Abstract
Purpose of review
Persistent transforming growth factor β (TGF-β) signaling is the major factor contributing to scleroderma (SSc) fibrosis. This review will summarize recent progress on the noncanonical TGF-β signaling pathways and their role in SSc fibrosis.
Recent findings
Canonical TGF-β signaling involves activation of the TGF-β receptors and downstream signal transducers Smad2/3. The term noncanonical TGF-β signaling includes a variety of intracellular signaling pathways activated by TGF-β independently of Smad2/3 activation. There is evidence that these pathways play important role in SSc fibrosis. In a subset of SSc fibroblasts, a multiligand receptor complex consisting of TGF-β and CCN2 receptors drives constitutive activation of the Smad1 pathway. CCN2 is also a primary effector of this pathway, thus establishing an autocrine loop that amplifies TGF-β signaling. SSc fibroblasts also demonstrate reduced expression of endogenous antagonists of TGF-β signaling including transcriptional repressors, Friend leukemia integration-1 and perixosome proliferator-activated receptor-γ, as well as inhibitor of Smad3 phosphorylation, PTEN. PTEN is a key mediator of the cross-talk between the sphingosine kinase and the TGF-β pathways.
Summary
Discovery of the role of noncanonical TGF-β signaling in fibrosis offers new molecular targets for the antifibrotic therapies. Due to the heterogeneous nature of SSc, knowledge of these pathways could help to tailor the therapy to the individual patient depending on the activation status of a specific profibrotic pathway.
Keywords: CCN2, endoglin, Fli1, perixosome proliferator-activated receptor-γ, PTEN, Smad1
Introduction
Scleroderma (SSc) is a clinically heterogeneous connective tissue and autoimmune disease of unknown cause characterized by severe and often progressive cutaneous and visceral fibrosis, pronounced alterations in the microvasculature, and numerous cellular and humoral immune abnormalities [1]. Although excessive scarring due to overproduction of extracellular matrix (ECM) proteins is a hallmark of SSc, recent studies suggest that different molecular mechanisms may underlie fibrotic process in different patient populations [2,3]. Understanding these mechanisms is of primary importance in developing clinically useful biomarkers and designing rational and effective therapies for SSc. Transforming growth factor β (TGF-β) is one of the most potent inducers of ECM production and is considered a central player in the process of fibrogenesis in SSc and other fibrotic diseases. Canonical TGF-β signaling is initiated by ligand binding to a heteromeric complex of transmembrane serine/threonine kinases, type I (ALK5) and type II, and a subsequent activation of transcriptional co-regulators, Smad2 and Smad3 [4]. The role of Smad3 in fibrosis has previously been summarized [5] and will not be discussed herein. However, this relatively simple mode of signaling could not explain the plethora of biological effects elicited by TGF-β under physiological conditions and in numerous pathological situations. It is now well recognized that in addition to Smad2/3 signaling, TGF-β can directly or indirectly activate various other intracellular signaling pathways, which in parallel with Smad2/3 regulate TGF-β function [6]. This review article will summarize recent progress in this area focusing on signaling pathways with relevance to the process of fibrosis in SSc.
Activation of Smad1 signaling pathway contributes to scleroderma fibrosis
The conventional view of the TGF-β receptor signaling implies strict specificity of the receptor–Smad interactions, with Smad 2 and 3 serving as substrates for the TGF-β and activin receptors, and Smad 1, 5, and 8 serving as substrates for the bone morphogenetic proteins (BMP) receptors [4]. Surprisingly, however, the studies of ten Dijke and colleagues revealed that in endothelial cells TGF-β signals through both types of receptors, including its cognate receptor, ALK5, which results in activation of Smad2/3, as well as a novel receptor complex consisting of ALK5 and a BMP receptor, ALK1, which results in activation of Smad1. Subsequent work by these investigators has shown that an accessory TGF-β receptor, endoglin, which is expressed predominantly in vascular endothelial cells, promotes ALK1/Smad1 signaling, while inhibiting ALK5/Smad3 signaling, suggesting that the endoglin levels determine the balance between Smad2/3 and Smad1 pathways [7]. Studies in other cell types have revealed that activation of Smad1 by TGF-β is not restricted to endothelial cells, but also occurs in fibroblasts [8] and in a number of epithelial cell lines [9,10]. Interestingly, although studies in fibroblasts suggest that activation of Smad1 occurs in a manner similar to that reported for endothelial cells through ALK5/ALK1 receptor complex [8], in some other cell types, Smad1 is activated directly by ALK5, independently of BMP receptors [9,10].
