Abstract
Objective
To assess the role of HIV and monocytes/macrophages in adipose tissue dysregulation.
Methods
Cross-sectional study in 5 groups: HIV seronegative, HIV+ antiretroviral therapy (ART)-naive, HIV+ nonlipoatrophic on zidovudine- and/or stavudine-containing ART, HIV+ lipoatrophic on similar ART, and HIV+ on abacavir- or tenofovir-containing ART. HIV DNA in circulating monocyte subsets was quantitated by real-time polymerase chain reaction. Biopsied subcutaneous fat was examined for macrophage content by CD68 staining. Isolated adipocytes and macrophages were cultured and the supernatant assayed for secretory products by Luminex multiplex cytokine technology.
Results
Sixty-nine subjects were enrolled. Lipoatrophic subjects had higher median HIV DNA levels (270.5 copies/106 cells) in circulating peripheral CD14+CD16+ co-expressing monocyte subsets compared with subjects who were ART-naive (25.0 copies), non-lipoatrophic (15.0 copies), or on abacavir/tenofovir (57.5 copies), P < 0.01. Group differences in adipocytes and adipose macrophage content were marginal. Although adipocyte secretory products were similar, HIV-infected subjects had higher adipose macrophage–derived interleukin (IL)-12p40, IL-6, IL-8, and monocyte inflammatory protein 1 alpha and lower eotaxin and interferon gamma levels than HIV seronegative subjects (P < 0.05). Within HIV-infected subjects, adipose macrophage secretory products were comparable between subjects naive with ART versus those on ART.
Conclusions
Circulating HIV-infected and proinflammatory CD14+CD16+ monocyte subsets contribute to the pathogenesis of HIV-associated lipoatrophy. Among HIV-infected individuals, macrophages, rather than adipocytes, are the primary source of low-grade inflammation in subcutaneous adipose tissue. HIV infection modifies these macrophages to a more proinflammatory phenotype, and these changes are not substantially mitigated by the use of ART.
Keywords: lipoatrophy, HIV, monocyte, macrophage, adipose
INTRODUCTION
In addition to its role as an energy reservoir, adipose tissue exerts widespread paracrine and endocrine effects involved in the control of energy homeostasis, glucose and lipid metabolism, and inflammatory processes. These effects influence risk of chronic complications including cardiovascular disease or severe insulin resistance.1–3 Adipose tissue contains connective tissue matrix, nerve tissue, vascular cells, and immune cells.4 Adipose tissue not only responds to afferent signals from traditional hormone systems and the central nervous system but also expresses and secretes factors with important endocrine functions.5
Abnormalities of adipose tissue content and function are common among HIV-infected individuals on antiretroviral therapy (ART). Most notably, loss of subcutaneous (SC) adipose tissue in the form of lipoatrophy is a major side effect associated strongly with the use of zidovudine (ZDV) or stavudine (d4T), 2 older nucleoside reverse transcriptase inhibitors (NRTI) still commonly used for the treatment of HIV in developing countries. Although the pathogenesis of lipoatrophy is attributed primarily to the tendency of these older NRTIs to induce mitochondrial toxicity,6,7 HIV and the inflammatory response that it induces have been hypothesized to synergize and contribute to its development.8,9 Dysregulation of adipose tissue in HIV is also suggested by the higher rates of insulin resistance, impaired glucose tolerance, and diabetes among HIV-infected individuals on potent ART. Such risks have similarly been attributed not only to the use of various antiretroviral medications but also to the effects of HIV as well.10
Monocytes and macrophages are components of the innate immune system. These cells are important sources of soluble immune mediators, including cytokines and chemokines, and therefore may be instrumental in mediating systemic effects in various tissues. By international consensus classification, circulating monocytes, which are characterized by their surface marker CD14, are further subdivided by the immunoglobulin G Fc (FcR III) surface receptor, CD16.11 In comparison with CD14+CD16− monocytes, CD14+CD16+ monocytes constitutively produce higher levels of proinflammatory cytokines and express a number of adhesion molecules that facilitate their arrest, diapedesis, transendothelial migration, and recruitment into the tissues.12 CD14+CD16+ monocytes are increased in HIV-infected individuals and are preferentially infected by HIV.13–15
Bone marrow–derived monocytes infiltrate various tissues, including adipose tissue, and differentiate into macrophages that participate in the paracrine activities within that tissue. HIV lipoatrophy is characterized by signs of macrophage infiltration and inflammation.16 Studies in the general population have demonstrated that macrophages are responsible for the secretion of most proinflammatory cytokines within adipose tissue, including tumor necrosis factor alpha, and that these cells play a crucial role in obesity-related insulin resistance.17,18
In this study, we sought to understand the contributing role of HIV and monocyte/macrophage populations in adipose tissue dysregulation among HIV-infected individuals on potent ART by conducting a cross-sectional study of subjects differing by HIV infection, use of NRTI, and the presence of lipoatrophy. We quantitated the degree of HIV infection (HIV DNA) within circulating blood monocyte subsets. After biopsy of SC fat, we analyzed the tissue for macrophage content within SC fat and assessed the secretory profiles of adipocytes and macrophages obtained from this tissue. We believe that this is the first study to examine the role of HIV-infected monocyte subsets and the secretory characteristics of SC adipocyte macrophages and adipocytes in adipose tissue dysregulation that occurs in the context of HIV. We found that circulating HIV-infected CD16 expressing monocytes and cytokines and chemokines from macrophages in SC fat contribute to adipose tissue dysregulation in HIV.
