-fertile animals exhibited a low death rate for the first 8 days before rising substantially on day 9 (Fig. 1C). By the end of mated reproduction on day 12, the mated and self-fertile populations exhibited nearly the same age-specific mortality rates. The increase in mortality rate in the population of mated animals between days 5 and 9 was due to MH, since the first death in mated animals that was not caused by MH occurred on day 8 (Fig. 1A, supplemental data).
Matricidal hatching occurs primarily in animals reproducing after day 5
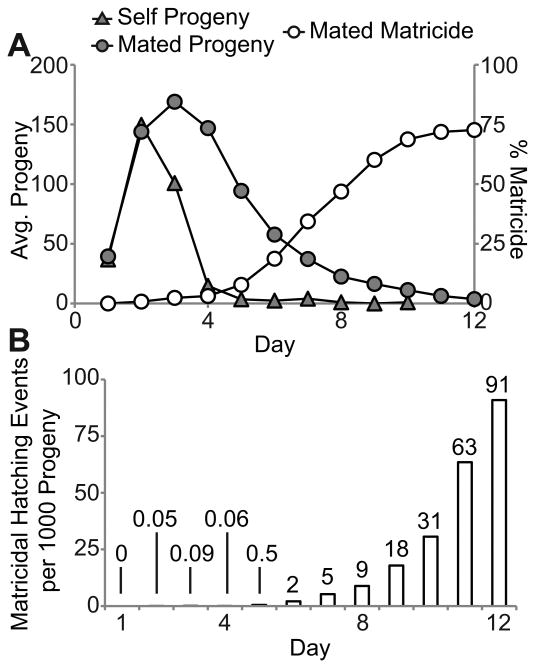
To die by MH, a hermaphrodite must generate viable progeny. Self-fertile animals displayed a peak of progeny production on day 2 and ceased progeny production about day 5 or 6 due to self-sperm depletion (Fig. 2A). Mated animals displayed peak progeny production on days 2-4 and ceased progeny production about day 12 due to age-related degeneration of the reproductive system (Fig. 2A) (Hughes et al. 2007). As of day 5, less than 10% of mated hermaphrodites had died by MH. After day 5, the fraction increased progressively, achieving a plateau at day 10 of nearly 70% (Fig. 2A). Thus, MH occurs primarily during the reproductive decline phase, days 5-10, long after maximal daily progeny production. The low incidence of MH in self-fertile animals may reflect the fact that these animals cease generating progeny after day 5 due to sperm depletion.
Figure 2.
Matricidal hatching occurs during the declining phase of progeny production. A) Daily average progeny production of mated and self-fertile WT hermaphrodites and the cumulative incidence of MH in mated WT hermaphrodites. B) Number of MH events per 1000 progeny generated by mated WT hermaphrodites. See Table 1 for N values.
To determine the risk of laying eggs at different maternal ages, we calculated the number of MH events each day divided by the number of progeny produced the same day. This ratio evaluates the risk of a fertilized egg hatching internally. For the first 5 days the risk was very low for mated WT hermaphrodites, since less than one MH event occurred per 1000 progeny (Fig. 2B). Starting on day 6, the number increased progressively, peaking at 91 MH events per 1000 progeny on day 12 (Fig. 2B). Thus, each fertilized egg in a day 12 hermaphrodite has an ∼9% chance of hatching internally, resulting in matricide. These data indicate that fertilized eggs have a progressively higher chance of hatching internally as hermaphrodites age.
The level of early progeny production is not predictive for matricidal hatching
Two models could explain the age-related increase in frequency of MH. The use-dependent model postulates that the process of egg laying or mating damages the egg-laying system, and the accumulation of this damage causes the egg-laying system to become dysfunctional. This model predicts that the extent of reproduction or copulation is a predictive risk factor for MH. Alternatively, the use-independent model postulates that the egg-laying system undergoes age-related degeneration independent of egg laying or copulation. This model predicts that the extent of MH is not associated with the level of progeny production or the number of copulation events.
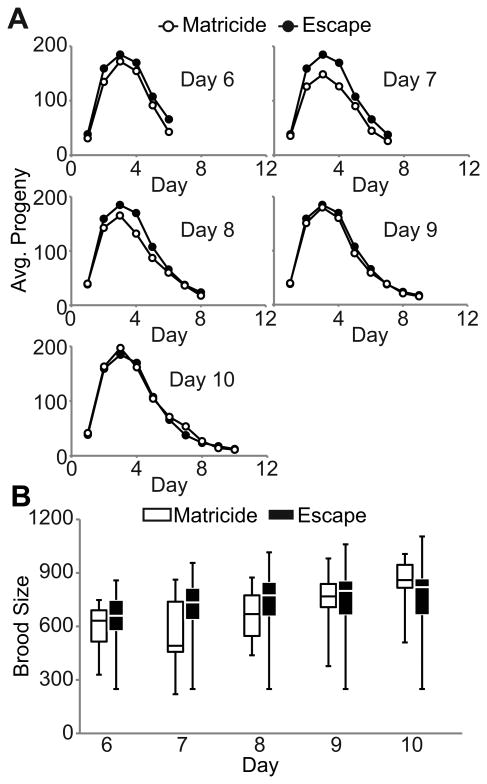
To distinguish between these models, we monitored daily progeny production of mated WT hermaphrodites that succumbed to MH and escaped. We focused the analysis on days 6-10, since few matricidal deaths occurred before or after this time interval. Compared to animals that escaped MH, animals that died by MH on days 6-10 had similar or slightly lower levels of average daily progeny production prior to death (Fig. 3A). These data were used to analyze cumulative progeny production. Compared to animals that escaped MH, the lower quartile, median, and upper quartile of cumulative brood sizes for were slightly lower for animals that died by MH on days 6-8, similar for animals that died on day 9, and slightly higher for animals that died on day 10 (Fig. 3B). Together, these data indicate that there is no consistent trend between daily or cumulative progeny production and MH, consistent with the use-independent model.