There is increasing evidence that activation of Smad1 signaling may play a pivotal role in the process of fibrosis [11,12]. Consistent with this notion, blockade of Smad1 by siRNA completely abrogated TGF-β -stimulated collagen and CCN2 upregulation in dermal fibroblasts [8]. Additional mechanistic studies, which employed mutational analysis of the CCN2 promoter and a chromatin immunoprecipitation (ChiP) assay, have determined that a GC-rich motif in the proximal region of the CCN2 promoter constitutes a Smad1-binding site [13•]. This observation is especially relevant to SSc, because this DNA-binding site was previously shown to mediate a constitutive upregulation of CCN2 gene in SSc fibroblasts [14]. Although that previous study has suggested that Sp1 interacts with the proximal GC-rich motif, the possible explanation is that analogous to Smad3, which interacts with Sp1 on the COL1A2 promoter [15], both transcription factors form a protein–protein complex on the CCN2 promoter. The role of Smad1 in mediating upregulation of CCN2 and collagen in SSc fibroblasts was supported by the observation that a majority of cultured SSc fibroblasts expressed elevated levels of total Smad1 and phospho-Smad1 and that depletion of Smad1 by siRNA normalized CCN2 and collagen levels in SSc fibroblasts [13•]. However, it is important to point out that only a proportion of fibroblasts in SSc biopsies showed the presence of phosphorylated Smad1 [13•], which was consistent with in-vitro findings from the human telomerase reverse transcriptase-immortalized SSc clonal cell lines [2]. An intriguing possibility is that a Smad1-positive subset of lesional fibroblasts originates from the endothelial cells through the process of endothelial–mesenchymal transformation, a phenomenon that was recently documented in several experimental models of fibrosis [16]. Additional observations such as enhanced expression of endoglin in SSc fibroblasts [17] support this possibility. Similar to endothelial cells, endoglin is required for the Smad1 activation in SSc fibroblasts and depletion of endoglin results in normalization of CCN2 and collagen levels in these cells [18]. In contrast, in healthy fibroblasts, depletion of endoglin has no effect or even leads to upregulation of collagen.
CCN2 – Smad1 autocrine loop amplifies and prolongs transforming growth factor β signaling
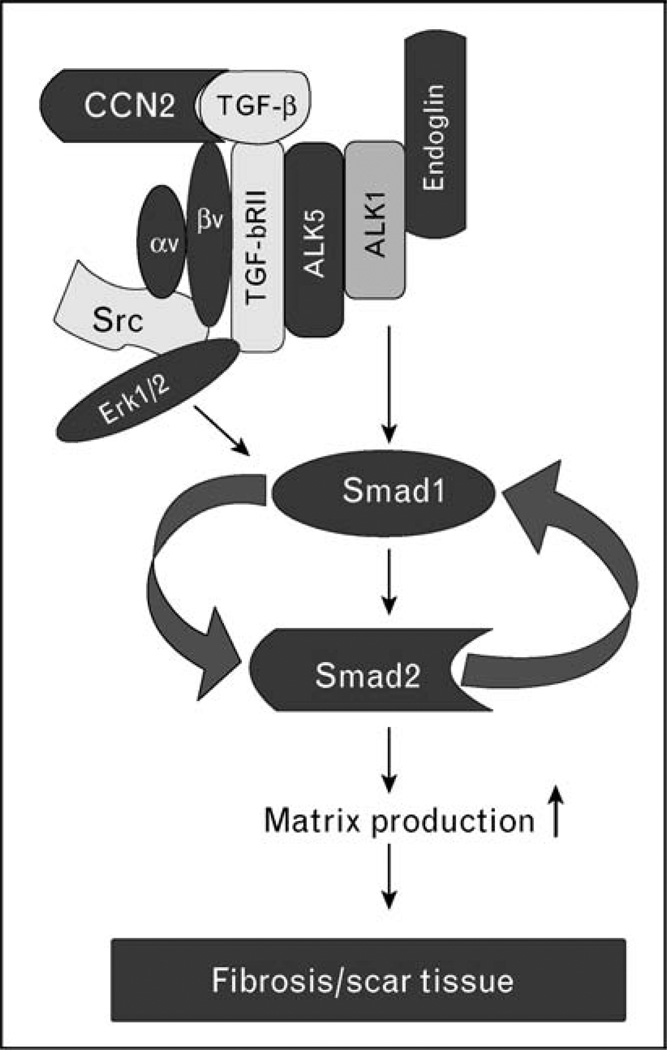
CCN2, a member of the CCN family of matricellular proteins, plays a critical role during physiological and pathological processes, including tissue repair and fibrosis [19]. Although overexpression of CCN2 is almost universally associated with organ fibrosis, the role of CCN2 in the process of fibrosis has not been clearly defined. The majority of published studies support the view of cooperation between CCN2 and TGF-β in the process of a sustained fibrotic response [20,21]. Kothapalli et al. [22] were the first to show that CCN2 is induced by TGF-β and that it is required for the TGF-β induction of collagen. Recent studies [23] from our laboratory have provided additional mechanistic insights into this process. We obtained the evidence that in fibroblasts CCN2 is required for the activation of Smad1, but it is dispensable for the activation of the Smad3 pathway. Specifically, CCN2 through binding to the αvβ3 integrin receptor activates Src and Erk1/2 signaling pathways, which are required for the TGF-β-induced activation of Smad1. However, alone, CCN2 is not able to activate Smad1 and is only a weak inducer of collagen (Fig. 1). Although additional details of this pathway still need to be elucidated, these recent observations further clarify the mechanism of constitutively activated TGF-β signaling in SSc fibroblasts. As depicted in the hypothetical model in Fig. 1, endogenously produced TGF-β signals through the receptor complex consisting of the TGF-βRI (ALK5), ALK1 and endoglin, leading to activation of Smad1 and its downstream target CCN2. Concurrently, CCN2 activates Erk1/2 in a Src-dependent manner, which is required for the sustained activation of Smad1 [8]. It is likely that αvβ3 integrin is in complex with TGF-β receptors, thus allowing the simultaneous activation of the downstream signaling. Formation of such complexes was described in other experimental models [24]. Given that CCN2 and TGF-β can bind to each other [20], it is plausible that a dual ligand–receptor complex is responsible for activation of Smad1/Erk1/2 signaling and downstream profibrotic effects in SSc. Although, TGF-βRI and endoglin appear to be the key components of this dysregulated TGF-β signaling, the mechanism leading to their elevated expression in SSc fibroblasts is unknown.
