Abstract
This review provides a summary of the global epidemiology of inflammatory bowel diseases (IBD). It is now clear that IBD is increasing worldwide and has become a global emergence disease. IBD, which includes Crohn’s disease (CD) and ulcerative colitis (UC), has been considered a problem in industrial-urbanized societies and attributed largely to a Westernized lifestyle and other associated environmental factors. Its incidence and prevalence in developing countries is steadily rising and has been attributed to the rapid modernization and Westernization of the population. There is a need to reconcile the most appropriate treatment for these patient populations from the perspectives of both disease presentation and cost. In the West, biological agents are the fastest-growing segment of the prescription drug market. These agents cost thousands of dollars per patient per year. The healthcare systems, and certainly the patients, in developing countries will struggle to afford such expensive treatments. The need for biological therapy will inevitably increase dramatically, and the pharmaceutical industry, healthcare providers, patient advocate groups, governments and non-governmental organizations should come to a consensus on how to handle this problem. The evidence that IBD is now affecting a much younger population presents an additional concern. Meta-analyses conducted in patients acquiring IBD at a young age also reveals a trend for their increased risk of developing colorectal cancer (CRC), since the cumulative incidence rates of CRC in IBD-patients diagnosed in childhood are higher than those observed in adults. In addition, IBD-associated CRC has a worse prognosis than sporadic CRC, even when the stage at diagnosis is taken into account. This is consistent with additional evidence that IBD negatively impacts CRC survival. A continuing increase in IBD incidence worldwide associated with childhood-onset of IBD coupled with the diseases’ longevity and an increase in oncologic transformation suggest a rising disease burden, morbidity, and healthcare costs. IBD and its associated neoplastic transformation appear inevitable, which may significantly impact pediatric gastroenterology and adult CRC care. Due to an infrastructure gap in terms of access to care between developed vs. developing nations and the uneven representation of IBD across socioeconomic strata, a plan is needed in the developing world regarding how to address this emerging problem.
Keywords: Inflammatory bowel disease, industrial-urbanized-societies, colorectal cancer
Background
Inflammatory bowel diseases (IBD) include ulcerative colitis (UC) and Crohn’s disease (CD),1 two chronic, relapsing, and remitting conditions that have no permanent drug cure and can result in significant long-term morbidity. UC affects only the colon and is primarily confined to the mucosal and to a lesser degree, the submucosal compartments. In contrast, CD can involve any component of the gastrointestinal tract from the oral cavity to the anus and may involve all layers of the gut.2 Although the factors that contribute to the development of IBD remain elusive, these associated diseases have long been considered a problem of Western societies, with the Western lifestyle largely contributing to their pathogenesis.1–4 The incidence of IBD is now rising in developing countries and is increasingly considered an emerging global disease (Figs. 1–3).3,4 Despite limited epidemiological data from developing nations, it is now clear that both the incidence and prevalence of IBD are increasing worldwide.5 Younger populations in industrial urbanized societies are more affected.6 IBD is also known to be associated with a substantial increase in the risk of colorectal cancer (CRC), especially after 8–10 years of active disease.7–9
Figure 1.
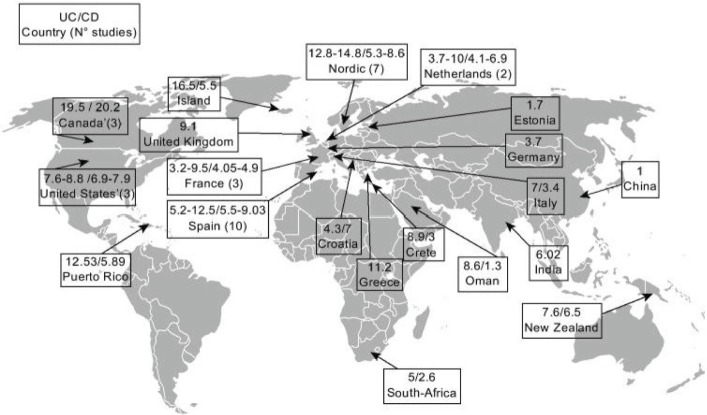
Incidence of reported CD and UC worldwide. Reproduced with permission from the publisher: Rogler et al. Gut. 2012;61:706–712.34
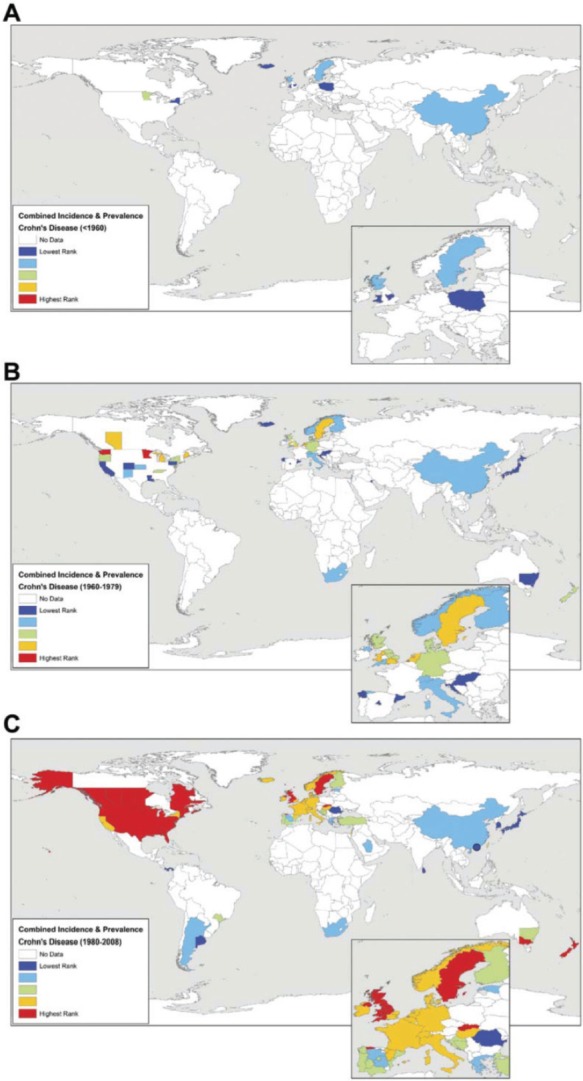
Figure 3.
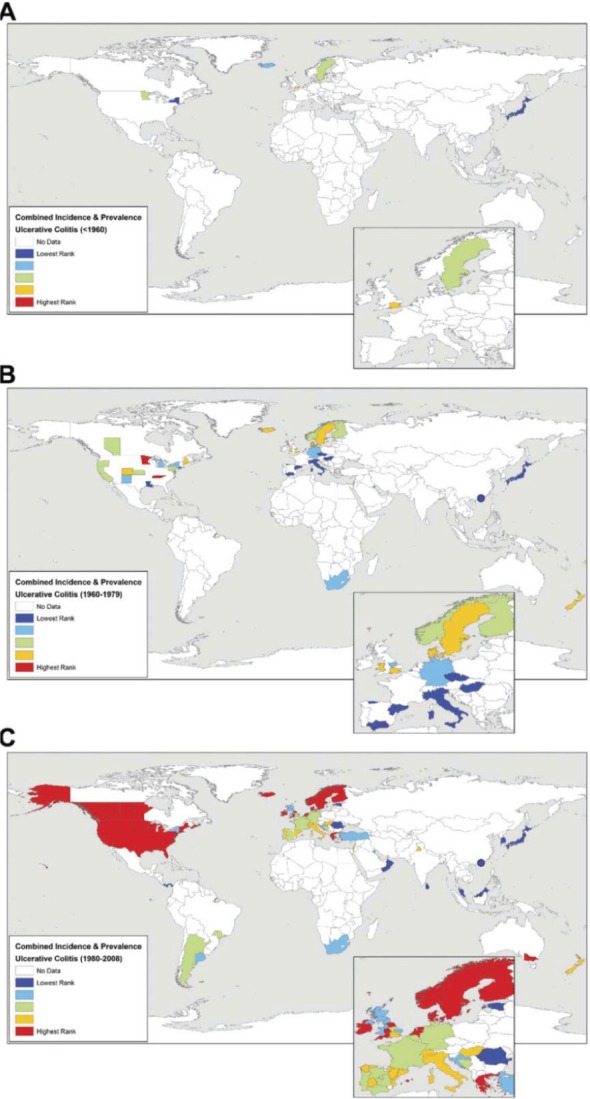
Worldwide UC incidence rates and/or prevalence for countries reporting data: (A) before 1960, (B) from 1960 to 1979, and (C) after 1980. Incidence and prevalence values were ranked into quintiles representing low (dark and light blue) to intermediate (green) to high (yellow and red) occurrence of disease. Reproduced with permission from the publisher: Molodecky et al. Gastroenterology. 2012;142:46–54, e42.5
UC and CD disorders have distinct pathologic and clinical characteristics, but their etiology and biopathogenesis remains poorly understood. Clinically, CD is distinguished from UC by disease proximal to the colon, perineal disease, fistulas, histologic granulomas, and full-thickness as opposed to largely mucosa-submucosa limited disease. In CD, granulomas are evident in up to 50% of patients and fistulas in 25%. Although IBD susceptibility genes (such as NOD2 gene variants)10,11 have been identified, advances in defining specific environmental risk factors involved primarily include abundant indirect evidence suggesting that smoking, oral contraceptives, diet, appendectomy, breast feeding, antibiotics, vaccination, infections, and childhood hygiene12–14 may be involved. However, to date, none of these factors completely explain the environmental determinants of IBD and most studies report inconsistent observations, making additional studies necessary to better understand the etiology and bio-pathophysiology of IBD. IBD is thought to result from the interaction between a genetically-susceptible host and environmental factors which influence the normal gut flora and trigger an inappropriate mucosal immune system response (antibody-antigen reaction against the normal mucosal resistance).13–17 Recent data suggest that children and adolescents show the highest incidence of IBD,6,18 as approximately 25 to 30 percent of patients with CD and 20 percent of patients with UC present before the age of 20 years.2,18,19
Patients with IBD have significant social, psychological, and financial repercussions20–22 along with an impaired health-related quality of life (HRQoL).23,29 Since the incidence of IBD is increasing worldwide, there will be an ever-increasing economic impact/burden on the healthcare system and the economy as a whole. Therefore, it is important to start planning for the major needs of patients with IBD in the developing world, particularly with respect to proper diagnosis and care,30,31 and with consideration of the added impact of the high cost of emerging drug therapies.32 Longobardi et al33 reported that patients diagnosed with IBD for less than 5 years have more frequent emergency room visits (odds ratio [OR] 2.41; 95% confidence interval [CI] 1.49–3.88), hospitalizations, and surgical interventions (OR 2.34; 95% CI 1.09–4.19) compared to non-IBD controls. There is also a clear infrastructure gap between urban population centers and rural areas in terms of access to care.34 In this review, I summarize the global epidemiology of IBD and discuss the role of the industrial urbanized environment and developing nations as possible factors promoting IBD.
