Abstract
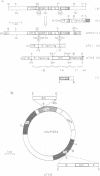
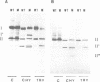
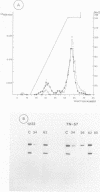
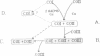
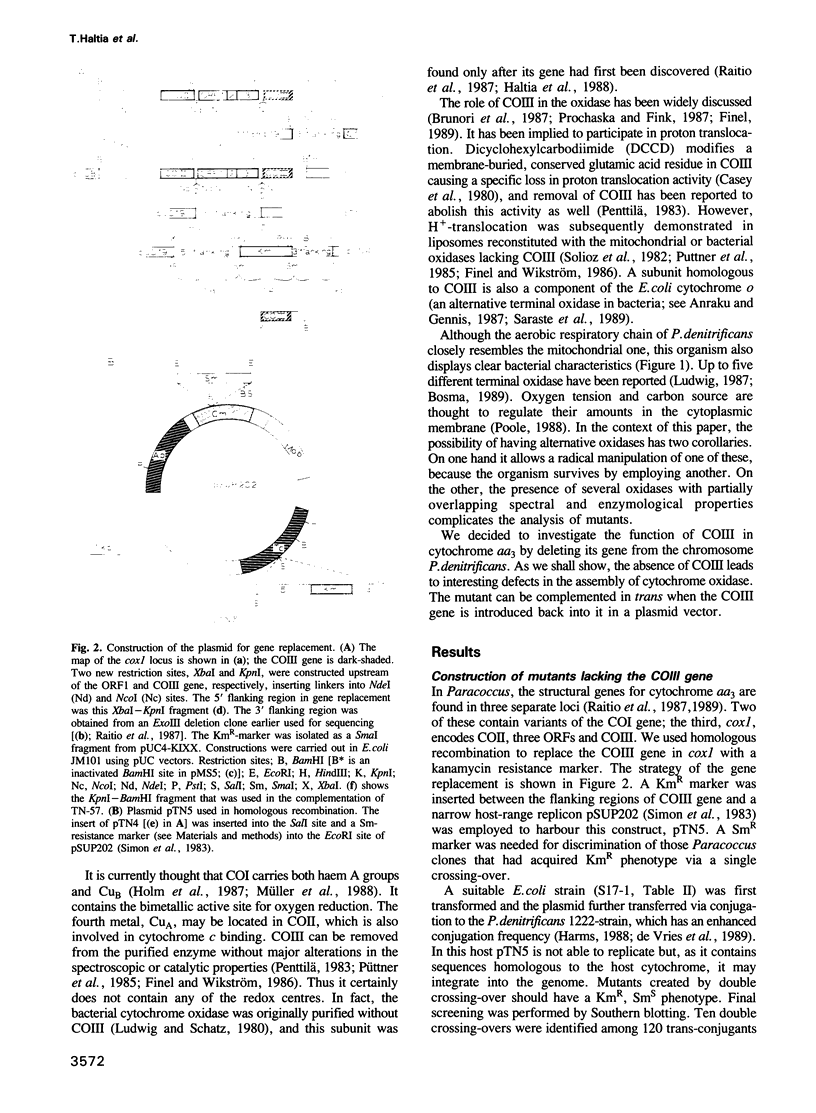
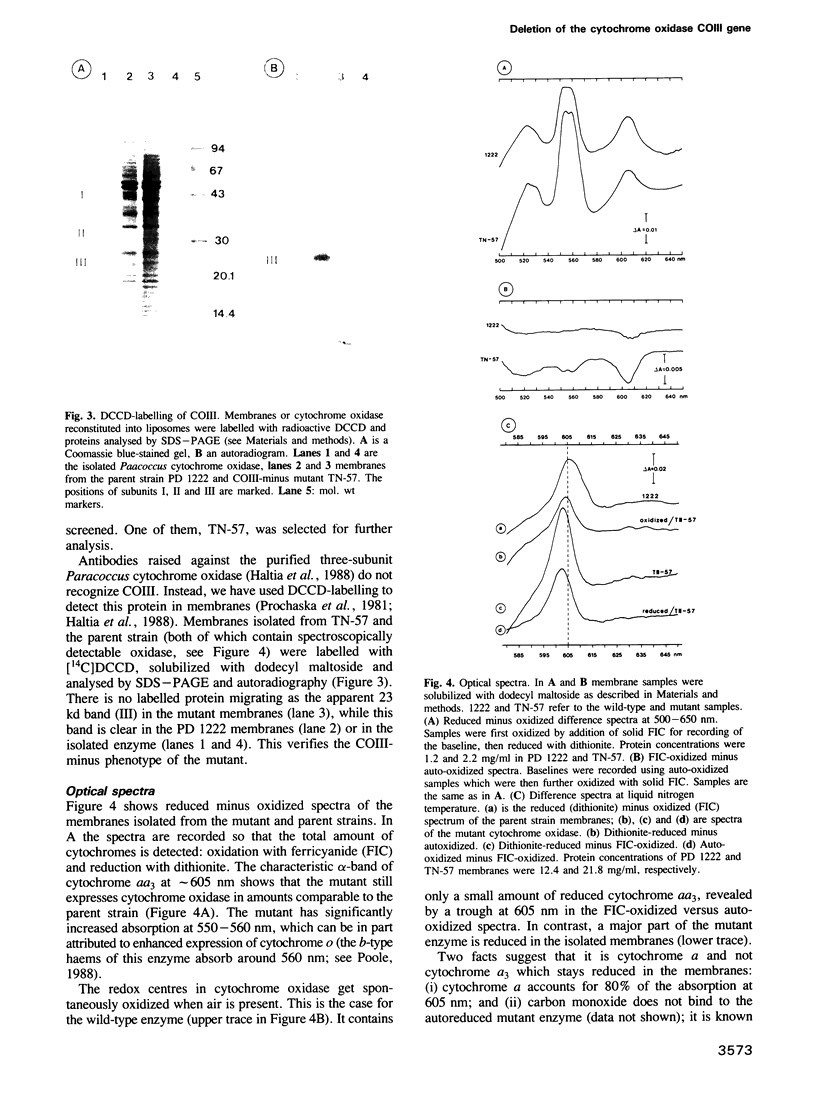
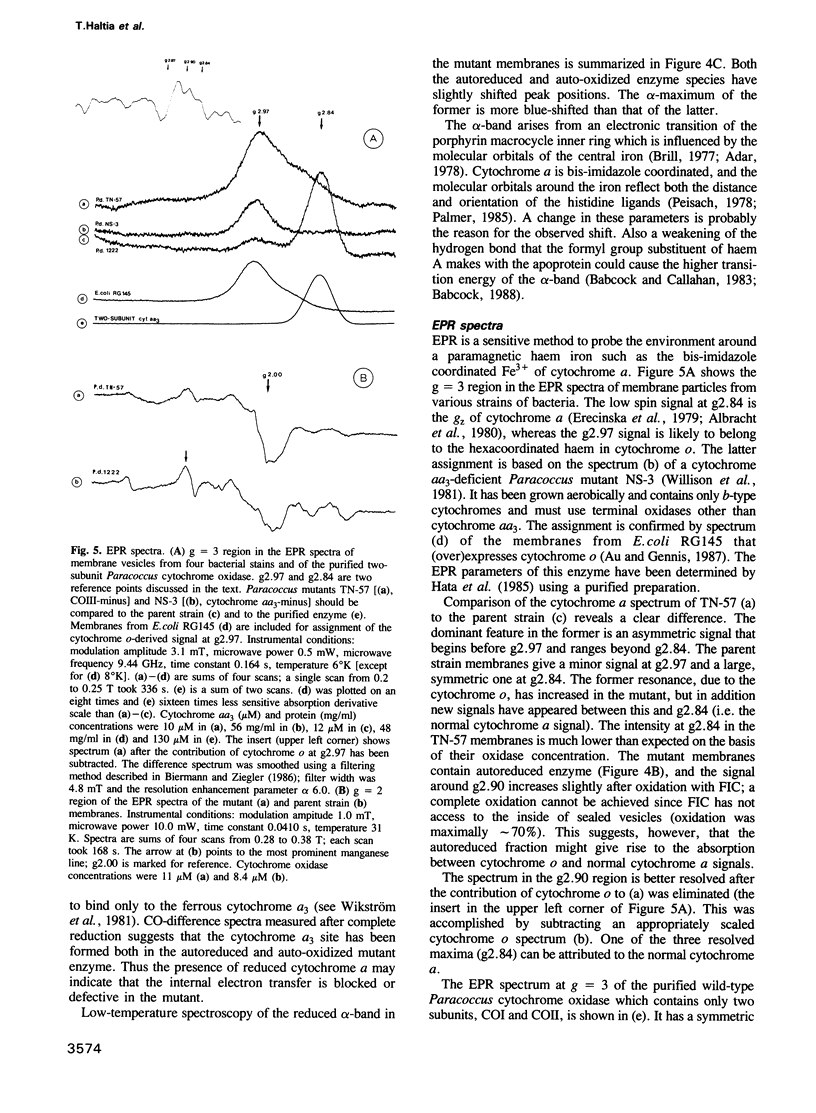
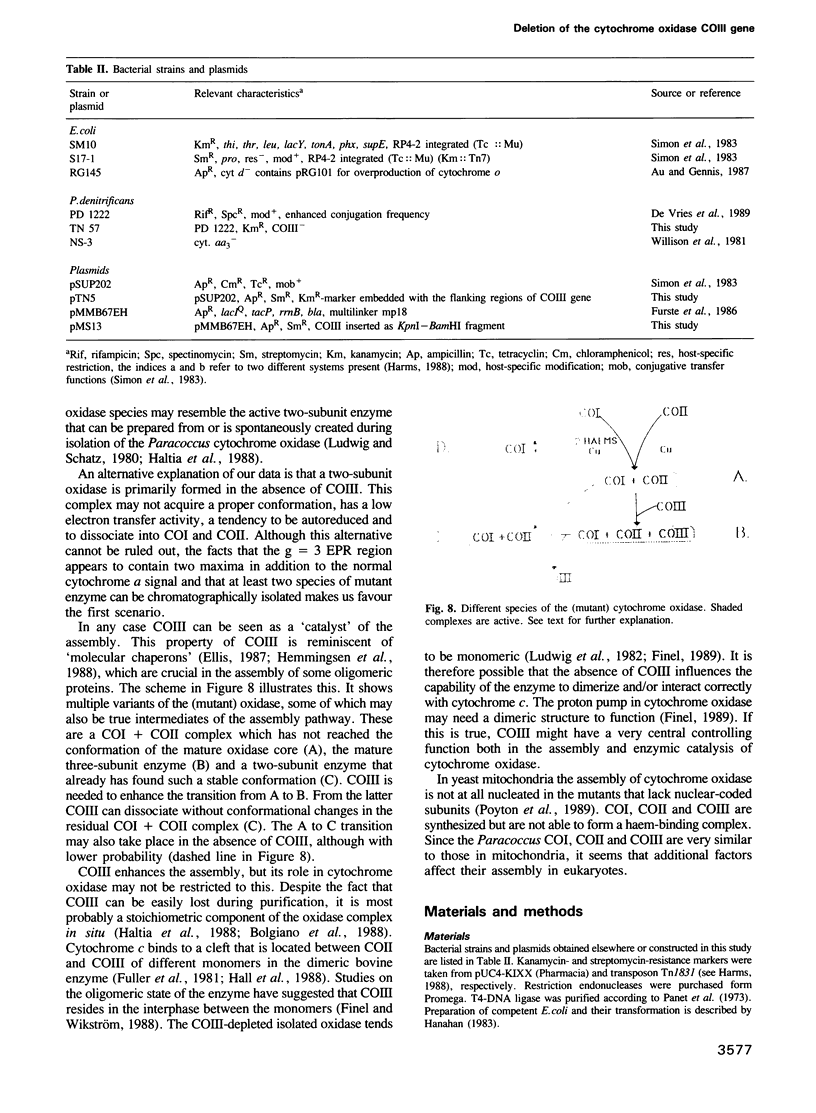
COIII is one of the major subunits in the mitochondrial and a bacterial cytochrome c oxidase, cytochrome aa3. It does not contain any of the enzyme's redox-active metal centres and can be removed from the enzyme without major changes in its established functions. We have deleted the COIII gene from Paracoccus denitrificans. The mutant still expresses spectroscopically detectable enzyme almost as the wild-type, but its cytochrome c oxidase activity is much lower. From 50 to 80% of cytochrome a is reduced and its absorption maximum is 2-3 nm blue-shifted. The EPR signal of ferric cytochrome a is heterogeneous indicating the presence of multiple cytochrome a species. Proteolysis of the membrane-bound oxidase shows new cleavage sites both in COI and COII. DEAE-chromatography of solubilized enzyme yields fractions that contain a COI + COII complex and in addition haem-binding, free COI as well as free COII. The mutant phenotype can be complemented by introducing the COIII gene back to cells in a plasmid vector. We conclude that cytochrome oxidase assembles inefficiently in the absence of COIII and that this subunit may facilitate a late step in the assembly. The different oxidase species in the mutant represent either accumulating intermediates of the assembly pathway or dissociation products of a labile COI + COII complex and its conformational variants.
