Abstract
Purpose
The guideline purpose is to provide the urologist with a framework for the early detection of prostate cancer in asymptomatic average risk men.
Materials and Methods
A systematic review was conducted and summarized evidence derived from over 300 studies that addressed the predefined outcomes of interest (prostate cancer incidence/mortality, quality of life, diagnostic accuracy and harms of testing). In addition to the quality of evidence, the panel considered values and preferences expressed in a clinical setting (patient-physician dyad) rather than having a public health perspective. Guideline statements were organized by age group in years (age <40; 40 to 54; 55 to 69; >70).
Results
With the exception of prostate-specific antigen (PSA)-based prostate cancer screening, there was minimal evidence to assess the outcomes of interest for other tests. The quality of evidence for the benefits of screening was moderate, and evidence for harm was high for men age 55 to 69 years. For men outside this age range, evidence was lacking for benefit, but the harms of screening, including over diagnosis and over treatment, remained. Modeled data suggested that a screening interval of two years or more may be preferred to reduce the harms of screening.
Conclusions
The Panel recommended shared decision-making for men age 55 to 69 years considering PSA-based screening, a target age group for whom benefits may outweigh harms. Outside this age range, PSA-based screening as a routine could not be recommended based on the available evidence. The entire guideline is available at www.AUAnet.org/education/guidelines/prostate-cancer-detection.cfm
Keywords: prostate cancer, prostate specific antigen, screening, early detection
PURPOSE
This guideline addresses prostate cancer early detection for the purpose of reducing prostate cancer mortality with the intended user as the urologist. This document does not make a distinction between early detection and screening for prostate cancer; both imply detection of disease at an early, pre-symptomatic stage when an individual would have no reason to seek medical care.1 In the US, early detection is driven by prostate specific antigen (PSA)-based screening followed by prostate biopsy for diagnostic confirmation. This document does not address detection of prostate cancer in symptomatic men whose symptoms could be related to locally advanced or metastatic prostate cancer (e.g., new onset bone pain and/or neurological symptoms involving the lower extremities, etc.).
METHODOLOGY
A systematic review and meta-analysis of the published literature on prostate cancer detection and screening was conducted to identify published studies relevant to prostate cancer detection and screening. The search focused on digital rectal exam (DRE), serum biomarkers (PSA, PSA Isoforms, PSA kinetics, free PSA, complexed PSA, proPSA, prostate health index, PSA velocity, PSA doubling time), urine biomarkers (PCA3, TMPRSS2:ERG fusion), imaging (TRUS, MRI, MRS, MR-TRUS fusion), genetics (SNPs), shared decision-making and prostate biopsy. The outcomes of interest were a priori determined by the Panel and included prostate cancer incidence and mortality, quality of life, the diagnostic performance of each of the tests and the harms of testing (premature death and complications from testing and biopsy). The systematic review included over 300 eligible studies that addressed the questions of interest published between 1995 and 2013.
The AUA nomenclature system explicitly links statement type to body of evidence strength and the Panel's judgment regarding the balance between benefits and risks/burdens.2 For a complete discussion of the methodology and evidence grading, please refer to the unabridged guideline available at www.AUAnet.org/education/guidelines/prostate-cancer-detection.cfm
BACKGROUND
Guideline Framework and Evidence Interpretation
The literature supporting the efficacy of DRE and biomarkers other than PSA for screening average risk men was of low to moderate quality, was more relevant to cancer detection in higher risk men than true average risk population screening and did not address outcomes important to patients, such as mortality or quality of life. Therefore, this document focuses only on the efficacy of PSA screening for the early detection of prostate cancer with the specific intent to reduce prostate cancer mortality and not secondary tests often used after screening to determine the need for a prostate biopsy or a repeat prostate biopsy (e.g., PSA isoforms, PCA3, imaging).