Figure 3.
Progeny production is not predictive for matricidal hatching. A) Daily average progeny production of mated WT hermaphrodites that died by MH on the indicated day or escaped MH. For days 6, 8, 9, and 10, p>0.05. For day 7, p<0.01. Each graph displays a unique group of animals that exhibited MH and the same group of animals that escaped MH. B) Box-plot of cumulative brood sizes for mated WT hermaphrodites that died by MH and those that escaped through the indicated day. The bottom, internal line, and top of the box represent the 25th percentile, median, and 75th percentile, respectively. The lower and upper bars represent the minimum and maximum brood size, respectively. Matricide N= 10-20, Escape N= 34.
To determine if early reproduction influences MH, we analyzed progeny production of mated WT hermaphrodites on days 1-5. This analysis was comprehensive, since almost every animal in the experiment completed early reproduction. Cumulative progeny production on days 1-2, 1-3, 1-4, and 1-5 and daily progeny production on days 1, 2, 3, 4, or 5 of animals that died by MH was the same or slightly lower than animals that escaped MH, but the differences were not significant (Table S1). To determine if the level of early progeny production is correlated with the timing of MH, we used Spearman rank correlation to analyze the longitudinal data (Huang et al. 2004). For mated WT animals that died by MH, there was no significant correlation between the level of cumulative progeny production on days 1-2, 1-3, 1-4 or 1-5 or the level of daily progeny production on days 1, 2, 3, 4, or 5 and the matricidal life span (Fig. S1, Table S2). These data document that the level of early progeny production is not an indicator of the extent or timing of MH, suggesting that high levels of early progeny production do not increase the frequency or accelerate the onset of MH.
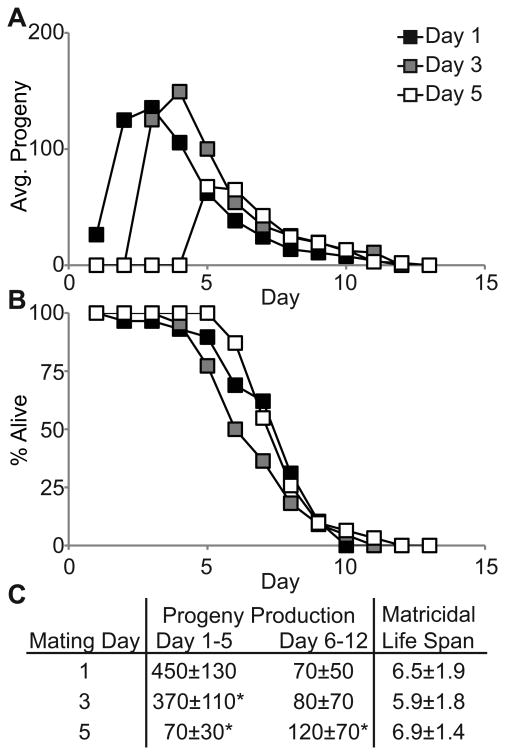
Mated C. elegans hermaphrodites lay more than 80% of their brood on days 1-5 (Hughes et al. 2007). If egg-laying during this period damages the egg-laying system in a manner that promotes delayed MH, then reducing reproduction on days 1-5 is predicted to reduce the incidence or delay the timing of MH. To test this prediction, we analyzed fog-2(q71) females that generate no self-sperm and lay very few unfertilized oocytes (Clifford et al. 2000). fog-2(q71) females mated on day 1 generated 450±130 progeny on days 1-5, whereas those mated on day 3 or 5 generated significantly fewer progeny: 370±110 and 70±30, respectively (Fig. 4A, C). However, the incidence of MH (74-86%, p>0.2) and the timing of MH (5.9 to 6.9 days, p>0.25) were not significantly different for animals mated on day 3 or 5 compared to day 1 (Fig. 4B, C). Together, the analysis of WT and fog-2(q71) animals indicates that the levels of progeny production on days 1-5 does not influence the incidence or timing of MH.
Figure 4.
Matricidal hatching is independent of early progeny production. A) Daily average progeny production and B) life span of fog-2(q71) females that mated to WT males on the indicated day and died by MH. C) Average early and late progeny production ± standard deviation and mean life spans of fog-2(q71) animals mated on the indicated day. Incidence of MH – Day 1 = 74%; Day 3 = 81%; Day 5 = 86%. N = 29, 22 and 31 for days 1, 3 and 5, respectively. *p<0.05 compared to mating day 1.