Full text
PDF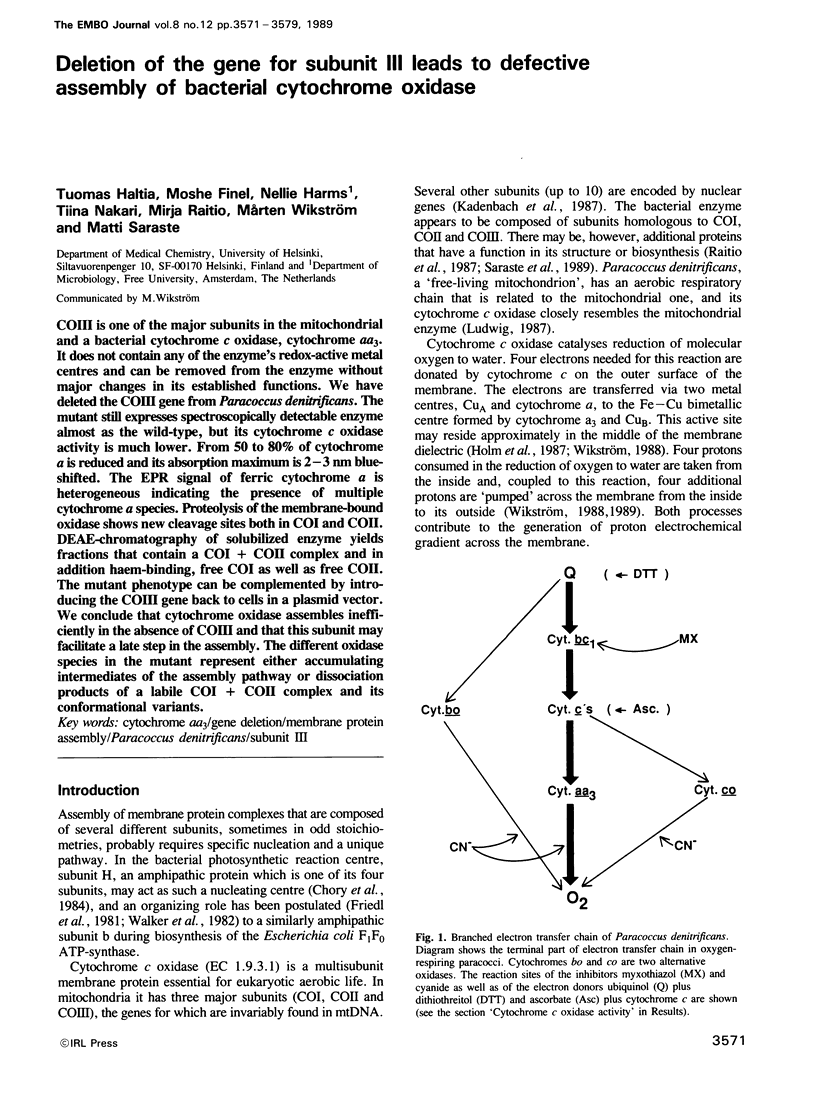




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P., van Verseveld H. W., Hagen W. R., Kalkman M. L. A comparison of the respiratory chain in particles from Paracoccus denitrificans and bovine heart mitochondria by EPR spectroscopy. Biochim Biophys Acta. 1980 Dec 3;593(2):173–186. doi: 10.1016/0005-2728(80)90055-9. [DOI] [PubMed] [Google Scholar]
- Au D. C., Gennis R. B. Cloning of the cyo locus encoding the cytochrome o terminal oxidase complex of Escherichia coli. J Bacteriol. 1987 Jul;169(7):3237–3242. doi: 10.1128/jb.169.7.3237-3242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M. Redox-linked hydrogen bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983 May 10;22(10):2314–2319. doi: 10.1021/bi00279a002. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J Biol Chem. 1985 Feb 25;260(4):2458–2467. [PubMed] [Google Scholar]
- Bolgiano B., Smith L., Davies H. C. Electron transport reactions in a cytochrome c-deficient mutant of Paracoccus denitrificans. Biochim Biophys Acta. 1989 Feb 28;973(2):227–234. doi: 10.1016/s0005-2728(89)80426-8. [DOI] [PubMed] [Google Scholar]
- Bolgiano B., Smith L., Davies H. C. Kinetics of the interaction of the cytochrome c oxidase of Paracoccus denitrificans with its own and bovine cytochrome c. Biochim Biophys Acta. 1988 Apr 22;933(2):341–350. doi: 10.1016/0005-2728(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Brunori M., Antonini G., Malatesta F., Sarti P., Wilson M. T. Cytochrome-c oxidase. Subunit structure and proton pumping. Eur J Biochem. 1987 Nov 16;169(1):1–8. doi: 10.1111/j.1432-1033.1987.tb13572.x. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Thelen M., Azzi A. Dicyclohexylcarbodiimide binds specifically and covalently to cytochrome c oxidase while inhibiting its H+-translocating activity. J Biol Chem. 1980 May 10;255(9):3994–4000. [PubMed] [Google Scholar]
- Chory J., Donohue T. J., Varga A. R., Staehelin L. A., Kaplan S. Induction of the photosynthetic membranes of Rhodopseudomonas sphaeroides: biochemical and morphological studies. J Bacteriol. 1984 Aug;159(2):540–554. doi: 10.1128/jb.159.2.540-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Blasie J. K. Studies of the orientation of the mitochondrial redox carriers. III. Orientation of the gx and gy axes of the hemes of cytochrome oxidase with respect to the plane of the membrane in oriented membrane multilayers. Biochim Biophys Acta. 1979 Feb 8;545(2):352–364. doi: 10.1016/0005-2728(79)90212-3. [DOI] [PubMed] [Google Scholar]