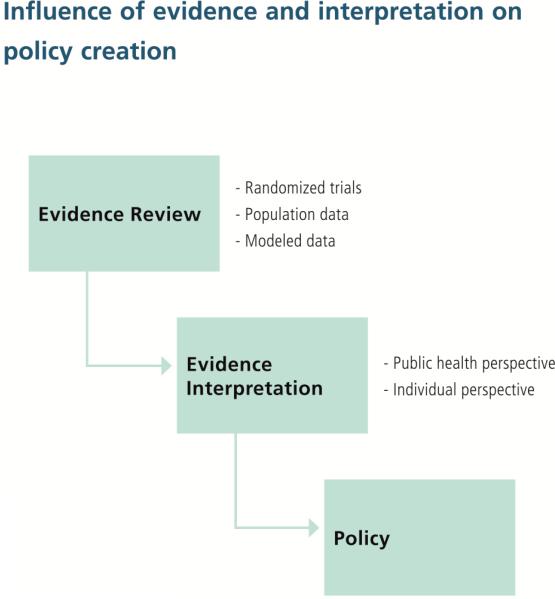
While the evidence that other guideline panels evaluate may be the same, the weighting of the evidence and the panels’ perspectives can be very different (e.g., public health versus individual perspectives) leading to differing interpretations of evidence and policy implications (Figure 1). The AUA Guideline Panel interpreted the evidence from the perspective of the individual with emphasis on the information, both benefits and harms, that an asymptomatic man would need to make an informed decision about prostate cancer screening.
Figur 1.
Influence of evidence and interpretation on policy creation
The Panel evaluated the best evidence from randomized trials of screening but did not assume that all trials were of equal relevance. For example, the Prostate, Lung, Colon, and Ovary (PLCO)3 and European Randomized Study of screening for Prostate Cancer (ERSPC)4 randomized trials ultimately addressed different questions: screening versus no or little screening in ERSPC as compared to annual screening versus usual care in the PLCO trial. By the time the PLCO trial began, usual care was opportunistic screening in the US and was, on average, every other year. The modest effect of PSA screening versus none in the ERSPC implies that a substantially larger study than PLCO would be needed to meaningfully test more versus less frequent screening. Thus, the PLCO was underpowered to address the question of organized versus opportunistic screening. The Panel interprets the randomized evidence to indicate that the ERSPC trial best reflects the effect of PSA screening in a situation with low background screening.
The bulk of the information on PSA-based screening comes from men age 55 to 69 years. The evidence from screening men under age 50 or over 69 years is very scarce; additionally, there is no evidence concerning the benefits of screening men of differing ethnicities. There are no data from head-to-head comparisons of differing screening intervals. The main evidence is from the ERSPC that included both two and four year intervals.
The Panel utilized population data as supporting evidence for a beneficial effect of screening and used modeling studies to fill gaps in knowledge. This use of modeling was felt to be important given the follow-up of 11 to 13 years provided by current randomized trial results and the paucity of data regarding the benefits of screening outside the age range of 55 to 69 years.
The evidence reviewed by the Panel clearly shows that the current practice of prostate cancer screening in asymptomatic men with comorbidities that limit life expectancy and treatment of virtually all men after diagnosis, even those with non-aggressive features and limited life expectancy, results in substantial harm. Thus, the Panel focused on both shared decision-making in the face of uncertainty5 and approaches to early detection of prostate cancer that would reduce harms while maintaining the benefits.
Index Groups
The guideline statements listed in this document target men at average risk, defined as a man without risk factors, such as a family history of prostate cancer in multiple generations and/or family history of early onset below age 55 years or African American race. Because the benefit/harm profile of PSA-based prostate cancer screening is highly age dependent, guideline statements included in this document target four index groups; these age ranges were chosen to correspond to age ranges tested in randomized trials and data from population and simulation studies.
Men <40 years of age
Men age 40 to 54 years
Men age 55 to 69 years
Men age 70+ years
Evidence Summary and Limitations of the Literature
In brief, five well known randomized trials addressed the question of mortality benefit of prostate cancer screening.6 Considering various methodological limitations and biases, the best estimate for the effect of screening (versus no screening) on prostate cancer-specific mortality was obtained from the ERSPC.4 The quality of the evidence was moderate for benefits and high for harms in men aged 55 to 69 years. Follow-up was limited, and quality of evidence was low on screening benefits in men outside of this age range, population subgroups with greater than average risk of the disease and screening protocols different from those used in the ERSPC.
Ample evidence was available to support the use of various shared decision-making processes that increased men's knowledge scores, reduced their decisional conflict and promoted greater involvement in decision-making.