The duration of male exposure is not predictive of matricidal hatching
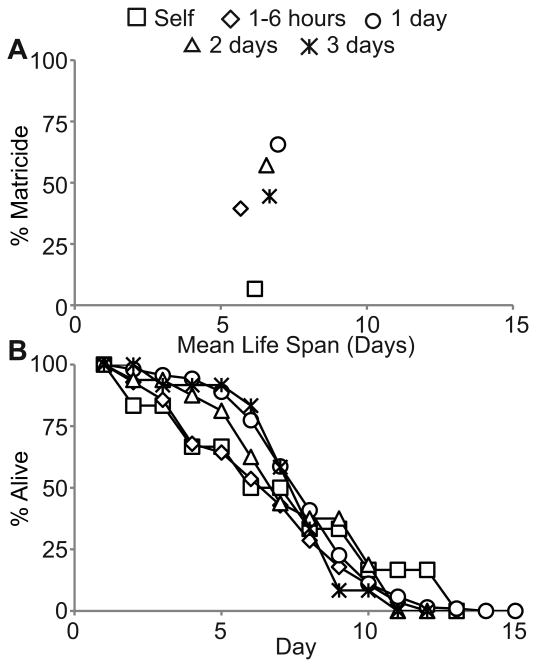
The higher incidence of MH in mated hermaphrodites compared to self-fertile hermaphrodites is likely to be caused by transfer of male sperm that results in an extended period of reproduction, but it might result from another aspect of male exposure such as trauma caused by copulation. The majority of MH in mated WT hermaphrodites occurred 4 to 9 days after male exposure ended (Fig. 1). Thus, if copulation trauma is causative, then it must act in a manner that results in delayed MH. To determine the role of copulation trauma, we analyzed relationships between the duration of male exposure and MH. We reasoned that the number of mating events is proportional to the amount of time hermaphrodites were exposed to males (Barr & Garcia 2006). Our standard mating protocol consists of 24 hours of male exposure on day 1. To minimize male exposure, we identified hermaphrodites that had copulated only once using a sex-specific Mitotracker (MT) mating assay and isolated these animals to limit male exposure to less than 6 hours (see Experimental Procedures) (Stanfield & Villeneuve 2006). To increase male exposure, we cultured hermaphrodites daily with fresh, young-adult males for 48 or 72 hours. For hermaphrodites that engaged in only one copulation event, the incidence of MH was ∼40% compared to 68% for hermaphrodites mated for 1 day (p<0.001), and the mean life span was 5.7±2.9 days compared to 7.2±2.2 days for hermaphrodites mated for 1 day (p=0.006) (Fig. 5A, B). For hermaphrodites exposed to males for 2 or 3 days, the incidence of MH was ∼50% and the mean life spans were 6.6±2.7 and 6.7±2.0 days, respectively (Fig. 5A, B). Compared to animals mated for 1 day, animals mated for 2 or 3 days had a similar incidence of MH and life span (p>0.1). These data indicate there is no consistent relationship between the amount of time hermaphrodites were exposed to males and the incidence or timing of MH.
Figure 5.
Matricidal hatching is not strongly affected by the duration of the mating period. A) A scatter plot showing the average incidence of MH and the average matricidal life spans of WT hermaphrodites that were self-fertile and mated to WT males for the indicated times. B) Life span curves of WT hermaphrodites that died by MH. Self N = 90; 1-6 hours N = 71; 1 day N = 295; 2 days N = 28; 3 days N = 27.
Dietary restriction delays the timing of matricidal hatching
The age-related increase in MH might be explained by an age-related degeneration of the egg-laying system. This model predicts that mutations that delay age-related degeneration and extend longevity might also delay MH. Mutations that disrupt insulin/IGF-1 signaling (IIS), mitochondrial function, or TGF-β signaling extend life span and also delay age-related degeneration of specific tissues (Herndon et al. 2002; Huang et al. 2004; Hughes et al. 2007; Luo et al. 2009). To determine if these mutations also delay MH, we used WT males to mate hermaphrodites carrying the daf-2(e1370), age-1(hx546), or daf-16(mu86) mutations that affect IIS (Friedman & Johnson 1988; Kenyon et al. 1993; Ogg et al. 1997), the clk-1(qm30) or isp-1(qm150) mutations that affect electron transport (Lakowski & Hekimi 1996; Feng et al. 2001), and the sma-2(e502) or sma-3(e491) mutations that affect TGF-β signaling (Shaw et al. 2007). Attempts to use mutant males in these experiments were not successful, because mutant males did not mate efficiently. Total progeny production was reduced significantly in all mutant strains, indicating that these mutations reduce fertility (Fig. S2D-F, Table 2). The incidence of MH was increased, and the difference was significant for daf-2, sma-2 and sma-3 mutant animals (Table 2). Furthermore, starting about day 6, the rate of MH for each progeny produced was higher in age-1, clk-1, sma-2 and sma-3 mutant animals compared to WT hermaphrodites (Fig. S2G-I). The mean life spans of mutant hermaphrodites mated to WT males that died by MH were usually shorter but not significantly different compared to WT hermaphrodites mated to WT males (p>0.1), which may reflect the combination of reduced progeny production and an increased rate of MH for each progeny produced in the mutant strains. (Fig. S2A-C, Table 2). These mutant strains displayed an increased rate of MH for each progeny produced and an overall increase in the rate of MH suggesting that these mutations cause pleiotropic defects that impair the function of the egg-laying system. Because of these pleiotropic defects, these data do not establish whether these mutations delay age-related degeneration of the egg-laying system.
