Abstract
Background
Understanding intensive care unit (ICU) triage decisions for high-risk surgical patients may ultimately facilitate resource allocation and improve outcomes. The surgical Apgar score (SAS) is a simple score that uses intraoperative information on hemodynamics and blood loss to predict postoperative morbidity and mortality, with lower scores associated with worse outcomes. We hypothesized that the SAS would be associated with the decision to admit a patient to the ICU postoperatively.
Methods
Retrospective cohort study of adults undergoing major intra-abdominal surgery from 2003 to 2010 at an academic medical center. We calculated the SAS (0 – 10) for each patient based on intraoperative heart rate, mean arterial pressure, and estimated blood loss. Using logistic regression, we assessed the association of the SAS with the decision to admit a patient directly to the ICU after surgery.
Results
The cohort consisted of 8,501 patients, with 72.7% having a SAS of 7-10 and less than 5% a SAS of 0-4. A total of 8.7% of patients were transferred immediately to the ICU postoperatively. After multivariate adjustment, there was a strong association between the SAS and the decision to admit a patient to the ICU (adjusted odds ratio 14.41 [95% CI 6.88 – 30.19, P < 0.001] for SAS 0-2, 4.42 [95% CI 3.19 – 6.13, P <0.001] for SAS 3-4, and 2.60 [95% CI 2.08 – 3.24, P < 0.001] for SAS 5-6 compared with SAS 7-8).
Conclusions
The SAS is strongly associated with clinical decisions regarding immediate ICU admission after high-risk intra-abdominal surgery. These results provide an initial step towards understanding whether intraoperative hemodynamics and blood loss influence ICU triage for post-surgical patients.
INTRODUCTION
Triage of high-risk surgical patients to intensive care may impact outcomes in those with the highest likelihood of postoperative complications and death. In one large study in the United Kingdom, patients undergoing high-risk surgical procedures accounted for 12.5% of hospital admissions but over 80% of postoperative deaths, with less than 15% admitted to the intensive care unit (ICU) after surgery.1 Another British study showed that high-risk patients admitted to the ICU immediately after surgery had greatly improved survival compared with patients who were admitted to the ICU after a delay.2 Appropriately identifying patients who may require intensive care postoperatively may facilitate resource allocation and ultimately improve postoperative outcomes.
Limitations on postoperative ICU admission may be due in part to high demand relative to scarce ICU resources3, 4 or may be related to the perception that intensive care is unnecessary. Therefore, intensivists, surgeons, and anesthesiologists must make postoperative triage decisions on whether a patient should be admitted to intensive care, and high-risk patients appropriate for ICU admission must be identified by the end of surgery. Triaging physicians may consider many perioperative factors when deciding whether to admit a patient to the ICU after surgery, including preoperative patient characteristics, surgical procedure, and postoperative concerns. Specific patient and surgical factors that may compel triaging physicians to opt for postoperative ICU admission include advanced age, the presence of multiple comorbidities, emergency procedures, and high surgical complexity, which have all been associated with poor postoperative outcomes.1
Intraoperative factors can also affect postoperative outcomes. The surgical Apgar score (SAS) was developed as a predictor of morbidity and mortality after surgery, incorporating three intraoperative variables – heart rate, mean arterial pressure, and estimated blood loss – to identify patients at highest risk of postoperative complications and death.5 The SAS takes on values from 0 to 10, with lower scores associated with worse outcomes. In theory, these intraoperative variables reflect a combination of surgical complexity and the individual patient’s response to surgical stress. The major benefit of using this score lies in the simplicity of its calculation; other perioperative scoring systems that use intraoperative factors to predict outcomes are more complicated to calculate.6
Ultimately, an easily calculated score could potentially be used to assist in ICU triage decisions at the end of surgery. As an initial step to this end, we chose to examine the relationship between the SAS and the clinical decision for immediate ICU admission after surgery. We hypothesized that the SAS is strongly associated with the decision to admit a patient to the ICU, irrespective of other patient or surgical factors.
METHODS
Patient selection
We performed a retrospective cohort study of adult patients age 18 years or older undergoing major intra-abdominal surgical procedures at Columbia University Medical Center (CUMC) from March 2003 through January 2010. This study was reviewed and approved by the CUMC Institutional Review Board (IRB-AAAF2559), and the requirement for written informed consent was waived by the IRB. Our goal was to select a group of patients with a relatively high frequency of postoperative ICU admission. We included patients who underwent surgery on any portion of the gastrointestinal tract, pancreas, spleen, hepatobiliary system, adrenal gland, urologic and gynecologic organs, and major vessels. Using information extracted from the electronic anesthesia record, we collected data on patient characteristics including age, gender, body mass index (BMI), type of procedure, whether it was an emergency, the anesthetic duration, and the American Society of Anesthesiologists’ (ASA) status. The ASA, a widely used marker of postoperative risk, is a simple preoperative scoring system that describes the overall physical status of the patient.7 Only the first high-risk surgical procedure during a single hospital admission was included. We excluded procedures on other organ systems, procedures that were outpatient and minor intra-abdominal surgeries, and those that involved cardiopulmonary bypass or organ transplantation (see Appendix 1 in Supplemental Data for a full list of exclusion criteria). We then used information from the CUMC electronic clinical information system (WebCIS) to determine the occurrence of immediate ICU admission, later ICU admission, and in-hospital mortality.
