Summary
Background
Clathrin-mediated endocytosis in budding yeast requires the regulated recruitment and disassociation of over 60 proteins at discrete plasma membrane punctae. Post-translational modifications such as ubiquitination may play important regulatory roles in this highly processive and ordered process. However, while ubiquitination plays an important role in cargo selection, functions for ubiquitination of the endocytic machinery are not known.
Results
We identified the deubiquitinase (DUB) Ubp7 as a late arriving endocytic protein. Deletion of the DUBs Ubp2 and Ubp7 resulted in elongation of endocytic coat protein lifetimes at the plasma membrane and recruitment of endocytic proteins to internal membranes. These phenotypes could be replicated by expressing a permanently ubiquitinated version of Ede1, the yeast Eps15 homolog, which is implicated in endocytic site initiation, while EDE1 deletion partially suppressed the deubiquitinase deletion phenotype. Both DUBs are capable of deubiquitinating Ede1 in vitro.
Conclusions
Deubiquitination regulates formation of endocytic sites and stability of the endocytic coat. This regulation appears to occur through Ede1, since permanently ubiquitinated Ede1 phenocopies deletion of UBP2 and UBP7. Moreover, incomplete suppression of the ubp2Δ ubp7Δ phenotype by ede1Δ indicates that ubiquitination and deubiquitination are likely to regulate additional components of the endocytic machinery.
INTRODUCTION
Clathrin-mediated endocytosis in yeast and mammals requires the coordinated actions of over 60 different proteins at plasma membrane foci [1–3] (Figure S5A shows a schematic of yeast endocytosis). While the order of arrival and departure of these proteins has been described with high precision, the role of post-translational modifications in regulating the assembly and disassembly of endocytic machinery proteins has been less fully explored. A role for the Ark1/Prk1 kinases has been established [4–8], yet how dynamic ubiquitination and deubiquitination might regulate the endocytic machinery is not known.
A role for ubiquitin as a signal for internalization of cargo molecules is well established [9–12], as is a role for deubiquitinating enzymes in the sorting of internalized receptors [13, 14]. The role of arrestin-like proteins and other endocytic proteins as specific adaptors that promote ubiquitination and internalization of specific cargos has also been explored [15]. However, while there is some evidence that ubiquitination and ubiquitin-binding domains may help hold components of the endocytic machinery together [16], how ubiquitination affects the dynamics and function of the endocytic machinery has not been as well studied. A mutant of Rvs167 that lacks a ubiquitinated lysine was reported to have no endocytic phenotype [17], while ubiquitination of Eps15 (homologous to yeast Ede1) has been proposed to control an intramolecular interaction [18–20]. The ubiquitin-like domain of the E3 ligase parkin has been shown to bind to Eps15 in an UIM dependent manner and to be required for Eps15 ubiquitination, a modification that delays cargo internalization [20].
Since the yeast genome encodes 43 E3 ubiquitin ligases and 19 deubiquitinases [21], assigning endocytic functions to any given enzyme can be complicated as a result of compensatory effects from other enzymes when one is mutated, as well as secondary effects due to participation of enzymes in multiple processes. The yeast Nedd4 homologue, Rsp5, a HECT domain E3 ligase, is known to ubiquitinate endocytic proteins and endocytic cargos [16, 17, 22–24]. Rsp5's C2 domain can bind to lipids and its WW domains bind to proteins, including adaptors such as the arrestin-like proteins [15, 25, 26]. Temperature-sensitive mutations in Rsp5 cause fluid-phase endocytosis defects [25]. Rsp5 reportedly has physical interactions with Rvs167, Las17, Lsb1 and Lsb2 and genetic interactions with arp2, end3, sla2, las17 and ede1 mutants. RSP5 mutants are sensitive to the actin drug latrunculin A and have actin organization defects [27, 28]. These data suggest that Rsp5 has a role in endocytosis beyond modifying cargo as a signal for internalization. Rsp5 performs a coupled monoubiquitination of Vps9 wherein the substrate binds to a ubiquitin modification on the ligase before being ubiquitinated [29], and Rsp5 may likewise ubiquitinate Ede1 after it binds to the modified ligase, as has been proposed for Nedd4 and Eps15 [19]. Rsp5 is an essential protein also involved in RNA export, regulation of fatty acid biosynthesis, mitochondrial organization, and DNA repair [30–33]. Rsp5 localization to the plasma membrane, to Abp1-RFP punctae and to intracellular punctae has been reported for the plasmid-based over-expression of the full-length protein and for fragments containing the C2 and WW domains [27, 31, 34]. Localization to invaginations was observed by immuno-EM of plasmid-expressed HA-tagged Rsp5 [34]. Cortical localization of the full-length protein expressed at approximately endogenous levels was observed in a temperature-sensitive SLA2 mutant, which is severely defective in endocytosis [34]. These observations suggest that Rsp5 is located at the plasma membrane, at least transiently.
Rsp5 can monoubiquitinate substrates and preferentially assembles K63 chains over K48 chains, an action that is antagonized by the physically associated DUB Ubp2 [35, 36]. Ubp2 specifically degrades K63 chains, which are known to be important for endocytosis [35–40], making Ubp2 a strong candidate for regulating non-proteasome related ubiquitination of the endocytic machinery. The DUB Ubp7 was identified via phage display as an interaction partner for the SH3 domains of multiple endocytic proteins [41], and it was enriched with many well characterized endocytic proteins when Las17 (yeast WASP)-coated beads from which actin tails were assembled in yeast extracts were isolated [42].