Table 2. Summary statistics for mated hermaphrodites.
| Genotype | Brood Size† | % Matricide‡ | Matricide Life Span§ | N¶ |
|---|---|---|---|---|
| WT | 690±200 | 68 | 7.2±2.2 | 257 |
| eat-2(ad465) | 310±90*** | 60 | 10.9±3.5*** | 35 |
| eat-13(ad522) | 130±50*** | 80 | 8.5±2.0** | 15 |
| tph-1(mg280) | 380±140*** | 91* | 5.4±1.2*** | 33 |
| daf-2(e1370) | 340±120*** | 93* | 7.3±2.0 | 29 |
| age-1(hx546) | 460±70*** | 90 | 6.3±1.1 | 22 |
| daf-16(mu86) | 470±170*** | 82 | 6.3±1.9 | 22 |
| clk-1(qm30) | 480±110*** | 79 | 6.4±1.1 | 33 |
| isp-1(qm150) | 260±80*** | 86 | 6.7±1.6 | 22 |
| sma-2(e502) | 170±60*** | 96* | 6.0±1.9 | 25 |
| sma-3(e491) | 190±70*** | 92* | 6.3±1.6 | 25 |
All hermaphrodites were mated for ∼24 hours to WT males on day 1 of adulthood.
The average number of progeny laid by an animal until the end of reproduction ± the standard deviation.
The percentage of animals that died by MH.
The average life span in days of animals that died by MH ± the standard deviation measured in days.
The number of mated animals examined. The N value for WT animals applies to the % matricide and matricide life span. A subset of this population (N = 128) was analyzed for brood size.
Compared to WT,
p<0.05,
p<0.01,
p<0.001.
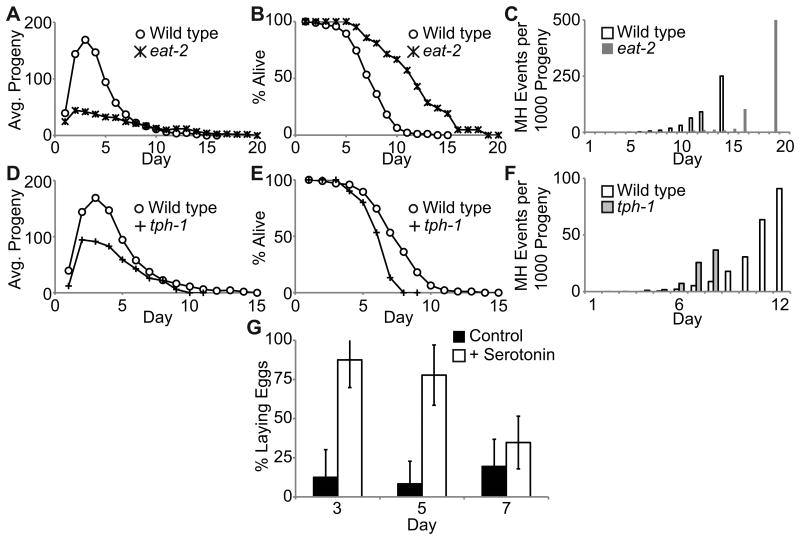
Dietary restriction in C. elegans reduces progeny production, delays age-related degeneration of somatic and reproductive function, and extends life span (Lakowski & Hekimi 1998; Huang et al. 2004; Hughes et al. 2007). The eat-2(ad465) and eat-13(ad522) mutations cause dietary restriction by slowing pharyngeal pumping (Lakowski & Hekimi 1998). The mean life spans of eat-2(ad465) and eat-13(ad522) animals mated to WT males that died by MH (10.9±3.5 days and 8.5±2.0 days, respectively) were significantly longer than WT animals mated to WT males (7.2±2.2 days; p<0.001) (Fig. 6B, Fig. S3B, Table 2), whereas total progeny production of mutant animals was reduced significantly (p<0.001) (Fig. 6A, Fig. S3A, Table 2). Furthermore, eat-2(ad465) hermaphrodites, but not eat-13(ad522) hermaphrodites, mated to WT males displayed a delay in the age-related increase in the rate of MH for each progeny produced (Fig. 6C, Fig. S3C). Together, these data indicate that dietary restriction delays MH by reducing the rate of internal egg hatching in older hermaphrodites, suggesting that age-related degeneration of the egg-laying system that is influenced by dietary restriction may be a cause of MH.
Figure 6.
The function of the egg-laying system declines in an age-related manner. WT, eat-2(ad465) and tph-1(mg280) hermaphrodites were mated to WT males. A), D) Daily average progeny production B), E) life span curves of animals that died by MH and C), F) the rate of MH per progeny generated. See Table 2 for summary statistics and N values. G) Percentage ± standard deviation of mated WT hermaphrodites laying eggs in M9 medium alone (control) or M9 medium supplemented with 10mM serotonin (+ serotonin). Age is days of adulthood. + serotonin N ≥22; control N ≥7.
Serotonin-induced egg-laying displays an age-related decline
Egg-laying is a complex neuromuscular process that is mediated by serotonin signaling and controlled by nutrient availability (Schafer 2005). Animals with impaired serotonin signaling or vulval muscle defects are egg-laying defective and frequently die by MH (Trent et al. 1983; Weinshenker et al. 1995). To determine the role of serotonin signaling in age-related MH, we examined tph-1(mg280) hermaphrodites. TPH-1 is a tryptophan hydroxylase necessary for production of serotonin in C. elegans, and the tph-1(mg280) allele results in an extended life span and a weak egg-laying defect (Sze et al. 2000). Mated tph-1(mg280) hermaphrodites displayed reduced daily progeny production and significantly smaller broods (p<0.001) (Fig. 6D, Table 2). Despite reduced progeny production, these hermaphrodites displayed a significantly shorter matricidal life span of 5.4±1.2 days (p<0.001) compared to mated WT hermaphrodites (Fig. 6E, Table 2), an acceleration of the age-related increase in the rate of MH for each progeny produced (Fig. 6F) and a significantly higher MH incidence (91%, p=0.03) (Table 2). These results indicate that robust serotonin signaling is necessary to delay the timing of MH in mated hermaphrodites.