Immediate ICU admission was defined as transfer directly from the operating room to the ICU, while later ICU admission comprised patients who initially went to the post-anesthesia care unit (PACU), the step-down unit (SDU), or the floor prior to being admitted to the ICU. Patients who undergo high-risk intra-abdominal surgery at our institution either go from the operating room immediately to the ICU or to the PACU; the latter patients then get transferred to the ICU, a SDU, or the floor. The ICU allows 1:1 or 1:2 nurse-to-patient ratios with the ability to provide mechanical ventilation, renal replacement therapy, and administration of vasopressor and inotropic drugs. While none of these types of organ support is available in our SDU or floor units, mechanical ventilation and administration of vasopressors are allowed in our PACU. Transfer to the ICU is warranted for PACU patients with prolonged need for this level of care. Decisions about patient location at the end of surgery are generally made by the anesthesiologist and surgeon in conjunction with the intensivist.
Data collection
We extracted intraoperative data from the electronic anesthesia record (CompuRecord©, Philips Medical Systems, Andover, MA) to compute the SAS, comprising lowest heart rate (HR), lowest mean arterial pressure (MAP), and estimated blood loss (EBL) during the operation (Table 1). Using the electronic data acquisition algorithm described by Regenbogen et al, we excluded extraphysiologic values for HR (less than 20 or greater than 200 beats per minute) and MAP (less than 25 or greater than 180 mm Hg), and we used the median of the remaining HR and MAP values out of each 5-minute period.8 The intraoperative data were retrieved from the computerized anesthesia record system using Structured Query Language (SQL) in Microsoft® Visual Studio® 2008 (Microsoft Corporation, Redmond, Washington, USA). The data were then imported into R statistical software version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria) for subdivision into 5-minute epochs and determination of median values. Using these raw data, we then assigned appropriate points to the absolute values for HR, MAP, and EBL and calculated the SAS from these assigned points.
Table 1.
Calculation of the surgical Apgar score, range from 0 to 10 points, with lower scores associated with worse postoperative outcomes.
| Surgical Apgar Score, number of points | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Estimated blood loss, ml | > 1000 | 601-1000 | 101-600 | ≤ 100 | |
| Lowest MAP, mm Hg | < 40 | 40-54 | 55-69 | ≥ 70 | |
| Lowest HR, beats/minute | > 85 | 76-85 | 66-75 | 56-65 | ≤ 55 |
Abbreviations: ml = milliliters; MAP = mean arterial pressure; HR = heart rate.
To verify the accuracy of the electronic data acquisition algorithm, we chose to manually review approximately 1% of included patients, leading to 84 randomly selected electronic records for which we compared the manually calculated SAS results with those obtained through use of the algorithm. Manual calculation of lowest HR and MAP was performed by scrolling through vital sign data automatically collected every 15 seconds throughout each operative case by the electronic anesthesia record. HR data were manually extracted from the pulse rate (plethysmography) rather than from the electrocardiogram (ECG) given the possible interference of electrocautery with the ECG. Blood pressure data were preferentially recorded from invasive measurements over noninvasive measurements by the electronic anesthesia record. To exclude erroneous values, HR and MAP values were disregarded in the manual extraction if they differed by greater than 5 points (beats per minute or mm Hg) from the preceding and subsequent values. HR values extracted from plethysmography that differed by more than 5 beats per minute from the electrocardiographic HR values were also excluded. Computation of the Spearman rank correlation coefficient demonstrated very strong agreement between algorithm-generated and manually-determined point assignments for HR, MAP, EBL, and SAS (Appendix 2 in Supplemental Data).
Statistical analysis
We first summarized patient characteristics and outcomes for the entire cohort. We grouped continuous variables into appropriate categories to improve discriminative power and to use standard clinical categories. Age was divided into < 50, 50-59, 60-69, 70-79, ≥ 80 years, and BMI was grouped as < 18.5, 18.5-24.9, 25.0-29.9, and ≥ 30. ASA classes IV and V were combined into one class. Anesthetic duration was considered as < 2, 2-6, and > 6 hours. SAS was divided into groups of scores from 0-2, 3-4, 5-6, 7-8, and 9-10. As in Haynes et al, the median SAS of 7-8 was chosen as the reference group.9 Differences between patient and procedure characteristics and outcomes were assessed using the chi-square test for categorical variables.
We created univariate logistic regression models to evaluate each variable’s potential association with the clinical decision to postoperatively admit a patient to the ICU, including age, gender, BMI, ASA physical status, type of procedure, emergency procedure, anesthetic duration, and SAS. A multivariate logistic regression model was then developed to evaluate adjusted odds ratios (OR) including variables with P-value < 0.2 in the univariate models. Calibration of the multivariate model was evaluated with the Pearson χ2 test10 and discrimination was assessed with receiver operating characteristic (ROC) curves and the c-statistic. The first multivariate model included procedure type; we then generated models stratified by individual procedure type to evaluate the association of the SAS with specific types of procedures.
To evaluate the group of patients with later admission to the ICU, we first examined the distribution of these patients by SAS. We then performed an additional analysis of all the patients who did not immediately go to the ICU postoperatively to assess whether there was an association between the SAS and the outcome of later admission to the ICU. We examined the area under the curve (AUC) and determined the sensitivity and specificity of different cut-off values of the SAS with regard to the outcome of later ICU admission.