The systematic review and guideline process identified clear gaps in the available evidence base. Data are needed to clarify the harm/benefit balance of screening in men younger and older than those enrolled in the available randomized trials. Even for the age groups enrolled, critical outcomes, such as over diagnosis and the additional number needed to treat, are not easily estimated from empirical trial data. Data on the harm/benefit balance are needed in men with known risk factors, such as family history of prostate cancer and men of various ethnicities. Outcomes of newer screening tests used alone or in combination with PSA need to be determined. Men contemplating screening will need outcome data based on follow-up that exceeds the 11 to 13 year horizon currently available in the literature.
Extrapolating results from one population to another must be done cautiously since the benefits of screening are dependent on the baseline incidence of and mortality from cancer without screening, the specific screening protocol, biopsy referral criteria and compliance with biopsy recommendations. The mortality from prostate cancer in the absence of screening is higher in the Netherlands and Sweden as compared to the US,7 and these were the only two countries of the seven participating in the ERSPC trial where a mortality benefit was observed. Thus, the benefits of PSA-based screening seen in these two countries may not be generalizable to the US population. Further, the screening protocol, criteria for biopsy referral and compliance with biopsy recommendations differed considerably in the US population and ERSPC trial settings. Given the knowledge gaps in the literature, the Panel considered both modeled and population data as circumstantial evidence for addressing the benefits and harms of PSA-based screening.
GUIDELINE STATEMENTS AND RATIONALE
Guideline Statement 1
The Panel recommends against PSA screening in men under age 40 years. (Recommendation; Evidence Strength Grade C)
- In this age group there is a low prevalence of clinically detectable prostate cancer, no evidence demonstrating benefit of screening and likely the same harms of screening as in other age groups.
The prevalence of prostate cancer in men under age 40 years is extremely low. Population based studies reveal the prevalence of prostate cancer in men below age 40 years to be about 0.1% with numbers as low as 700 cases being reported to the SEER registry between 2001 and 2007.8 Prior autopsy studies have been able to identify clinically undetected cases of prostate cancer in men as young as 20 years of age, but the prevalence has been low even in these retrospective studies of small cohorts of men.9 U.S. studies reveal a higher prevalence of 2% to 29% of undiagnosed cancer at autopsy even in men under age 40 years, particularly African-Americans, compared to studies from Europe and Asia.9 The prevalence among European men in their 20's is <5% while it rises to 5% to 10% in men in their 30's.10 Even in men under age 40 years who are found to have prostate cancer at autopsy, the disease tends to be of low volume and low Gleason grade.
None of the prospective randomized studies evaluating the benefits of PSA-based screening for prostate cancer included men under age 40 years. Hence there are no data available to estimate the benefit of prostate cancer screening in this population. However, the harms that can accrue from screening, which include the side effects of diagnostic biopsies and perhaps subsequent treatment, will certainly apply to men in this age group who would be subject to screening. Therefore, due to the relatively low prevalence of clinically detectable prostate cancer in men below age 40 years, the absence of any evidence demonstrating benefits of screening and the known harms, screening is discouraged for men under age 40 years of age.
Guideline Statement 2
The Panel does not recommend routine screening in men between ages 40 to 54 years at average risk. (Recommendation; Evidence Strength Grade C)
- For men between ages 40 to 54 years at higher risk (e.g., positive family history or African American race), decisions regarding prostate cancer screening should be individualized.
The Panel recommends that screening, as routine practice, not be encouraged in men age 40 to 54 years who are not at increased risk for the disease based on family history and race, for example. There is no high-quality evidence to support this practice in the general population. Specifically, the two large randomized clinical trials (PLCO3 and the core group of the ERSPC4) did not include men under age 55 years and, therefore, do not inform the decision. While there is some lower-quality evidence (Grade C) that an absolute reduction in prostate cancer mortality may be associated with population-wide screening of men in their 40's at average risk, the benefit is relatively small. Howard et al.11 estimated that annual PSA screening of men in their 40's was associated with a 10-year prostate cancer-specific mortality rate of 0.037 deaths/1,000 men compared to 0.041 deaths/1,000 men if no screening was performed. While the evidence of benefit of screening of men age 40 to 55 years indicates that the effect size is marginal at best, at least in terms of prostate cancer-specific mortality, the weight and quality of the evidence demonstrating the harms of screening remains high. The Panel concludes that the harms of screening in this population are at least equal to the benefits, if not higher and, to this end, recommends that screening should not be routine practice.