Methods
A systematic literature search for the global epidemiology of IBD was performed using a predetermined protocol and in accordance with the quality of reporting meta-analyses of observational studies (MOOSE).35,36 Two computer-stored databases, MEDLINE (1950–2010) and EMBASE (Excerpta Medica Database; 1980–2013), were searched for studies investigating the epidemiology of IBD. Inclusion criteria included relevant epidemiological literature available regarding the incidence and prevalence of IBD worldwide, IBD in industrialized and developing nations, immigration and IBD incidence, pediatric IBD, and management challenges of IBD in industrialized and in developing countries. The search was not limited by language in order to ensure capture all relevant papers. Reference lists of relevant articles were also reviewed. An initial screen of identified abstracts and titles was conducted. Abstracts were eliminated in this initial screen if they were not observational and did not investigate the epidemiology of IBD. Studies that did not report original data were also excluded. Abstracts meeting these criteria were eligible for full-text review. Articles were independently considered for inclusion in the review if they reported an incidence and/or prevalence rate of UC and/or CD or if they had adequate information to calculate these rates. UC and CD were required to be reported separately for inclusion in the systematic report. Prevalence studies were identified to highlight the burden of IBD globally, whereas studies reporting incidence assessed the temporal evolution of disease diagnosis as well as patient characteristics at diagnosis, including age and gender. Data incidence rates per 100,000 person-years with 95% confidence intervals for the overall study time period were collected. The prevalence per 100,000 populations with 95% confidence intervals for the overall study period was documented including: (i) time trends, (ii) age groups, and (iii) gender ratios. The incidence of IBD was summarized using incidence rates and defined as the numbers of new cases in a population. An average incidence rate was calculated when incidence rates were reported separately for male and female subjects, for race/ethnicity, or over multiple years. The prevalence was defined as the number of persons with IBD in the population. Incidence rates were adjusted for confounding factors, such as socioeconomic class, age, diet, gender, household size, and genetic factors, to estimate cases in a defined region per 100,000 persons. All studies were organized by geographic region.
Results
To date, there are 3,028 publications from six of the seven continents reporting the incidence or prevalence for IBD worldwide (569 from Asia/Middle East, 102 from Africa, 692 from North America, 60 from South America, 1,507 from Europe, 98 from Australia, and 0 from Antarctica). The results showed that the incidence and prevalence of IBD has been increasing worldwide. Since the 19th century, the incidence of IBD has increased steadily in North America and Europe until stabilizing in the middle and latter part of the 20th century to 2–15 per 100,000 person-years for UC and 3–15 per 100,000 person-years for CD. The annual incidence rates vary by geographic region as depicted in Table 1 and Figures 1, 2, and 3. Table 2 describes the ranges in incidence and prevalence stratified into quintiles levels for CD and UC. The highest annual incidence of UC is currently 24.3 per 100,000 person-years in Europe, 6.3 per 100,000 person-years in Asia and the Middle East, and 19.2 per 100,000 person-years in North America. The highest annual incidence of CD is reported to be 12.7 per 100,000 person-years in Europe, 5.0 per 100,000 person-years in Asia and the Middle East, and 20.2 per 100,000 person-years in North America. The highest reported prevalence values for IBD were in Europe (UC, 505 per 100,000 persons; CD, 322 per 100,000 persons) and North America (UC, 249 per 100,000 persons; CD, 319 per 100,000 persons). In time-trend analyses, 75% of CD studies and 60% of UC studies showed increased incidence rates over times that were of statistical significance (P < 0.05). Incidence rates stratified by gender were reported in 50 UC and 59 CD studies. The female to male ratio varied from 0.51 to 1.58 for UC studies and 0.34 to 1.65 for CD studies, suggesting that IBD diagnosis was not gender-specific.5
Table 1.
Incidence and prevalence of UC and CD in Europe, Asia and Middle East and North America for the study period, 1930 to 2008.
| Regions | Incidence
|
Prevalence
|
Study period, range | ||
|---|---|---|---|---|---|
| Ulcerative colitis | Crohn’s disease | Ulcerative colitis | Crohn’s disease | ||
| Europe | 0.6–24.3/100,000 | 0.3–12.7/100,000 | 4.9–505/100,000 | 0.6–322/100,000 | 1930–2008 |
| Asia & Meddle East | 0.1–6.3/100,000 | 0.04–5.0/100,000 | 4.9–168.3/100,000 | 0.88–67.9/100,000 | 1950–2008 |
| North America | 0–19.2/100,000 | 0–20.2/100,000 | 37.5–248.6/100,000 | 16.7–318.5/100,00 | 1930–2004 |
Figure 2.
Worldwide CD incidence rates and/or prevalence for countries reporting data: (A) before 1960, (B) from 1960 to 1979, and (C) after 1980. Incidence and prevalence values were ranked into quintiles representing low (dark and light blue) to intermediate (green) to high (yellow and red) occurrence of disease. Reproduced with permission from the publisher: Molodecky et al. Gastroenterology. 2012;142:46–54, e42.5
Table 2.
Incidence and prevalence ranges stratified into quintiles for CD and UC.
| Quintile rank (percentile) | Crohn’s disease
|
Ulcerative colitis
|
||
|---|---|---|---|---|
| Incidence per 100,000 |
Prevalence per 100,000 |
Incidence per 100,000 |
Prevalence per 100,000 |
|
| 0 to 19th (dark blue) | 0.0–0.80 | 0.6–6.75 | 0.0–1.85 | 2.42–21.0 |
| 20th to 39th (light blue) | 0.81–1.94 | 6.76–25.0 | 1.86–3.09 | 21.1–44.3 |
| 40th to 59th (green) | 1.95–3–76 | 25.1–48.0 | 3.10–4.97 | 44.4–100.9 |
| 60th to 80th (yellow) | 3.77–6.38 | 48.1–135.6 | 4.98–7.71 | 101.0–198.0 |
| 80th to 100th (red) | 6.39–29.3 | 135.7–318.5 | 7.72–19.2 | 198.1–298.5 |
Note: Ranges (as denoted by color) correspond to ranking of incidence and or prevalence in Figures 2 and 3. Quintile ranges were developed from 260 published studies on incidence and/or prevalence of IBD. Reproduced with permission from the publisher: Molodecky et al. Gastroenterology. 2012;142:46–54, e42.5
While the incidence of UC increased in Western countries in the 1960s and 1970s, it then plateaued. In contrast, the incidence of UC continued to increase in previously low-incidence areas in Eastern Europe, Asia32,37–39 and developing countries, such as Africa.40–59 Additionally, the incidence of CD is high in Canada and New Zealand, intermediate in Western Europe and the USA, and lower in Israel and South Africa. Although the overall rates are low, they appear to be increasing in some parts of Asia and South America.4,32,39,60,61 Interestingly the incidence/prevalence rates in China appeared to be stable over the time periods shown (Fig. 2). Finally, in the past 20 years, CD has generally matched or overtaken UC in incidence and certainly has overtaken UC in prevalence in developed countries, except for Scandinavia.39
There also is a continuing trend in the increased incidence and prevalence rates of IBD across parts of Asia. There is also an emerging increase in the incidence and prevalence of IBD across Africa.40,41,43,51,53,54,56,57,59 Wright et al studied IBD incidence rates observed in Cape Town’s GI Clinic of Groote Schuur Hospital, South Africa (SA), from 1975–1980. In regards to CD and UC incidences, they reported 117 and 220 cases each, respectively, with a mean ± SEM follow-up of 6.1 ± 0.5 and 7.7 ± 0.4 years. Of these patients, 72% and 60% were white, 37% and 37% mixed race and 1% and 3% black, respectively. The incidence for mixed-race and white population groups was 0.4 and 0.9 and 1.3 and 2.4 per 100,000 person-years during 1970–1974 and 1.3 and 1.2 and 1.6 and 2.1 per 100,000 person-years during 1975–1980, respectively. These differences with time in SA were significant (P < 0.05). Despite an increased number of reports stemming from the continent of Africa,40–59 there was insufficient data to calculate the incidence rate for the entire African population.