We hypothesized that serotonin signaling or a further downstream component in the egg-laying system degenerates in an age-related manner. To test this hypothesis, we compared the ability of young and aged hermaphrodites to lay eggs in response to exogenous serotonin. Hermaphrodites transferred to M9 buffer with no food typically cease egg-laying, but addition of serotonin results in vulval muscle contraction and egg-laying (Horvitz et al. 1982; Trent et al. 1983; Weinshenker et al. 1995). WT hermaphrodites were mated to WT males on day 1 and tested for the ability to lay eggs in M9 supplemented with 10mM serotonin on days 3, 5, and 7. Day 3 animals were highly responsive to serotonin: whereas only ∼13% laid eggs in M9 buffer alone, ∼90% laid eggs in response to serotonin (Fig. 6G). The percentage of day 5 and 7 animals that responded to serotonin decreased to ∼77% and ∼35%, respectively (Fig. 6G). Day 7 animals displayed similar egg-laying with or without serotonin, indicating that day 7 hermaphrodites were largely insensitive to serotonin. Hermaphrodites that were not responsive to exogenous serotonin did contain fertilized eggs in the uterus, suggesting that the lack of egg-laying was not due to a lack of eggs. These data indicate that the responsiveness of the vulval muscles to serotonin or a further downstream component in the egg laying system degenerates in an age-related manner, supporting the hypothesis that age-related degenerative changes in the egg-laying system underlie the age-related increase in MH in mated hermaphrodites.
Discussion
Matricidal hatching is a frequent, age-related cause of death in mated hermaphrodites
C. elegans hermaphrodites exhibit robust physiological processes for the first ∼5 days of adulthood. A variety of age-related degenerative changes are readily observable on days 4-6, including declines in the level of cross-progeny production (Hughes et al. 2007; Andux & Ellis 2008; Mendenhall et al. 2011), coordinated body movement (Herndon et al. 2002), and the rate of pharyngeal pumping (Huang et al. 2004). The progressive declines in complex physiological processes presumably reflect progressive declines of the underlying organs, tissues, cells and/or subcellular components, although in most cases these connections are not well established experimentally (Collins et al. 2008b). Here we demonstrate that MH is an age-related cause of death in mated WT C. elegans hermaphrodites that affects about 70% of the population. Our results indicate that MH is an indicator of the age-related decline of the egg-laying system. This analysis elucidates the age-related decline of a vital physiological process that has not been previously characterized, which is important because C. elegans is a leading model system for genetic studies of aging, and the characterization of age-related changes in this animal is an essential step in connecting genes that control longevity with underlying physiological processes.
In mated WT hermaphrodites, MH rarely occurred before day 5 of adulthood, increased from day 6-12, and ended with the cessation of cross-progeny production. Individual hermaphrodites either undergo MH or escape, and the outcome appears to depend on the rates of two age-related declines. First, the ability to generate and fertilize oocytes displays an age-related decline, and this decreases the chances of MH. When reproductive aging results in the complete cessation of progeny production, the hermaphrodite is no longer at risk of MH. Second, the function of the egg-laying system declines, and this increases the chances of MH. Here we measured this decline and showed the rate of MH increases more than 1000-fold from 0.05 events per 1000 progeny on day 2 to 91 events per 1000 progeny on day 12. For self-fertile hermaphrodites, the ability to fertilize oocytes typically fails due to self-sperm depletion prior to failure of the egg-laying system, and thus most self-fertile animals escape MH. For mated hermaphrodites, the ability to generate fertilized oocytes typically persists past the time the egg-laying system fails, and thus most mated hermaphrodites display MH.
Gems and Riddle (1996) demonstrated that continuous male exposure reduced the life span of WT hermaphrodites to 9.6 days. Consistent with these results, we observed a mean life span of 9.8 days for the combined population of mated WT hermaphrodites. By monitoring individual hermaphrodites and distinguishing between matricidal and senescent death, we were able to advance the understanding of this phenomenon—the reduced life span of the population is caused by MH of about 70% of the animals. By comparing hermaphrodites that experienced different durations of male exposure, we showed that the duration of male exposure is not predictive of the incidence or timing of MH. Furthermore, self-fertile hermaphrodites that were never exposed to males display MH, albeit at a low frequency, indicating that male exposure is not necessary to cause internal hatching. These findings do not exclude the possibility that trauma caused by male contact influences the pattern of MH, but our results did not reveal clear evidence for this association. We conclude that male mating increases the rate of MH as a result of sperm transfer that causes extended progeny production, thereby decreasing the mean life span of the mated population.