In making this recommendation, the Panel recognizes that there may be other benefits associated with screening that we either did not consider or have not been demonstrated by the current literature. Effectively, we acknowledge that the “absence of evidence does not constitute evidence of absence” and, as such, we are not explicitly stating that screening should be actively discouraged in this group of men. The literature in this area is quite dynamic, and future studies may document additional benefits in this younger population. For example, Lilja et al.12 have documented in a large study of 21,277 men from Malmo, Sweden, that a single PSA measurement taken between age 33 to 50 years was highly predictive of subsequent prostate cancer diagnosis and advanced stage at diagnosis. Whether or not this information would lead to a decrease in morbidity or mortality from the disease is uncertain. To this end, the benefit of this early risk stratification is uncertain.
The Panel recognizes that certain subgroups of men age 40 to 54 years may realize added benefit from earlier screening. For example, men at increased risk for prostate cancer, such as those with a strong family history or those of African-American race, may benefit from earlier detection, given their higher incidence of disease.13 These men should be informed of both the known harms and the potential benefits of screening at an earlier age, and shared decision-making should ensue with an understanding that there are no comparative data to demonstrate that men at higher than average risk for prostate cancer will benefit more from screening when compared to those at average risk.
In summary, given the Panel's interpretation of the evidence concerning the benefits and harms of annual screening in men age 40 to 55 years who are not at an increased risk for prostate cancer and the rarity of fatal prostate cancers arising in this age group, we do not recommend this practice as a routine. The reader is advised to remember that this does not imply that there is absolutely no benefit to screening this age group, rather that there are significant enough harms associated with screening that the benefits likely are not great enough to outweigh the harms.
Guideline Statement 3
For men ages 55 to 69 years the Panel recognizes that the decision to undergo PSA screening involves weighing the benefits of preventing prostate cancer mortality in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment. For this reason, the Panel strongly recommends shared decision-making for men age 55 to 69 years that are considering PSA screening and proceeding based on a man's values and preferences. (Standard; Evidence Strength Grade B)
- The greatest benefit of screening appears to be in men ages 55 to 69 years.
Although there are considerable harms associated with screening the Panel determined that in men age 55 to 69 years, there is sufficient evidence that the benefits of screening could outweigh the harms. Therefore, a recommendation of shared decision-making in this age group is justified. The Panel believes that the test should not be offered in a setting where this is not practical, for example community-based screening by health systems or other organizations.
Evidence for screening benefit in this setting is moderate and is derived from a large randomized controlled trial (RCT). Specifically, results from ERSPC document a relative risk reduction of prostate cancer-specific death of 21% at a median follow-up of 11 years.14 While the absolute reduction in prostate cancer-specific mortality was relatively small (0.10 deaths per 1,000 person-years or 1.07 deaths per 1,000 men randomized), this may represent an underestimate of the ultimate benefit given the length of follow-up of the study and some degree of non-compliance in the intervention arm. The Panel acknowledges that the prostate component of PLCO failed to show a benefit to screening with a median follow-up of 13 years15 but attributes this finding to high rates of screening in the control arm diluting the study's results toward the null.
Any discussion of the benefits and harms of prostate cancer screening in men age 55 to 69 years should consider a man's individual life expectancy. Prior studies have documented that men with less than a 10 to 15 year life expectancy are unlikely to realize a benefit from aggressive treatment for localized prostate cancer16 and, as such, it follows that the earlier disease detection associated with screening in these patients likely will be less beneficial, if beneficial at all. To this end, shared decision-making should include a discussion of a man's baseline mortality risk from other co-morbid conditions, his individual risk for prostate cancer given his race/ethnicity and family history, the degree to which screening might influence his overall life expectancy and chance of experiencing morbidity from prostate cancer or its treatment.
Guideline Statement 4
To reduce the harms of screening, a routine screening interval of two years or more may be preferred over annual screening in those men who have participated in shared decision-making and decided on screening. As compared to annual screening, it is expected that screening intervals of two years preserve the majority of the benefits and reduce over diagnosis and false positives. (Option; Evidence Strength Grade C)
- Additionally, intervals for rescreening can be individualized by a baseline PSA level.