Although this emergence is occurring among developing nations, it is also occurring in Japan, an advanced country from a socioeconomic perspective.4,61 Although the prevalence of IBD in Japan is still much lower than in Western countries,62 the age-standardized prevalence of UC in Japan in 2005 was 63.6 per 100,000 persons, and that of CD was 21.2 per 100,000 persons. IBD patient numbers have been steadily increasing over time. The age distribution also differed between the two diseases, with CD primarily affecting younger people. In both UC and CD, more than 50% of patients were male and over 80% of patients were classified as having mild to moderate disease in terms of severity.63
Because of these observations, it is concerning that the rise in IBD in developing countries may be met with a lack of necessary medical support, particularly in severely ill patients. Ethical discussions regarding the limited availability of treatment in these countries and how this may impact clinical practice accessibility in the foreseeable future are lacking.
Pediatric IBD
The epidemiologic patterns of pediatric IBD have evolved over the past several decades, with a significant increase in both incidence and prevalence values.64 A recent statewide IBD survey from Wisconsin in the United States reported that the state’s incidence of IBD was 5 to 11 per 100,000 children with rate of diagnosis as 4.56 per 100,000 for CD and 2.14 per 100,000 for UC.65 Canadian studies66 indicated that new pediatric IBD diagnoses rose from 9.5 per 100,000 in 1994 to 11.4 per 100,000 in 2005. The most significant accelerations were among younger age groups, with the incidence increasing 5% annually in children younger than 4 years of age and 7.6% annually in children ages 5 to 9 years.66
Similar observations were reported for the US as a whole. In the US, the mean age at diagnosis of pediatric IBD is 12.5 years,65 with 20% of children diagnosed before the age of 19 years and fewer than 5% diagnosed before age of 5 years. Males appeared to be overrepresented in new cases of pediatric CD, although an equal number of males and females received a UC diagnosis.67 This is in contrast to a French study6 which showed that the number of younger females newly diagnosed with CD was higher than that of males. In US children, according to Kappelman et al68 the prevalences of CD and UC are 43 and 28 per 100,000 persons, respectively, and in US adults the prevalences of CD and UC are 201 and 238 per 100,000, respectively.68 When comparing healthcare utilization between children and adults in the US, Kappelman et al69 found that healthcare utilization was disproportionate higher in younger IBD patients.69
Regional variations in IBD incidence provide additional support for hypothesis that environmental factors influence IBD pathogenesis in children.5 For instance, there is considerable geographical variation in the incidence of pediatric IBD, with rates ranging from 11.4 per 100,000 person-years in Canada to 0.25 per 100,000 person-years in Spain.66,70–72 A study conducted in Scotland73 reported rates of 7.82 per 100,000 person-years in children younger than 16 years of age. The same study observed an overall 76% increase in pediatric IBD cases between 1990 to 1995 and 2003 to 2008.73 Another recent UK report74 of adult IBD services revealed that 39% of adult sites actually care for IBD patients ages 16 years and younger.
Studies on pediatric IBD in Scandinavian countries (Sweden and Norway) showed similar results. Hildebrand et al75 observed a changing pattern in pediatric IBD in northern Stockholm (1990–2001) and reported a marked increase in the overall occurrence of pediatric IBD, corresponding to an overall incidence of 7.4 per 100,000 person per-years. The incidence of CD was 4.9, UC was 2.2, and indeterminate colitis (IC) was 0.2. From the same investigators,76 a later study (2002–2007) showed the standardized incidence to be 9.2 (95% Cl 7.5–11.2) for CD and 2.8 (95% CI 1.9–4.0) for UC. A significant increasing incidence in UC (P < 0.05) was observed. No temporal trend was observed for the incidence of CD. The incidence rate of pediatric IBD in the northern Stockholm was significantly higher in 2002–2007 than that observed in early study conducted from 1990–2001. Another retrospective population based study77 as an epidemiological update was conducted in Stockholm on CD during 1990–2001. They reported a mean incidence rate for the period of 8.3 per 100,000 (95% Cl 7.9–8.8) children per-years. There was no difference between genders. The mean annual incidence for the entire study period for colorectal disease and ileocecal disease was 4.4 (95% Cl 4.0–4.7) and 2.4 (95% Cl 2.2–2.6) per 100,000 children per-year, respectively. The prevalence of CD was 213 per 100,000 inhibitants.
A Norwegian study78 was conducted on the incidence and clinical presentation of IBD in children (1993–2004) to compare prospective and retrospective data in a selected Norwegian population. The total incidence of pediatric IBD did not change over time, whereas a trend towards an increase in CD and a reduction in UC was observed. The rates of CD for the two periods were, respectively, 1.95 and 3.64, and for UC 3.67 and 2.05 per 100,000 children-year. Total incidence rates of IBD for the two periods were 5.6 and 5.7, respectively, similar to the finding of the IBSEN study of 1990–1994.79
Immigrants from India residing in England
There has been a significant increase in incidence rates and hospitalizations related to IBD in first-generation of immigrants moving from developing countries to industrialized nations.70,80 In developing nations in which IBD is emerging, UC typically is more common than CD.34 In India, for example, the reported UC/CD ratio is 8:1.4.81 The overall UC incidence rate for adults in India is 6.02 per 100,000 person-years (95% CI 1.2–17.6) with a crude prevalence rate of 44.3 per 100,000 inhabitants (January to March, 1999). The number of childhood IBD cases in India is largely unknown. At the Pediatric Gastroenterology section of PGIMER, Chandigarh, Mehta et al82 reported that 15 of 294 children (5%) admitted for colonic disorders were diagnosed with UC. We found no data as to whether the rates were similar or different for Hindus versus Muslims or Sikhs living in India, although some of these groups present with different UC rates once relocated to England (see below).
An epidemiological analysis was performed which reported UC rates in immigrants from India versus the indigenous population of Leicestershire, UK70 over two study periods: 1972–1980 and 1981–1989. Between 1972 and 1980, there were 75 new cases of UC in the indigenous group versus 38 new cases in Indian immigrants among the 280,000 inhabitants of the city. Given their relative numbers, immigrants from India had a significantly higher incidence of UC than those already living in the area (X = 1.96, P < 0.05). The excess was among Hindu immigrants, in particular, in whom the standardized incidence was 13.4 per 100,000 person-years (X = 1.98, P < 0.05). The relative risk for Hindus was 3.9. The standardized incidence in Muslims, however, was similar to that in the rest of the population (Z = 1.4, NS).
From 1981–1989, there were 105 new cases of UC in the general population and 61 in recent immigrants from India. During this period, the standardized incidence in the general population had risen significantly (Z2 = 8.5, P < 0.005) to 5.3 per 100,000 person-years, while in those that had recently moved from India, the standardized incidence was stable (X2 = 0.6) at 10.8 per 100,000 person-years. However, the incidence of UC in Indian immigrants was significantly higher than that of the general population (Z = 2.15, P < 0.05). Additionally, during this period, both Hindus and Sikhs had significantly more UC than the general population (Z = 2.0, P < 0.05 and Z = 3.9, P < 0.001, respectively).70 The relative risk to those who had emigrated from India was 2.45, while these values for Hindus and Sikhs were 1.9 and 2.9, respectively. The standardized incidence in Muslims was similar to that in the general population in Leicester.70
Data from the city of Leicester were also compared with those from the rest of England. During the 1970s, there were 282 new cases of UC in England and 46 in migrants from India. Indians had a significantly greater standardized incidence than the general population in the rest of England (Z = 3.7, P < 0.001). The standardized incidence of UC in Britain remained unchanged in the 1980s at 4.38 cases per 100,000 person-years. The standardized incidence in immigrant Indians, however, fell marginally to 9.95 cases per 100,000 per year, but this group remained at a significantly greater risk of UC than Europeans (Z = 3.5, P < 0.001). The fall in standardized incidence in Indians was not statistically significant (X2 = 0.45). There was no difference in the incidence of UC in those individuals originally coming from India but who came directly from India of via Eastern Africa (Z = 1.4, NS).70
Pediatric IBD in children who came before adolescence or born in Britain to parents who emigrated from India
The same study described above from Leicestershire County70 evaluated the incidence of UC in children born in Britain following the immigration of their parents from India compared to the incidence in children in the general population. The incidence fell in children in the general population (ages 11–15 years), from 3.3 cases per 100,000 per year (95% CI 1.8–5.4 per 100,000 per year) in the 1970s (when there were 15 cases), to 1.5 cases per 100,000 per year (95% CI 0.4–2.9 per 100,000 per year) in the 1980s (when there were seven cases). This change was not significant (X2 = 2.9). The incidence in children of the same age born in England of immigrant parents also fell from 4.7 cases per 100,000 per year (95% CI 0–10.2 per 100,000 per year) to 3.0 cases per 100,000 per year (X2 = 0.2).70 Further, the incidence of UC in the general population of children in the County (aged 0–10 years) was 0.3 cases per 100,000 person-years (95% CI 0–0.6 per 100,000 per year). There were five cases in children whose parents had emigrated from India for whom the incidence was 1.0 case per 100,000 person-years (95% CI 0–5.5 per 100,000 per year) or roughly 3 times higher than that observed in the general population.