METHODS
Subject Selection
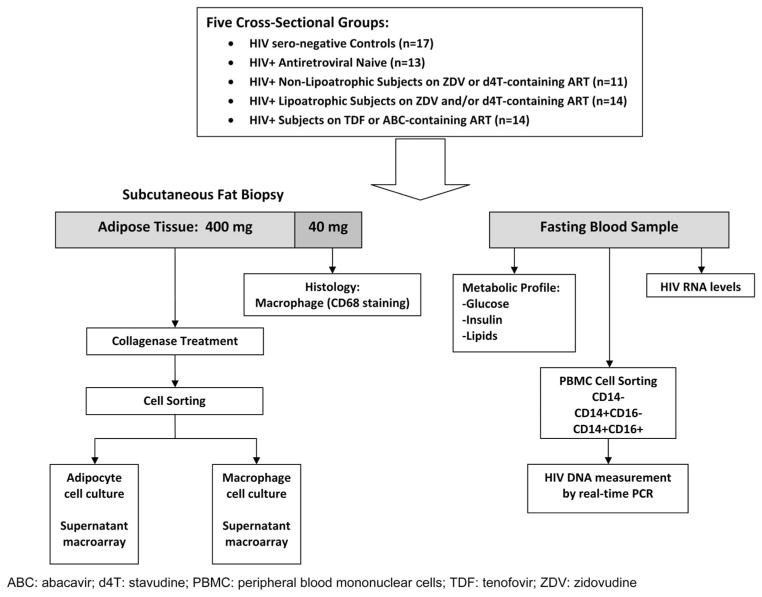
This study was approved by the University of Hawaii Committee on Human Subjects and was conducted between 2007 and 2012. Subjects were cross-sectionally recruited into 5 groups: (1) HIV seronegative healthy subjects, (2) ART-naive subjects: HIV-infected subjects naive to ART, (3) nonlipoatrophic subjects: HIV-infected subjects on ZDV- or d4T-containing ART for ≥48 weeks before study entry without lipoatrophy, (4) lipoatrophic subjects: HIV-infected subjects on similar ZDV- or d4T-containing ART for ≥48 weeks before study entry, but with lipoatrophy assessed by self-report and study physician concurrence, and (5) subjects on abacavir (ABC)/tenofovir (TDF): HIV-infected subjects on ABC- or TDF-based ART for ≥48 weeks before study evaluation without regard to body composition changes and without use of concomitant ZDV or d4T.
Subjects receiving medications likely to impact metabolism, increase metabolic rate, or impact mitochondrial function within 12 weeks before enrollment were excluded. These medications included androgens, glucocorticoids, niacin and related compounds, rosiglitazone or other thiazolidinediones, any appetite stimulant, growth hormone, vitamin or antioxidant preparation in doses above standard multivitamin preparations, cytokines or cytokine inhibitors, immunomodulator therapy, and experimental medications. Subjects with history of diabetes mellitus, defined as history of random serum glucose >200 mg/dL, fasting serum glucose on study screening of ≥126 mg/dL, or current or past receipt of diabetic medications, were excluded. Subjects with body mass index (BMI) <18 or >35 kg/m2, subjects on antiretroviral structured treatment interruption, and subjects with other chronic diseases or acute intercurrent illnesses were also excluded.
Study Evaluations
The study schema used is shown in Figure 1. After informed consent and screening procedures, subjects who met eligibility criteria were asked to return for a one time study visit. Subjects were evaluated between 8 and 11 AM and asked to refrain from exercise or unusual exertion for 48 hours before clinical visit. Weight and height were obtained in triplicate and averaged. Extremity and truncal fat were quantitated by dual energy x-ray absorptiometry (DXA) using Lunar Prodigy scanner (GE Medical Systems, Inc, Milwaukee, WI). Blood samples were drawn fasting, defined as nothing by mouth except water for ≥12 hours before the blood draw and couriered to a CLIA- and CAP-certified laboratory (Diagnostic Laboratory Services, Inc, Honolulu, HI) for analysis of routine metabolic parameters (glucose, insulin, lactate, and total, low-density lipoprotein, and high-density lipoprotein cholesterol), T-cell subset and plasma HIV RNA performed initially by the COBAS Amplicor HIV-1 polymerase chain reaction (PCR) test and later by the COBAS TaqMan HIV-1 real-time PCR test (Roche Diagnostics, Inc, Indianapolis, IN). Blood for glucose and lactate were drawn in sodium fluoride tubes. Insulin resistance was assessed as homeostatic model assessment of insulin resistance 2 (HOMA-IR2), calculated using the HOMA Calculator version 2.2 (Diabetes Trials Unit University of Oxford, Oxford Center for Diabetes, Endocrinology and Metabolism. Available at: http://www.dtu.ox.ac.uk/homacalculator/index.php). Blood for cellular HIV DNA analysis was processed as viable peripheral blood mononuclear cells (PBMCs), stored at −80°C, and analyzed in batches.
FIGURE 1.
Summary of methods: peripheral blood analysis and SC fat biopsy processing.
Quantification of HIV DNA in PBMCs
HIV DNA copy numbers were measured from PBMCs and cellular subsets as previously described.19 Briefly, frozen PBMCs were separated into monocyte and nonmonocyte fractions using the Human Monocyte Enrichment Kit without CD16 Depletion (StemCell Technologies, Inc, Vancouver, BC) as per manufacturer’s guidelines, which is a negative selection leaving the CD14 cells untouched. The CD14 monocyte fractions were then separated into CD16+ and CD16− fractions. CD14+-isolated monocytes were further labeled with the anti-CD16-biotinylated monoclonal antibody (BioLegend Inc, San Diego, CA) and magnetically separated by streptavidin magnetic particles (Chemicell GmbH, Berlin, Germany). Quality control for efficiency of the cell separation demonstrated >95% purity. After DNA extraction using the QIAamp DNA Micro Extraction kit (Qiagen, Valencia, CA), multiplex real-time PCR using HIV gag and β-globin primers was performed as previously published.19 The copy numbers of each sample gene were analyzed against standard curves and the HIV DNA copy number per 1 × 106 cells determined.