While RCT's have used both two- and four-year screening intervals,4 there is no direct evidence supporting a specific screening interval. The available evidence is mostly based on modeling, and some evidence may be gleaned from randomized trials, although none of these trials actually randomized patients to different intervals as a primary objective. Modeling studies17 have projected that screening men every two years preserves the majority (at least 80%) of prostate cancer deaths prevented compared with annual screening while materially reducing the number of tests, the chance of a false positive test and over diagnoses.
The two largest screening trials have provided some indirect evidence about the likely benefits of more versus less frequent screening. In the ERSPC, a comparison between the Rotterdam section (interscreening interval four years) and the Swedish section (interscreening interval two years) suggested that a two-year screening interval significantly reduced the incidence of advanced disease.18 Evidence on the comparison of a two-year screening interval with annual screening was provided by the PLCO trial. This trial compared annual screening with a control group that had screening rates similar to those in the US population that corresponded to screening on average every two years.19 Prostate cancer mortality rates were similar in the two groups through 13 years of follow-up, suggesting little benefit from screening more frequently than every two years. In addition, data from a randomized trial (Goteborg) and a case-control study suggest that a rescreening interval of four years is not likely to miss a curable prostate cancer among men with a PSA below 1.0 ng/ml.20,21
Guideline Statement 5
The Panel does not recommend routine PSA screening in men over age 70 years or any man with less than a 10 to 15 year life expectancy. (Recommendation; Evidence Strength Grade C)
- Some men over age 70 years who are in excellent health may benefit from prostate cancer screening.
The Panel recognizes that some men over age 70 years can have a life-expectancy over 10 to 15 years and that a small subgroup of men over age 70 years who are in excellent health may benefit from PSA screening, but evidence to support the magnitude of benefit in this age group is extremely limited. Men in this age group who choose to be screened should recognize that there is strong evidence that the ratio of harm to benefit increases with age and that the likelihood of over diagnosis is extremely high, particularly among men with low-risk disease.
Evidence for screening benefit in this setting is unclear and indirect. An absolute reduction in mortality is possible but likely small with a quality rating of Grade C. The quality of the evidence for harm remains high or at least higher than that of the benefit. The certainty in the balance of harm and benefit justifies a recommendation against routine PSA-based screening.
The rationale for this recommendation is based on the absence of evidence of a screening benefit in this population with clear evidence of harms. In the ERSPC randomized trial of screening, there was no reduction in mortality among men age 70 years or older.14 Although men in this age group have a higher prevalence of prostate cancer and a higher incidence of fatal tumors, they also have increased competing mortality compared to younger men22 and no compelling evidence of a treatment benefit, especially in men with a limited life expectancy below 10 to 15 years.16,23 Therefore, given the lack of direct evidence for benefit of screening beyond age 70 years, and especially beyond age 74 years, as well as higher quality data regarding harms, the Panel discourages routine screening in this age group.
Men age 70 years and older who wish to be screened should do so after an understanding that the ratio of benefit to harm declines with age, although there is evidence that men with high-risk disease in this age range may benefit from early diagnosis and treatment over a decade or less.16 In order to identify the older man more likely to benefit from treatment if screening takes place, the Panel recommends two approaches. First, increasing the prostate biopsy threshold based on evidence that men with a PSA level above 10 ng/ml are more likely to benefit from treatment of prostate cancer when compared to those with a PSA below 10 ng/ml.16 Second, discontinuation of PSA screening among men with a PSA below 3 ng/ml, given evidence that these men have a significantly lower likelihood of being diagnosed with a lethal prostate cancer during the remaining years of life when compared to men with a PSA above 3 ng/ml.24
The likelihood of over diagnosis increases as men age and is particularly high for older men with low-risk disease. Modeling studies of over diagnosis in the US population have estimated that among men aged 70 to 79 years, half or more of cases detected by PSA screening with PSA less than 10 and Gleason score 6 or below are over diagnosed. Among men over age 80 years, three-fourths or more of cases detected by PSA screening with PSA less than 10 and Gleason score 6 or below are over diagnosed.25 Because of the harms of biopsy, over treatment and over diagnosis in this population, shared decision-making and consideration of individual values, preferences and quality of life goals is paramount among men expressing interest in screening.
