Abstract
Faces convey a multitude of information in social interaction, among which are trustworthiness and attractiveness. Humans process and evaluate these two dimensions very quickly due to their great adaptive importance. Trustworthiness evaluation is crucial for modulating behavior toward strangers; attractiveness evaluation is a crucial factor for mate selection, possibly providing cues for reproductive success. As both dimensions rapidly guide social behavior, this study tests the hypothesis that both judgments may be subserved by overlapping brain networks. To this end, we conducted an activation likelihood estimation meta-analysis on 16 functional magnetic resonance imaging studies pertaining to facial judgments of trustworthiness and attractiveness. Throughout combined, individual, and conjunction analyses on those two facial judgments, we observed consistent maxima in the amygdala which corroborates our initial hypothesis. This finding supports the contemporary paradigm shift extending the amygdala’s role from dominantly processing negative emotional stimuli to processing socially relevant ones. We speculate that the amygdala filters sensory information with evolutionarily conserved relevance. Our data suggest that such a role includes not only “fight-or-flight” decisions but also social behaviors with longer term pay-off schedules, e.g., trustworthiness and attractiveness evaluation.
Keywords: fMRI, Meta-analysis, Attractiveness, Trustworthiness, Amygdala
Introduction
The social brain hypothesis states that the emergence of computationally more powerful brains cannot be explained solely by the ensuing advantage in solving problems posed by the physical environment. Rather, the advantage of being able to form, and successfully cope with, increasingly complex social systems may represent a key factor driving the evolution of mammalian brains (Dunbar 1998; Byrne and Whiten 1988; Dunbar and Shultz 2007b). It has been hypothesized that human ancestors were more likely to be killed by conspecifics than by individuals of other species (Wrangham and Peterson 1996; De Waal 2005; Öhman 2009). This prompted the hypothesis that being able to infer the states of mind in members of the own species to predict forthcoming behavior represented a distinct evolutionary advantage (Frith and Frith 2010). Darwin (1872) pioneered the idea that facial expressions of primates represent biologically evolved adaptations to sending social information. The human facial musculature thus seems to have coevolved with the corresponding decoding machinery in the brain and behavioral responses (Dimberg and Öhman 1996). Hence, being able to efficiently process socially relevant information is an optimal adaptation to complex social systems. Indeed, it has been shown that facial judgments may be processed in less than 100 ms (Bar et al. 2006; Willis and Todorov 2006).
Trustworthiness and attractiveness in a face convey particularly pivotal social information. Assessing facial trustworthiness is decisive because trusting an untrustworthy individual could have severe negative consequences, whereas not trusting a trustworthy one means a missed opportunity for cooperation (Cosmides and Tooby 1992, 2000). Assessing attractiveness is relevant for estimating the reproductive fitness of a potential mating partner, a critical factor when considering a prospective long-term commitment (Dunbar and Dunbar 1980; Pawlowski and Dunbar 1999; Schillaci 2006), which is a particularly risky and demanding social decision (Dunbar and Shultz 2007a). Moreover, the fact that humans appear to have universal standards of facial attractiveness within and across cultures (Cunningham et al. 1995; Perrett et al. 1994) implies a biologically encoded mechanism rather than an effect of enculturation (Thornhill and Gangestad 1999).
Mutual trust forms the basis for engagement in cooperation which is integral to daily life (Rilling et al. 2002) and a prerequisite for cultural and social evolution. Assessing an individual’s trustworthiness might be related to a broader categorization into ‘good guy/bad guy’ (Todorov 2008), guiding approach versus avoidance behavior (Chen and Bargh 1999; Cosmides and Tooby 2000). An overestimation of others’ approachability has been reported in cases of bilateral amygdala (AM) lesion in monkeys (Aggleton and Passingham 1981; Emery et al. 2001; Amaral 2003) and humans (Adolphs et al. 1998). Together with hyperorality and hypersexuality, this became known as the Klüver–Bucy syndrome (Devinsky et al. 2009; Klüver and Bucy 1939). Correlations between facial trustworthiness and various other facial judgments, e.g., how caring, happy or dominant a person is (Todorov et al. 2008b; Adolphs 2002; Todorov and Duchaine 2008), indicate that trustworthiness judgments may summarize numerous derived trait inferences. It is highly unlikely, though, that emotion alone drives the decision on trustworthiness because the latter social judgment has been shown to be separable from the effects of facial emotion (Winston et al. 2002; Adolphs 2002).
Besides trust, attractiveness deeply shapes the way we behave toward other people. For instance, attractive people are more likely to receive favorable treatment from others, earn higher salaries, and are expected to have better personality traits (Langlois et al. 2000). The ‘attractiveness halo effect’ describes this advantageous overgeneralization of beauty to an individual’s personality (Zebrowitz and Montepare 2008). This may be a consequence of the Darwinian mate value perceived in attractive humans, motivating positively biased behavior in social interaction (Cloutier et al. 2008). In fact, activation in the nucleus accumbens (NA) was demonstrated in heterosexual males in response to beautiful female, but not male, faces suggesting a dissociation between the esthetic and (sexual) reward assessment (Aharon et al. 2001; Franklin and Adams 2010). In sum, attractiveness evaluation may not be a subjective judgment of esthetics, such as that regarding objects, but rather might reflect a purposeful social-evolutionary adaptation of human behavior and brain circuits.
It is possible that both trustworthiness and attractiveness appraisal of others’ faces could be interwoven with the brain’s reward circuitry, including the NA (Walter et al. 2005). That is, reward mechanisms may not only modulate behavior toward basic survival needs, such as food and sex, but also toward salient social cues (cf. Kampe et al. 2001; Cardinal et al. 2002; Schilbach et al. 2010). Trusting behavior has been studied in dyadic cooperation using economic games. This repeatedly showed that activation in the reward system seems to reflect the social relevance of encountered cooperators and cooperative interaction (Rilling et al. 2002; Decety et al. 2004; Singer et al. 2004). The literature on attractiveness judgments, on the other hand, directly related the appraisal of attractive faces to increased reward processing (Aharon et al. 2001; Bray and O’Doherty 2007; Kranz and Ishai 2006). Therefore, we expect that a quantitative meta-analysis on trustworthiness and attractiveness judgments likely reveals the NA as a point of convergence. Confirming this hypothesis would provide another argument that the reward circuitry does, indeed, bridge the investigated two social judgments, and thus perhaps, also links social judgments on faces with motivation and behavioral relevance.
Trustworthiness and attractiveness ratings are positively correlated (Todorov et al. 2008b). That is, attractive people are likely to be evaluated as trustworthy, and vice versa. As far as we know, however, no neuroimaging study has conjointly investigated trustworthiness and attractiveness judgments yet. Consequently, the present paper tests whether the reported psychological relation can be extended to a neural relation between both judgments by pooling results of many individual functional magnetic resonance imaging (fMRI) experiments. The scarcity of neuroimaging research on other social judgments, such as intelligence or self-confidence, currently precludes comparison with a third complex evaluation. Note that neurological patient studies recently demonstrated that trustworthiness and attractiveness judgments may be selectively impaired by lesions involving the posterior superior temporal sulcus (pSTS) and fusiform face area, respectively (Iaria et al. 2008; Todorov and Duchaine 2008). This dissociation in early face processing suggests that both judgments are to some degree differently implemented on the neuronal level, contrasting the many similarities.