Database management and statistical analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) and Stata 10.0 (StataCorp LP, College Station, Texas, USA). A P-value of < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
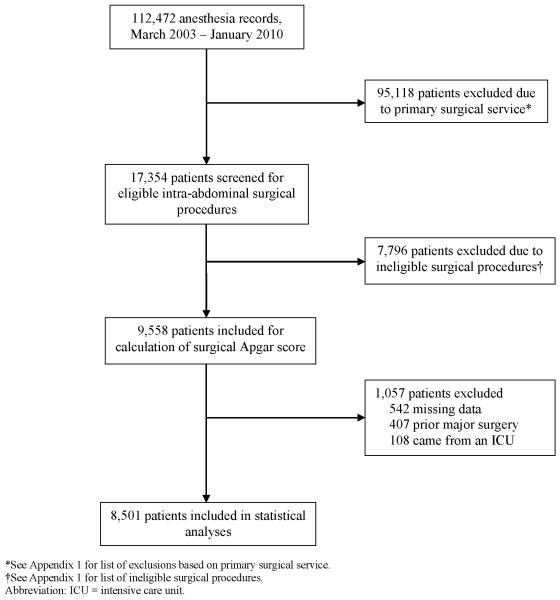
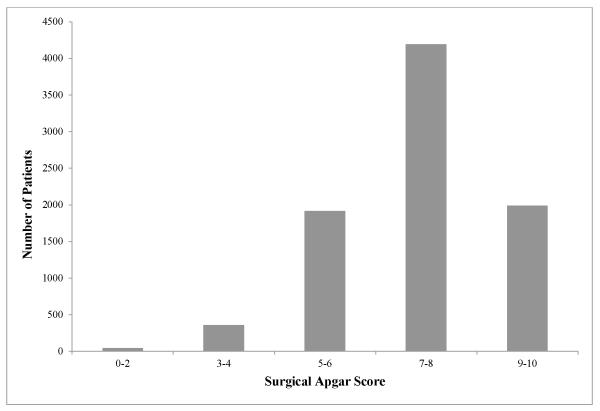
Between March 2003 and January 2010, 8,501 adult patients underwent primary major intra-abdominal surgical procedures and were included in our cohort (Figure 1). The cohort had a mean age of 59 years (± 15.6), 48.6% were female, 92.0% were elective cases, and almost one-third were assigned an ASA classification of III or higher. Approximately one-quarter of the patients were obese (BMI ≥ 30), while only 3.1% were considered underweight (BMI < 18.5). Anesthesia duration lasted between 2 and 6 hours in 73.5% of the cases and longer than 6 hours in 24.8%. The most common operations performed were bowel surgery, nephrectomy/adrenalectomy, prostatectomy, and gynecologic oncology procedures (Table 2). Approximately one-quarter of the entire cohort had a SAS of 9-10, and less than 5% of patients had a SAS of 0-4 (Figure 2). The overall hospital mortality for the cohort was 1.6%. As expected, the SAS was strongly associated with hospital mortality, with a lower SAS associated with higher mortality rates (SAS 0-2: 8.7%; SAS 3-4: 7.0%; SAS 5-6: 2.9%; SAS 7-8: 1.0%; SAS 9-10: 0.5%; P < 0.001).
Figure 1.
Flowsheet of cohort showing exclusions.
Table 2.
Patient characteristics and frequency of admission to the ICU.
| Patient Characteristics | Total number of patients, n (% of all patients) |
Immediate ICU admission, n (% of total in each stratum) |
P-value | ||
|---|---|---|---|---|---|
| Total number of patients, n | 8501 | 737 (8.7) | |||
| Age, years | < 50 | 2214 (26.0) | 117 (5.3) | < 0.001 | |
| 50-59 | 1917 (22.6) | 143 (7.5) | |||
| 60-69 | 2146 (25.2) | 179 (8.3) | |||
| 70-79 | 1467 (17.3) | 186 (12.7) | |||
| ≥ 80 | 757 (8.9) | 112 (14.8) | |||
| Female | 4133 (48.6) | 301 (7.3) | < 0.001 | ||
| Male | 4368 (51.4) | 436 (10.0) | |||
| ASA | I | 724 (8.5) | 31 (4.3) | < 0.001 | |
| II | 5185 (61.0) | 245 (4.7) | |||
| III | 2434 (28.6) | 382 (15.7) | |||
| IV-V | 158 (1.9) | 79 (50.0) | |||
| Emergency procedure | 682 (8.0) | 124 (18.2) | < 0.001 | ||
| Elective procedure | 7819 (92.0) | 613 (7.8) | |||
| BMI * | < 18.5 | 261 (3.1) | 38 (14.6) | 0.003 | |
| 18.5-24.9 | 2873 (33.8) | 258 (9.0) | |||
| 25.0-29.9 | 2988 (35.1) | 246 (8.2) | |||
| ≥ 30 | 2354 (27.7) | 190 (8.1) | |||
| Anesthesia duration | < 2 hours | 139 (1.6) | 1 (0.7) | < 0.001 | |
| 2-6 hours | 6251 (73.5) | 220 (3.5) | |||
| > 6 hours | 2111 (24.8) | 516 (24.4) | |||
|
Type of
surgery |
Esophageal | Esophagectomy | 140 (1.6) | 79 (56.4) | < 0.001 |
| Other esophageal | 230 (2.7) | 3 (1.3) | |||
| Gastric | Gastric bypass/gastrectomy | 561 (6.6) | 7 (1.2) | ||
| Hepatobiliary and Pancreatic | Pancreatectomy/splenectomy | 407 (4.8) | 43 (10.6) | ||
| Whipple | 445 (5.2) | 147 (33.0) | |||
| Hepatectomy | 351 (4.1) | 128 (36.5) | |||
| Other hepatobiliary | 145 (1.7) | 16 (11.0) | |||
| Intestinal | Bowel surgery | 1506 (17.7) | 77 (5.1) | ||
|
Renal, Adrenal,
and Urologic |
Nephrectomy/adrenalectomy | 1316 (15.5) | 29 (2.2) | ||
| Cystectomy | 179 (2.1) | 38 (21.2) | |||
| Prostatectomy | 1211 (14.2) | 1 (0.1) | |||
| Other urologic | 157 (1.8) | 0 (0) | |||
|
Gynecologic
Oncology |
Gynecologic oncology | 927 (10.9) | 26 (2.8) | ||
| Vascular | Major vascular | 108 (1.3) | 64 (59.3) | ||
| Other | Exploratory laparotomy | 596 (7.0) | 75 (12.6) | ||
| Laparoscopy | 222 (2.6) | 4 (1.8) | |||
| SAS | 0-2 | 46 (0.5) | 26 (56.5) | < 0.001 | |
| 3-4 | 357 (4.2) | 115 (32.2) | |||
| 5-6 | 1915 (22.5) | 363 (19.0) | |||
| 7-8 | 4194 (49.3) | 212 (5.1) | |||
| 9-10 | 1989 (23.4) | 21 (1.1) | |||
Patients with missing or non-physiologic height and/or weight were excluded.