- Finel M. Proteolysis of Paracoccus denitrificans cytochrome oxidase by trypsin and chymotrypsin. FEBS Lett. 1988 Aug 29;236(2):415–419. doi: 10.1016/0014-5793(88)80068-1. [DOI] [PubMed] [Google Scholar]
- Finel M., Wikström M. Monomerization of cytochrome oxidase may be essential for the removal of subunit III. Eur J Biochem. 1988 Sep 1;176(1):125–129. doi: 10.1111/j.1432-1033.1988.tb14259.x. [DOI] [PubMed] [Google Scholar]
- Finel M., Wikström M. Studies on the role of the oligomeric state and subunit III of cytochrome oxidase in proton translocation. Biochim Biophys Acta. 1986 Aug 13;851(1):99–108. doi: 10.1016/0005-2728(86)90253-7. [DOI] [PubMed] [Google Scholar]
- Friedl P., Bienhaus G., Hoppe J., Schairer H. U. The dicyclohexylcarbodiimide-binding protein c of ATP synthase from Escherichia coli is not sufficient to express an efficient H+ conduction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6643–6646. doi: 10.1073/pnas.78.11.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., Darley-Usmar V. M., Capaldi R. A. Covalent complex between yeast cytochrome c and beef heart cytochrome c oxidase which is active in electron transfer. Biochemistry. 1981 Nov 24;20(24):7046–7053. doi: 10.1021/bi00527a043. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Hall J., Moubarak A., O'Brien P., Pan L. P., Cho I., Millett F. Topological studies of monomeric and dimeric cytochrome c oxidase and identification of the copper A site using a fluorescence probe. J Biol Chem. 1988 Jun 15;263(17):8142–8149. [PubMed] [Google Scholar]
- Haltia T., Puustinen A., Finel M. The Paracoccus denitrificans cytochrome aa3 has a third subunit. Eur J Biochem. 1988 Mar 15;172(3):543–546. doi: 10.1111/j.1432-1033.1988.tb13923.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hata A., Kirino Y., Matsuura K., Itoh S., Hiyama T., Konishi K., Kita K., Anraku Y. Assignment of ESR signals of Escherichia coli terminal oxidase complexes. Biochim Biophys Acta. 1985 Oct 29;810(1):62–72. doi: 10.1016/0005-2728(85)90206-3. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig B. Cytochrome c oxidase from Paracoccus denitrificans. Methods Enzymol. 1986;126:153–159. doi: 10.1016/s0076-6879(86)26017-6. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Grabo M., Gregor I., Lustig A., Regenass M., Rosenbusch J. P. Solubilized cytochrome c oxidase from Paracoccus denitrificans is a monomer. J Biol Chem. 1982 May 25;257(10):5576–5578. [PubMed] [Google Scholar]
- Ludwig B., Schatz G. A two-subunit cytochrome c oxidase (cytochrome aa3) from Paracoccus dentrificans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):196–200. doi: 10.1073/pnas.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Müller M., Schlapfer B., Azzi A. Preparation of a one-subunit cytochrome oxidase from Paracoccus denitrificans: spectral analysis and enzymatic activity. Biochemistry. 1988 Sep 20;27(19):7546–7551. doi: 10.1021/bi00419a055. [DOI] [PubMed] [Google Scholar]