Figure 1. Activation of the autocrine CCN2–Smad1 signaling in scleroderma fibroblasts: a hypothetical model.
Activation of the Smad1 pathway requires cooperation between two ligands, transforming growth factor β (TGF-β) and CCN2, signaling through their cognate receptors. Elevated levels of endoglin [17] promote TGF-β signaling through a receptor complex also consisting of ALK5, ALK1, and TGF-β RII, which leads to induced phosphorylation of Smad1 [7,8]. Concurrent activation of ERK1/2 is required for Smad1 phosphorylation, although the specific role of ERK1/2 in this process has not yet been elucidated [8]. Binding of CCN2 to the αvβ3 integrin receptor activates ERK1/2 in a Src-dependent manner. As CCN2 has been shown to bind TGF-β and enhance TGF-β receptor binding and signaling [20], activation of Smad1/Erk1/2 signaling can occur in a spatially and temporally coordinated manner. Smad1 and Erk1/2 activate CCN2 gene expression leading to elevated CCN2 production, which then contributes to sustained activation of this pathway. Several components of this signaling pathway are expressed at elevated levels in scleroderma (SSc) fibroblasts, including ALK5, endoglin, αvβ3 integrin, Erk1/2, Smad1, and CCN2.
Dysregulation of transcriptional repressors of collagen gene in scleroderma fibroblasts
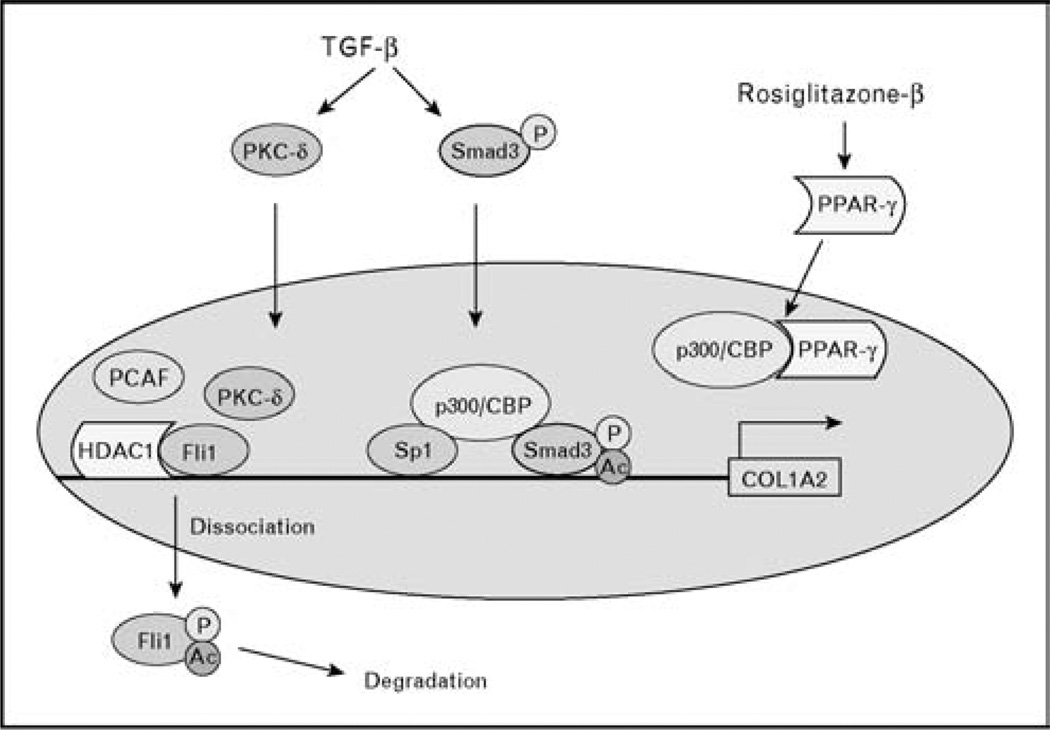
Recent studies, which uncovered the presence of endogenous suppressors of collagen gene expression, brought to light a new aspect of TGF-β signaling with direct relevance to SSc fibrosis. The best characterized examples of such suppressor molecules are Friend leukemia integration-1 (Fli1) and perixosome proliferator-activated receptor-γ (PPAR-γ). Fli1 is a member of the E26 transformation-specific (ETS) transcription factor family characterized by the presence of the evolutionarily conserved DNA-binding domain, which recognizes purine-rich core sequence [25]. Several earlier studies performed in cultured fibroblasts have established that Fli1 inhibits expression of type I collagen gene and counteracts TGF-β stimulation of collagen [26,27]. In quiescent fibroblasts, Fli1 occupies the collagen promoter and represses its transcription through recruitment of the histone deacetylase 1 (HDAC1) (Asano, Kapanadze, and Trojanowska, unpublished data). Initiation of collagen gene transcription requires displacement of this complex. In the case of TGF-β, this is achieved through the posttranslational modification of Fli1. In response to TGF-β stimulation, PKC-δ translocates to the nucleus in which it phosphorylates Fli1 on threonine 312 [28•]. This phosphorylation is required for the subsequent acetylation of Fli1 on lysine 380 by PCAF, which leads to Fli1 dissociation from the DNA and a de-repression of collagen gene transcription [29] (Fig. 2). Downregulation of Fli1 may also occur through transcriptional mechanisms, as was reported for other profibrogenic factors such as adenosine and IL-13 [30].
Figure 2. Regulation of collagen gene transcription by endogenous repressors.
Transcription factor Friend leukemia integration-1 (Fli1) functions as a constitutive repressor of collagen gene transcription by recruiting HDAC1 to the collagen promoter, which leads to reduced levels of histone acetylation (Asano, Kapanadze, and Trojanowska, unpublished observations). Activation of collagen gene transcription requires inactivation of Fli1. Transforming growth factor β (TGF-β) inactivates Fli1 through the mechanism that involves phosphorylation of Fli1 on threonine 312 by PKC-δ, subsequent acetylation of Fli1 on lysine 380 by PCAF, and dissociation of Fli1 from the DNA [28•,29]. perixosome proliferator-activated receptor-γ (PPAR-γ) functions as an inducible repressor of collagen gene transcription. In response to agonist stimulation PPAR-γ translocates to the nucleus in which it competes with other transcriptional activators, such as Smad2/3, for the limited amount of histone acetyltransferase p300.