Abbreviations: ICU = intensive care unit; ASA = American Society of Anesthesiologists’ physical status; BMI = body mass index; SAS = surgical Apgar score.
Figure 2.
Frequency distribution of patients in cohort by surgical Apgar score.
Frequency of admission to ICU
Out of the total cohort, 737 (8.7%) were transferred directly from the operating room to the ICU after surgery, and 8.4% of these patients with immediate ICU admission died during hospitalization. Individual patient characteristics significantly affected the frequency of admission to ICU (Table 2). Significantly higher rates of ICU admission occurred in patients who were older, had higher ASA physical status, were underweight, had emergent procedures, and had longer anesthetic durations. Frequency of ICU admission also varied by surgical procedure: over half of patients who underwent esophagectomy and major vascular surgery were admitted to the ICU postoperatively, while only 0.1% of those who underwent prostatectomy went to the ICU after surgery (Table 2). The rate of immediate ICU admission increased progressively as SAS decreased, from 1.1% for patients with a SAS of 9-10 to 56.5% for those with a SAS of 0-2 (P < 0.001, Table 2).
Variables associated with immediate ICU admission
After multivariate modeling, a number of variables were found to be associated with the decision to admit a patient to the ICU immediately after surgery (Table 3). Patients with an ASA physical status of IV or V were more than eight times as likely to go to the ICU after surgery than patients with ASA I (adjusted OR 8.48 [95% CI 4.48 - 16.05, P < 0.001]). Those patients who underwent emergency procedures were almost five times as likely to be admitted to the ICU after surgery than those who underwent elective operations (adjusted OR 4.91 [95% CI 3.42 - 7.06, P < 0.001]), while patients with anesthetic duration > 6 hours were over four times as likely to go to the ICU postoperatively as those with duration 2 – 6 hours (adjusted OR 4.11 [95% CI 3.24 - 5.21, P < 0.001]). Certain surgical procedures were associated with higher rates of ICU admission when compared to the reference procedure of bowel surgery. Significantly greater proportions of patients who underwent esophagectomy (adjusted OR 26.71 [95% CI 16.53 - 43.19, P < 0.001]), Whipple (adjusted OR 5.76 [95% CI 3.99 - 8.33, P < 0.001]), hepatectomy (adjusted OR 12.90 [95% CI 8.75 - 19.01, P < 0.001]), and major vascular cases (adjusted OR 12.75 [95% CI 7.44 - 21.84, P < 0.001]) were admitted to the ICU postoperatively compared with the reference procedure. On the other hand, prostatectomy patients were significantly less likely to go the ICU when compared to patients undergoing bowel surgery (adjusted OR 0.04 [95% CI 0.01 - 0.31, P = 0.002]). After adjusting for other factors, there was a clear association between the SAS and the decision to admit a patient to the ICU. Patients with a SAS of 0-2 were fourteen times as likely to be admitted to the ICU compared with the reference group of patients with a SAS of 7-8 (adjusted OR 14.41 [95% CI 6.88 - 30.19, P < 0.001]).
Table 3.
Variables associated with immediate ICU admission as determined by univariate and multivariate analysis, with unadjusted and adjusted odds ratio (OR) and 95% confidence interval (CI).