FUTURE DIRECTION AND RESEARCH NEEDS
Unlike many interventions in which the ratio of benefit to harm is high and the choice is clear, prostate cancer screening is a preference-sensitive intervention for which there are reasonable choices to make. Optimal methods (pictograms, text, computerized) that best communicate uncertainty to patients and allow individualized decisions regarding screening are needed. Further, improved tools for estimating life expectancy would help identify those men more likely to benefit from screening. Assessment of the absolute benefits of PSA-based prostate cancer screening relative to the rates of over diagnosis and over treatment of disease among different populations is an important area for future research. Evaluation of the optimal management of screen detected cancers and the cost effectiveness of these options will be important to understand before making broad policy decisions regarding prostate cancer screening. In addition, clarification of the genetic and epigenetic basis of disease development and progression may provide biomarkers and/or panels of biomarkers with improved specificity that allow targeted screening of those men at greatest risk of harm from prostate cancer. Targeted screening would reduce unnecessary testing, false positive tests and the burden of over diagnosis and over treatment. An improved understanding of the interaction between inherited risk alleles and the environment (lifestyle choices) could provide a potential means of prevention of prostate cancer.
GUIDELINES DISCLAIMER
This document was written by the Detection of Prostate Cancer Guidelines Panel of the American Urological Association Education and Research, Inc., which was created in 2011. The Practice Guidelines Committee (PGC) of the AUA selected the committee chair. Panel members were selected by the chair. Membership of the committee included urologists, primary care physicians, radiation and medical oncologists and epidemiologists. The mission of the committee was to develop recommendations that are analysis-based or consensus-based, depending on Panel processes and available data, for optimal clinical practices in the detection of prostate cancer.
Funding of the committee was provided by the AUA. Committee members received no remuneration for their work. Each member of the committee provides an ongoing conflict of interest disclosure to the AUA.
While these guidelines do not necessarily establish the standard of care, AUA seeks to recommend and to encourage compliance by practitioners with current best practices related to the condition being treated
As medical knowledge expands and technology advances, the guidelines will change. Today these evidence-based guidelines statements represent not absolute mandates but provisional proposals for treatment under the specific conditions described in each document. For all these reasons, the guidelines do not pre-empt physician judgment in individual cases.
Treating physicians must take into account variations in resources, and patient tolerances, needs, and preferences. Conformance with any clinical guideline does not guarantee a successful outcome. The guideline text may include information or recommendations about certain drug uses (‘off label‘) that are not approved by the Food and Drug Administration (FDA), or about medications or substances not subject to the FDA approval process. AUA urges strict compliance with all government regulations and protocols for prescription and use of these substances. The physician is encouraged to carefully follow all available prescribing information about indications, contraindications, precautions and warnings. These guidelines and best practice statements are not in-tended to provide legal advice about use and misuse of these substances.
Although guidelines are intended to encourage best practices and potentially encompass available technologies with sufficient data as of close of the literature review, they are necessarily time-limited. Guidelines cannot include evaluation of all data on emerging technologies or management, including those that are FDA-approved, which may immediately come to represent accepted clinical practices.
For this reason, the AUA does not regard technologies or management which are too new to be addressed by this guideline as necessarily experimental or investigational.
Abbreviations
- DRE
Digital Rectal Exam
- ERSPC
European Randomized Study of screening for Prostate Cancer
- PLCO
Prostate, Lung, Colon, and Ovary
- PSA
Prostate Specific Antigen
- RCT
Randomized Controlled Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURES
All panel members completed COI disclosures. Relationships that have expired (more than one year old) since the panel's initial meeting, are listed. Those marked with (C) indicate that compensation was received; relationships designated by (U) indicate no compensation was received.