Development of mice with the homozygous deletion of C-terminal domain of Fli1 (Fli1 ΔCTA) provided additional insights into the role of Fli1 in collagen homeostasis in the skin [31]. The absence of functional Fli1 resulted in transcriptional de-repression of all fibrillar collagen genes, thus validating the central role of Fli1 in maintaining collagen homeostasis in the skin in vivo. The mouse studies also revealed that Fli1 influences expression of other genes important for the process of fibrillogenesis, including decorin, lumican, and fibromodulin; however, further studies are needed to elucidate the full spectrum of the biological roles of Fli1. Nonetheless, these studies firmly establish the key role of Fli1 in maintaining collagen genes in a repressed state and the necessity to downregulate Fli1 during physiological and pathological matrix remodeling. Such a role of Fli1 is also supported by the in-situ analyses of skin fibroblasts in healthy individuals and in patients with SSc, which found an inverse correlation between Fli1 and type I collagen levels [32].
PPAR-γ, a member of the nuclear hormone receptor superfamily, is a ligand-activated intracellular transcription factor and an important regulator of glucose and lipid homeostasis [33]. Ligands for PPAR-γ include 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2), as well as fatty acids, eiconosanoids, and oxidized lipids. Importantly, PPAR-γ has been shown to be a target of the insulin-sensitizing drugs represented by thiazolidinediones. A potent PPAR-γ agonist, rosiglitazone (Avandia; Glaxo-SmithKline, London, UK), has also been widely used to characterize its biological function. Recent studies have provided new insights into the antifibrotic properties of PPAR-γ. The study by Xu et al. [34] has delineated the molecular mechanism involved in the repression of collagen gene transcription by interferon-γ (IFN-γ). Their studies show that IFN-γ suppresses collagen transcription through recruitment to the collagen transcription start site of a multiprotein repressor complex containing PPAR-γ, class II trans-activator (CIIA), and regulatory factor for X-box 5 (RFX5). A different molecular mechanism was proposed to explain the antagonistic effect of PPAR-γ on the profibrotic effects of TGF-β. It was shown that PPAR-γ blocks the transcriptional activity of Smad2/3 by preventing the recruitment of the key co-activator, histone acetyltransferase p300, to the collagen promoter, thus resulting in a reduced histone H4 hyperacetylation and a reduced collagen gene transcription [35•] (Fig. 2). The role for PPAR-γ as an effective inhibitor of dermal fibrosis was recently demonstrated in an animal model of scleroderma, bleomycin-induced skin fibrosis [36]. Administration of rosiglitazone significantly diminished inflammation and fibrosis in this model. Specifically, rosiglitazone reduced monocyte infiltration and prevented collagen upregulation and myofibroblast accumulation. Consistent with the role of PPAR-γ in adipogenesis, rosiglitazone reversed bleomycininduced lipoatrophy.