- Palmer G. The electron paramagnetic resonance of metalloproteins. Biochem Soc Trans. 1985 Jun;13(3):548–560. doi: 10.1042/bst0130548. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Penttilä T. Properties and reconstitution of a cytochrome oxidase deficient in subunit III. Eur J Biochem. 1983 Jun 15;133(2):355–361. doi: 10.1111/j.1432-1033.1983.tb07470.x. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Inhibition of cytochrome c oxidase function by dicyclohexylcarbodiimide. Biochim Biophys Acta. 1981 Sep 14;637(2):360–373. doi: 10.1016/0005-2728(81)90175-4. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Fink P. S. On the role of subunit III in proton translocation in cytochrome c oxidase. J Bioenerg Biomembr. 1987 Apr;19(2):143–166. doi: 10.1007/BF00762722. [DOI] [PubMed] [Google Scholar]
- Puettner I., Carafoli E., Malatesta F. Spectroscopic and functional properties of subunit III-depleted cytochrome oxidase. J Biol Chem. 1985 Mar 25;260(6):3719–3723. [PubMed] [Google Scholar]
- Puustinen A., Finel M., Virkki M., Wikström M. Cytochrome o (bo) is a proton pump in Paracoccus denitrificans and Escherichia coli. FEBS Lett. 1989 Jun 5;249(2):163–167. doi: 10.1016/0014-5793(89)80616-7. [DOI] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Raitio M., Jalli T., Chepuri V., Lemieux L., Gennis R. B. Cytochrome o from Escherichia coli is structurally related to cytochrome aa3. Ann N Y Acad Sci. 1988;550:314–324. doi: 10.1111/j.1749-6632.1988.tb35346.x. [DOI] [PubMed] [Google Scholar]
- Seelig A., Ludwig B., Seelig J., Schatz G. Copper and manganese electron spin resonance studies of cytochrome c oxidase from Paracoccus denitrificans. Biochim Biophys Acta. 1981 Jul;636(2):162–167. doi: 10.1016/0005-2728(81)90089-x. [DOI] [PubMed] [Google Scholar]
- Solioz M., Carafoli E., Ludwig B. The cytochrome c oxidase of Paracoccus denitrificans pumps protons in a reconstituted system. J Biol Chem. 1982 Feb 25;257(4):1579–1582. [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. E. coli F1-ATPase interacts with a membrane protein component of a proton channel. Nature. 1982 Aug 26;298(5877):867–869. doi: 10.1038/298867a0. [DOI] [PubMed] [Google Scholar]
- Wikström M. Identification of the electron transfers in cytochrome oxidase that are coupled to proton-pumping. Nature. 1989 Apr 27;338(6218):776–778. doi: 10.1038/338776a0. [DOI] [PubMed] [Google Scholar]