SC Adipose Tissue Analyses
Approximately 600 mg of SC adipose tissue was collected from the gluteal fold of the lateral thigh by open biopsy. Immediately after sample collection, a small ~25 mg of section was fixed in 10% buffered formalin solution and stored at 4°C, and the remaining tissue was used later for cellular analyses.
Immunohistochemistry
Tissue for histology was processed for paraffin embedding. Four millimeter of serial sections were immunohistochemically labeled for macrophages using the IHC Select Kit and anti-CD68 monoclonal antibody (clone KP1; Chemicon/ Millipore, Temecula, CA). Reactions in the absence of primary antibody and with isotype antibody only served as negative controls. Quantitation of macrophages/field was conducted by visual quantitation by a board-certified pathologist of the entire cross-section (Carlaz Biotechnology, Inc, San Diego, CA).
Cell Sorting of SC Adipose Tissue and Adipocyte and Macrophage Cell Culture
Adipose tissue was digested with collagenase (Sigma-Aldrich Co, St Louis, MO), filtered through a 300-μm nylon mesh and centrifuge spun at 400g. The adipocytes floated and the pellet consisted of preadipocytes, erythrocytes, fibroblasts, endothelial cells, and macrophages. An aliquot of adipocytes were stained with Oil Red O (Sigma-Aldrich Co, LLC) and visualized with an inverted microscope to confirm that the cells were adipocytes. Adipocytes were counted using the Countess automated cell counter (Life Technologies Corp, Grand Island, NY). Adipocytes, ~500,000 cells per well (n = 2), were cultured in 96 well tissue culture plates at 37° C and 5% of CO2 incubator for cytokine expression in adipocyte medium (http://www.zen-bio.com/products.html). Supernatant fractions were acquired at 24 hours after plating, quick-frozen in liquid nitrogen, and stored at −80°C for Luminex analyses.
Erythrocytes were removed from the remaining pellet using erythrocyte lysis buffer (ebioscience, San Diego, CA). The cell pellet was washed and, after Fc-block, labeled with CD11b-APC (CALTAG, Buckingham, United Kingdom) and CD34-biotin (Abcam, Cambridge, MA) followed by strep-APC-Cy7 (BD Biosciences, San Jose, CA). The 2 labeled cell fractions were separated using a FACS ARIA cell sorter (Becton, Dickinson Co, San Jose, CA). Macrophages, 5000 cells per well (based on sorter event counts) (n = 2), were plated in a 384 well tissue culture plates and incubated for 24 hours in a 37°C and 5% of CO2 incubator. Isolated macrophages were cultured in 25% of RPMI media (Sigma-Aldrich Co, LLC), Iscove modified Dulbecco medium (Irvine Scientific, Santa Ana, CA), 4 mM of L-glutamine (Sigma-Aldrich Co, LLC), 100 units per milliliter of penicillin and 100 mg/mL of streptomycin (Sigma-Aldrich Co, LLC), and 10% of normal human serum (Sigma-Aldrich Co, LLC) at 37°C and 5% of CO2 incubator. Supernatant were quick-frozen and stored at −80°C.
Adipocyte and Macrophage Supernatant Analyses
Adipocyte cytokines were assayed using a human adipocyte panel with a Luminex 200× PONENT system (EMD Millipore Corp, Billerica, MA). The adipocyte supernatant protein levels were measured using bicinchoninic acid protein assay (Thermo Fisher Scientific Inc, Rockford, IL) and the adipocyte data were normalized because adipocyte sizes vary and this may have effected counting. Macrophage cytokines were assayed using a 22-plex human cytokine multiplex immunoassay kit. Resulting data were analyzed with MILLIPLEX Analyst 5.1 software (EMD Millipore).
Statistical Analyses
Kruskal–Wallis test was used to compare the distribution of continuous variables among groups. Pairwise comparisons of distribution between 2 specific groups were assessed by Wilcoxon rank sum test. P value <0.05 was considered significant. STATA IC 12.0 (College Station, TX) was used.
RESULTS
Demographic, Body Composition, and Metabolic Parameters of the Cohort
A total of 69 subjects were enrolled. Clinical characteristics by groups are summarized in Table 1. The groups did not differ significantly by gender or ethnicity. In terms of ethnicity, the study population consisted of 49.3% Whites, 15.9% Asian, 11.6% Hispanic, 7.3% African American, 4.3% Hawaiian/ Pacific Islander, and 11.6% others. Overall median age (Q1, Q3) was 47 (40, 56) years. Lipoatrophic subjects were older compared with other groups. Among HIV+ subjects, the median current CD4 did not significantly differ among groups. Duration of ART was longest among lipoatrophic subjects, whereas nadir CD4 count was lowest among nonlipoatrophic subjects on ZDV/d4T. ZDV was the NRTI used by the overwhelming majority of subjects in both the nonlipoatrophic and the lipoatrophic groups, whereas TDF was the NRTI used by most subjects in the ABC/TDF group.
TABLE 1.