Based on the evolutionary importance, social impact, and highly correlated choice preferences, the present study tested the hypothesis that common brain networks subserve facial judgments of trustworthiness and attractiveness. To this end, we conducted contrast and conjunction meta-analyses according to the activation likelihood estimation (ALE) algorithm on 16 functional magnetic resonance imaging (fMRI) studies. The resulting foci of activation were anatomically localized using probabilistic cytoarchitectonic maps.
Materials and methods
Data used for the meta-analysis
We searched the Pubmed database (http://www.pubmed.org) for PET and fMRI studies investigating the neural correlates of evaluating facial trustworthiness and attractiveness. Both keyword searches (search strings: “attractiveness,” “attractive,” “beauty,” “beautiful,” “trust,” “trustworthiness,” “PET,” “fMRI”) and reference tracing were performed. Inclusion criteria comprised full brain coverage as well as absence of pharmacological manipulations, brain lesions or mental disorders. Additionally, studies were only considered if they reported results of whole-brain group analyses as coordinates corresponding to a standard reference space (Talairach/Tournoux, MNI). Accordingly, a number of neuroimaging studies, although within the thematic scope, were excluded from the present meta-analysis because of inseparability from unrelated psychological processes (Kirk et al. 2009), unobtainable coordinates (Kampe et al. 2001), analyses that were restricted to a priori defined regions of interest (Pinkham et al. 2008a, b; Rupp et al. 2009a; Aharon et al. 2001; Iaria et al. 2008; Ishai 2007; Liang et al. 2010) or lack of suitable experiments (Platek et al. 2009; Rupp et al. 2009b; Tsukiura and Cabeza 2010a, b; Roberts et al. 2008; Smith et al. 2010). The searches yielded a total of 16 eligible fMRI studies (7 on trustworthiness; 9 on attractiveness) with 43 experiments (18 on trustworthiness; 25 on attractiveness), assessing 390 subjects and reporting 268 foci of activity.
Experiments were divided into two main categories “Trustworthiness” (144 subjects, 18 experiments, 96 foci) and “Attractiveness” (246 subjects, 25 experiments, 172 foci). Additionally, all experiments were also divided into two categories “Implicit” (281 subjects, 18 experiments, 71 foci) and “Explicit” (262 subjects, 25 experiments, 197 foci) independent of the type of judgment to be made. If an experiment was performed with the subject knowing the target of investigation (facial judgments of trustworthiness or attractiveness), the experiment was classified as “Explicit”. If, on the other hand, the neurobiological response to the assessment of those two facial judgments was examined unknowingly to the subjects, the experiment was classified as “Implicit”. See Table 1 for details.
Table 1.
Overview of the 16 studies (43 experiments) included in the meta-analysis on judgments of facial trustworthiness and facial attractiveness
| Study | Category | Subjects | Mode | Instruction | Experiment | Rep. foci |
Stimuli | Evaluation |
|---|---|---|---|---|---|---|---|---|
| Baas et al. (2008) Neuroimage | Trustworthiness | 21 | fMRI | Indicate whether face is older/younger than 30 years, indicate whether face is trustworthy or untrustworthy | Task > rest | 16 | Neutral faces rated on trustworthiness and emotional valence | Explicit |
| (T-rest) AND (Age-rest) | 4 | |||||||
| T > A | 3 | |||||||
| T ~ BOLD | 3 | |||||||
| T ~ BOLD | 1 | |||||||
| Bray and O’Doherty (2007) | Attractiveness | 28 | fMRI | Press left/right button according to where fractal image appears (classical conditioning with face images) | Prediction error A > prediction error UA | 9 | Male/female attractive/unattractive faces shown simultaneously with abstract fractal images | Implicit |
| pC between A and prediction error | 4 | |||||||
| nC between A and prediction error | 1 | |||||||
| A > UA | 6 | |||||||
| Chatterjee et al. (2009) | Attractiveness | 13 | fMRI | Indicate whether face is more or less attractive than average | A > UA | 14 | Artificial male/female face pairs at different morph percentages created by GenHead | Explicit |
| Indicate whether two faces are identical | A > UA | 3 | Implicit | |||||
| Cloutier et al. (2008) | Attractiveness | 48 | fMRI | Indicate attractiveness level from 1 to 4 | A > UA | 19 | Unfamiliar faces representing a range of attractiveness | Explicit |
| UA > A | 8 | |||||||
| Engell et al. (2007) | Trustworthiness | 16 | fMRI | Report whether the identity of the test face was shown in a previous block | C with T consensus rating | 11 | Emotionally neutral faces rated for T | Implicit |
| C with T idiosyncratic rating | 1 | |||||||
| Kawabata et al. (2008) J Neurophysiol | Attractiveness | 18 | fMRI | Indicate desirability from 1 to 10 | Face > non-face | 8 | Various faces, activities and objects | Explicit |
| Kim et al. (2007) | Attractiveness | 28 | fMRI | Indicate “preferred face” | Preferred > not preferred | 8 | Face pairs created by FaceGen | Explicit |
| Decide which face is rounder | Preferred > not preferred | 2 | Implicit | |||||
| Kranz and Ishai (2006) | Attractiveness | 40 | fMRI | Indicate whether face is attractive, neutral or unattractive | Face > rest | 21 | Male/female faces for evaluation, additional viewing of unfamiliar/famous/emotional faces | Explicit |
| O’Doherty et al. (2003) | Attractiveness | 25 | fMRI | Indicate gender | A > UA | 5 | Single male/female faces rated for attractiveness (1–7) | Implicit |
| UA > A | 4 | |||||||
| A ~ face gender | 1 | |||||||
| Subject gender differences in response to opposite-sex A | 3 | |||||||
| A ~ Happiness | 1 | |||||||
| Own A ratings ~ BOLD | 1 | |||||||
| Said et al. (2009) | Trustworthiness | 32 | fMRI | Rate faces on trustworthiness from 1 to 4 | T > UT (linear) | 4 | Male/female composite faces rated on trustworthiness as LSF (low spatial frequency), BSF (broad spatial frequency) and HSF (high spatial frequency) version | Explicit |
| UT > T (linear) | 2 | |||||||
| T > UT (quadratic) | 6 | |||||||
| Todorov et al. (2008a) | Trustworthiness | 14 | fMRI | Report whether the identity of the test face was as any of the faces in previously shown block | T > UT | 5 | Trustworthy and untrustworthy faces | Implicit |
| Todorov and Engell (2008) Soc Cogn Affect Neurosci | Trustworthiness | 15 | fMRI | Report whether the identity of the test face was as any of the faces in previously shown block | Faces > rest | 8 | Standardized faces with neutral expression | Implicit |
| Turk et al. (2004) Neuroimage | Attractiveness | 18 | fMRI | Select same-sex face, select face they would like to go for a dinner date | Same-sex face > viewing facese | 8 | Same-sex and opposite-sex faces | Explicit |
| Opposite-sex face > viewing faces | 9 | |||||||
| AND conjunction of the two above | 6 | |||||||
| Opposite-sex face > same-sex face | 7 | |||||||
| Same-sex face > opposite-sex face | 2 | |||||||
| Verosky and Todorov (2009) Neuroimage | Trustworthiness | 30 | fMRI | Indicate whether face looks more like one-self or more like some other person | Self (quadratic) ~ T | 1 | Artificial male/female (un-)trustworthy faces morphed with participants photo by FaceGen | Implicit |
| Winston et al. (2002) | Trustworthiness | 16 | fMRI | Indicate whether high school or university student, indicate whether trustworthy or untrustworthy | Explicit T > implicit T | 11 | Neutral face images at different levels of trustworthiness | Explicit |
| Implicit T > explicit T | 2 | |||||||
| UT > T | 14 | |||||||
| T > UT | 2 | |||||||
| T ~ task (without effects from facial emotion) | 2 | |||||||
| Winston et al. (2007) | Attractiveness | 28 | fMRI | Indicate level of attractiveness from 1 to 3 in one task, indicate age as young, medium or old in a second task | A > age | 16 | Single male/female faces rated for attractiveness (1–7) | Explicit |
| A ~ task | 5 | Implicit |
A attractive faces/judgments of facial attractiveness, UA unattractive faces/judgments of facial unattractiveness, T trustworthy faces/judgments of facial trustworthiness, UT untrustworthy faces/judgments of facial untrustworthiness, BOLD relative change in BOLD response, pC/nC positive/negative correlation, ~: interaction
Our goal was to examine the core network of each judgment in an unbiased fashion. Therefore, we aimed at the consideration of heterogeneous experiments, such as contrasts looking at differently pre-rated stimulus material, correlations between psychological traits and the BOLD signal, as well as explicit task-driven and implicit stimulus-driven experiments.