Matricidal hatching is not caused by use-dependent damage to the egg-laying system
According to the use-dependent model of MH, each egg that is laid has the potential to damage the egg-laying system, and the accumulation of this damage causes an age-related decline of function. This model predicts that the number of eggs laid will be predictive of the extent of damage to the egg-laying system and the frequency of MH. We observed no consistent differences between the levels of egg laying for animals that displayed MH and animals that escaped, indicating that use-related damage may not be a cause of MH. Furthermore, fog-2(q71) females that produced no progeny for the first two or four days of adulthood nonetheless displayed the same pattern of MH as fog-2(q71) females that produced progeny starting on day one. These results indicate that the age-related decline of the egg-laying system is not related to the number of eggs laid early in the reproductive period, but rather seems to be determined by a mechanism that is independent of early progeny production. These findings do not exclude the possibility that damage caused by late progeny production might contribute to MH. We previously demonstrated that the age-related decline of the reproductive system was independent of the number of progeny generated (Hughes et al. 2007). Together, these findings indicate that there is an age-related decline of the reproductive system that generates fertilized eggs and the egg-laying system that deposits embryos into the environment, but in neither case does the age-related functional decline correlate with using the system early in the reproductive period.
Age-related matricidal hatching is delayed by dietary restriction and may be caused by an age-related decline of vulval muscle function
Some mutations that extend life span also delay age-related degenerative changes in muscles, the reproductive system, and other tissues (Collins et al. 2008b). If a life-span extending mutation delays the age-related decline of the egg-laying system, then MH is predicted to be delayed. Consistent with this prediction, two mutations that extend life span by causing dietary restriction significantly extended the matricidal life span. These results indicate the nutritional status of hermaphrodites is an important determinant of the timing of age-related degeneration of the egg-laying system. By contrast, mutations that disrupt insulin/IGF-1 signaling, mitochondrial function, or TGF-β signaling did not delay the timing of MH. However, these mutations reduced progeny production and increased the incidence of MH, suggesting that these mutations cause pleiotropic defects that impair the egg-laying system. Consistent with this interpretation, daf-2 mutations cause an increase in the incidence of MH in self-fertile animals (Gems et al. 1998). Thus, these results do not establish whether these genes influence the age-related decline of the egg-laying system.
The C. elegans egg-laying system has been genetically dissected based on mutations that cause egg retention in young adult animals (Trent et al. 1983). These studies revealed that efficient egg laying requires proper development of the vulva, vulval muscles, and neurons that innervate these muscles (Ferguson & Horvitz 1985; Schafer 2005). To identify elements of the egg-laying system that undergo an age-related decline in function and may contribute to age-related MH, we focused on the vulval muscles. Serotonin is a key neurotransmitter that is released by HSN neurons to stimulate vulval muscles, and exogenous serotonin treatment can stimulate vulval muscle contractions and ameliorate defects in egg-laying caused by defective HSN neurons (Trent et al. 1983; Weinshenker et al. 1995; Zhang et al. 2008). We showed that WT hermaphrodites exhibited an age-related decline in the ability to lay eggs in response to exogenous serotonin, indicating that there is an age-related decline in vulval muscle responsiveness to serotonin. We hypothesize that an age-related degeneration of the vulval muscles or a process further downstream in the egg laying system is the underlying cause of age-related MH. Consistent with this model, tph-1(mg280) hermaphrodites that are defective in serotonin synthesis (Sze et al. 2000) displayed accelerated MH. These data indicate that robust serotonin signaling delays MH, consistent with the model that an age-related decline in the effectiveness of serotonin signaling contributes to age-related MH.
The role of age-related matricidal hatching in the natural environment
The destruction of the mother to provide nutrients to the offspring is common among nematodes and an example of efficient transfer of resources between generations (Luc et al. 1979; Kirkwood & Cremer 1982; Johnigk & Ehlers 1999; Luong et al. 1999). In C. elegans, MH provides sufficient nutrition for progeny to reach the developmentally quiescent dauer stage (Chen & Caswell-Chen 2004), thereby promoting survival in over-crowded, nutrient-deprived conditions (Golden & Riddle 1982). However, because MH terminates the reproductive capacity and life of the hermaphrodite, it is likely to be carefully regulated. One form of regulation that has been characterized is MH that occurs in response to nutrient deprivation (Chen & Caswell-Chen 2004). Fertile hermaphrodites that are shifted to nutrient deprived conditions will cease laying eggs and experience MH, and the final set of progeny will receive adequate nutrition to develop to the dauer larvae stage. This is likely to be an evolutionarily selected behavioral response; presumably it is more adaptive to sacrifice the hermaphrodite than continue to lay eggs into an environment that lacks nutrients. Here we document that age-related MH is the predominant cause of death in mated hermaphrodites. Although the percentage of mated versus self-fertile hermaphrodites in the wild is unknown, we speculate age-related MH may be adaptive because it promotes the survival of the final group of progeny while causing only a small reduction in the brood size.
Matricidal hatching as a model of myometrial degeneration
Much remains to be learned about muscular degeneration in the context of organ function. While MH may be primarily nematode-specific, the underlying causes of age-related muscle decline are relevant for many animals. For example, in humans the smooth muscle cells that line the uterus, the myometrium, contract in a coordinated fashion during labor and delivery. Furthermore, age-related degeneration of myometrial efficiency has been implicated in complications that result in high rates of cesarean sections in older mothers (Cleary-Goldman et al. 2005). Age-related MH in C. elegans has intriguing similarities to age-related decline of human myometrial efficiency and thus may serve as a useful model system. C. elegans vulval muscles and the myometrium are smooth muscles that are required to expel fertilized offspring, and reproduction after these muscles have degenerated is potentially lethal. The results described here provide evidence that C. elegans MH may be a model of human reproductive complications, and understanding age-related degenerative events with catastrophic consequences will be of great interest to C. elegans and human biology.