Because there is no recently published data available, the risk of IBD in certain immigrants, and in particular, their children, was even greater than that in the remainder of the population of Leicester.70 This geographical and immigrant data suggest that factors attributable to a Western lifestyle or environment (diet, environmental exposures, and/or intestinal microbiota) predispose these individuals to IBD70 in Westernized countries compared with those in their country of origin.
Utilization of healthcare resources by patients with IBD
Different drug categories are used for the management of IBD like aminosalicylates,83 corticosteroids,84 anti-tumor necrosis alpha (anti TNF-α) drugs,85,86 antibiotics,87,88 probiotics,89–91 and immunosuppressants.92,93 Because of a lack of desirable efficacy and poor tolerability of these drugs, surgical intervention may be indicated.94,95 The healthcare utilization associated with IBD is different between nations and appears related to sociodemographic factors.69,96–100 In the US, healthcare costs are higher than in any other country.97 One systematic review101 showed that the cost of CD in particular is more expensive in the US than in other Western nations such the UK and France. For patients with CD living in the US, direct medical costs were estimated to be US $18,022–18,932 per patient per year (data from 2008) compared to approximately US $4,000–10,000 in Europe (converted Euros to US dollars).101 A second analysis from MarketScan102 (1999–2005) was used to measure the cost burden of CD and UC. Commercially insured CD and UC patients in the US had annual medical expenditures of US $18,963 and US $15,020, respectively, which is significantly more than the $5,000 estimated for patients in the matched comparison group of similar patients living outside the US (specifically compared to the UK). In a third analysis (2003–2004) of insurance claim data, direct costs were measured in children and adults by analyzing 87 different health plans in 33 US states.103 The estimated mean annual cost was US $8,265 for CD and US $5,066 for UC. The discrepancy between this observation and the previous two studies is perhaps due to the reporting of actual reimbursements rather than charges to the insurer. Further observations also revealed that costs for patients under 20 years of age were disproportionately higher per year than those for adults over 20 years of age.69 This suggests that a focus on a more effective management of IBD in pediatric patients could yield significant cost-saving and of course health benefits. More than one-third of the total IBD-related costs were attributed to inpatient care of disease, suggesting that reducing hospitalization through optimal maintenance of remission would decrease the overall cost-burden.
Indeed a multinational European and Israel study104 showed that majority of IBD-related healthcare expenditures were for inpatient medical and surgical management. A Canadian study105 showed that general medical inpatient costs for CD and UC were identical, but surgical costs were more for those patients with UC than those with CD. A larger proportion of total charges for IBD were therefore attributable to surgical care.26 These two studies, however, which were reported over two decades ago, did not take into account the impact of biologics such as infliximab, adalimumab, certolizumab pegol and natalizumab on the current medical options for IBD.
In the UK,98–100 it was consistently found that hospital inpatient costs were incurred by a minority of patients but they accounted for approximately half of the total cost burden of IBD, while drug costs contributed to less than a quarter of the total healthcare costs. Indirect IBD cost burdens falling on society exceeded medical expenditures.100 Conservative estimates for caring for the estimated 240,000 IBD patients in the UK cost the National Health Service in excess of GBP £1 billion (equivalent to US $1.6 billion) per year, with these calculations made largely in a pre-biological era and when incidence and prevalence rates were lower.5,100
A search for cost-effectiveness studies from developing nations did not identify any relevant papers that examined surveillance for management costs in those with IBD. However, during the search, 671 studies did report IBD incidence and prevalence data from Asia/Middle and Africa.46–48,51,52,54–57
Discussion
Since the 1950s, epidemiologic studies have been undertaken in an attempt to identify risk factors for IBD genesis.106 Ethnic groups identified to have a greater risk included people of Jewish background.107 Ashkenazi Jews were found to have a 5–8-fold increased risk of CD and UC compared to non-Jews.108 Despite the fact that CD and UC share several clinical features, they have different causes, mechanisms of tissue damage, and treatment options. Therefore, an accurate diagnosis of IBD phenotype is of paramount importance for providing evidence-based, personalized medical care. The distinction between CC and UC is made based on a review of clinical, radiologic, endoscopic, and pathologic data, but the two IBD cannot be differentiated in up to 15–30% of IBD patients. Therefore, correct management of IBD depends upon an accurate diagnosis.30,31
Further, the incidence and prevalence numbers for IBD are increasing worldwide. The rapidly increasing incidence of IBD in North America and Western Europe since the 1950s is higher than can be explained by genetic factors alone. The incidence of IBD has substantially increased over the latter part of the 20th century and continues to rise in developing countries revealing its emergence as a global disease.12,13,109 Until recently, IBD was considered a disease seen primarily in Caucasians and, in particular, those of Jewish origins.4,5,110 Genetics is thought to play a role, as evidenced by the greater prevalence of CD in Ashkenazi than Sephardic Jewish populations.110 Now, it is a global disease and its incidence in developing nations is rising.42,111–113 Despite inadequate epidemiological data from developing parts of the world, the prevalence and incidence among blacks, Asians, and Hispanics has been reported with increasing frequencies40–42,111,114 suggesting that the trend is indeed increasing worldwide.42,52,112–116 Traditionally, developing nations have reported a lower prevalence of IBD, but the incidence is currently rising in many of these countries as they become more industrialized.114,115
Of significant concern is the escalation in incidence rates in children and adolescents.66,75–78,117–123 Notably, a significant fraction of these youth are among first-generation of non-white and non-Jewish individuals immigrating from developing countries to industrialized nations.3,66,75,78,111,120–123 These are school age populations 4–20 years old (Figs. 4 and 5).6 These observations suggest that in these populations there is a strong environmental component that may not be linked to genetic factors alone.19,59,61,75,78,120,121,123 Only approximately 10% of patients with IBD report a family history of IBD.124 It is therefore evident that there is a partial complex relationship between genetic susceptibility and environment and that this heritability is likely to be polygenic in nature in most patients with IBD. The association of IBD with industrial and urbanized regions as compared to rural regions is evident.125–128 The increase in IBD incidence in this young population may be based on the lack of predisposition to environmental factors that can establish a defensive immunity. Thus, once exposed to new and different environmental factors, these individuals could develop autoimmune morbidity, such as IBD. The industrialization and urbanization of societies are associated with changes in sanitation, occupations, microbiota, diet, lifestyle behaviors, medications, and pollution exposures, which have all been implicated as potential environmental risk factors for IBD.12 In developing nations, IBD was largely unknown; however, as these nations are transitioning to more industrial and urbanized societies, the incidence of IBD has begun to increase.42,114,115
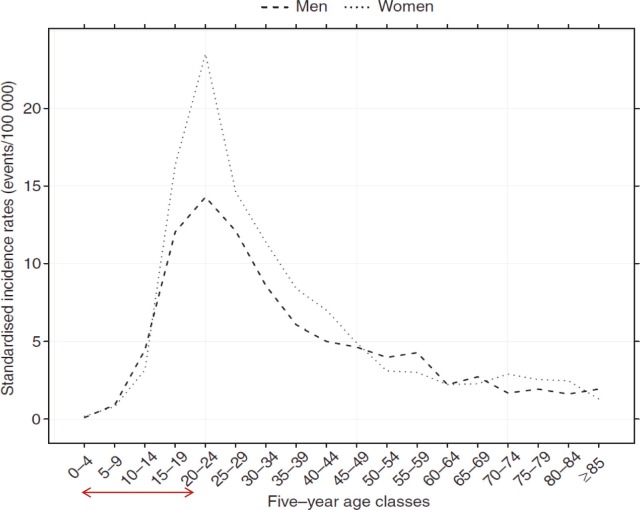
Figure 4.
Incidence rate of CD by gender and age in Northern France from 1988 to 2007. Reproduced with permission from the publisher: Chouraki et al, Aliment Pharmacol Ther. 2011;33:1133–1142.6 Study shows a higher newly diagnosed young population between the ages of 4–20 years (shown by arrow on abscissa). Young female is higher than male. The increase in CD in this school-age population can be speculated to be based on the lack of predisposition to environmental factors that can establish a defensive immunity.
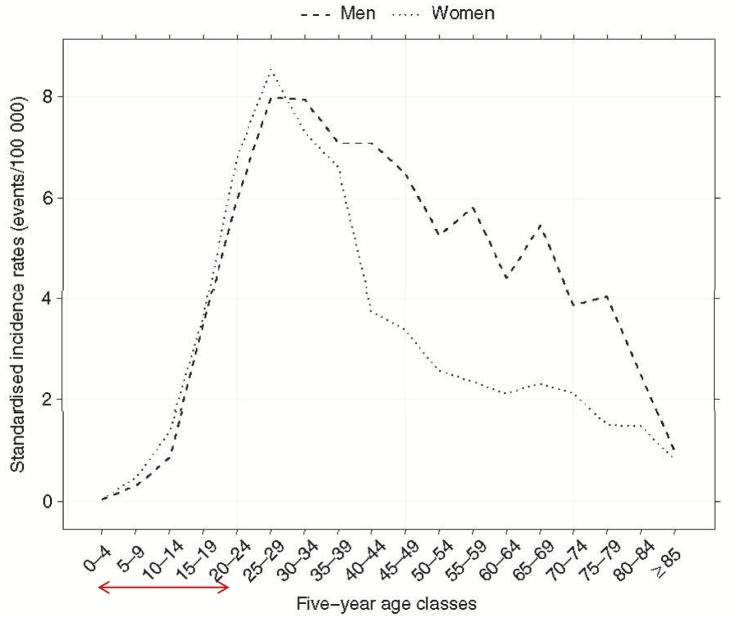
Figure 5.