Methodological foundation of the meta-analysis algorithm
The reported coordinates were analyzed for topographic convergence using the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff et al. 2009; Turkeltaub et al. 2002; Laird et al. 2009a). The goal of coordinate-based meta-analysis of neuroimaging data is to identify brain areas in which the reported foci of activation converge across different published experiments. To this end, the meta-analysis determines if the clustering is higher than expected under the null distribution of a random spatial association of results from the considered experiments while acknowledging the spatial uncertainty associated with neuroimaging foci.
As the first step, reported foci were interpreted as centers for 3D Gaussian probability distributions that capture the spatial uncertainty associated with each focus. This uncertainty is mostly a function of between-subject (due to small sample sizes) and between-template variance (attributable to different normalization strategies and templates across laboratories). Defining this uncertainty was previously the major drawback of ALE algorithms, since the size of the modeled Gaussian distribution was set subjectively by the investigator (Laird et al. 2005). This drawback has been overcome in the revised implementation of the algorithm, based on empirical estimates of between-subject variability and gauging the between-subject variance by the number of examined subjects (Eickhoff et al. 2009). The between-template variance was assessed in an empirical fashion based on nine common normalization approaches (Eickhoff et al. 2009) leading to an estimation of uncertainty attributable to differences in normalization algorithms between neuroimaging laboratories.
In a second step, the probabilities of all activation foci in a certain experiment were combined for each voxel, yielding a modeled activation map (MA map). Voxel-wise ALE scores resulted from the union across these MA maps that delineated the convergence across experiments at each particular location.
The third and last step distinguished between random and ‘true’ convergence by comparing the ensuing ALE scores against an empirical null distribution reflecting a random spatial association between the experiments’ MA maps. The within-experiment distribution of foci, however, was regarded to be fixed (Eickhoff et al. 2009; Laird et al. 2009b). Thus, a random-effects inference was invoked, focusing on the above-chance convergence between different experiments (Eickhoff et al. 2009; Caspers et al. 2010; Kurth et al. 2010). The resulting ALE scores were tested against the earlier calculated ‘true’ ALE scores and cut-off at a cluster-level-corrected threshold of p < 0.05.
Additional conjunction and difference analyses were conducted to explore how different meta-analyses relate to each other. Conjunction analyses testing for convergence between different meta-analyses employed inference by the minimum statistic, i.e., computing intersection of the thresholded Z-maps (Caspers et al. 2010). Difference analyses calculated the difference between corresponding voxels’ ALE scores for two sets of experiments. Then, the experiments contributing to either analysis were pooled and randomly divided into two analogous sets of experiments. Voxel-wise ALE scores for these two sets were calculated and subtracted from each other. Repeating this process 10,000 times yielded a null distribution of recorded differences in ALE scores between two sets of experiments. The ‘true’ difference in ALE scores was then tested against these differences obtained under the null distribution yielding voxel-wise p values for the difference. These resulting non-parametric p values were thresholded at p < 0.001.
For cluster level correction, the statistical image of uncorrected voxel-wise p values was first cut off by the cluster-forming threshold. Then, the size of the suprathreshold clusters was compared against a null distribution of cluster sizes derived from simulating 1,000 datasets with the same properties (number of foci, uncertainty, etc.) as the original experiments but random location of foci. The p value associated with each cluster was then given by the proportion of clusters arising from randomly generated pseudo-experiments.
Applying the ALE algorithm to selected studies
To examine the main effect of facial assessment, we determined brain areas with consistent activation across all studies on judgments of facial trustworthiness and facial attractiveness considered together. The judgment-specific convergence of results from experiments on trustworthiness or attractiveness, respectively, was subsequently delineated by separate ALE analyses. We then carried out a conjunction analysis across the results for trustworthiness and attractiveness judgments to determine the intersecting neurobiological correlates of both facial judgments. Finally, to investigate significant differences in the consistent activity patterns of trustworthiness judgments and attractiveness judgments, we conducted a difference analysis with the separate analyses of both facial judgments. As evidence points to an efficient and automatic assessment of others’ facial trustworthiness and facial attractiveness, we were also interested in characterizing brain activity in automatic implicit facial judgments as opposed to facial judgments by explicit demand. Accordingly, we conducted separate ALE analyses, conjunction analysis, and difference analyses on the same experiment pool divided into the Implicit and Explicit categories. For all results, the significance threshold was set at p < 0.05, corrected for multiple comparisons at the cluster level.
Conducting a valid ALE meta-analysis critically depends on choosing the appropriate input. As to this study, for instance, one might object that any maximum revealed in a brain area related to face processing may just result from all input experiments employing facial stimuli. It should be noted, though, that the majority of the incorporated contrasts were derived from subtraction between a target conditions and a high-level control condition, which excluded a priori the basal activity of the neural face-processing circuitry (see Table 1 for details). Hence, converging activity should be specifically related to the assessment of facial trustworthiness and attractiveness rather than face processing per se.
The anatomical localizations were obtained using the SPM Anatomy Toolbox (Eickhoff et al. 2005a, 2007). By means of a maximum probability map (MPM), activations were assigned to the most likely cytoarchitectonic area. Those maps are based on earlier studies about cytoarchitecture, intersubject variability as well as quantitatively defined borders of the areas. The cytoarchitectonic map of the amygdala (Amunts et al. 2005) is the most important one for the current analysis.