Experimental Procedures
Nematode Methods
C. elegans were maintained as described previously (Brenner 1974). Worms were cultured at 20°C on 6cm dishes with nematode growth medium (NGM) and a lawn of E. coli OP50. The wild-type strain (WT) was N2-Bristol (Brenner 1974), and the following mutations were used: daf-16(mu86) I (Ogg et al. 1997); age-1(hx546) II (Friedman & Johnson 1988); eat-2(ad465) II (Avery 1993); tph-1(mg280) II (Sze et al. 2000); clk-1(qm30) III (Lakowski & Hekimi 1996); daf-2(e1370) III (Kimura et al. 1997); sma-2(e502) III (Savage et al. 1996); sma-3(e491) III (Savage et al. 1996); isp-1(qm150) IV (Feng et al. 2001); fog-2(q71) V (Clifford et al. 2000); and eat-13(ad522) X (Avery 1993).
Life Span Assays
One L4-stage hermaphrodite was placed on a dish with no males (self-fertile) or three WT L4 or young adult males (mated). After 24 hours, the hermaphrodite was transferred to a fresh dish lacking males (1 day mating) or containing 3 new WT L4 or young adult males (2 or 3 day mating). After 24 hours, the hermaphrodite was transferred to a fresh dish lacking males (2 day mating) or containing 3 new WT L4 or young adult males (3 day mating). Hermaphrodites were transferred to fresh dishes daily until the animal died or egg laying ceased. We defined the L4 stage as day 1. We monitored male cross progeny of mated hermaphrodites, and animals that generated only self progeny or switched from cross progeny to self progeny were excluded from the analysis.
For experiments with fog-2(q71) females, one L4-stage female was placed on a dish with three WT L4 or young adult males for 24 hours (mated Day 1) or with no males. Females were transferred to fresh dishes daily until the animal died or egg laying ceased. On days 3 and 5, unmated fog-2(q71) females were individually transferred to a fresh dish with 3 WT L4 or young adult males for 24 hours (mated Day 3 or Day 5, respectively).
Phenotypes were determined by observation using a dissecting microscope. MH was defined by the presence of internally hatched progeny. Progeny production was measured by manual counting of live progeny present on the surface or edge of the dish 2-4 days after the hermaphrodite was removed.
Serotonin Response Assays
On day 1, four L4-stage hermaphrodites were placed on a dish with 12 WT L4 or young adult males. On day 2, mated hermaphrodites were pooled on a single plate. On days 4 and 6, surviving hermaphrodites were transferred to fresh dishes away from their progeny. On days 3, 5, and 7, egg-laying in response to serotonin was determined as previously described (Collins et al. 2008a).
Mitotracker Labeling and Mating Assays
Mitotracker labeling of males was adapted from Stanfield and Villeneuve (2006). Mitotracker-CMX Ros (Invitrogen) was dissolved in DMSO, diluted in 1X phosphate-buffered saline (137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 2mM KH2PO4, pH 7.4), applied to NGM dishes with a lawn of E. coli OP50 to a final concentration of 400nM and allowed to dry. WT L4 or young adult males were placed on Mitotracker dishes and incubated in the dark at 20°C overnight. The next day, males were transferred to a standard NGM dish for 5min to remove excess label. Twelve Mitotracker-labeled males and 4 unlabeled, young-adult hermaphrodites were then transferred to a standard NGM dish. Hermaphrodites were monitored hourly for the presence of Mitotracker-labeled sperm in the spermatheca or uterus—an indicator of a successful mating event—by observation using an Olympus SZX12 fluorescent dissecting microscope. Mitotracker-positive hermaphrodites were transferred to a fresh dish and monitored as described above.
Statistical Analyses
Hermaphrodites or females that were sterile or died due to desiccation on the side of the dish or extrusion of intestines through the vulva (rupture) were excluded from the final analysis (Huang et al. 2004; Hughes et al. 2007). Additionally, experiments that ended with fewer than ten animals were excluded to avoid possible skewing of the dataset from the small sample size. For the calculation of average progeny production, only animals that were generating progeny were included in the calculations. Animals that had ceased generating progeny were omitted.
The mortality rate was calculated with the equation qj=dj/(nj-1) where j indicates the day, qj is the mortality on a given day, dj is the number of dead on day j, and nj-1 indicates the number of live animals on the previous day. The natural log of this value was determined for graphing purposes.
Student t-tests were used for pairwise comparisons unless otherwise noted.
Supplementary Material
Figure S1: Scatter plot of day 1-5 cumulative progeny production versus matricidal life span of individual mated WT hermaphrodites. Spearman rank correlation analysis showed a small positive correlation (rS = 0.15) that was not statistically significant (p = 0.18).
Figure S2: Mutations that extend life span but do not affect the timing of matricidal hatching. WT and mutant hermaphrodites were mated to WT males. Matricidal life spans, daily average progeny production and the rate of MH per progeny generated for A), D) G) daf-2(e1370), age-1(hx546), and daf-16(mu86); B), E) H) clk-1(qm30) and isp-1(qm150); and C), F) I) sma-2(e502) and sma-3(e491). See Table 2 for summary statistics and N values.
Figure S3: Matricidal hatching is delayed in eat-13(ad522) hermaphrodites. WT and eat-13 hermaphrodites were mated to WT males. A) Daily average progeny production; B) matricidal life spans; and C) rate of MH per progeny generated. See Table 2 for summary statistics and N values.