Incidence rate of UC by gender and age in Northern France from 1988–2007. Reproduced with permission from the publisher: Chouraki et al, Aliment Pharmacol Ther. 2011;33:1133–1142.6 This figure represents several observations from current epidemiological studies. Young females are at higher rate than males. The age with highest incidence is 4–20 years of age (shown by arrow on abscissa). The increase in UC in this school-age population can be speculated to be based on the lack of predisposition to environmental factors.
The increasing evidence that IBD is affecting a younger population (Figs. 4 and 5) presents additional concerns. Although the global incidence rates stratified by gender appear to be similar5 (suggesting that the diagnosis of IBD is not gender-specific), Chouraki et al6 found that younger females were more affected by CD than males in France (Fig. 4). In contrast, while the incidence rates for UC are similar for male/female youths in France (Fig. 5), males had an increased UC incidence rate for adults 40–75 years compared to females. Meta-analyses conducted in patients acquiring IBD at a young age show an increased risk of developing colorectal cancer (CRC), as the cumulative incidence rate in CRC in IBD patients diagnosed in childhood is higher than in adults.129,130 There are strong data suggesting that CRC in IBD patients is related to the duration of disease. For instance, it is generally accepted that the risk of CRC in IBD patients becomes appreciable after 10 years of disease.131 In addition, IBD patients who have had prophylactic surgery (proctocolectomy) do not always eliminate their cancer transformation risk, as malignancies have been reported postoperatively in these patients.131–135
Additionally, developing countries cover two-thirds of the earth’s surface, with a population of 3–5 billion inhabitants, constituting three-quarters of all humans. Therefore the need for a cost-effective biological therapy for IBD will increase dramatically, and the pharmaceutical industry, healthcare providers, patient advocate groups, governments, and non-governmental organizations should consider how to manage this. This dialogue should begin now with regard to: (1) the major needs of patients with complicated IBD in developing countries; (2) the potential need for a biological therapy for developing countries to treat IBD; (3) the necessary infrastructure for selecting patients with IBD who require biological therapy; and (4) the medical/ethical issues limiting the use of biological therapy.
While the West continues to improve ambulatory care delivery136–138 to meet high-quality, safety, efficacy, coordination of care, and evidenced-based care in IBD patients,139–141 developing nations have health service constraints to meet the required standard of care,34 and doctors and personnel are not trained for treating these diseases. Further, for cost-effectiveness considerations and recommendations by the World Health Organization (WHO) and the World Gastroenterology Organization,142 developing countries will be hit the hardest. In 1998, the WHO published threshold values for intervention cost-effectiveness by region.142 Medical intervention is regarded as being ‘very cost-effective’ if it costs <1 gross domestic product (GDP)/capita; it is ‘cost-effective’ in a range of 1–3 GDP/capita, and not cost-effective if it exceeds 3 GDP/capita. In US dollars, between 1998–2000, the GDP/capita was US $39,950 in North America, US $30,493 in Europe, US $10,208 in the Eastern Mediterranean region, US $4,608 in South America, US $4,959 in South East Asia, and US $1,695 in Africa.142,143 Subsequently, the WHO launched a ‘CHOICE’ project with the objective of ‘providing policy makers with the evidence for deciding on the interventions and programmes which maximize health for the available resources’.142 WHO-CHOICE reported the costs and effects of a wide range of health interventions in the 14 epidemiological sub-regions. The results of these cost-effectiveness analyses were collected in regional databases, by which policy makers could adapt to their specific country setting. It is very important to carefully adjust those cost-effectiveness models to country-specific conditions. For instance, they must be modified to reflect the increased risk of infectious complications in specific regions. Given these risks, it is possible that overall quality-adjusted life years may not be higher for users of biological agents. Treatments regarded to be cost-effective in North America and Europe may not be cost-effective in developing countries, which must be taken into consideration when making local resource consumption decisions. Anti-TNF therapy has been determined to be effective, but the jury is still out as to whether it is cost-effective in the West.144–146
The intentions of IBD treatment are to eliminate symptoms, prevent flare-ups (maintain long-term remission), and restore patients’ HRQoL. For most people, medications control symptoms and promote HRQoL and healing. Surgery is usually required only if medications fail to improve symptoms or if precancerous transformations in the colon or other serious complications occur. In such cases, surgery can be performed on an emergency or elective basis.147,148
In conclusion, the observations of rates increase of IBD worldwide, particularly in younger populations coupled with the longevity of colitides disease is likely to be associated with increased CRC rates in these patients. Predictably, over the next few decades, the world will face a significant increase in IBD morbidity with associated neoplastic transformation, social, psychological, and financial repercussions and impaired patient HRQoL. As a result, IBD will most likely be a front-line morbidity, seen first in pediatric gastroenterology.147,148 Access to medical care and utilization of medical services is critically important when discussing IBD as some ethnicities are unevenly represented across socioeconomic strata.
Summary
IBD represents a group of idiopathic chronic relapsing and remitting diseases of unknown etiology. The two disease categories include CD and UC with both overlapping and distinct clinical and pathological features, making differentiation often difficult to diagnose. The global incidence of IBD differs geographically. Although the incidence has been increasing in Western nations since the Second World War, rates are beginning to level off in these countries. However, IBD has been increasing in previously low-incidence areas in Eastern Europe, Asia, and other developing areas. The prevalence appears to be higher in urban areas than in rural areas, as well as in higher socio-economic classes. Individuals who immigrate to industrial urbanized developed nations before adolescence and those immigrants who initially belonged to a low-incidence population show a significant higher incidence of IBD. This is particularly true for the first-generation of families born in an industrialized country with an already high incidence. Thus, IBD has become a global disease. Caring for patients with IBD can be challenging due to the heterogeneous nature of the disease and the lack of consensus in many areas of practice. Variation in practice is therefore unavoidable and does not necessarily imply deficiencies in quality. The healthcare systems, both at the level of primary care and referral in most developing countries, face significant challenges as they often lack regular clinical supervision and laboratory assessments needed for monitoring patients and will increasingly have difficulty affording expensive treatment (medical and surgical) to meet the medical needs of these patients.
Acknowledgements
I acknowledge all the scientists who made contributions to the areas of research that are reviewed here but were not cited owing to space constraints. I am grateful to Diana Marver, Ph.D. and Naji Abumrad, M.D., for critical reading of the manuscript.
Footnotes
Author Contributions
Conceived and designed the experiments: AEMK. Analyzed the data: AEMK. Wrote the first draft of the manuscript: AEMK. Developed the structure and arguments for the paper: AEMK. Made critical revisions and approved final version: AEMK. The author reviewed and approved of the final manuscript.
Funding
NIH/NIDDK 1R21DK095186–01A1; 3U54 CA091408–09S1; U54RR026140/U54MD007593; UL1 RR02 4975; Research Foundation, American Society of Colon and Rectal Surgeons, Limited Project Grant (LPG-086).