Results
ALE analyses on facial judgments of trustworthiness and attractiveness
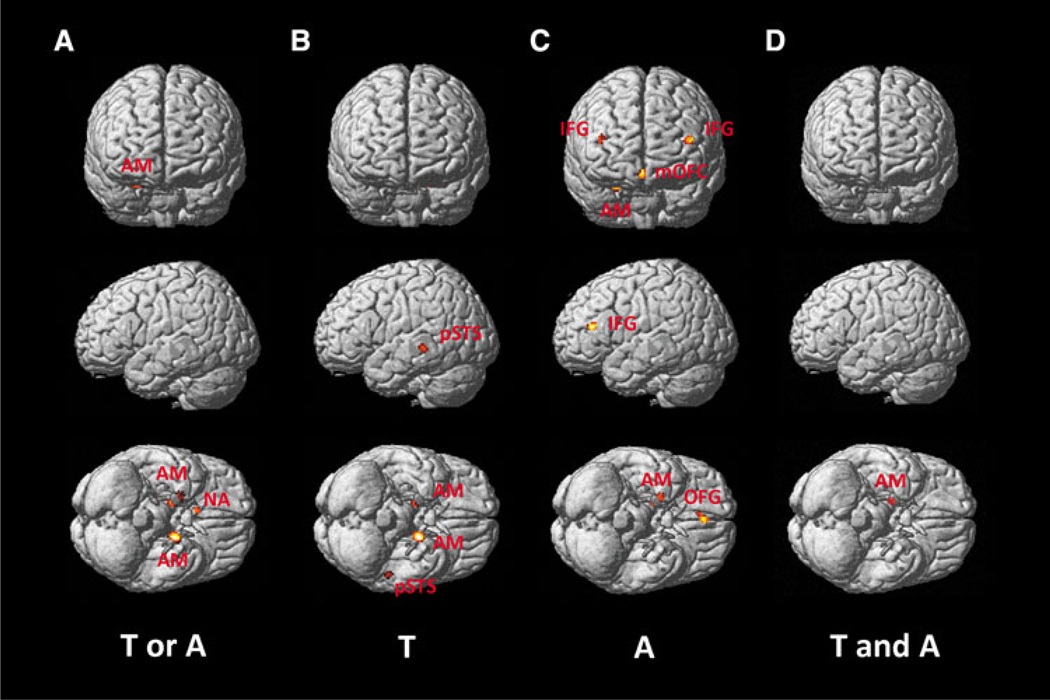
Brain activity converging across both trustworthiness and attractiveness judgments was found (cf. Fig. 1a; Table 2) in the bilateral amygdala and right NA. Judgments of facial trustworthiness consistently evoked activation in the right and left AM, as well as left pSTS (Fig. 1b; Table 2). For judgments of attractiveness, the analysis revealed converging activation across studies in the right AM, right OFC, as well as left and right IFG (Fig. 1c; Table 2). The conjunction analysis across judgments of trustworthiness and attractiveness revealed an overlap of activity in the right AM (Fig. 1d; Table 2).
Fig. 1.
Significant meta-analysis results displayed on the frontal, left and bottom surface view of the MNI single subject template for a the main effect of facial assessment, b facial judgments of trustworthiness, c facial judgments of attractiveness, and d conjunction analysis of both facial judgments
Table 2.
Peaks of activations for the main effect of facial assessment, analyses on the “Trustworthiness” and “Attractiveness” categories, as well as conjunction and difference analyses between these two categories
| Macroanatomical location | Cytoarchitectonic location | MNI coordinates | ||
|---|---|---|---|---|
| x | y | z | ||
| Trustworthiness OR Attractiveness | ||||
| R amygdala | Amygdala (LB, 60%) | 26 | 0 | −22 |
| R amygdala | Amygdala (SF, 50%) | 18 | −8 | −14 |
| L amygdala | Amygdala (SF, 100%) | −18 | −6 | −18 |
| R nucleus accumbens | – | 10 | 16 | −2 |
| Trustworthiness | ||||
| R amygdala | Amygdala (SF, 50%) | 18 | −8 | −12 |
| L amygdala | Amygdala (SF, 100%) | −18 | −6 | −18 |
| L posterior superior temporal sulcus | – | −54 | −34 | −4 |
| Attractiveness | ||||
| R amygdala | Amygdala (LB, 60%) | 26 | 0 | −22 |
| R amygdala | Amygdala (SF, 80%) | 20 | −6 | −16 |
| R medial orbitofrontal cortex | – | 2 | 42 | −14 |
| R inferior frontal gyrus | – | 38 | 28 | 18 |
| L inferior frontal gyrus | – | −38 | 42 | −14 |
| Trustworthiness AND Attractiveness | ||||
| R amygdala | Amygdala (SF, 60%) | 18 | −6 | −14 |
| Attractiveness–Trustworthiness | ||||
| – | – | – | – | – |
| Trustworthiness–Attractiveness | ||||
| – | – | – | – | – |
All peaks are assigned to the most probable brain areas as revealed by the SPM Anatomy Toolbox (Eickhoff et al. 2005b, 2007; Amunts et al. 2005; Geyer 2004)
Convergence in the NA was significant for the pooled experiments of the trustworthiness and attractiveness group, but not in either group. This indicates that only the combined foci of both judgments sufficed to survive statistical correction. Hence, this maximum was due to a roughly balanced contribution of foci from both experiment groups.
Contrasting the likelihood of activations in experiments assessing judgments of trustworthiness and attractiveness, respectively, yielded no significant regional effects (Table 2). That is, there was no region that was significantly more strongly associated with the trustworthiness, respectively, attractiveness task in the current set of studies.
ALE analyses on implicit and explicit facial judgments
Please note that the number of considered studies did not allow us to investigate the implicit and explicit categories in the individual pools of trustworthiness and attractiveness experiments. Implicit facial judgments, orthogonal to the trustworthiness and attractiveness categories, consistently evoked activation in the right and left AM, as well as right and left inferior frontal gyrus (IFG) (Table 3). Experiments pertaining to explicit facial judgments converged likewise in the right and left AM (Table 3). The conjunction analysis across implicit and explicit facial judgments revealed an overlap of activity in the left AM (Table 3). Contrasting the likelihood of activations in experiments concerning implicit or explicit facial judgments, respectively, showed two maxima in the right AM in the Implicit > Explicit contrast (Table 3). However, no significant regional effect was found in the Explicit > Implicit contrast.
Table 3.