| Unadjusted Odds Ratio | Adjusted Odds Ratio | |||||
|---|---|---|---|---|---|---|
| Patient Characteristics | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Age, years | < 50 | Reference | Reference | |||
| 50-59 | 1.44 (1.12,1.86) | 0.004 | 1.68 (1.21, 2.31) | 0.002 | ||
| 60-69 | 1.63 (1.28, 2.08) | < 0.001 | 1.60 (1.16, 2.20) | 0.004 | ||
| 70-79 | 2.60 (2.04, 3.31) | < 0.001 | 1.61 (1.15, 2.24) | 0.005 | ||
| ≥ 80 | 3.11 (2.37, 4.09) | < 0.001 | 2.25 (1.56, 3.26) | < 0.001 | ||
| Female | Reference | Reference | ||||
| Male | 0.71 (0.61, 0.83) | < 0.001 | 0.84 (0.69, 1.04) | 0.103 | ||
| ASA | I | Reference | Reference | |||
| II | 1.11 (0.76, 1.62) | 0.597 | 0.73 (0.45, 1.18) | 0.2 | ||
| III | 4.16 (2.86, 6.06) | < 0.001 | 1.44 (0.88, 2.37) | 0.149 | ||
| IV-V | 22.35 (13.89, 35.99) |
< 0.001 | 8.48 (4.48, 16.05) | < 0.001 | ||
| Elective procedure | Reference | Reference | ||||
| Emergency procedure | 2.61 (2.11, 3.23) | < 0.001 | 4.91 (3.42, 7.06) | < 0.001 | ||
| BMI | < 18.5 | 1.73 (1.20, 2.49) | 0.004 | 1.24 (0.77, 1.99) | 0.376 | |
| 18.5-24.9 | Reference | Reference | ||||
| 25.0-29.9 | 0.91 (0.76, 1.09) | 0.308 | 0.87 (0.69, 1.10) | 0.236 | ||
| ≥ 30 | 0.89 (0.73, 1.08) | 0.243 | 1.11 (0.87, 1.43) | 0.403 | ||
| Anesthesia duration | < 2 hours | 0.20 (0.03, 1.43) | 0.108 | 0.10 (0.01, 0.79) | 0.028 | |
| 2-6 hours | Reference | Reference | ||||
| > 6 hours | 8.87 (7.50, 10.48) | < 0.001 | 4.11 (3.24, 5.21) | < 0.001 | ||
| Type of surgery | Esophageal | Esophagectomy | 24.03 (16.03, 36.04) |
< 0.001 | 26.71 (16.53, 43.19) |
< 0.001 |
|
Other
esophageal |
0.25 (0.08, 0.78) | 0.018 | 0.82 (0.24, 2.79) | 0.753 | ||
| Gastric |
Gastric bypass /
gastrectomy |
0.23 (0.11, 0.51) | < 0.001 | 0.56 (0.24, 1.33) | 0.19 | |
|
Hepatobiliary
and Pancreatic |
Pancreatectomy
/ splenectomy |
2.19 (1.48, 3.24) | < 0.001 | 2.40 (1.52, 3.78) | < 0.001 | |
| Whipple | 9.15 (6.76, 12.39) | < 0.001 | 5.76 (3.99, 8.33) | < 0.001 | ||
| Hepatectomy | 10.65 (7.77, 14.61) |
< 0.001 | 12.90 (8.75, 19.01) |
< 0.001 | ||
|
Other
hepatobiliary |
2.30 (1.30, 4.06) | 0.004 | 2.74 (1.43, 5.24) | 0.002 | ||
| Intestinal | Bowel surgery | Reference | Reference | |||
|
Renal, Adrenal,
and Urologic |
Nephrectomy /
adrenalectomy |
0.42 (0.27, 0.65) | < 0.001 | 1.17 (0.72, 1.90) | 0.529 | |
| Cystectomy | 5.00 (3.27, 7.65) | < 0.001 | 2.55 (1.57, 4.16) | < 0.001 | ||
| Prostatectomy | 0.02 (0.00, 0.11) | < 0.001 | 0.04 (0.01, 0.31) | 0.002 | ||
| Other urologic | - | - | - | - | ||
|
Gynecologic
Oncology |
Gynecologic
oncology |
0.54 (0.34, 0.84) | 0.007 | 1.19 (0.71, 1.99) | 0.501 | |
| Vascular | Major vascular | 26.99 (17.26, 42.21) |
< 0.001 | 12.75 (7.44, 21.84) |
< 0.001 | |
| Other | Exploratory laparotomy | 2.67 (1.91, 3.73) | < 0.001 | 1.50 (1.00, 2.26) | 0.049 | |
| Laparoscopy | 0.34 (0.12, 0.94) | 0.038 | 0.42 (0.14, 1.25) | 0.121 | ||
| SAS | 0-2 | 24.42 (13.41, 44.45) |
< 0.001 | 14.41 (6.88, 30.19) |
< 0.001 | |
| 3-4 | 8.93 (6.87, 11.59) | < 0.001 | 4.42 (3.19, 6.13) | < 0.001 | ||
| 5-6 | 4.39 (3.67, 5.26) | < 0.001 | 2.60 (2.08, 3.24) | < 0.001 | ||
| 7-8 | Reference | Reference | ||||
| 9-10 | 0.20 (0.13, 0.31) | < 0.001 | 0.39 (0.24, 0.65) | < 0.001 | ||
Abbreviations: ICU = intensive care unit; ASA = American Society of Anesthesiologists’ physical status; BMI = body mass index; SAS = surgical Apgar score.
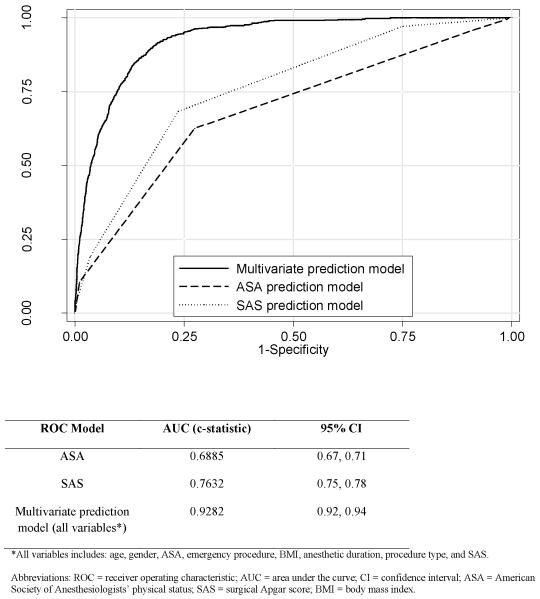
The multivariate model showed good discrimination regarding the postoperative decision for immediate ICU admission. ROC curve analysis demonstrated that the SAS alone was strongly associated with immediate admission to the ICU (c-statistic 0.7632 [95% CI 0.75 - 0.78]) and outperformed ASA physical status alone (c-statistic 0.6885 [95% CI 0.67 - 0.71]) (Figure 3). The model that included all statistically significant variables – SAS, ASA, age, gender, emergency procedure, BMI, type of surgery, and anesthetic duration – had excellent accuracy in distinguishing those patients who were admitted to the ICU from those who were not (c-statistic 0.93 [95% CI 0.92 - 0.94]), with good calibration (Pearson χ2(3170) = 3040.86, P = 0.95).