A cross-talk between PTEN and sphingosine kinase metabolites regulates transforming growth factor β signaling
Sphingosine kinase is a lipid kinase that catalyzes formation of two bioactive lipid mediators, sphingosine-1 phosphate (S1P) and dihydrosphingosine-1 phosphate (dhS1P). Sphingosine kinase and its metabolite, S1P, have emerged as important regulators of a wide range of biological processes, including cell growth and proliferation, cell survival and apoptosis, calcium homeostasis, angiogenesis, and vascular remodeling [37]. S1P is abundant in plasma and plays an important role in the immune system by regulating lymphocyte egress from the thymus and secondary lymphoid organs [37]. S1P mimetic (FTY720, fingolimod) interferes with the immunomodulatory function of S1P and has been shown in phase 2 clinical trials to be effective in the treatment of relapsing multiple sclerosis [38]. Recent evidence suggests that S1P may also play an important role in fibrosis through a cross-talk with the TGF-β pathway. Activation of TGF-β/Smad signaling in response to S1P stimulation has been reported in several experimental systems including keratinocytes [39], mesangial cells [40], and dermal fibroblasts [41], suggesting a universal nature of this cross-talk. Interestingly, similar to S1P, FTY720 has a potent profibrotic effect, suggesting that despite its immunosuppressive function, it may not be suitable as a treatment for SSc. Although the specific mechanism by which S1P activates TGF-β signaling is presently unknown, studies from keratinocytes and mesangial cells indicate that S1P response requires functional TGF-β receptors [39,40]. Other studies have demonstrated that sphingosine kinase is upregulated during bleomycin-induced lung fibrosis and contributes to the TGF-β-induced myofibroblast differentiation of lung fibroblasts [42]. We observed that dermal fibroblasts obtained from patients with SSc are significantly more responsive to the profibrotic effects of S1P, suggesting that sphingosine kinase may contribute to activation of TGF-β signaling in SSc [43]. Given the potent profibrotic effects of S1P, it would be important to evaluate distribution of S1P in plasma of SSc patients, as well as the expression level and/or activation status of sphingosine kinase in SSc fibroblasts in vitro and in vivo.
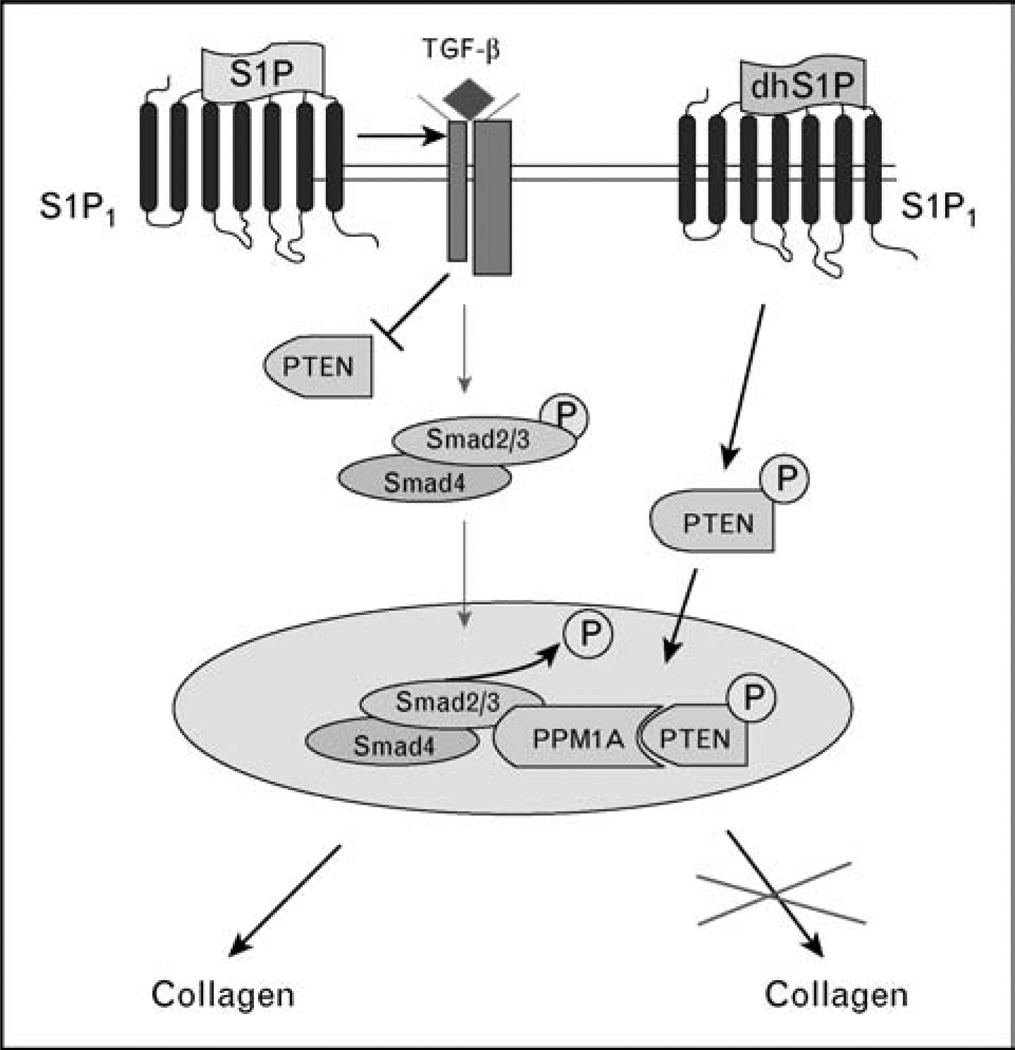
Recent studies [41] from our laboratory demonstrated that in contrast to the profibrotic function of S1P, dhS1P elicits a potent antifibrotic effect through the mechanism that involves tumor suppressor, phosphatase and tensin homolog, PTEN. PTEN encodes a lipid phosphatase that dephosphorylates PtdIns(3,4,5)P3 (PIP3) leading to the inhibition of PI3 kinase/Akt signaling. There is also evidence that PTEN, through the protein–protein interaction involving its C terminal