Clinical and Body Composition/Metabolic Characteristics of the Cohort
| HIV Negative | HIV-Infected Subjects
|
P | ||||
|---|---|---|---|---|---|---|
| ART-Naive | ZDV/d4T Nonlipoatrophic | ZDV/d4T Lipoatrophic | ABC/TDF | |||
| Sample size | 17 | 13 | 11 | 14 | 14 | — |
| Current antiretrovirals regimen | — | — | 10 ZDV, 1 combined ZDV + d4T | 13 ZDV, 1 d4T | 1 ABC, 11 TDF, 1 combined ABC + TDF | — |
| Age, yrs | 43.2 (39.8, 50.8) | 43.7 (33.7, 48.1) | 47.7 (42.2, 62.8) | 57.1 (48.4, 59.7) | 45.0 (31.1, 51.3) | 0.05 |
| Male, n (%) | 16 (94.1) | 13 (100) | 10 (90.9) | 14 (100) | 13 (92.9) | 0.82 |
| Duration ART (yrs) | — | 0 | 7 (5, 10) | 9 (4, 13) | 1.9 (1, 3) | <0.01 |
| CD4 (cells/mm3) | — | 414.0 (319.0, 488.0) | 410.5 (329.0, 634.0) | 510.5 (379.0, 737.0) | 450.5 (362.0, 660.0) | 0.55 |
| % HIV RNA <50 copies/mL | — | 0 | 80.0 | 92.9 | 78.6 | 0.62* |
| Nadir CD4 (cells/mm3)† | — | 386.0 (318.0, 400.0) | 21.0 (16.0, 199.0) | 105.0 (65.0, 200.0) | 201.0 (80.0, 216.0) | <0.01 |
| BMI (kg/m2) | 24.6 (22.8, 28.0) | 26.1 (24.7, 27.5) | 26.7 (21.9, 28.2) | 25.5 (23.6, 26.8) | 24.6 (21.4, 26.0) | 0.39 |
| DXA extremity fat (kg) | 7.2 (5.3, 9.7) | 7.6 (5.3, 10.2) | 5.9 (3.0, 8.6) | 4.4 (2.2, 7.7) | 5.7 (4.1, 7.4) | 0.10 |
| DXA trunk fat (kg) | 10.7 (7.0, 16.1) | 9.8 (8.3, 14.3) | 14.6 (8.0, 15.9) | 10.1 (8.3, 16.7) | 9.6 (7.0, 14.5) | 0.68 |
| DXA lean Mass (kg) | 5.3 (46.2, 56.5) | 53.7 (50.8, 61.7) | 50.3 (47.6, 61.3) | 55.1 (52.1, 65.3) | 54.3 (48.7, 56.4) | 0.47 |
| Extremity fat/trunk fat | 0.67 (0.58, 0.81) | 0.70 (0.61, 0.89) | 0.5 (0.37, 0.68) | 0.40 (0.28, 0.47) | 0.65 (0.52, 0.79) | <0.01 |
| Lactic acid (mEq/L) | 1.0 (0.7, 1.2) | 0.8 (0.6, 0.9) | 1.4 (1.1, 1.8) | 1.2 (0.9, 1.5) | 1.0 (0.8, 1.2) | <0.01 |
| Total cholesterol (mg/dL) | 183.0 (162.0, 208.0) | 152.0 (141.0, 174.0) | 199.0 (159.0, 245.0) | 166.0 (150.0, 197.0) | 160.0 (135.0, 210.0) | 0.04 |
| HDL cholesterol (mg/dL) | 46.0 (39.0, 58.0) | 36.0 (29.0, 41.0) | 44.0 (35.0, 52.0) | 37.5 (31.0, 57.0) | 41.0 (31.0, 52.0) | 0.07 |
| LDL cholesterol (mg/dL) | 120.0 (94.0, 134.0) | 104.0 (86.0, 123.0) | 116.5 (93.0, 164.0) | 107.5 (58.0, 119.0) | 92.0 (77.0, 114.0) | 0.22 |
| Triglycerides (mg/dL) | 88.0 (63.0, 123.0) | 104.0 (72.0, 136.0) | 117.0 (102.0, 215.0) | 131.5 (78.0, 218.0) | 143.0 (100.0, 163.0) | 0.24 |
| Fasting glucose (mg/dL) | 89.0 (83.0, 93.0) | 84.0 (81.0, 89.0) | 93.0 (90.0, 99.0) | 92.0 (84.0, 99.0) | 87.5 (80.0, 92.0) | 0.20 |
| Fasting insulin (uU/mL) | 5.2 (3.1, 7.3) | 5.3 (3.8, 16.0) | 6.3 (4.1, 10.0) | 11.2 (6.7, 18.8) | 4.9 (4.7, 10.0) | 0.02 |
| HOMA-IR2‡ | 0.7 (0.4, 0.9) | 0.7 (0.5, 2.0) | 0.8 (0.5, 1.3) | 1.4 (0.9, 2.4) | 0.6 (0.6, 1.3) | 0.01 |
All values reported are median (Q1, Q3) except for % plasma HIV RNA <50 copies per milliliter and gender. Kruskal–Wallis test or Fisher exact test were used as appropriate.
Fisher exact test restricted to patients on antiretroviral therapy.
By self-report, data reported on 7 nonlipoatrophic, 7 lipoatrophic, and 10 ABC/TDF subjects.
HOMA-IR2 calculated using HOMA Calculator version 2.2 (Diabetes Trials Unit University of Oxford. The Oxford Center for Diabetes, Endocrinology and Metabolism. Available at: http://www.dtu.ox.ac.uk/homacalculator/index.php).
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The median BMI of this cohort was 24.9 kg/m2. Lipoatrophic subjects had the least extremity fat by DXA at 4.4 kg. Although expected trends were seen, significant differences by groups were not noted by BMI or by DXA extremity fat or truncal fat content, as absolute values or as adjusted for weight or height. However, extremity fat/truncal fat ratios by DXA differentiated lipoatrophic subjects from seronegative (P < 0.001), ART-naive (P < 0.001), and ABC/TDF (P = 0.006) subjects, but not from nonlipoatrophic subjects (P = 0.30).
Distributions of fasting insulin levels, HOMA-IR2, and lactic acid were statistically different among groups. The highest fasting insulin levels and calculated HOMA-IR2 were seen among lipoatrophic subjects, and the highest lactic acid levels were among nonlipoatrophic subjects.