Board Member, Officer, Trustee: Philip Kantoff, BIND Biosciences (C) (Expired)
Consultant/Advisor: Peter C. Albertsen, Blue Cross/Blue Shield (C), Dendreon Corporation (C), ; Glaxo Smith Kline (C)(Expired), Johnson & Johnson (C)(Expired); Stephen J. Freedland, Amgen (C), Medivation (C), Bayer (C), Mitomics (C), Astellas (C), AstraZeneca (C), Dendreon (C), Janssen (C), Glaxo Smith Kline (C) (Expired); Philip Kantoff, Bellicum (C), BIND Biosciences (C), Blend (C), BN-IT (C), Dendreon (C), Dendreon (C), Johnson and Johnson (C), Metamark (C), Oncocellmdx (C), Sanofi (C), Sotio (C), Tokai (C), Amgen (C)(Expired), Genentech (C)(Expired); Badrinath R. Konety, Allergan(C), Axogen Inc.(U), Dendreon (C), Endo Pharmaceuticals (C), Spectrum Pharmaceuticals (C), Centocor Ortho Biotech (C)(Expired)
Investigator: Peter C. Albertsen, Agency Health Care Quality (C) (Expired), National Cancer Institute (C) (Expired), Sanofi (C)(Expired)
Leadership Position: Anthony L. Zietman,
American Board of Radiology (U), American Society for Radiation Oncology (U), National Cancer Institute, GU Steering Committee (C)
Meeting Participant or Lecturer: Peter C. Albertsen, Ferring Pharmaceuticals, (C), Stephen J. Freedland, Amgen (C)(Expired), AstraZeneca (C)(Expired), Centocor Ortho Biotech (C)(Expired); Badrinath R. Konety, Amgen (C)
Scientific Study or Trial: Peter C. Albertsen, Agency Health Care Quality (C); Stephen J. Freedland, Glaxo Smith Kline
REFERENCES
- 1.Gordis L. The Epidemiologic Approach to Evaluating Screening Programs. Epidemiology. (4th edition) 2009 [Google Scholar]
- 2.Faraday M, Hubbard H, Kosiak B, et al. Staying at the cutting edge: a review and analysis of evidence reporting and grading; the recommendations of the American Urological Association. BJU Int. 2009;104:294. doi: 10.1111/j.1464-410X.2009.08729.x. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Grubb RL, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366:780. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 6.Ilic D, O'Connor D, Green S, et al. Screening for prostate cancer: an updated Cochrane systematic review. BJU Int. 2011;107:882. doi: 10.1111/j.1464-410X.2010.10032.x. [DOI] [PubMed] [Google Scholar]
- 7.GLOBOCAN . Section of Cancer Information. GLOBOCAN (IARC); 2008. Prostate cancer incidence and mortality worldwide in 2008: summary. http://globocan.iarc.fr/factsheets/cancers/prostate.asp. [Google Scholar]
- 8.Li J, Djenaba JA, Soman A, et al. Recent trends in prostate cancer incidence by age, cancer stage, and grade, the United States, 2001-2007. Prostate Cancer. 2012;2012:691380. doi: 10.1155/2012/691380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo. 1994;8:439. [PubMed] [Google Scholar]
- 10.Sánchez-Chapado M, Olmedilla G, Cabeza M, et al. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54:238. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- 11.Howard K, Barratt A, Mann GJ, et al. A model of prostate-specific antigen screening outcomes for low- to high-risk men: information to support informed choices. Arch Intern Med. 2009;169:1603. doi: 10.1001/archinternmed.2009.282. [DOI] [PubMed] [Google Scholar]
- 12.Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117:1210. doi: 10.1002/cncr.25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 14.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilt TJ, Brawer MK, Jones KM, et al. Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen--based prostate cancer screening strategies: model estimates of potential benefits and harms. Ann Intern Med. 2013;158:145. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leeuwen PJ, Roobol MJ, Kranse R, et al. Towards an optimal interval for prostate cancer screening. Eur Urol. 2012;61:171. doi: 10.1016/j.eururo.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Pinsky PF, Blacka A, Kramer BS, et al. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clin Trials. 2010;7:303. doi: 10.1177/1740774510374091. [DOI] [PubMed] [Google Scholar]
- 20.Vickers AJ, Cronin AM, Björk T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aus G, Damber JE, Khatami A, et al. Individualized screening interval for prostate cancer based on prostate-specific antigen level: results of a prospective, randomized, population-based study. Arch Intern Med. 2005;165:1857. doi: 10.1001/archinte.165.16.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 24.Schaeffer EM, Carter HB, Kettermann A, et al. Prostate specific antigen testing among the elderly--when to stop? J Urol. 2009;181:1606. doi: 10.1016/j.juro.2008.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati R, Wever EM, Tsodikov A, et al. What if I don't treat my PSA-detected prostate cancer? Answers from three natural history models. Cancer Epidemiol Biomarkers Prev. 2011;20:740. doi: 10.1158/1055-9965.EPI-10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]