domain, has cellular functions that do not depend on its lipid phosphatase activity [44]. Our studies uncovered a novel function of PTEN as a co-factor of Smad3 phosphatase, protein phosphatase 1A (PPM1A). PPM1A is rapidly degraded in response to TGF-β signaling; however, co-stimulation with dhS1P prevents its degradation through the mechanism that involves translocation of PTEN into the nucleus and formation of the PPM1A/PTEN complexes. This, in turn, allows PPM1A to dephosphorylate nuclear Smads [41] (Fig. 3). Interestingly, PTEN deficiency has recently been linked to lung fibrosis [45]. It was shown that expression of PTEN is diminished in myofibroblasts in vivo in lungs of patients with idiopathic pulmonary fibrosis. The authors demonstrated that pharmacologic inhibition of PTEN resulted in worsened manifestation in an experimental model of pulmonary fibrosis. Furthermore, fibroblasts derived from pten−/− mice showed elevated collagen and α-SMA levels suggesting that PTEN negatively regulates myofibroblast differentiation. We observed that cultured SSc fibroblasts exhibit a markedly reduced expression level of PTEN [43]. A significant reduction of PTEN in a large proportion of fibroblasts was also observed in lesional SSc skin in vivo (Asano and Trojanowska, unpublished observations). As TGF-β downregulates PTEN expression, the reduced levels of PTEN in SSc fibroblasts are likely due to the activation of TGF-β signaling. Importantly, addition of dhS1P normalized PTEN levels in SSc fibroblasts, together with a reduction of collagen synthesis, upregulation of MMP1, and a decrease of the levels of phosphorylated Smad3. More importantly, SSc, but not control fibroblasts, were responsive to the antifibrotic effects of dhS1P [43], suggesting a potential role for this sphingolipid, or its derivative, as a therapeutic agent.
Figure 3. Antagonistic effects of sphingosine-1 phosphate and dihydrosphingosine-1 phosphate on the transforming growth factor β/Smad signaling pathway.
Sphingosine-1 phosphate (S1P) treatment reproduces profibrotic effects of transforming growth factor β (TGF-β) through the enhancement of the endogenous TGF-β ligand/receptor signaling (Bu and Trojanowska, unpublished data), but the specific details of this process remain to be clarified. In contrast, dihydrosphingosine-1 phosphate (dhS1P) suppresses TGF-β signaling through the TGF-β receptor-independent, PTEN-dependent mechanism [41]. DhS1P treatment triggers rapid phosphorylation of PTEN and its subsequent translocation to the nucleus, in which PTEN facilitates Smad2/3 dephosphorylation by stabilizing Smad1/2 phosphatase, PPM1A. Interestingly, both sphingolipid mediators utilize the same receptor, S1P1, suggesting that additional, presently unknown components of these pathways contribute to the distinct postreceptor signaling.