Circulating Peripheral Blood Monocyte and Nonmonocyte HIV DNA Levels
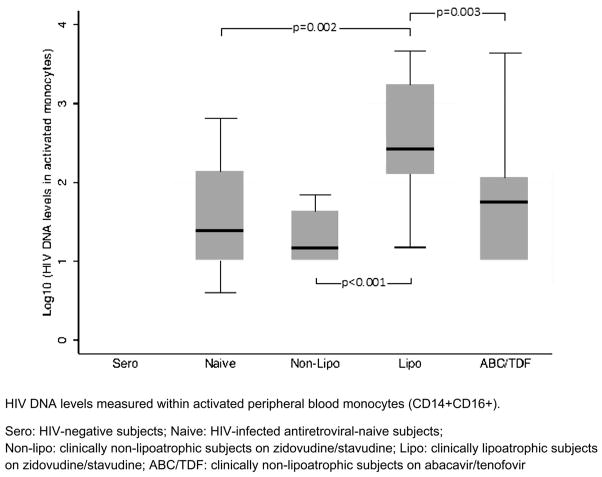
The distribution of HIV DNA did not differ among the 4 groups of HIV-infected subjects within the circulating CD14+CD16− monocyte subset or within the nonmonocyte (CD14−) cell subset. Differences in distribution, however, were seen among the 4 groups in HIV DNA within the CD14+CD16+ monocyte subset. In pairwise comparisons, lipoatrophic subjects had significantly higher median HIV DNA levels (270.5 copies/106 cells) in the CD14+CD16+ monocyte subset compared with ART-naive subjects (25.0 copies; P = 0.002), nonlipoatrophic subjects on ZDV/d4T (15.0 copies/106 cells; P < 0.001), and subjects on ABC/TDF (57.5 copies/106 cells; P = 0.003) (Fig. 2). Restricting the analysis to HIV patients on ART with undetectable HIV RNA levels (<50 copies/mL; N = 32), the distribution of CD14+CD16+ HIV DNA levels remained significantly different among groups (P = 0.001). CD14+CD16+ HIV DNA levels remained significantly higher among lipoatrophic subjects compared with non-lipoatrophic subjects on ZDV/d4T (P < 0.001) and subjects on ABC/TDF (P = 0.008).
FIGURE 2.
HIV DNA in peripheral blood CD14+CD16+ monocytes by cohort.
Fat Biopsy Findings
Levels of various adipocyte and macrophage cytokines and chemokines are shown in Table 2. Median (Q1, Q3) SC adipose macrophage contents [cells per high-power field (hpf)] by cohort were as follows: 4.2 (2.3, 5.7) in seronegatives, 4.4 (3.6, 5.5) in ART-naives, 7.2 (4.9, 8.3) in nonlipoatrophics on ZDV/d4T, 4.3 (3.6, 6.5) in lipoatrophics on ZDV/ d4T, and 3.9 (2.3, 5.6) in subjects on ABC/TDF. This distribution of SC adipose macrophage content was not significantly different among the 5 groups (P = 0.08). However, when subjects on ZDV/d4T (lipoatrophic and nonlipoatrophic groups) were combined, subjects on ZDV/d4T had marginally higher macrophage content compared with subjects on ABC/ TDF (5.4 cells/hpf vs 3.9 cells/hpf, respectively, P = 0.05).
TABLE 2.
SC Adipose Tissue Macrophage and Adipocyte Secretory Products by Cohort
| HIV Negative | HIV-Infected Subjects
|
P | ||||
|---|---|---|---|---|---|---|
| ART-Naive | ZDV/d4T Nonlipoatrophic | ZDV/d4T Lipoatrophic | ABC/TDF | |||
| Macrophage secretory products (pg/mL) | ||||||
| Sample size | 16 | 13 | 11 | 13 | 13 | — |
| Eotaxin | 4.8 (3.8, 5.9) | 3.8 (3.2, 6.7) | 3.8 (2.9, 4.9) | 3.7 (2.0, 3.8) | 3.6 (1.4, 4.4) | 0.23 |
| GM-CSF | 1.1 (0.1, 2.3) | 1.3 (0.3, 4.7) | 1.9 (0.4, 6.0) | 1.1 (0.1, 3.0) | 1.7 (0.6, 4.8) | 0.74 |
| IL-1α | 1.6 (0, 2.3) | 0.8 (0, 5.5) | 4.0 (0.6, 47.0) | 0.4 (0, 58.1) | 1.2 (0, 26.1) | 0.67 |
| IL-1β | 0.4 (0.1, 3.5) | 1.5 (0.1, 1.8) | 0.8 (0.1, 3.3) | 1.6 (0, 3.5) | 0.6 (0, 2.8) | 0.93 |
| IL-3 | 1.3 (0.6, 2.5) | 2.7 (1.1, 4.8) | 1.2 (0, 2.9) | 0.7 (0, 2.5) | 2.5 (1.2, 4.9) | 0.13 |
| IL-5 | 1.2 (1, 1.2) | 1.0 (1.0,1.2) | 1.2 (0.8, 1.3) | 1.2 (1.2,1.2) | 1.2 (1, 1.2) | 0.91 |
| IL-6 | 2.9 (1.8, 6.3) | 7.6 (1.7, 21.8) | 6.8 (3.0, 74.5) | 5.6 (2.6, 33.1) | 7.6 (4.2, 19.9) | 0.24 |
| IL-7 | 0 (0, 3.4) | 0 (0, 1.3) | 0 (0, 4.8) | 0 (0, 0) | 0 (0, 5.1) | 0.90 |
| IL-8 | 442.0 (79.4, 645.0) | 2020.0 (301.0, 3750.0) | 2320.0 (609.0, 8460.0) | 1920.0 (389.0, 3800.0) | 1540.7 (199.0, 1890.0) | 0.01 |
| IL-10 | 19.4 (8.4, 21.9) | 11.2 (4.2, 20.0) | 23.2 (7.3, 37.6) | 12.0 (10.9, 17.7) | 1.9 (1.0, 12.0) | 0.01 |
| IL-12p40 | 0 (0,0) | 0 (0, 7.2) | 0 (0, 4.8) | 0 (0, 4.4) | 0 (0, 7.0) | 0.05 |
| IL-15 | 0 (0, 0.1) | 0 (0,0) | 0 (0, 2.3) | 0 (0, 0) | 0 (0, 1.4) | 0.98 |
| IFN-γ | 2.4 (0, 2.8) | 0 (0,0) | 0 (0, 2.7) | 0 (0,0) | 0 (0,0) | 0.11 |
| IP-10 | 6.6 (0, 26.4) | 4.8 (3.9, 7.1) | 4.5 (0, 8.4) | 2.8 (0, 7.4) | 4.2 (0, 6.1) | 0.83 |