Peaks of activations for the analyses on the “Implicit” and “Explicit” categories, as well as conjunction and difference analyses between these two categories
| Macroanatomical location |
Cytoarchitectonic location |
MNI coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Implicit | ||||
| R amygdala | Amygdala (LB, 60%) | 26 | 0 | −22 |
| L amygdala | Amygdala (SF, 100%) | −18 | −4 | −18 |
| R inferior frontal gyrus | – | 38 | 28 | 18 |
| L inferior frontal gyrus | – | −38 | 34 | 16 |
| Explicit | ||||
| R amygdala | Amygdala (SF, 50%) | 18 | −8 | −14 |
| L amygdala | Amygdala (SF, 80%) | −16 | −8 | −16 |
| Implicit and Explicit | ||||
| L amygdala | Amygdala (SF, 90%) | −16 | −6 | −18 |
| Implicit–Explicit | ||||
| R amygdala | Amygdala (LB, 50%) | 28 | 2 | −26 |
| R amygdala | Amygdala (LB, 50%) | 24 | −2 | −20 |
| Explicit–Implicit | ||||
| – | – | – | – | – |
All peaks are assigned to the most probable brain areas as revealed by the SPM Anatomy Toolbox (Eickhoff et al. 2005b, 2007; Amunts et al. 2005; Amunts et al. 1999)
Probabilistic anatomical labeling
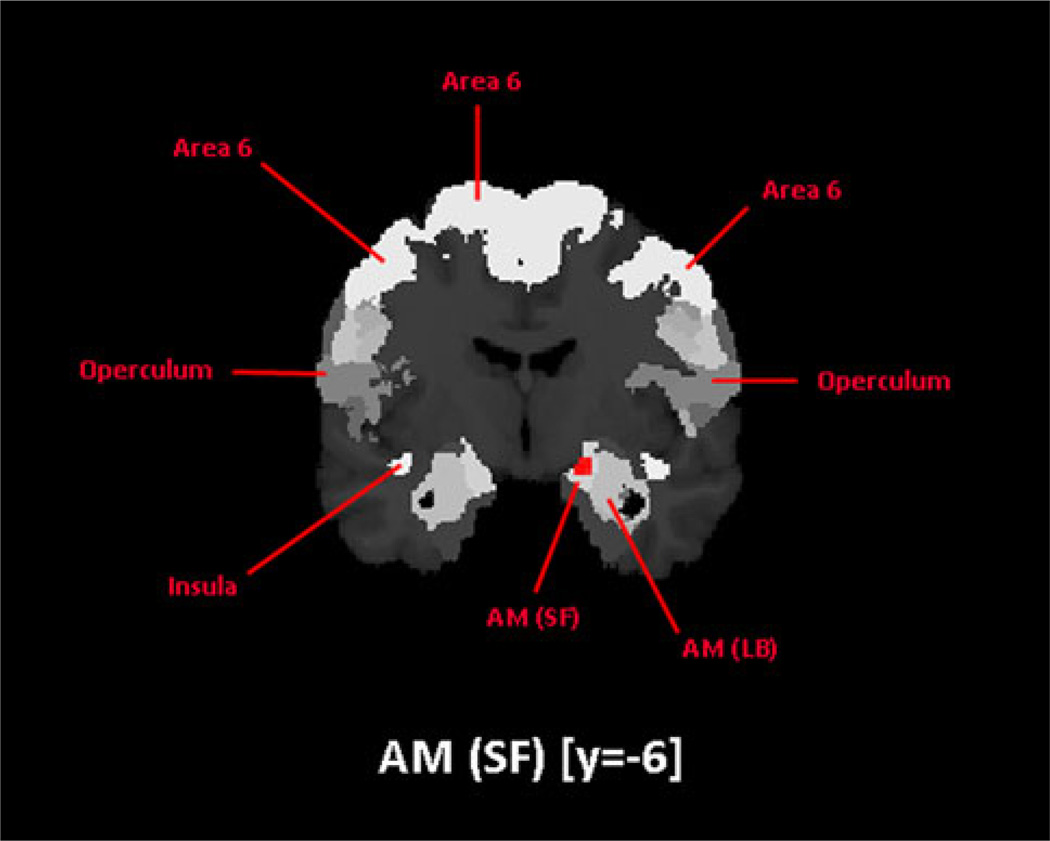
In all meta-analyses, we observed one consistent maximum in the amygdala. The maxima of activation from analyses of the Trustworthiness and Attractiveness categories was consistently assigned to the cytoarchitectonically defined (Amunts et al. 2005) superficial nuclei group (SF) of the amygdala (Fig. 2) using the SPM Anatomy Toolbox (Eickhoff et al. 2005a). Only the main effect of facial assessment and the meta-analysis on the attractiveness experiments showed an additional maximum that was assigned to the laterobasal nuclei group (LB) of the amygdala (Table 2). The maxima in the analyses of the Implicit and Explicit categories were also assigned to nuclei of the amygdala: LB and SF were identified as cytoarchitectonic loci in the separate analysis of the Implicit category, SF in the separate analysis of the Explicit category, SF in the conjunction analysis of the Implicit and Explicit categories, and LB in the Implicit > Explicit contrast.
Fig. 2.
One coronal sections through the T1-weighted MNI single subject template at y = −6 in anatomical MNI space. Using the SPM Anatomy toolbox, the resulting maxima in the amygdala have been mapped onto the superficial nuclei group across all analyses of the Trustworthiness and Attractiveness categories
Moreover, foci in the amygdala have been assigned probabilities between 50 and 100%, which increases the confidence of convergence in this region in the corresponding meta-analyses.
Discussion
Coordinate-based meta-analysis of functional neuroimaging data has recently emerged as a novel tool to condense the wealth of published neuroimaging experiments (for review, see Wager et al. 2007). Using the ALE meta-analysis approach on experiments related to judgments of facial trustworthiness and facial attractiveness, we consistently observed a convergence of reported activation foci in the amygdala across all analyses. This corroborates the hypothesis that these two socially and evolutionarily important judgments based on facial aspects may be sub-served by a common neural substrate. To the best of our knowledge, this is the first neuroimaging-based integration of trustworthiness and attractiveness evaluations and also the first summary of the emerging literature in the field of social judgments. We provide evidence that the behavioral correlation of both judgments is related to common neural correlates.
Judgments of facial trustworthiness
Our meta-analysis of experiments on judgments of facial trustworthiness yielded overlapping activation in the pSTS and AM. Activity in the pSTS is well in line with the putative role of this region in mentalizing about other’s likely intentions (Fletcher et al. 1995; Gallagher et al. 2000; Brothers 1990), a key factor governing approach decisions. On the other hand, the pSTS is also believed to specifically process unstable facial features, such as emotion (Allison et al. 2000). In the AM, facial features are apparently appraised with larger AM activation being evoked the more extreme (in either direction) a face is ranked on the trustworthiness scale, i.e., the higher this trait’s saliency (Said et al. 2009; Todorov et al. 2008a). Yet, untrustworthy faces elicit slightly stronger BOLD responses in the AM than equally trustworthy ones (Adolphs 2002; Winston et al. 2002; Pinkham et al. 2008b). Further, Singer (2004) and Rilling (2002) reported enhanced activation of the AM and reward circuitry during exchange with individuals previously experienced as fair versus unfair. The authors concluded that the social saliency of fair cooperators promotes mutual cooperation in human societies by inherent reward. This interpretation is consistent with our results, because the main effect of facial assessment included activation in the NA, an important node of the reward circuitry. Thus, the behavioral consequence of perceiving trustworthy versus untrustworthy faces appears to be influenced by the NA. Taken together, we demonstrate that the pSTS, AM, and NA seem to provide a core network for guiding behavior in trust and cooperation.
Judgments of facial attractiveness
The analysis of fMRI experiments on attractiveness judgments revealed converging activity in the medial orbitofrontal cortex (OFC), IFG, and AM. Facial beauty might be appraised in the OFC area according to reward value. IFG activation was implicated in semantic aspects of face processing (Chatterjee et al. 2009; Ishai et al. 2000, 2002; Leveroni et al. 2000). Paralleling facial trustworthiness evaluation, the AM strongly reacts not only to very attractive faces but also to very unattractive ones (Winston et al. 2007). Taken together, the AM generally responds to both trustworthiness and attractiveness evaluation processes leading to a convergence in this region.