Figure 3.
Receiver operating characteristic (ROC) curves with area under the curve (AUC) and 95% confidence interval (CI) for models predicting ICU admission that included ASA alone, SAS alone, and the full multivariate prediction model.
Specific surgical procedures
Stratified by individual surgical procedure, the relationship between the SAS and the clinical decision for immediate ICU admission remained consistent across all of these procedures, with higher rates of ICU admission for patients with lower SAS. However, the number of patients in the lowest SAS group was often very small. The table of individual procedures and numbers of patients, the adjusted OR of ICU admission, AUC, and 95% confidence interval (CI) is included in Appendix 3 in Supplemental Data.
Patients with later ICU admission
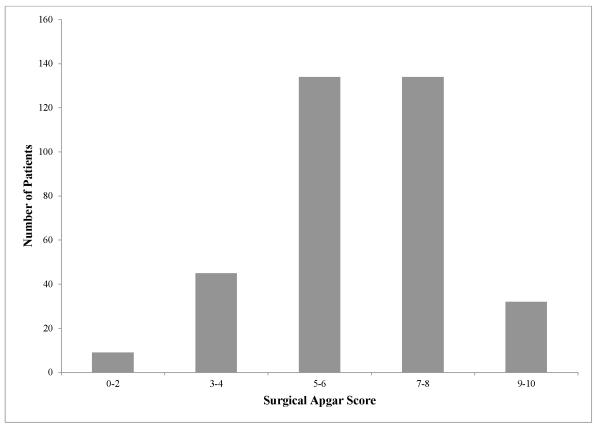
Of the 7,764 patients who did not receive immediate ICU admission, 354 (4.6%) had a subsequent later admission to the ICU. The frequency distribution by SAS for the patients with later ICU admission is shown in Figure 4. These patients are distributed widely across all the SAS strata, with the majority of patients having a SAS of 5-6 (37.9%) and 7-8 (37.9%). For the cohort of patients who did not initially get admitted to the ICU, we examined the sensitivity and specificity of different cut-off values of the SAS to assess the utility of the score for identifying patients who required later admission to the ICU (Table 4). The c-statistic of 0.69 (95% CI 0.66 – 0.72) demonstrates only moderate performance, and there is clearly no cut-off value that provides an adequate balance between sensitivity and specificity.
Figure 4.
Distribution of patients with later ICU admission by surgical Apgar score.
Table 4.
Sensitivity and specificity of different SAS cutoff values in predicting later ICU admission in those patients not immediately admitted to the ICU after surgery.
| Surgical Apgar Score Cutoff Value |
Sensitivity (%) | Specificity (%) |
|---|---|---|
| ≤ 2 | 2.5 | 99.8 |
| ≤ 4 | 15.3 | 97.2 |
| ≤ 6 | 53.1 | 78.1 |
| ≤ 8 | 91.0 | 26.1 |
AUC 0.69 (95% CI 0.66 – 0.72)
Abbreviations: SAS = surgical Apgar score; ICU = intensive care unit; AUC = area under the curve; CI = confidence interval.
DISCUSSION
This study demonstrates that the SAS is associated with the clinical decision to admit a patient to the ICU immediately after surgery, with a low SAS significantly associated with a higher likelihood of immediate postoperative ICU admission. The association between the SAS and ICU admission after high-risk surgery was far from perfect, however, as the SAS showed only moderate discrimination by itself, indicating that further exploration is needed to understand why some patients who experience hypotension, bleeding, and/or tachycardia intraoperatively are not perceived as requiring intensive care after surgery. Moreover, there was little association between the SAS and later ICU admission, suggesting that intraoperative changes in hemodynamics or blood loss may play less of a role in the subsequent deterioration of some postoperative patients.
The SAS has previously been shown to predict postoperative complications and mortality in certain populations. In the original development of the score, Gawande et al found that the SAS was associated with major surgical complications and death within 30 days for a cohort of general and vascular surgery patients,5 with similar results in a much larger validation cohort at a different institution.8 Other studies have demonstrated the ability of the SAS to predict outcomes in a wide range of international settings9, 11 and after a variety of surgical procedures.12,13,14,15,16 Some studies noted that the SAS may not comprehensively predict outcomes by itself,11, 17 but in the development and validation of the SAS, researchers intentionally chose an objective score that would be easy to calculate in real time,5 despite better discrimination achieved by using more complicated models.8 It is clear from our final model that incorporation of pre- and intraoperative factors provides the strongest association with current clinical decisions. In addition, the SAS may be of questionable utility, as it cannot be calculated until the completion of the surgical procedure. However, postponing final decision-making in order to consider the intraoperative course is helpful and may sometimes be necessary for current ICU triage practices, despite the inconvenience for early bed allocation.