| MCP-1 | 27.8 (12.0, 57.8) | 33.9 (9.9, 175.7) | 56.4 (43.0, 197.8) | 37.4 (7.1, 121.0) | 46.2 (7.2, 97.5) | 0.29 |
| MIP-1α | 15.7 (6.4, 71.6) | 64.5 (10.8, 232.5) | 146.0 (51.3, 361.0) | 122.5 (32.8, 156.0) | 54.5 (11.4, 84.9) | 0.047 |
| TNF-α | 1.65 (1.25, 2.0) | 2.1 (1.2, 5.9) | 2.7 (1.5, 6.4) | 2.7 (1.5, 8.1) | 1.7 (1.1, 2.8) | 0.37 |
| Adipocyte secretory products (pg/mL/μg protein) | ||||||
| Sample size | 16 | 8 | 10 | 11 | 10 | — |
| Adiponectin | 1324.6 (1024.9, 1869.0) | 4333.4 (2520.4, 6505.1) | 1438.8 (185.5, 3741.3) | 804.5 (450.5, 2181.2) | 1065.6 (465.3, 1617.1) | 0.09 |
| IL-1β | 0 (0.1) | 0 (0, 0.2) | 0 (0, 0.1) | 0 (0, 0.4) | 0 (0, 0) | 0.74 |
| IL-6 | 41.2 (6.4, 127.2) | 78.1 (2.1, 405.4) | 4.3 (0.1, 63.2) | 2.6 (0, 131.8) | 4.4 (1.3, 51.0) | 0.44 |
| IL-8 | 70.7 (14.6, 140.5) | 28.3 (2.1, 406.9) | 40.3 (0.3, 134.4) | 13.0 (0.9, 337.2) | 9.2 (0.8, 68.9) | 0.59 |
| Leptin | 3.0 (0, 4.0) | 2.2 (0.6, 2.8) | 4.3 (1.4, 5.2) | 1.5 (1.0, 2.6) | 1.8 (1.2, 3.2) | 0.67 |
| MCP-1 | 57.8 (9.2, 92.5) | 37.8 (0.8, 262.8) | 8.0 (0.2, 114.8) | 7.7 (0.2, 264.8) | 3.0 (0.5, 37.8) | 0.48 |
| PAI-1 | 62.4 (14.2, 101.9) | 71.6 (2.3, 178.6) | 10.0 (0.9, 83.9) | 1.4 (0.2, 118.0) | 3.05 (1.8, 54.8) | 0.31 |
| Resistin | 0.3 (0.1, 0.7) | 0.4 (0, 0.7) | 0.1 (0, 0.3) | 0 (0, 0.7) | 0.3 (0.1, 0.4) | 0.43 |
| TNF-α | 0.8 (0.2, 1.4) | 0.2 (0, 2.3) | 0.4 (0, 0.7) | 0.3 (0, 0.9) | 0.2 (0, 0.5) | 0.39 |
All values reported are median (Q1, Q3). Kruskal–Wallis test was used for analyses.
GM-CSF, granulocyte–macrophage-colony stimulating factor; IFN-γ, interferon gamma; IP-10, interferon gamma-induced protein 10; MCP-1, monocyte chemotactic protein 1; PAI-1, plasminogen activator inhibitor 1; TNF-α, tumor necrosis factor alpha.
The distribution of all adipocyte cytokines and chemokines assessed (Table 2) were similar among the 5 groups. In contrast, differences were found among the groups for macrophage secretory products, with significant differences in distribution for interleukin (IL)-10, IL-8, IL-12p40, and monocyte inflammatory protein 1 alpha (MIP-1α) levels.
Study participants were grouped in different ways to enable pairwise comparisons of the impact of HIV-infected versus noninfected, ART-naive vs on ART, on ZDV/d4T-containing ART versus ABC/TDF-containing ART, and lipoatrophic versus nonlipoatrophic. The 4 groups of HIV-infected subjects combined (constituting a group of all HIV-infected subjects) had higher macrophage IL-12p40 (P < 0.01), IL-6 (P = 0.03), IL-8 (P < 0.01), and MIP-1α (P = 0.01) levels and lower macrophage eotaxin (P = 0.04) and interferon gamma (P = 0.01) levels than HIV seronegative subjects. Adipocyte secretory levels were similar between these 2 groups. Among all HIV-infected subjects, levels of all macrophage cytokines and chemokines assessed were comparable between subjects naive with ART and those on ART (non-lipoatrophic, lipoatrophic, and ABC/TDF groups combined), whereas lower adipocyte adiponectin levels were found among HIV-infected subjects on ART compared with ART-naive subjects (P = 0.03). Compared by NRTI regimens, patients on ZDV/d4T (nonlipoatrophic and lipoatrophic combined) had significantly higher macrophage secretion of IL-10 (P < 0.01), marginally higher macrophage IL-8 (P = 0.05), and marginally lower macrophage IL-3 (P = 0.05) compared with subjects on ABC/TDF. No differences were found in adipocyte secretory products. Among HIV-infected subjects on ZDV/d4T, there was no significant difference between lipoatrophic and nonlipoatrophic groups in either macrophage or adipocyte cytokines and chemokines.