From molecular targets to the antifibrotic therapies for scleroderma
Because of its established pathological role in fibrotic diseases, TGF-β is the most obvious candidate for therapeutic intervention in SSc. A comprehensive review of the strategies to block TGF-β signaling has recently been published [46]. One of the best characterized inhibitors of the noncanonical TGF-β signaling pathway is imatinib mesylate (Gleevec, Novartis Pharmaceuticals, Basel, Switzerland), a small molecule inhibitor of the Bcr-Abl, which has been successfully used for treatment of chronic meylogenous leukemia. The studies by Daniels et al. [47] have shown that the nonreceptor tyrosine kinase c-Abl mediates the profibrotic effects of TGF-β in a Smad3-independent manner and that imatinib inhibits these effects in vitro and in a bleomycin model of pulmonary fibrosis. Recent studies [48,49] have also shown amelioration of dermal fibrosis in animal models using imatinib derivatives, desatinib and nilotinib. Imatinib mesylate has also been tried in a small number of SSc patients, but so far the results have been inconsistent [50]. One possible explanation is that this therapy was only beneficial for patients with fibrosis due to the activated c-Abl kinase. If that assumption was correct, it would be important to select patients based on the evidence of activation of this pathway. Some of the recently identified downstream mediators of c-Abl, which can serve as markers of activation of this pathway, include Erk1/2, Egr1, and Smad1 [13•,51]. Other potential therapeutic targets that were recently evaluated include Src [52] and Rho-associated kinase [53]. The list of potential antifibrotic agents is rapidly growing and also includes antifibrotic agents that do not directly target TGF-β receptors or components of the TGF-β signaling pathway. For example adenosine A2A receptor antagonists and α-melanocyte-stimulating hormone were shown to be effective in ameliorating dermal fibrosis in animal models [54,55].
Conclusion
Characterization of the noncanonical TGF-β signaling pathways extends the repertoire of the possible anti-fibrotic therapeutic targets beyond TGF-β ligand and its signaling receptors. As these signaling molecules represent common pathways activated in other diseases, such as cancer, there are available inhibitors that have been evaluated in preclinical or clinical studies for many of these molecules. One of the main limitations in testing antifibrotic drugs for SSc is an absence of an animal model that reproduces the chronic fibrotic process specific to this disease. The most commonly used animal model of dermal fibrosis is based on bleomycin injection, which causes acute inflammation and transient fibrosis. It is possible that more recently developed animal models, which are based on perturbation of the TGF-β signaling, will more adequately represent SSc fibrosis [1]. So far, however, these models have not been fully characterized and are not widely used for testing potential therapies.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 663).
- 1.Varga JA, Trojanowska M. Fibrosis in systemic sclerosis. Rheum Dis Clin N Am. 2008;34:115–143. vii. doi: 10.1016/j.rdc.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapanadze B, Morris E, Smith E, Trojanowska M. Establishment and characterization of scleroderma fibroblast clonal cell lines by introduction of the hTERT gene. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00773.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sargent JL, Milano A, Connolly MK, Whitfield ML. Scleroderma gene expression and pathway signatures. Curr Rheumatol Rep. 2008;10:205–211. doi: 10.1007/s11926-008-0034-5. [DOI] [PubMed] [Google Scholar]
- 4.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 5.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Pannu J, Nakerakanti S, Smith E, et al. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–10413. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 9.Liu IM, Schilling SH, Knouse KA, et al. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrighton KH, Lin X, Yu PB, Feng XH. Transforming growth factor {beta} can stimulate Smad1 phosphorylation independently of bone morphogenic protein receptors. J Biol Chem. 2009;284:9755–9763. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe H, Matsubara T, Iehara N, et al. Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J Biol Chem. 2004;279:14201–14206. doi: 10.1074/jbc.M310427200. [DOI] [PubMed] [Google Scholar]
- 12.Wiercinska E, Wickert L, Denecke B, et al. Id1 is a critical mediator in TGF-beta-induced transdifferentiation of rat hepatic stellate cells. Hepatology. 2006;43:1032–1041. doi: 10.1002/hep.21135. [DOI] [PubMed] [Google Scholar]
- 13. Pannu J, Asano Y, Nakerakanti S, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58:2528–2537. doi: 10.1002/art.23698. This study provides evidence for the activation of the Smad1 pathway in SSc fibroblasts in vitro and in vivo and demonstrates the functional relevance of this pathway to SSc fibrosis.