The implication of the NA in attractiveness judgments was strengthened by the main effect of facial assessment. Neuroimaging research ascribes complex reward functions to the NA, such as the evaluation of reward expectancy in social, monetary, or drug rewards (Schultz et al. 1997; Rademacher et al. 2010; Kampe et al. 2001). However, a NA response to socially rewarding attractive faces was found only in some (Cloutier et al. 2008; Aharon et al. 2001; Kampe et al. 2001; Bray and O’Doherty 2007; Kim et al. 2007) but not all (O’Doherty et al. 2003; Winston et al. 2007; Chatterjee et al. 2009; Kranz and Ishai 2006) studies. Taking a broader perspective, overlapping activation in the NA in trustworthiness and attractiveness judgments, found in the main effect of facial assessment, suggests a general role of the reward circuitry in social judgments.
The amygdala in social judgments
The AM, OFC, and temporal poles have early been theorized to comprise the neural network for social information processing (Brothers 1990). The AM expanded significantly in size during primate evolution (Öhman 2009; Barton and Aggleton 2000), and consequently has a more complex anatomical structure than, e.g., the rodent AM (Barton and Aggleton 2000; Crosby and Humphrey 1944; Stephan et al. 1987; Amaral 2002). This evolvement has been conjectured to be closely related to the primate’s complex social environment (Amaral 2002). Needless to say, ALE meta-analysis cannot directly test AM’s specialization for information with evolutionary salience. However, we would like to stress that the demonstrated convergence of two complex social judgments in the AM resonates very well with the AM’s ensuing critical importance in social decisions making, earlier accounts on amygdalar enlargement and the evolution in the non-human primate societies. Nevertheless, research on the AM has been strongly driven by the importance of this structure for emotion processing; especially, emotionally negative stimuli such as fearful and threatening faces (Adolphs 1999; Morris et al. 1996; Phan et al. 2002; LeDoux 2000; Gallese et al. 2004). In contrast, the involvement of the AM in processing positive and appetitive sensory information, such as trustworthy and attractive faces, has been explored more slowly (Amaral 2002; Bonda et al. 1996; Schneider et al. 1997).
Although we here concentrate on facial cues given that most of the included studies used visual stimulation, amygdalar responses are known to extend to non-visual input modalities. Among others, this includes tastes (O’Doherty et al. 2001), odorants (Wicker et al. 2003), hearing one’s own name while asleep (Portas et al. 2000), laughing and crying sounds (Sander and Scheich 2001), as well as changing sound intensity (Bach et al. 2008). This is in line with the AM being involved in relevance detection regardless of the type of sensory input. Echoing its reaction to attractive and unattractive faces, the AM also responds to both pleasant and unpleasant music (Koelsch 2005; Ball et al. 2007). It thus seems that the AM detects the socio-emotional value (“beauty”) of sensory stimuli across visual and auditory input modalities.
The AM’s laterobasal nuclei group (LB) is probably a gatekeeper for visual, auditory, gustatory, and somatosensory information (Solano-Castiella et al. 2010). The AM’s superficial nuclei group (SF), however, was argued to be closely related to processing social communication (Goossens et al. 2009). This is supported by the SF’s involvement in olfaction-based intraspecies communication in lower non-primate animals (Moreno and Gonzalez 2007). Concurrent with the LB’s role as input channel, we found this nuclei group to be more active in bottom-up-driven, i.e. implicit, fMRI experiments. That is, LB activation was observed in the meta-analysis on implicit but not on explicit experiments. Additionally, the difference analysis between these two approaches suggests that the LB is more implicated in implicit experiments. Moreover, the specific overlap between trustworthiness and attractiveness judgments in the SF further attests to the importance of this part of the AM in deciphering social signals.
The low number of studies focusing on the processing of positive and social stimuli in the AM might be explained by the less obvious long-term outcome of some social decisions (Williams 2006). That is, positive trustworthiness evaluation encourages direct investment in cooperative social exchange, although this might amortize only years later. In a similar vein, the adaptive attractiveness evaluation appears to eventually aim at stable partnerships in which the work load of maintaining the family will be distributed fairly due to the partners’ genetic fitness. On the other hand, negatively valenced traits, untrustworthiness and unattractiveness, cause a more tangible direct avoidance response without long-term outcomes. We, therefore, extend earlier accounts on AM function by emphasizing that the AM might also mediate approach behavior that tends to only pay off in the long run.
A speculative evolutionary perspective
Disentangling ‘emotional’ and ‘social’ influences on the human brain and behavior might be so difficult because these concepts might poorly reflect reality as they overlap in the goal of obtaining a better grasp of our environment in order to increase pleasure, reduce pain, survive, and reproduce (Williams 2006). The AM may potentially be specialized to identify cues relevant to the human race for obtaining this goal. This would explain why so many ostensibly heterogeneous functions such as reward evaluation (Baxter and Murray 2002), conditioning (LaBar et al. 1998), emotion (Phillips et al. 2003), and attention (Whalen 1998) all intersect in this brain area. Consistently, the AM’s widespread anatomical connections predispose for these heterogeneous and interwoven functions (LeDoux 2000; Pessoa 2008).
Sander et al. (2003) thus advocated a broader view of the AM as a general significance detector. Ousdal et al. (2008) recently proposed that the AM might privilege sensory information that requires a behavioral response, in addition to sensory information with general self-relevance. In this context, it is not surprising that a large part of the AM’s detector role is devoted to social stimuli (Goossens et al. 2009), since accumulating evidence suggests social interaction as the prime selection pressure in primate evolution (Dunbar and Shultz 2007a). In line with such a paramount role of the AM in complex social behavior, our results confirm an important role of the AM in the appraisal of facial (un-)trustworthiness, a basis for whether or not to engage in cooperation, and of facial (un-) attractiveness, a basis for more stable pair bonds.
Given the AM’s rich anatomical connections and its apparent functional omnipresence in the modulation of social behavior (Adolphs 2010), one could infer that it facilitates those social behaviors that are most appropriate. This interpretation would be in keeping with several other findings. For instance, ratings on facial attractiveness are consistent across cultures (Cunningham et al. 1995), and mean post hoc ratings on facial trustworthiness across an entire group predict neural activation in the AM more accurately than individual ratings (Engell et al. 2007). These findings together with the demonstrated converging activation across both facial judgments point to an adaptation formed by the advantage in natural selection gained from judicious social behavior. This would even hold true if the AM responded solely to the valence in very (un-)trustworthy or (un-)attractive faces. Yet, the valence hypothesis has been repeatedly challenged as a global description of AM function (Ousdal et al. 2008; Cunningham et al. 2004; Herry et al. 2007; Bach et al. 2008; Schiller et al. 2009; Sergerie et al. 2008). For instance, the AM has been shown to respond to rising but hardly to falling sound intensities (Bach et al. 2008), questioning the notion that amygdalar activity is generally triggered by events “off normalcy”.
Methodological considerations
Several limitations of our results should be addressed. Importantly, the amount of studies included in our meta-analysis was limited, due to the rather recent development of research on the neurobiology of social judgments on faces. The majority of included studies were published since 2007 (see Table 1 for details). Nevertheless, all results reported here were statistically significant and survived correction for multiple comparisons at the cluster level.