DISCUSSION
Data from our cross-sectional study suggest that high circulating levels of HIV-infected CD14+CD16+ monocytes increase risk of lipoatrophy among subjects maintained on ART-containing mitochondrial-toxic medications. Furthermore, this study indicates SC fat macrophages rather than adipocytes as the likely source of the chronic low-level inflammation found in adipose tissue of HIV-infected individuals. The study found that HIV infection is associated with substantial alterations in SC fat macrophage secretory products that are not substantially altered by the use of ART.
Many HIV-infected subjects fully suppressed in potent ART with undetectable plasma HIV RNA continue to harbor evidence of circulating HIV-infected cells (both lymphocytes and monocytes) in the bloodstream.20,21 High levels of HIV DNA within monocytes (CD14+ cells) and specifically within CD14+CD16+ monocytes have been correlated to the development of cognitive impairment19,22,23 and to brain atrophy.24 Our current study implicates, for the first time, this HIV-infected and proinflammatory monocyte subset as a potential contributing factor in the development of lipoatrophy. The association was specific to the subset, with no HIV DNA associations found using PBMCs as a whole or in nonmonocyte (CD14− cell) or CD14+CD16− monocyte subsets. The CD14+CD16+ monocyte subset is expanded in HIV-infected individuals.13 The strong association of HIV with the CD14+CD16+ subset may be secondary to the fact that this subset is most easily infected by HIV.14 We hypothesize that HIV infection alters the characteristics of CD14+CD16+ monocytes making these cells more conducive to in situ inflammation on entering adipose tissue. HIV infection of monocytes have been reported to lead to defective phagocytic capacity25 and impaired chemotaxis26 both of which may increase exposure of these cells to cellular debris likely to trigger an increased inflammatory response. Perhaps more importantly, HIV infection of monocytes in vitro has been reported to result in upregulation and constitutive secretion of proinflammatory cytokines.27 Furthermore, monocytes from HIV-infected subjects differentially upregulate the expression of proinflammatory cytokines IL-1β, IL-6, IL-8, and tumor necrosis factor alpha in response to stimulation by common inflammatory triggers when compared with monocytes from HIV seronegative subjects,28 underscoring the possible relevance of HIV DNA content within monocytes.
It is of particular importance that HIV DNA in CD14+CD16+ monocytes was significantly different between subjects with or without lipoatrophy despite being on the same identical ZDV- and/or d4T-containing ART. This suggests that high levels of these HIV-infected monocytes increase the risk of lipoatrophy among individuals placed on such mediations. Because loss of fat over time cannot be documented in a cross-sectional study, we elected to define lipoatrophy by patient self-report and physician concurrence. Although less than ideal, it is reassuring that the subjective assessments were consistent with extremity fat/truncal fat ratio using DXA fat content.
In contrast to the results in peripheral blood, the difference in SC fat macrophage content among the groups was marginal, with differences seen only when the non-lipoatrophic and lipoatrophic subjects on ZDV and/or d4T were combined and compared with subjects on ABC- or TDF-based therapy. Increase of SC fat macrophages in subjects with lipoatrophy has been consistently reported.16,29 In our study, the highest level of SC fat macrophage content was seen among our nonlipoatrophic subjects. Lipoatrophy likely occurs over a continuum, and we speculate that the lower macrophage content in our lipoatrophic subjects may indicate that many in this cohort had “burnt-out” disease with increased fibrosis replacing an inflammatory picture. An increase in fibrosis has been reported to occur in association with lipoatrophic changes.16 Our study did not characterize macrophages in the fat tissue obtained, and it is possible that simple identification of macrophages is insufficient to assess alterations attributable to macrophages. In the obesity literature, it has been hypothesized that macrophages migrating to adipose tissue on high-fat feeding may differ from those that reside there under normal diet conditions and are more proinflammatory.30,31
Evidence for increased proinflammatory cytokines both in plasma and in SC adipose tissue have been demonstrated among HIV-infected subjects with lipoatrophy.16,32,33 Our study contributes to this literature by indicating macrophages as a source of the inflammation. Although the distribution of adipocyte secretory products did not differ overall among the 5 groups, substantial differences were seen in macrophage cytokines and chemokines. Levels of IL-8 and MIP-1α were over 3 times higher in subjects who were HIV infected compared with subjects who were HIV seronegative. Interestingly, levels of these cytokines did not differ significantly among the HIV-infected groups and were generally similar whether they were ART-naive or treated. The use of different types of ART, whether ZDV/d4T-based or ABC/TDF-based ART, makes minimal difference in the levels of macrophage secretory products. Similarly, studies based on human plasma have found evidence of proinflammatory cytokines among HIV-infected individuals regardless of the specific combination of ART.34 Macrophage secretion of IL-10, an anti-inflammatory cytokine, was significantly higher, and IL-8 marginally higher, in subjects on ZDV/d4T (nonlipoatrophic and lipoatrophic combined), compared with subjects on ABC/ TDF, suggesting that the choice of NRTI does, however, impact levels of these cytokines and chemokines.
This study has several important limitations including its relatively small sample size and cross-sectional design. Without serial measurements starting before use of antiretro-viral medications, the study cannot demonstrate that high monocyte HIV DNA levels preceded use of such drugs or that it contributed to the development of lipoatrophy possibly by intensifying the effect of ZDV/d4T-induced mitochondrial toxicity on adipocytes or via other effects on peripheral monocytes or adipose-resident macrophages. Our study unfortunately did not quantitate the number of cells within each monocyte subset. Thus, although we could demonstrate an association between CD14+CD16+ monocyte HIV DNA and lipoatrophy, whether this subset was expanded in our lipoatrophic subjects is unknown. Additionally, subjects on ZDV and/ or d4T had lower nadir CD4 and longer self-reported lifetime use of ART compared with subjects on ABC or TDF, which may have affected results. Also, the cytokine data may have been affected by minor cellular contamination from preadipocyte, endothelial cells, or fibroblast cells because no cellular sorting or isolation is absolute. Preadipocyte cytokine levels were not examined because there were insufficient cells to evaluate by Luminex.