Competing Interests
Author discloses no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North American Society for Pediatric Gastroenterology H, Nutrition, Colitis Foundation of America, Bousvaros A et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57(9):1185–1191. doi: 10.1136/gut.2007.122143. [DOI] [PubMed] [Google Scholar]
- 4.Goh K, Xiao SD. Inflammatory bowel disease: a survey of the epidemiology in Asia. J Dig Dis. 2009;10(1):1–6. doi: 10.1111/j.1751-2980.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 5.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 6.Chouraki V, Savoye G, Dauchet L, et al. The changing pattern of Crohn’s disease incidence in northern France: a continuing increase in the 10- to 19-year-old age bracket (1988–2007) Aliment Pharmacol Ther. 2011;33(10):1133–1142. doi: 10.1111/j.1365-2036.2011.04628.x. [DOI] [PubMed] [Google Scholar]
- 7.Terzíc J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35(11):1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jess T, Riis L, Jespersgaard C, et al. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am J Gastroenterol. 2005;100(11):2486–2492. doi: 10.1111/j.1572-0241.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 11.Gaya DR, Russell RK, Nimmo ER, Satsangi J. New genes in inflammatory bowel disease: lessons for complex diseases? Lancet. 2006;367(9518):1271–1284. doi: 10.1016/S0140-6736(06)68345-1. [DOI] [PubMed] [Google Scholar]
- 12.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6(5):339–346. [PMC free article] [PubMed] [Google Scholar]
- 13.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 14.Halfvarson J, Jess T, Magnuson A, et al. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis. 2006;12(10):925–933. doi: 10.1097/01.mib.0000228998.29466.ac. [DOI] [PubMed] [Google Scholar]
- 15.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petnicki-Ocwieja T, Hrncir T, Liu YJ, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106(37):15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakobsen C, Paerregaard A, Munkholm P, et al. Pediatric inflammatory bowel disease: increasing incidence, decreasing surgery rate, and compromised nutritional status: A prospective population-based cohort study 2007–2009. Inflamm Bowel Dis. 2011;17(12):2541–2550. doi: 10.1002/ibd.21654. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol. 2004;18(3):509–523. doi: 10.1016/j.bpg.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Ross SC, Strachan J, Russell RK, Wilson SL. Psychosocial functioning and health-related quality of life in paediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53(5):480–488. doi: 10.1097/MPG.0b013e31822f2c32. [DOI] [PubMed] [Google Scholar]
- 21.Pallis AG, MI Psychosocial functioning and health-related quality of life in paediatric inflammatory bowel disease. Ann Gastroenteol. 2002;15:143–147. [Google Scholar]
- 22.Mouzas IA, PA Assessing quality of life in medical trials on patients with inflammatory bowel disease. Ann Gastroenterol. 2000:261–3. [Google Scholar]
- 23.Graff LA, Walker JR, Lix L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006;4(12):1491–1501. doi: 10.1016/j.cgh.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Feagan BG, Vreeland MG, Larson LR, Bala MV. Annual cost of care for Crohn’s disease: a payor perspective. Am J Gastroenterol. 2000;95(8):1955–1960. doi: 10.1111/j.1572-0241.2000.02261.x. [DOI] [PubMed] [Google Scholar]
- 25.Blomqvist P, Ekbom A. Inflammatory bowel diseases: health care and costs in Sweden in 1994. Scand J Gastroenterol. 1997;32(11):1134–1139. doi: 10.3109/00365529709002993. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117(1):49–57. doi: 10.1016/s0016-5085(99)70549-4. [DOI] [PubMed] [Google Scholar]
- 27.Ebinger M, Leidl R, Thomas S, et al. Cost of outpatient care in patients with inflammatory bowel disease in a German University Hospital. J Gastroenterol Hepatol. 2004;19(2):192–199. doi: 10.1111/j.1440-1746.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- 28.Hay JW, Hay AR. Inflammatory bowel disease: costs-of-illness. J Clin Gastroenterol. 1992;14(4):309–317. doi: 10.1097/00004836-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Pallis AG, Vlachonikolis IG, Mouzas IA. Assessing health-related quality of life in patients with inflammatory bowel disease, in Crete, Greece. BMC Gastroenterol. 2002;2:1. doi: 10.1186/1471-230X-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M’Koma AE, Seeley EH, Washington MK, et al. Proteomic profiling of mucosal and submucosal colonic tissues yields protein signatures that differentiate the inflammatory colitides. Inflamm Bowel Dis. 2011;17(4):875–883. doi: 10.1002/ibd.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeley EH, Washington MK, Caprioli RM, M’Koma AE. Proteomic patterns of colonic mucosal tissues delineate Crohn’s colitis and ulcerative colitis. Proteomics Clin Appl. 2013 doi: 10.1002/prca.201200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52(11):1587–1590. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longobardi T, Jacobs P, Bernstein CN. Utilization of health care resources by individuals with inflammatory bowel disease in the United States: a profile of time since diagnosis. Am J Gastroenterol. 2004;99(4):650–655. doi: 10.1111/j.1572-0241.2004.04132.x. [DOI] [PubMed] [Google Scholar]
- 34.Rogler G, Bernstein CN, Sood A, et al. Role of biological therapy for inflammatory bowel disease in developing countries. Gut. 2012;61(5):706–712. doi: 10.1136/gutjnl-2011-300613. [DOI] [PubMed] [Google Scholar]
- 35.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 37.Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8(3):121–127. doi: 10.1111/j.1443-9573.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 38.El Mouzan MI, Abdullah AM, Al Habbal MT. Epidemiology of juvenile-onset inflammatory bowel disease in central Saudi Arabia. J Trop Pediatr. 2006;52(1):69–71. doi: 10.1093/tropej/fmi039. [DOI] [PubMed] [Google Scholar]
- 39.Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian J Gastroenterol. 2007;26(6):285–289. [PubMed] [Google Scholar]
- 40.Agoda-Koussema LK, Anoukoum T, Djibril AM, et al. Ulcerative colitis: a case in Togo. Med Sante Trop. 2012;22(1):79–81. doi: 10.1684/mst.2012.0012. French. [DOI] [PubMed] [Google Scholar]
- 41.Mebazaa A, Aounallah A, Naija N, et al. Dermatologic manifestations in inflammatory bowel disease in Tunisia. Tunis Med. 2012;90(3):252–257. [PubMed] [Google Scholar]
- 42.Ukwenya AY, Ahmed A, Odigie VI, Mohammed A. Inflammatory bowel disease in Nigerians: still a rare diagnosis? Ann Afr Med. 2011;10(2):175–179. doi: 10.4103/1596-3519.82067. [DOI] [PubMed] [Google Scholar]
- 43.Senbanjo IO, Oshikoya KA, Onyekwere CA, Abdulkareem FB, Njokanma OF. Ulcerative colitis in a Nigerian girl: a case report. BMC Res Notes. 2012;5:564. doi: 10.1186/1756-0500-5-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouzid D, Fourati H, Amouri A, et al. The CREM gene is involved in genetic predisposition to inflammatory bowel disease in the Tunisian population. Hum Immunol. 2011;72(12):1204–1209. doi: 10.1016/j.humimm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Kallel L, Feki M, Sekri W, et al. Prevalence and risk factors of hyperhomocysteinemia in Tunisian patients with Crohn’s disease. J Crohns Colitis. 2011;5(2):110–114. doi: 10.1016/j.crohns.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Zaahl MG, Winter TA, Warnich L, Kotze MJ. The –237C-->T promoter polymorphism of the SLC11A1 gene is associated with a protective effect in relation to inflammatory bowel disease in the South African population. Int J Colorectal Dis. 2006;21(5):402–408. doi: 10.1007/s00384-005-0019-z. [DOI] [PubMed] [Google Scholar]
- 47.Zaahl MG, Winter T, Warnich L, Kotze MJ. Analysis of the three common mutations in the CARD15 gene (R702W, G908R and 1007fs) in South African colored patients with inflammatory bowel disease. Mol Cell Probes. 2005;19(4):278–281. doi: 10.1016/j.mcp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 48.O’Keefe EA, Wright JP, Froggatt J, Cuming L, Elliot M. Medium-term follow-up of ulcerative colitis in Cape Town. S Afr Med J. 1989;76(4):142–145. [PubMed] [Google Scholar]
- 49.O’Keefe EA, Wright JP, Froggatt J, Zabow D. Medium-term follow-up of Crohn’s disease in Cape Town. S Afr Med J. 1989;76(4):139–141. [PubMed] [Google Scholar]
- 50.Segal I. Ulcerative colitis in a developing country of Africa: the Baragwanath experience of the first 46 patients. Int J Colorectal Dis. 1988;3(4):222–225. doi: 10.1007/BF01660719. [DOI] [PubMed] [Google Scholar]
- 51.Wright JP, Marks IN, Jameson C, Garisch JA, Burns DG, Kottler RE. Inflammatory bowel disease in Cape Town, 1975–1980 Part I. Ulcerative colitis. S Afr Med J. 1983;63(7):223–226. [PubMed] [Google Scholar]
- 52.Wright JP, Froggatt J, O’Keefe EA, et al. The epidemiology of inflammatory bowel disease in Cape Town 1980–1984. S Afr Med J. 1986;70(1):10–15. [PubMed] [Google Scholar]
- 53.Brom B, Bank S, Marks IN, Barbezat GO, Raynham B. Crohn’s disease in the Cape: a follow-up study of 24 cases and a review of the diagnosis and management. S Afr Med J. 1968;42(41):1099–1107. [PubMed] [Google Scholar]
- 54.Segal I, Tim LO, Hamilton DG, Walker AR. The rarity of ulcerative colitis in South African blacks. Am J Gastroenterol. 1980;74(4):332–336. [PubMed] [Google Scholar]
- 55.Wright JP, Marks IN, Jameson C, Garisch JA, Burns DG, Kottler RE. Inflammatory bowel disease in Cape Town, 1975–1980 Part II. Crohn’s disease. S Afr Med J. 1983;63(7):226–229. [PubMed] [Google Scholar]