Meta-analyses, however, are necessarily based on the available literature and may hence be affected by the potential publication-bias disfavoring null results (Rosenthal 1979). Furthermore, all neuroimaging studies and consequently meta-analyses thereof may be influenced by intersubject variability, e.g., personality traits (Simon et al. 2010), gender or genotype (Hariri and Holmes 2006). Randomization of these factors in sufficiently large samples is rare in neuroimaging research due to logistical challenges.
Since the ALE meta-analysis approach is based on the reported peak activations, a large part of spatial information necessarily needs to be discarded. Image-based meta-analysis overcomes this issue but the required full statistic image data of all eligible experiments are seldom available (Schilbach et al. 2008). Moreover, results of this method are in good agreement with coordinate-based meta-analysis approaches (Salimi-Khorshidi et al. 2009). This suggests that coordinate-based meta-analysis algorithms such as ALE are currently the most comprehensive approach for summarizing neuroimaging findings in a particular field.
Conclusion
Convergent findings across fMRI experiments on judgments of facial trustworthiness and facial attractiveness were analyzed using ALE meta-analysis. We observed a maximum of convergence in the AM across all analyses. We thus demonstrated, probably for the first time, that the known psychological commonalities of both judgments are backed up by a conjointly engaged neurobiological substrate, namely the right amygdala. These results also support the contemporary paradigm shift in the AM literature extending the conceptualized role of this region from detecting and evaluating negative to positive stimuli, from emotional to social stimuli and from specific to behaviorally relevant stimuli. We then went on to argue for the plausibility of this concept of AM function from an evolutionary perspective. Although the AM is traditionally viewed as a modulator of behavior in short-term fight-or-flight-like settings, we tentatively extent this account by suggesting an amygdalar influence on rather long-term social behavioral tendencies, such as social exchange and mate choice.
Acknowledgments
This study was supported by the German Research Council (DFG, IRTG 1328, KZ, SBE, DB), the Human Brain Project (R01-MH074457-01A1), and the Helmholtz Initiative on Systems-Biology “The Human Brain Model” (KZ, SBE).
Footnotes
Conflict of interest The authors declare no conflict of interest.
Contributor Information
D. Bzdok, Department of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany Institute of Neuroscience and Medicine (INM-2), Research Center Jülich, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany; International Research Training Group “Schizophrenia and Autism” (IRTG 1328), Aachen, Germany; Jülich-Aachen Research Alliance (JARA)-Translational Brain Medicine, Aachen, Germany.
R. Langner, Department of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany Institute of Neuroscience and Medicine (INM-2), Research Center Jülich, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany; Jülich-Aachen Research Alliance (JARA)-Translational Brain Medicine, Aachen, Germany.
S. Caspers, Institute of Neuroscience and Medicine (INM-2), Research Center Jülich, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany Jülich-Aachen Research Alliance (JARA)-Translational Brain Medicine, Aachen, Germany.
F. Kurth, Department of Psychiatry, Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine at University of California, Los Angeles, CA, USA
U. Habel, Department of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany International Research Training Group “Schizophrenia and Autism” (IRTG 1328), Aachen, Germany; Jülich-Aachen Research Alliance (JARA)-Translational Brain Medicine, Aachen, Germany.
K. Zilles, Institute of Neuroscience and Medicine (INM-2), Research Center Jülich, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany Jülich-Aachen Research Alliance (JARA)-Translational Brain Medicine, Aachen, Germany; C. & O. Vogt Institute of Brain Research, University of Düsseldorf, Düsseldorf, Germany.
A. Laird, Research Imaging Institute, University of Texas Health Sciences Center, San Antonio, TX, USA
Simon B. Eickhoff, Email: S.Eickhoff@fz-juelich.de, Department of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany; Institute of Neuroscience and Medicine (INM-2), Research Center Jülich, Forschungszentrum Jülich GmbH, 52425 Jülich, Germany; International Research Training Group “Schizophrenia and Autism” (IRTG 1328), Aachen, Germany; Jülich-Aachen Research Alliance (JARA)-Translational Brain Medicine, Aachen, Germany.
References
- Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3(12):469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Trust in the brain. Nat Neurosci. 2002;5(3):192–193. doi: 10.1038/nn0302-192. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) J Comp Physiol Psychol. 1981;95(6):961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4(7) doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51(1):11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210(5–6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Vink M, Ramsey NF, de Haan EH, Kahn RS. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. Neuroimage. 2008;40(2):719–727. doi: 10.1016/j.neuroimage.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Bach DR, Schachinger H, Neuhoff JG, Esposito F, Di Salle F, Lehmann C, Herdener M, Scheffler K, Seifritz E. Rising sound intensity: an intrinsic warning cue activating the amygdala. Cereb Cortex. 2008;18(1):145–150. doi: 10.1093/cercor/bhm040. [DOI] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One. 2007;2(3):e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6(2):269–278. doi: 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Barton RA, Aggleton JP. Primate evolution and the amygdala. In: Aggleton JP, editor. The amygdala: a functional analysis. New York: Oxford University Press; 2000. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci. 1996;16(11):3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, O’Doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. J Neurophysiol. 2007;97(4):3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Byrne RW, Whiten A, editors. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford: Oxford University Press; 1988. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Thomas A, Smith SE, Aguirre GK. The neural response to facial attractiveness. Neuropsychology. 2009;23(2):135–143. doi: 10.1037/a0014430. [DOI] [PubMed] [Google Scholar]
- Chen M, Bargh JA. Consequences of automatic evaluation: immediate behavioral predispositions to approach or avoid the stimulus. Pers Soc Psychol. 1999;B 25(2):215–224. [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci. 2008;20(6):941–951. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides L, Tooby J, editors. The adapted mind: evolutionary psychology and the generation of culture. London: Oxford University Press; 1992. Cognitive adaptations for social exchange. [Google Scholar]
- Cosmides L, Tooby J. The cognitive neuroscience of social reasoning. In: Gazzaniga MS, editor. The new cognitive neuroscience. Cambridge: MIT Press; 2000. pp. 1259–1276. [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon. III. The amygdaloid complex in the shrew (Blarina brevicauda) J Comp Neurol. 1944;81:285–305. [Google Scholar]
- Cunningham MR, Roberts AR, Wu CH, Barbee AP, Druen PB. Their ideas of beauty are, on the whole, the same as ours—consistency and variability in the cross-cultural perception of female physical attractiveness. J Pers Soc Psychol. 1995;68(2):261–279. [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15(12):806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. New York: Oxford University Press; 1872. [Google Scholar]
- De Waal F. Our inner ape. New York: Riverhead Books; 2005. [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23(2):744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky J, Sacks O, Devinsky O. Kluver–Bucy syndrome, hypersexuality, and the law. Neurocase. 2009:1–6. doi: 10.1080/13554790903329182. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Öhman A. Behold the wrath: psychophysiological responses to facial stimuli. Motiv Emotion. 1996;20(2):149–182. [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6(5):178–190. [Google Scholar]
- Dunbar RIM, Dunbar EP. Pairbond in klipspringer. Anim Behav. 1980;28:219–229. [Google Scholar]
- Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007a;317(5843):1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Understanding primate brain evolution. Philos Trans R Soc B Biol Sci. 2007b;362(1480):649–658. doi: 10.1098/rstb.2006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005a;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005b;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. J Cogn Neurosci. 2007;19(9):1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “Theory of mind” in story comprehension. Cognition. 1995;57(2):109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Franklin RG, Jr, Adams RB., Jr The two sides of beauty: laterality and the duality of facial attractiveness. Brain Cogn. 2010;72(2):300–305. doi: 10.1016/j.bandc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C. The social brain: allowing humans to boldly go where no other species has been. Philos Trans R Soc Lond B Biol Sci. 2010;365(1537):165–176. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol. 2004;174:I–VIII. 1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp. 2009;30(10):3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27(22):5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Fox CJ, Waite CT, Aharon I, Barton JJ. The contribution of the fusiform gyrus and superior temporal sulcus in processing facial attractiveness: neuropsychological and neuroimaging evidence. Neuroscience. 2008;155(2):409–422. doi: 10.1016/j.neuroscience.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol. 2007;63(2):181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28(3):979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Ishai A, Haxby JV, Ungerleider LG. Visual imagery of famous faces: effects of memory and attention revealed by fMRI. Neuroimage. 2002;17(4):1729–1741. doi: 10.1006/nimg.2002.1330. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413(6856):589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Zeki S. The neural correlates of desire. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Adolphs R, O’Doherty JP, Shimojo S. Temporal isolation of neural processes underlying face preference decisions. Proc Natl Acad Sci USA. 2007;104(46):18253–18258. doi: 10.1073/pnas.0703101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Skov M, Christensen MS, Nygaard N. Brain correlates of aesthetic expertise: a parametric fMRI study. Brain Cogn. 2009;69(2):306–315. doi: 10.1016/j.bandc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy P. Preliminary analysis of functions of the temporal lobes in man. Arch Neurol Psychiatry. 1939;42:979–1000. [Google Scholar]
- Koelsch S. Investigating emotion with music. Ann N Y Acad Sci. 2005;1060(1):412–418. doi: 10.1196/annals.1360.034. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Curr Biol. 2006;16(1):63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25(1):155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009a;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009b;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychol Bull. 2000;126(3):390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20(2):878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, Zhang Y. Neural activation in the “Reward circuit” shows a nonlinear response to facial attractiveness. Soc Neurosci. 2010;5(3):320–334. doi: 10.1080/17470911003619916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno N, Gonzalez A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J Anat. 2007;211(2):151–163. doi: 10.1111/j.1469-7580.2007.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Öhman A. Of snakes and faces: an evolutionary perspective on the psychology of fear. Scand J Psychol. 2009;50(6):543–552. doi: 10.1111/j.1467-9450.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging go–nogo task. Neuroscience. 2008;156(3):450–455. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski B, Dunbar RI. Impact of market value on human mate choice decisions. Proc Biol Sci. 1999;266(1416):281–285. doi: 10.1098/rspb.1999.0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, May KA, Yoshikawa S. Facial shape and judgements of female attractiveness. Nature. 1994;368(6468):239–242. doi: 10.1038/368239a0. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in pet and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008a;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008b;34(4):688–697. doi: 10.1093/schbul/sbn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Krill AL, Wilson B. Implicit trustworthiness ratings of self-resembling faces activate brain centers involved in reward. Neuropsychologia. 2009;47(1):289–293. doi: 10.1016/j.neuropsychologia.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous eeg and fMRI monitoring in humans. Neuron. 2000;28(3):991–999. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Roberts GM, Newell F, Simoes-Franklin C, Garavan H. Menstrual cycle phase modulates cognitive control over male but not female stimuli. Brain Res. 2008;1224:79–87. doi: 10.1016/j.brainres.2008.05.061. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural activation in the orbitofrontal cortex in response to male faces increases during the follicular phase. Horm Behav. 2009a;56(1):66–72. doi: 10.1016/j.yhbeh.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural activation in women in response to masculinized male faces: mediation by hormones and psychosexual factors. Evol Hum Behav. 2009b;30(1):1–10. doi: 10.1016/j.evolhumbehav.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. J Cogn Neurosci. 2009;21(3):519–528. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45(3):810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res. 2001;12(2):181–198. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “Default system” of the brain. Conscious Cogn. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci. 2010;22(12):2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schillaci MA. Sexual selection and the evolution of brain size in primates. PLoS One. 2006;1(1) doi: 10.1371/journal.pone.0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12(4):508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Schneider F, Grodd W, Weiss U, Klose U, Mayer KR, Nagele T, Gur RC. Functional MRI reveals left amygdala activation during emotion. Psychiatry Res Neuroimag. 1997;76(2–3):75–82. doi: 10.1016/s0925-4927(97)00063-2. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich HC, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49(2):1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41(4):653–662. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30(7):2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD, Turner R. Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage. 2010;49(4):2958–2965. doi: 10.1016/j.neuroimage.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Stephan H, Frahm HD, Baron G. Comparison of brain structure volumes in insectivora and primates. 7. Amygdaloid components. J Hirnforsch. 1987;28(5):571–584. [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW. Facial attractiveness. Trends Cogn Sci. 1999;3(12):452–460. doi: 10.1016/s1364-6613(99)01403-5. [DOI] [PubMed] [Google Scholar]
- Todorov A. Evaluating faces on trustworthiness: an extension of systems for recognition of emotions signaling approach/ avoidance behaviors. Ann N Y Acad Sci. 2008;1124:208–224. doi: 10.1196/annals.1440.012. [DOI] [PubMed] [Google Scholar]
- Todorov A, Duchaine B. Reading trustworthiness in faces without recognizing faces. Cogn Neuropsychol. 2008;25(3):395–410. doi: 10.1080/02643290802044996. [DOI] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Soc Cogn Affect Neurosci. 2008;3(4):303–312. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Soc Cogn Affect Neurosci. 2008a;3(2):119–127. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Said CP, Engell AD, Oosterhof NN. Understanding evaluation of faces on social dimensions. Trends Cogn Sci. 2008b;12(12):455–460. doi: 10.1016/j.tics.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Remembering beauty: roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. Neuroimage. 2010a doi: 10.1016/j.neuroimage.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Shared brain activity for aesthetic and moral judgments: implications for the beauty-is-good stereotype. Soc Cogn Affect Neurosci. 2010b doi: 10.1093/scan/nsq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DJ, Banfield JE, Walling BR, Heatherton TF, Grafton ST, Handy TC, Gazzaniga MS, Macrae CN. From facial cue to dinner for two: the neural substrates of personal choice. Neuroimage. 2004;22(3):1281–1290. doi: 10.1016/j.neuroimage.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Verosky SC, Todorov A. Differential neural response to faces physically similar to the self as a function of their valence. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2(2):150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Abler B, Ciaramidaro A, Erk S. Motivating forces of human actions. Neuroimaging reward and social interaction. Brain Res Bull. 2005;67(5):368–381. doi: 10.1016/j.brainresbull.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol. 1998;7(6):177–188. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams LM. An integrative neuroscience model of “significance” processing. J Integr Neurosci. 2006;5(1):1–47. doi: 10.1142/s0219635206001082. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychol Sci. 2006;17(7):592–598. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45(1):195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wrangham R, Peterson D. Demonic males: apes and the origin of human violence. London: Bloomsbury; 1996. [Google Scholar]
- Zebrowitz LA, Montepare JM. Social psychological face perception: why appearance matters. Soc Pers Psychol Compass. 2008;2(3):1497–1517. doi: 10.1111/j.1751-9004.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]