In conclusion, this study implicates both HIV and monocytes/macrophages in adipose tissue dysregulation in HIV-infected individuals whereby the pathogenesis of HIV-associated lipoatrophy may involve CD14+CD16+ HIV-infected monocytes. Furthermore, we believe that macrophages, rather than adipocytes, are the source of low-grade inflammation in adipose tissue of HIV-infected individuals. We found that HIV infection alters the secretory function of SC fat macrophages to a more proinflammatory profile and that this alteration is not substantially mitigated by the use of ART.
Acknowledgments
The authors wish to express their gratitude to their study participants without whom this study would not have been possible. The authors would like to thank Drs L. E. Gerschen-son and C. A. Kruse from Carlaz Biotechnology, Inc, San Diego, CA, for their pathology services and expertise. They would also like to thank Ms J. Choi for her technical assistance with isolating adipocytes and macrophages from adipose tissue, tissue culture, and Luminex assays.
Supported by Department of Health and Human Services, National Institutes of Health grants: R01AI068525, R01HL095135, P20MD000173, U54RR026136, and U54MD007584 (C.M.S. and B.T.S.) and P20MD000173 and G12MD007601 (M.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Saint-Marc T, Partisani M, Poizot-Martin I, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 2.Mynarcik DC, McNurlan MA, Steigbigel RT, et al. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–321. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Vigouroux C, Maachi M, Nguyen TH, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS. 2003;17:1503–1511. doi: 10.1097/00002030-200307040-00011. [DOI] [PubMed] [Google Scholar]
- 4.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 5.Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64:163–169. doi: 10.1079/pns2005428. [DOI] [PubMed] [Google Scholar]
- 6.Shikuma CM, Hu N, Milne C, et al. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS. 2001;15:1801–1809. doi: 10.1097/00002030-200109280-00009. [DOI] [PubMed] [Google Scholar]
- 7.Nolan D, Hammond E, Martin A, et al. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS. 2003;17:1329–1338. doi: 10.1097/00002030-200306130-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kotler DP, Ionescu G, Johnson JA, et al. Studies of adipose tissue metabolism in human immunodeficiency virus-associated lipodystrophy. Clin Infect Dis. 2003;37(suppl 2):S47–S51. doi: 10.1086/375891. [DOI] [PubMed] [Google Scholar]
- 9.Grunfeld C, Tien P. Difficulties in understanding the metabolic complications of acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(suppl 2):S43–S46. doi: 10.1086/375886. [DOI] [PubMed] [Google Scholar]
- 10.Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–1383. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 12.Ancuta P, Moses A, Gabuzda D. Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions. Immunobiology. 2004;209:11–20. doi: 10.1016/j.imbio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Thieblemont N, Weiss L, Sadeghi HM, et al. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 14.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 15.Jaworowski A, Kamwendo DD, Ellery P, et al. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 16.Jan V, Cervera P, Maachi M, et al. Altered fat differentiation and adipo-cytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther. 2004;9:555–564. [PubMed] [Google Scholar]
- 17.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusao I, Shiramizu B, Liang CY, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonza S, Mutimer HP, Oelrichs R, et al. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS. 2001;15:17–22. doi: 10.1097/00002030-200101050-00005. [DOI] [PubMed] [Google Scholar]
- 21.Gibellini D, Borderi M, De Crignis E, et al. HIV-1 DNA load analysis in peripheral blood lymphocytes and monocytes from naive and HAART-treated individuals. J Infect. 2008;56:219–225. doi: 10.1016/j.jinf.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Valcour VG, Shiramizu BT, Sithinamsuwan P, et al. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiramizu B, Williams AE, Shikuma C, et al. Amount of HIV DNA in peripheral blood mononuclear cells is proportional to the severity of HIV-1-associated neurocognitive disorders. J Neuropsychiatry Clin Neurosci. 2009;21:68–74. doi: 10.1176/appi.neuropsych.21.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallianpur KJ, Kirk GR, Sailasuta N, et al. Regional Cortical Thinning associated with Detectable levels of HIV DNA. Cereb Cortex. 2011;19:19. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedzierska K, Azzam R, Ellery P, et al. Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy. J Clin Virol. 2003;26:247–263. doi: 10.1016/s1386-6532(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 26.Mastroianni CM, Lichtner M, Mengoni F, et al. Improvement in neutrophil and monocyte function during highly active antiretroviral treatment of HIV-1-infected patients. AIDS. 1999;13:883–890. doi: 10.1097/00002030-199905280-00003. [DOI] [PubMed] [Google Scholar]
- 27.Esser R, Glienke W, von Briesen H, et al. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with human immunodeficiency virus. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- 28.Jalbert E, D’Antoni ML, Keating SM, et al. IL-1b enriched monocytes mount massive IL-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable antiretroviral therapy at risk for cardiovascular disease. PLoS One. 2013;8:e75500. doi: 10.1371/journal.pone.0075500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievers M, Walker UA, Sevastianova K, et al. Gene expression and immunohistochemistry in adipose tissue of HIV type 1-infected patients with nucleoside analogue reverse-transcriptase inhibitor-associated lipoatrophy. J Infect Dis. 2009;200:252–262. doi: 10.1086/599986. [DOI] [PubMed] [Google Scholar]
- 30.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Deyoung SM, Bodzin JL, et al. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 32.Lihn AS, Richelsen B, Pedersen SB, et al. Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab. 2003;285:E1072–E1080. doi: 10.1152/ajpendo.00206.2003. [DOI] [PubMed] [Google Scholar]
- 33.Lindegaard B, Hansen AB, Pilegaard H, et al. Adipose tissue expression of IL-18 and HIV-associated lipodystrophy. AIDS. 2004;18:1956–1958. doi: 10.1097/00002030-200409240-00013. [DOI] [PubMed] [Google Scholar]
- 34.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]