- 56.Novis BH, Marks IN, Bank S, Louw JH. Incidence of Crohn’s disease at Groote Schuur Hospital during 1970–1974. S Afr Med J. 1975;49(17):693–697. [PubMed] [Google Scholar]
- 57.Sobel JD, Schamroth L. Ulcerative colitis in the South African Bantu. Gut. 1970;11(9):760–763. doi: 10.1136/gut.11.9.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis R, Schmaman A, Cosman B. Crohn’s disease in Transvaal blacks. A report of three cases with a review. S Afr Med J. 1974;48(14):580–586. [PubMed] [Google Scholar]
- 59.Giraud RM, Luke I, Schmaman A. Crohn’s disease in the Transvaal Bantu: a report of 5 cases. S Afr Med J. 1969;43(21):610–613. [PubMed] [Google Scholar]
- 60.Sood A, Midha V, Sood N, Bansal M. Long term results of use of azathioprine in patients with ulcerative colitis in India. World J Gastroenterol. 2006;12(45):7332–7336. doi: 10.3748/wjg.v12.i45.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouyang Q, Tandon R, Goh KL, et al. Management consensus of inflammatory bowel disease for the Asia-Pacific region. J Gastroenterol Hepatol. 2006;21(12):1772–1782. doi: 10.1111/j.1440-1746.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 62.Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44(7):659–665. doi: 10.1007/s00535-009-0057-3. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida Y, Murata Y. Inflammatory bowel disease in Japan: studies of epidemiology and etiopathogenesis. Med Clin North Am. 1990;74(1):67–90. doi: 10.1016/s0025-7125(16)30587-9. [DOI] [PubMed] [Google Scholar]
- 64.Glick SR, Carvalho RS. Inflammatory bowel disease. Pediatr Rev. 2011;32(1):14–24. doi: 10.1542/pir.32-1-14. quiz 25. [DOI] [PubMed] [Google Scholar]
- 65.Kugathasan S, Judd RH, Hoffmann RG, et al. Wisconsin Pediatric Inflammatory Bowel Disease Alliance Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143(4):525–531. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 66.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58(11):1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 67.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88(11):995–1000. doi: 10.1136/adc.88.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 69.Kappelman MD, Porter CQ, Galanko JA, et al. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):62–68. doi: 10.1002/ibd.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Probert CS, Jayanthi V, Pinder D, Wicks AC, Mayberry JF. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut. 1992;33(5):687–693. doi: 10.1136/gut.33.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17(1):423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez GN, BG, Ramos PE. Inflammaotory bowel disease in pediatric patients in Asturias, Spain 1993–2000. Acta Pediatr Esp. 2004;62:466–471. [Google Scholar]
- 73.Henderson P, Hansen R, Cameron FL, et al. Rising incidence of pediatric inflammatory bowel disease in Scotland. Inflamm Bowel Dis. 2012;18(6):999–1005. doi: 10.1002/ibd.21797. [DOI] [PubMed] [Google Scholar]
- 74.Fitzgerald M, Mitton SG, Protheroe A, et al. The organisation and structure of inflammatory bowel disease services for children and young people in the UK in 2010: significant progress but still room for improvement. Frontline Gastroenterology. 2013;4:25–31. doi: 10.1136/flgastro-2012-100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hildebrand H, Finkel Y, Grahnquist L, Lindholm J, Ekbom A, Askling J. Changing pattern of paediatric inflammatory bowel disease in northern Stockholm 1990–2001. Gut. 2003;52(10):1432–1434. doi: 10.1136/gut.52.10.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malmborg P, Grahnquist L, Lindholm J, Montgomery S, Hildebrand H. Increasing incidence of paediatric inflammatory bowel disease in northern stockholm county, 2002–2007. J Pediatr Gastroenterol Nutr. 2013;57(1):29–34. doi: 10.1097/MPG.0b013e31828f21b4. [DOI] [PubMed] [Google Scholar]
- 77.Lapidus A. Crohn’s disease in Stockholm County during 1990–2001 an epidemiological update. World J Gastroenterol. 2006;12(1):75–81. doi: 10.3748/wjg.v12.i1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perminow G, Frigessi A, Rydning A, Nakstad B, Vatn MH. Incidence and clinical presentation of IBD in children: comparison between prospective and retrospective data in a selected Norwegian population. Scand J Gastroenterol. 2006;41(12):1433–1439. doi: 10.1080/00365520600789891. [DOI] [PubMed] [Google Scholar]
- 79.Solberg IC, Vatn MH, Høie O, et al. IBSEN Study Group Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Sewell JL, Yee HF, Jr, Inadomi JM. Hospitalizations are increasing among minority patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2010;16(2):204–207. doi: 10.1002/ibd.21008. [DOI] [PubMed] [Google Scholar]
- 81.Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16(1):112–124. doi: 10.1002/ibd.21048. [DOI] [PubMed] [Google Scholar]
- 82.Mehta S. Inflammatory bowel disease in children: Indian perspective. Indian J Pediatr. 1999;66(1 Suppl):S87–S88. [PubMed] [Google Scholar]
- 83.Nikfar S, Rahimi R, Rezaie A, Abdollahi M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig Dis Sci. 2009;54(6):1157–1170. doi: 10.1007/s10620-008-0481-x. [DOI] [PubMed] [Google Scholar]
- 84.De Cassan C, Fiorino G, Danese S. Second-generation corticosteroids for the treatment of Crohn’s disease and ulcerative colitis: more effective and less side effects? Dig Dis. 2012;30(4):368–375. doi: 10.1159/000338128. [DOI] [PubMed] [Google Scholar]
- 85.Rahimi R, Nikfar S, Abdollahi M. Do anti-tumor necrosis factors induce response and remission in patients with acute refractory Crohn’s disease? A systematic meta-analysis of controlled clinical trials. Biomed Pharmacother. 2007;61(1):75–80. doi: 10.1016/j.biopha.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 86.Ehteshami-Afshar S, Nikfar S, Rezaie A, Abdollahi M. A systematic review and meta-analysis of the effects of infliximab on the rate of colectomy and post-operative complications in patients with inflammatory bowel disease. Arch Med Sci. 2011;7(6):1000–1012. doi: 10.5114/aoms.2011.26612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci. 2007;52(11):2920–2925. doi: 10.1007/s10620-007-9760-1. [DOI] [PubMed] [Google Scholar]
- 88.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. A meta-analysis of broad-spectrum antibiotic therapy in patients with active Crohn’s disease. Clin Ther. 2006;28(12):1983–1988. doi: 10.1016/j.clinthera.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 89.De Greef E, Vandenplas Y, Hauser B, Devreker T, Veereman-Wauters G. Probiotics and IBD. Acta Gastroenterol Belg. 2013;76(1):15–19. [PubMed] [Google Scholar]
- 90.Rahimi R, Nikfar S, Rahimi F, et al. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2008;53(9):2524–2531. doi: 10.1007/s10620-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 91.Ko JK, Auyeung KK. Inflammatory Bowel Disease: Etiology, Pathogenesis and Current Therapy. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- 92.Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):630–642. doi: 10.1038/ajg.2011.64. [DOI] [PubMed] [Google Scholar]
- 93.Ardizzone S, Cassinotti A, de Franchis R. Immunosuppressive and biologic therapy for ulcerative colitis. Expert Opin Emerg Drugs. 2012;17(4):449–467. doi: 10.1517/14728214.2012.744820. [DOI] [PubMed] [Google Scholar]
- 94.M’Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007;22(10):1143–1163. doi: 10.1007/s00384-007-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Riss S, Schuster I, Papay P, Mittlböck M, Stift A. Repeat intestinal resections increase the risk of recurrence of Crohn’s disease. Dis Colon Rectum. 2013;56(7):881–887. doi: 10.1097/DCR.0b013e31828cb80c. [DOI] [PubMed] [Google Scholar]
- 96.Benchimol EI, Cook SF, Erichsen R, et al. International variation in medication prescription rates among elderly patients with inflammatory bowel disease. J Crohns Colitis. 2012 doi: 10.1016/j.crohns.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 97.Park KT, Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis. 2011;17(7):1603–1609. doi: 10.1002/ibd.21488. [DOI] [PubMed] [Google Scholar]
- 98.Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53(10):1471–1478. doi: 10.1136/gut.2004.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindsay J, Punekar YS, Morris J, Chung-Faye G. Health-economic analysis: cost-effectiveness of scheduled maintenance treatment with infliximab for Crohn’s disease—modelling outcomes in active luminal and fistulizing disease in adults. Aliment Pharmacol Ther. 2008;28(1):76–87. doi: 10.1111/j.1365-2036.2008.03709.x. [DOI] [PubMed] [Google Scholar]
- 100.Luces C, Bodger K. Economic burden of inflammatory bowel disease: a UK perspective. Expert Rev Pharmacoecon Outcomes Res. 2006;6(4):471–482. doi: 10.1586/14737167.6.4.471. [DOI] [PubMed] [Google Scholar]
- 101.Yu AP, Cabanilla LA, Wu EQ, Mulani PM, Chao J. The costs of Crohn’s disease in the United States and other Western countries: a systematic review. Curr Med Res Opin. 2008;24(2):319–328. doi: 10.1185/030079908x260790. [DOI] [PubMed] [Google Scholar]
- 102.Gibson TB, Ng E, Ozminkowski RJ, et al. The direct and indirect cost burden of Crohn’s disease and ulcerative colitis. J Occup Environ Med. 2008;50(11):1261–1272. doi: 10.1097/JOM.0b013e318181b8ca. [DOI] [PubMed] [Google Scholar]