Acknowledgments
Strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. We thank Tim Schedl and members of the Kornfeld lab for providing critical comments on the manuscript. Support was provided by the National Science Foundation (IOBO446240), the National Institutes of Health (RO1AG02656101A1), and a Senior Scholar Award from the Ellison Medical Foundation to K.K. The authors report they have no conflict of interests.
Footnotes
Author Contributions: C.L.P. designed research, performed research, and analyzed data. C.L.P. and K.K. and wrote the paper.
Supporting Information: Supplemental dataset accounting for all animals
References
- Andux S, Ellis RE. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008;4:e1000295. doi: 10.1371/journal.pgen.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MM, Garcia LR. Male mating behavior. Wormbook. 2006:1–11. doi: 10.1895/wormbook.1.78.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Caswell-Chen EP. Facultative Vivipary is a Life-History Trait in Caenorhabditis elegans. J Nematol. 2004;36:107–113. [PMC free article] [PubMed] [Google Scholar]
- Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Saade GR, Eddleman KA, Klugman S, Dugoff L, Timor-Tritsch IE, Craigo SD, Carr SR, Wolfe HM, Bianchi DW, D'Alton M. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105:983–990. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- Clifford R, Lee MH, Nayak S, Ohmachi M, Giorgini F, Schedl T. FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development. 2000;127:5265–5276. doi: 10.1242/dev.127.24.5265. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Evason K, Pickett CL, Schneider DL, Kornfeld K. The anticonvulsant ethosuximide disrupts sensory function to extend C. elegans lifespan. PLoS Genet. 2008a;4:e1000230. doi: 10.1371/journal.pgen.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, Huang C, Hughes S, Kornfeld K. The measurement and analysis of age-related changes in Caenorhabditis elegans. Wormbook. 2008b:1–21. doi: 10.1895/wormbook.1.137.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Riddle DL. Longevity in Caenorhabiditis elegans reduced by mating by not gamete production. Nature. 1996;379:723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Greenstein D. Control of oocyte meiotic maturation and fertilization. Wormbook. 2005:1–12. doi: 10.1895/wormbook.1.53.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartge P. Genetics of reproductive lifespan. Nat Genet. 2009;41:637–638. doi: 10.1038/ng0609-637. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Nat Acad Sci. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnigk SA, Ehlers RU. Endotokia matricida in hermaphrodites of Heterorhabditis spp. and the effect of the food supply. Nematology. 1999;1:717–726. [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Cremer T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum Genet. 1982;60:101–121. doi: 10.1007/BF00569695. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science. 1996;272:1010–1013. doi: 10.1126/science.272.5264.1010. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Nat Acad Sci. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc M, Taylor DP, Netscher C. On Endotokia Matricida and Inta-Uterine Development and Hatching of Nematodes. Nematologica. 1979;25:268–274. [Google Scholar]
- Luo S, Shaw WM, Ashraf J, Murphy CT. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009;5:e1000789. doi: 10.1371/journal.pgen.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong LT, Platzer EG, De Ley P, Thomas WK. Morphological, molecular and biological characterization of Mehdinema alii (Nematoda: Diplogasterida) from the decorated cricket (Gryllodes sigillatus) J Parasitol. 1999;85:1053–1064. [PubMed] [Google Scholar]
- McCulloch D, Gems D. Evolution of male longevity bias in nematodes. Aging Cell. 2003;2:165–173. doi: 10.1046/j.1474-9728.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Mendenhall AR, Wu D, Park SK, Cypser JR, Tedesco PM, Link CD, Phillips PC, Johnson TE. Genetic dissection of late-life fertility in Caenorhabditis elegans. J Gerontol. 2011;66:842–854. doi: 10.1093/gerona/glr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Savage C, Das P, Finelli AL, Townsend SR, Sun CY, Baird SE, Padgett RW. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Nat Acad Sci. 1996;93:790–794. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer WR. Egg-laying. Wormbook. 2005:1–7. doi: 10.1895/wormbook.1.38.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17:1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield GM, Villeneuve AM. Regulation of sperm activation by SWM-1 is required for reproductive success of C. elegans males. Curr Biol. 2006;16:252–263. doi: 10.1016/j.cub.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chung SH, Fang-Yen C, Craig C, Kerr RA, Suzuki H, Samuel AD, Mazur E, Schafer WR. A self-regulating feed-forward circuit controlling C. elegans egg-laying behavior. Curr Biol. 2008;18:1445–1455. doi: 10.1016/j.cub.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Scatter plot of day 1-5 cumulative progeny production versus matricidal life span of individual mated WT hermaphrodites. Spearman rank correlation analysis showed a small positive correlation (rS = 0.15) that was not statistically significant (p = 0.18).
Figure S2: Mutations that extend life span but do not affect the timing of matricidal hatching. WT and mutant hermaphrodites were mated to WT males. Matricidal life spans, daily average progeny production and the rate of MH per progeny generated for A), D) G) daf-2(e1370), age-1(hx546), and daf-16(mu86); B), E) H) clk-1(qm30) and isp-1(qm150); and C), F) I) sma-2(e502) and sma-3(e491). See Table 2 for summary statistics and N values.
Figure S3: Matricidal hatching is delayed in eat-13(ad522) hermaphrodites. WT and eat-13 hermaphrodites were mated to WT males. A) Daily average progeny production; B) matricidal life spans; and C) rate of MH per progeny generated. See Table 2 for summary statistics and N values.