- 14.Holmes A, Abraham DJ, Sa S, et al. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Ou J, Inagaki Y, et al. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem. 2000;275:39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- 16.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leask A, Abraham DJ, Finlay DR, et al. Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002;46:1857–1865. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- 18.Morris E, Bujor A, ten Dijke P, Trojanowska M. The role of ALK1 and endoglin signaling in scleroderma fibrosis. Proceedings of the American Society for Cell Biology 48th Annual Meeting; San Francisco CA. 2008. abstract 103. [Google Scholar]
- 19.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori T, Kawara S, Shinozaki M, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Kothapalli D, Frazier KS, Welply A, et al. Transforming growth factor beta induces anchorage-independent growth of NRK fibroblasts via a connective tissue growth factor-dependent signaling pathway. Cell Growth Differ. 1997;8:61–68. [PubMed] [Google Scholar]
- 23.Nakerakanti S, Trojanowska M. CCN2-Smad1 Autocrine loop: key regulatory pathway in the TGF-beta induced fibrosis. Keystone Symposia on Molecular and Cellular Biology Keystone CO; Fibrosis. 2009. Abstract 224. [Google Scholar]
- 24.Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truong AH, Ben-David Y. The role of Fli-1 in normal cell function and malignant transformation. Oncogene. 2000;19:6482–6489. doi: 10.1038/sj.onc.1204042. [DOI] [PubMed] [Google Scholar]
- 26.Czuwara-Ladykowska J, Shirasaki F, Jackers P, et al. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem. 2001;276:20839–20848. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 27.Nakerakanti SS, Kapanadze B, Yamasaki M, et al. Fli1 and Ets1 have distinct roles in connective tissue growth factor/CCN2 gene regulation and induction of the profibrotic gene program. J Biol Chem. 2006;281:25259–25269. doi: 10.1074/jbc.M600466200. [DOI] [PubMed] [Google Scholar]
- 28. Asano Y, Trojanowska M. Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol Cell Biol. 2009;29:1882–1894. doi: 10.1128/MCB.01320-08. This study delineates molecular mechanism underlying inactivation of Fli1 by TGF-beta signaling.
- 29.Asano Y, Czuwara J, Trojanowska M. Transforming growth factor-beta regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J Biol Chem. 2007;282:34672–34683. doi: 10.1074/jbc.M703907200. [DOI] [PubMed] [Google Scholar]
- 30.Chan ES, Liu H, Fernandez P, et al. Modulation of IL-13 and Fli1 by adenosine: implication for scleroderma. Arthritis Rheum. 2009;58(Suppl) abstract 1811. [Google Scholar]
- 31.Asano Y, Markiewicz M, Kubo M, et al. Transcription factor Fli1 regulates collagen fibrillogenesis in mouse skin. Mol Cell Biol. 2009;29:425–434. doi: 10.1128/MCB.01278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo M, Czuwara-Ladykowska J, Moussa O, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Farmer SR, Smith BD. Peroxisome proliferator-activated receptor gamma interacts with CIITA × RFX5 complex to repress type I collagen gene expression. J Biol Chem. 2007;282:26046–26056. doi: 10.1074/jbc.M703652200. [DOI] [PubMed] [Google Scholar]
- 35. Ghosh AK, Bhattacharyya S, Wei J, et al. Peroxisome proliferator-activated receptor-{gamma} abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 2009 doi: 10.1096/fj.08-128736. [Epub ahead of print] This study clarifies the mechanism of the inhibitory effects of PPAR-γ on the TGF-β/Smad signaling pathway.
- 36.Wu M, Melichian DS, Chang E, et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 38.Takabe K, Paugh SW, Milstien S, Spiegel S. ‘Inside-out’ signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer B, Vogler R, von Wenckstern H, et al. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- 40.Xin C, Ren S, Kleuser B, et al. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- 41.Bu S, Kapanadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem. 2008;283:19593–19602. doi: 10.1074/jbc.M802417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kono Y, Nishiuma T, Nishimura Y, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 43.Bu S, Trojanowska M. DhS1P has a potent antifibrotic effect in Scleroderma fibroblasts via modulation of PTEN levels; Keystone Symposia for Molecular and Cellular Biology, Keystone CO; Fibrosis. 2009. abstract 109. [Google Scholar]
- 44.Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120:4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 45.White ES, Atrasz RG, Hu B, et al. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga J, Pasche B. Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr Opin Rheumatol. 2008;20:720–728. doi: 10.1097/BOR.0b013e32830e48e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhmetshina A, Dees C, Pileckyte M, et al. Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J. 2008;22:2214–2222. doi: 10.1096/fj.07-105627. [DOI] [PubMed] [Google Scholar]
- 49.Akhmetshina A, Venalis P, Dees C, et al. Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum. 2009;60:219–224. doi: 10.1002/art.24186. [DOI] [PubMed] [Google Scholar]
- 50.Distler JH, Distler O. Imatinib as a novel therapeutic approach for fibrotic disorders. Rheumatology (Oxford) 2009;48:2–4. doi: 10.1093/rheumatology/ken431. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharyya S, Ishida W, Wu M, et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene. 2009;28:1285–1297. doi: 10.1038/onc.2008.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skhirtladze C, Distler O, Dees C, et al. Src kinases in systemic sclerosis: central roles in fibroblast activation and in skin fibrosis. Arthritis Rheum. 2008;58:1475–1484. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- 53.Akhmetshina A, Dees C, Pileckyte M, et al. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum. 2008;58:2553–2564. doi: 10.1002/art.23677. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez P, Trzaska S, Wilder T, et al. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am J Pathol. 2008;172:1675–1682. doi: 10.2353/ajpath.2008.070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokot A, Sindrilaru A, Schiller M, et al. alpha-Melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma: melanocortin peptides as a novel treatment strategy for scleroderma? Arthritis Rheum. 2009;60:592–603. doi: 10.1002/art.24228. [DOI] [PubMed] [Google Scholar]