- 103.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135(6):1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Odes S, Vardi H, Friger M, et al. European Collaborative Study on Inflammatory Bowel Disease Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology. 2006;131(3):719–728. doi: 10.1053/j.gastro.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 105.Bernstein CN, Papineau N, Zajaczkowski J, Rawsthorne P, Okrusko G, Blanchard JF. Direct hospital costs for patients with inflammatory bowel disease in a Canadian tertiary care university hospital. Am J Gastroenterol. 2000;95(3):677–683. doi: 10.1111/j.1572-0241.2000.01845.x. [DOI] [PubMed] [Google Scholar]
- 106.Acheson ED. The distribution of ulcerative colitis and regional enteritis in United States veterans with particular reference to the Jewish religion. Gut. 1960;1:291–293. doi: 10.1136/gut.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roth MP, Petersen GM, McElree C, Feldman E, Rotter JI. Geographic origins of Jewish patients with inflammatory bowel disease. Gastroenterology. 1989;97(4):900–904. doi: 10.1016/0016-5085(89)91495-9. [DOI] [PubMed] [Google Scholar]
- 108.Mayberry JF, Judd D, Smart H, Rhodes J, Calcraft B, Morris JS. Crohn’s disease in Jewish people—an epidemiological study in south-east Wales. Digestion. 1986;35(4):237–240. doi: 10.1159/000199374. [DOI] [PubMed] [Google Scholar]
- 109.Logan RF. Inflammatory bowel disease incidence: up, down or unchanged? Gut. 1998;42(3):309–311. doi: 10.1136/gut.42.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levi Z, Shamiss A, Fraser GM, et al. The increasing prevalence of inflammatory bowel diseases among jewish adolescents and the sociodemographic factors associated with diagnosis. Inflamm Bowel Dis. 2013;19(9):1867–1871. doi: 10.1097/MIB.0b013e31828a3797. [DOI] [PubMed] [Google Scholar]
- 111.Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am J Gastroenterol. 2009;104(8):2100–2109. doi: 10.1038/ajg.2009.190. [DOI] [PubMed] [Google Scholar]
- 112.Penn KA, Whittle DO, Lee ML. Inflammatory bowel disease in Jamaica. Annals of Gastroenterology. 2013;26(3):1–4. [PMC free article] [PubMed] [Google Scholar]
- 113.Torres EA, Cruz A, Monagas M, et al. Inflammatory bowel disease in Hispanics: The University of Puerto Rico IBD Registry. Int J Inflam. 2012;2012:574079. doi: 10.1155/2012/574079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Desai HG, Gupte PA. Increasing incidence of Crohn’s disease in India: is it related to improved sanitation? Indian J Gastroenterol. 2005;24(1):23–24. [PubMed] [Google Scholar]
- 115.Zheng JJ, Zhu XS, Huangfu Z, Gao ZX, Guo ZR, Wang Z. Crohn’s disease in mainland China: a systematic analysis of 50 years of research. Chin J Dig Dis. 2005;6(4):175–181. doi: 10.1111/j.1443-9573.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 116.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;1238:6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010;50(1):27–31. doi: 10.1097/MPG.0b013e3181b99baa. [DOI] [PubMed] [Google Scholar]
- 118.Loftus CG, Loftus EV, Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13(3):254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 119.Gunesh S, Thomas GA, Williams GT, Roberts A, Hawthorne AB. The incidence of Crohn’s disease in Cardiff over the last 75 years: an update for 1996–2005. Aliment Pharmacol Ther. 2008;27(3):211–219. doi: 10.1111/j.1365-2036.2007.03576.x. [DOI] [PubMed] [Google Scholar]
- 120.Askling J, Grahnquist L, Ekbom A, Finkel Y. Incidence of paediatric Crohn’s disease in Stockholm, Sweden. Lancet. 1999;354(9185):1179. doi: 10.1016/S0140-6736(99)02625-2. [DOI] [PubMed] [Google Scholar]
- 121.Armitage E, Drummond HE, Wilson DC, Ghosh S. Increasing incidence of both juvenile-onset Crohn’s disease and ulcerative colitis in Scotland. Eur J Gastroenterol Hepatol. 2001;13(12):1439–1447. doi: 10.1097/00042737-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 122.Turunen P, Kolho KL, Auvinen A, Iltanen S, Huhtala H, Ashorn M. Incidence of inflammatory bowel disease in Finnish children, 1987–2003. Inflamm Bowel Dis. 2006;12(8):677–683. doi: 10.1097/00054725-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Phavichitr N, Cameron DJ, Catto-Smith AG. Increasing incidence of Crohn’s disease in Victorian children. J Gastroenterol Hepatol. 2003;18(3):329–332. doi: 10.1046/j.1440-1746.2003.02975.x. [DOI] [PubMed] [Google Scholar]
- 124.Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12(23):3668–3672. doi: 10.3748/wjg.v12.i23.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149(10):916–924. doi: 10.1093/oxfordjournals.aje.a009735. [DOI] [PubMed] [Google Scholar]
- 126.Ekbom A, Adami HO, Helmick CG, Jonzon A, Zack MM. Perinatal risk factors for inflammatory bowel disease: a case-control study. Am J Epidemiol. 1990;132(6):1111–1119. doi: 10.1093/oxfordjournals.aje.a115754. [DOI] [PubMed] [Google Scholar]
- 127.Klement E, Lysy J, Hoshen M, Avitan M, Goldin E, Israeli E. Childhood hygiene is associated with the risk for inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2008;103(7):1775–1782. doi: 10.1111/j.1572-0241.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 128.Radon K, Windstetter D, Poluda AL, Mueller B, von Mutius E, Koletzko S, Chronische Autoimmunerkrankungen und Kontakt zu Tieren (Chronic Autoimmune Disease and Animal Contact) Study Group Contact with farm animals in early life and juvenile inflammatory bowel disease: a case-control study. Pediatrics. 2007;120(2):354–361. doi: 10.1542/peds.2006-3624. [DOI] [PubMed] [Google Scholar]
- 129.Hernandez V, Clofent J. Intestinal cancer in inflammatory bowel disease: natural history and surveillance guidelione. Annals of Gastroenterology. 2012;25(3):193–200. [PMC free article] [PubMed] [Google Scholar]
- 130.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336(8711):357–359. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 131.M’Koma AE, Moses HL, Adunyah SE. Inflammatory bowel disease-associated colorectal cancer: proctocolectomy and mucosectomy do not necessarily eliminate pouch-related cancer incidences. Int J Colorectal Dis. 2011;26(5):533–552. doi: 10.1007/s00384-011-1137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Das P, Johnson MW, Tekkis PP, Nicholls RJ. Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2007;9(1):15–27. doi: 10.1111/j.1463-1318.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 133.Um JW, M’Koma AE. Pouch-related dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Tech Coloproctol. 2011;15(1):7–16. doi: 10.1007/s10151-010-0664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heppell J, Weiland LH, Perrault J, Pemberton JH, Telander RL, Beart RW. Fate of the rectal mucosa after rectal mucosectomy and ileoanal anastomosis. Dis Colon Rectum. 1983;26(12):768–771. doi: 10.1007/BF02554744. [DOI] [PubMed] [Google Scholar]
- 135.Hultén L, Willén R, Nilsson O, Safarani N, Haboubi N. Mucosal assessment for dysplasia and cancer in the ileal pouch mucosa in patients operated on for ulcerative colitis—a 30-year follow-up study. Dis Colon Rectum. 2002;45(4):448–452. doi: 10.1007/s10350-004-6218-9. [DOI] [PubMed] [Google Scholar]
- 136.Nguyen GC, Nugent Z, Shaw S, Bernstein CN. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology. 2011;141(1):90–97. doi: 10.1053/j.gastro.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 137.Rejler M, Tholstrup J, Elg M, Spångéus A, Gäre BA. Framework for assessing quality of care for inflammatory bowel disease in Sweden. World J Gastroenterol. 2012;18(10):1085–1092. doi: 10.3748/wjg.v18.i10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 139.Kappelman MD, Dorn SD, Peterson E, Runge T, Allen JI. Quality of care for gastrointestinal conditions: a primer for gastroenterologists. Am J Gastroenterol. 2011;106(7):1182–1187. doi: 10.1038/ajg.2011.118. [DOI] [PubMed] [Google Scholar]
- 140.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 141.Crandall WV, Margolis PA, Kappelman MD, et al. ImproveCareNow Collaborative Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics. 2012;129(4):e1030–e1041. doi: 10.1542/peds.2011-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Table: Threshold values for intervention cost-effectiveness by Region. World Health Organization; [Accessed date 2007/2008]. http://www.who.int/choice/costs/CER_levels/en/index.html. [Google Scholar]
- 143.Day H, Kinosian B. A tale of two walls. J Gen Intern Med. 1998;13(10):718–719. doi: 10.1046/j.1525-1497.1998.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2008;28(10):1230–1239. doi: 10.1111/j.1365-2036.2008.03839.x. [DOI] [PubMed] [Google Scholar]
- 145.Williams JG, Cheung WY, Russell IT, Cohen DR, Longo M, Lervy B. Open access follow up for inflammatory bowel disease: pragmatic randomised trial and cost effectiveness study. BMJ. 2000;320(7234):544–548. doi: 10.1136/bmj.320.7234.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nugent Z, Blanchard JF, Bernstein CN. A population-based study of health-care resource use among infliximab users. Am J Gastroenterol. 2010;105(9):2009–2016. doi: 10.1038/ajg.2010.139. [DOI] [PubMed] [Google Scholar]
- 147.Averboukh F, Ziv Y, Kariv Y, et al. Colorectal carcinoma in inflammatory bowel disease: a comparison between Crohn’s and ulcerative colitis. Colorectal Dis. 2011;13(11):1230–1235. doi: 10.1111/j.1463-1318.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 148.Kane S. Establishing an inflammatory bowel disease practice in an accountable world. Clin Gastroenterol Hepatol. 2012;10(12):1301–1304. doi: 10.1016/j.cgh.2012.09.022. [DOI] [PubMed] [Google Scholar]