Systematic ICU admission after high-risk surgery may improve postoperative outcomes, but there are no randomized clinical trials to support this assumption. In addition, the definition of a high-risk surgical patient remains elusive. Some studies define high-risk surgery as any procedure with a hospital mortality greater than 5%.1 Others focus on certain procedures known to have significant mortality, ranging from 3.0 to 8.0%.18 Our data suggest that current clinician triaging identifies a relatively high-risk group, as our hospital mortality rate for patients admitted to the ICU immediately after surgery was over 8%. However, it is also worth noting that the ultimate goal of care in the ICU is to decrease hospital mortality. For example, patients 75 years of age or younger who undergo coronary artery bypass grafting have very low in-hospital mortality (less than 2%)19 yet routinely receive intensive care to help achieve this low rate.
A scoring system that can improve upon physician decisions would more accurately match appropriate patients to the ICU postoperatively. One study in the United Kingdom suggested underutilization of intensive care resources in a high-risk surgical population. The mortality rate for those patients admitted directly to the ICU after surgery was significantly lower than for post-surgical patients who were either re-admitted to the ICU after premature discharge (mortality rate greater than 30%) or who were initially admitted to the ICU from the ward (mortality rate greater than 85%).2 Other researchers in the United States have examined variations across hospitals in rates of failure-to-rescue (i.e., the mortality rate of patients who experience a postoperative complication). While rates of complications for certain high-risk procedures were relatively similar amongst different hospitals, the mortality rates differed significantly,18 suggesting mismanagement of complications once they occurred. These studies illustrate the fallibility of physician decision-making and the potential role for a score that predicts which patients are likely to experience a complication.
Our study has a number of limitations. The patients in our cohort were from a single large academic medical center in the United States. There are few data on postoperative triage practices for comparison, so the generalizability of our findings to other institutions with different postoperative care systems and/or patient populations is unknown. ICU admission decisions are known to vary depending on patient comorbidities, family wishes, physician characteristics, ICU bed availability, institutional structure, and regional culture.20 Large registries of data relevant to perioperative care are currently being developed in the United States, such as the National Anesthesia Clinical Outcomes Registry (NACOR) and the Multicenter Perioperative Outcomes Group (MPOG),21 but they are still evolving and do not currently contain the detailed data required for this type of study. We also used the surgical procedure recorded in the anesthesia record, which may not have been as precise as the procedure described in the operative report. We limited our investigation to major intra-abdominal procedures, making generalization to other operations uncertain. However, we felt this group captured patients at a wide range of perioperative risk while simultaneously maintaining homogeneity. We also assumed that the surgical decision to operate was appropriate. This was a retrospective study that did not prospectively use the SAS in decisions regarding ICU admission after surgery. We also examined patients over a prolonged period of time (during which four additional surgical ICU beds were opened in 2006), and practices of ICU admission may have changed, although we are unaware of any systematic shifts in our ICU triage policies or practices. Finally, as mentioned above, the SAS and other intraoperative events can only be fully assessed at the conclusion of surgery, thus potentially limiting the utility of such data for certain triage decisions that may preferably occur earlier to allow for more advanced planning for ICU resource allocation.
We have shown that the SAS is significantly associated with the clinical decision to immediately admit patients to the ICU after high-risk intra-abdominal surgery across many surgical subtypes and that the SAS does not help to discriminate between patients who will or will not require later admission to the ICU. Given the dearth of guidelines and standards for ICU triage and the potential for both under- and over-use of the ICU for surgical patients, information regarding current clinician practice is essential. Moreover, there is a possible role for a score such as the SAS or a new predictive tool that could assist in standardizing decision-making and matching the patients most likely to benefit from ICU admission with intensive care after surgery. Future studies should focus on prospectively evaluating possible tools that may ultimately be used to help improve patient outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
Nanshi Sha, B.S., M.S., Ph.D., assisted with the extraction of intraoperative data from the electronic anesthesia record and the development of the electronic data acquisition algorithm.
Funding: Julia Sobol: Ruth L. Kirchstein National Research Service Award (NRSA) Institutional Research Training Grant (T32)
Hannah Wunsch: Award Number K08AG038477 from the National Institute on Aging
Guohua Li: Award Number R01AA09963 and R21DA029670 from the National Institutes of Health
Information for LWW regarding depositing manuscript into PubMed Central: This research was funded by National Institutes of Health grant number R01AA09963 and R21DA029670.
Footnotes
Role: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Conflicts: Julia B. Sobol reported no conflicts of interest
Attestation: Julia B. Sobol has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Role: This author helped analyze the data and write the manuscript
Conflicts: Hayley B. Gershengorn reported no conflicts of interest
Attestation: Hayley B. Gershengorn has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Role: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Conflicts: Hannah Wunsch reported no conflicts of interest
Attestation: Hannah Wunsch has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Role: This author helped design the study, conduct the study, and write the manuscript
Conflicts: Guohua Li reported no conflicts of interest
Attestation: Guohua Li has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
This report was previously presented, in part, at the Society of Critical Care Anesthesiologists annual meeting 10/14/2011 in Chicago, IL, and the American Society of Anesthesiologists annual meeting 10/15/2011 in Chicago, IL.
This manuscript describes human research.
IRB contact information: Susie Kim, Columbia University Medical Center IRB, 722 West 168th Street, 4th Floor Room #426, New York, NY 10032, (212) 305-5883 or (212) 342-3058 or sjk2142@columbia.edu.
The requirement for written informed consent was waived by the Institutional Review Board.
This report describes an observational clinical study.
This report describes cohort observational clinical study. The author states that the report includes every item in the STROBE checklist for cohort observational clinical studies.
Contributor Information
Julia B. Sobol, Department of Anesthesiology, College of Physicians & Surgeons, Columbia University, New York, NY, USA jbs2005@columbia.edu.
ayley B. Gershengorn, Albert Einstein College of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Beth Israel Medical Center, New York, NY USA hgershengorn@chpnet.org.
Hannah Wunsch, Department of Anesthesiology, College of Physicians & Surgeons, Columbia University, New York, NY, USA; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA hw2125@columbia.edu.
Guohua Li, Department of Anesthesiology, College of Physicians & Surgeons, Columbia University, New York, NY, USA; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA gl2240@mail.cumc.columbia.edu.
REFERENCES
- 1.Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, Grounds RM, Bennett ED. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia. 2008;63:695–700. doi: 10.1111/j.1365-2044.2008.05560.x. [DOI] [PubMed] [Google Scholar]
- 3.Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303:1371–2. doi: 10.1001/jama.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, de Keizer NF, Kersten A, Linde-Zwirble WT, Sandiumenge A, Rowan KM. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36:2787–93. e1–9. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 5.Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204:201–8. doi: 10.1016/j.jamcollsurg.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Mangam S, Marzouk D. A review of risk scoring systems utilised in patients undergoing gastrointestinal surgery. J Gastrointest Surg. 2009;13:1529–38. doi: 10.1007/s11605-009-0857-z. [DOI] [PubMed] [Google Scholar]
- 7.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–4. [Google Scholar]
- 8.Regenbogen SE, Ehrenfeld JM, Lipsitz SR, Greenberg CC, Hutter MM, Gawande AA. Utility of the surgical apgar score: validation in 4119 patients. Arch Surg. 2009;144:30–6. doi: 10.1001/archsurg.2008.504. discussion 7. [DOI] [PubMed] [Google Scholar]
- 9.Haynes AB, Regenbogen SE, Weiser TG, Lipsitz SR, Dziekan G, Berry WR, Gawande AA. Surgical outcome measurement for a global patient population: validation of the Surgical Apgar Score in 8 countries. Surgery. 2011;149:519–24. doi: 10.1016/j.surg.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Dupont WD. Statistical modeling for biomedical researchers : a simple introduction to the analysis of complex data. 2nd edition Cambridge University Press; Cambridge, UK ; New York: 2009. [Google Scholar]
- 11.Ohlsson H, Winso O. Assessment of the Surgical Apgar Score in a Swedish setting. Acta Anaesthesiol Scand. 2011;55:524–9. doi: 10.1111/j.1399-6576.2011.02424.x. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds PQ, Sanders NW, Schildcrout JS, Mercaldo ND, St Jacques PJ. Expansion of the surgical Apgar score across all surgical subspecialties as a means to predict postoperative mortality. Anesthesiology. 2011;114:1305–12. doi: 10.1097/ALN.0b013e318219d734. [DOI] [PubMed] [Google Scholar]
- 13.Prasad SM, Ferreria M, Berry AM, Lipsitz SR, Richie JP, Gawande AA, Hu JC. Surgical apgar outcome score: perioperative risk assessment for radical cystectomy. J Urol. 2009;181:1046–52. doi: 10.1016/j.juro.2008.10.165. discussion 52-3. [DOI] [PubMed] [Google Scholar]
- 14.Regenbogen SE, Bordeianou L, Hutter MM, Gawande AA. The intraoperative Surgical Apgar Score predicts postdischarge complications after colon and rectal resection. Surgery. 2010;148:559–66. doi: 10.1016/j.surg.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assifi MM, Lindenmeyer J, Leiby BE, Grunwald Z, Rosato EL, Kennedy EP, Yeo CJ, Berger AC. Surgical Apgar score predicts perioperative morbidity in patients undergoing pancreaticoduodenectomy at a high-volume center. J Gastrointest Surg. 2012;16:275–81. doi: 10.1007/s11605-011-1733-1. [DOI] [PubMed] [Google Scholar]
- 16.Zighelboim I, Kizer N, Taylor NP, Case AS, Gao F, Thaker PH, Rader JS, Massad LS, Mutch DG, Powell MA. “Surgical Apgar Score” predicts postoperative complications after cytoreduction for advanced ovarian cancer. Gynecol Oncol. 2010;116:370–3. doi: 10.1016/j.ygyno.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Wuerz TH, Regenbogen SE, Ehrenfeld JM, Malchau H, Rubash HE, Gawande AA, Kent DM. The Surgical Apgar Score in hip and knee arthroplasty. Clin Orthop Relat Res. 2011;469:1119–26. doi: 10.1007/s11999-010-1721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–34. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 19.Al-Alao BS, Parissis H, McGovern E, Tolan M, Young VK. Propensity analysis of outcome in coronary artery bypass graft surgery patients >75 years old. Gen Thorac Cardiovasc Surg. 2012;60:217–24. doi: 10.1007/s11748-011-0875-0. [DOI] [PubMed] [Google Scholar]
- 20.Capuzzo M, Moreno RP, Alvisi R. Admission and discharge of critically ill patients. Curr Opin Crit Care. 2010;16:499–504. doi: 10.1097/MCC.0b013e32833cb874. [DOI] [PubMed] [Google Scholar]
- 21.Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25:489–98. doi: 10.1016/j.bpa.2011.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.