This study reports that the DUB Ubp7 localizes to endocytic sites late in the pathway, and that both Ubp2 and Ubp7 are capable of deubiquitinating Ede1 in vitro. While Ede1 is more highly ubiquitinated in ubp2Δ ubp7Δ cells, and while both ubp2Δ ubp7Δ phenotypes are replicated by expression of permanently ubiquitinated Ede1, deletion of EDE1 in ubp2Δ ubp7Δ cells does not suppress a cytoplasmic punctae phenotype, suggesting that other components of the endocytic machinery involved in site initiation and coat stabilization are also regulated by ubiquitination.
RESULTS
Ubp7 localizes to endocytic sites and Rsp5 can be trapped at the plasma membrane
An N-terminal fusion of GFP to Ubp7, expressed at the UBP7 chromosomal locus from the endogenous promoter, localizes to dynamic plasma membrane punctae, as does a C-terminal fusion that truncates the UCH (catalytic) domain (Figure 1A-C, S1A). Because Ubp7 is expressed at very low levels [43], making detection challenging, and because the ΔUCH-GFP fusion is significantly brighter than the N-terminal fusion (Figure S1A), the latter fusion protein was often used in this study. The dynamics and internalization of Sla1-mCherry, an endocytic adaptor protein, are normal in strains expressing either GFP-tagged Ubp7 (Figure S1B). Ubp7ΔUCH-GFP punctae have a short lifetime of ~13 seconds and do not appear to internalize with the nascent vesicle, remaining at the plasma membrane for their entire lifetime (Figure 1C), a behavior shared with proteins involved in actin polymerization, such as Bzz1, Las17 and Myo3/5, but distinct from coat and adaptor proteins such as Sla1p, which internalize with the vesicle [1].
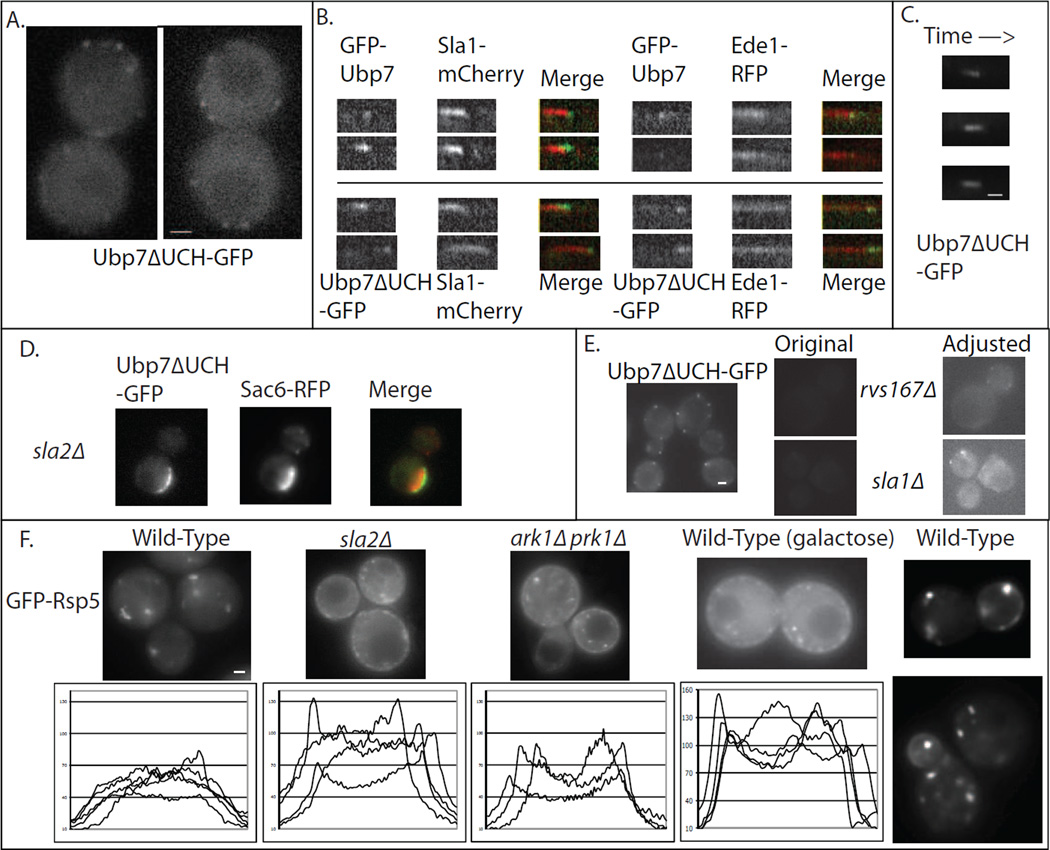
Figure 1. Localization of ubiquitination and deubiquitination machinery.
(A) Ubp7ΔUCH-GFP localizes to cortical punctae. (B) Dual-color microscopy reveals that Ubp7ΔUCH-GFP and GFP-Ubp7 colocalize with Ede1-RFP or Sla1-mCherry at endocytic sites and arrive late in the endocytic pathway after actin assembly begins. (C) Kymographs of Ubp7ΔUCH-GFP indicate that Ubp7 localizes to the plasma membrane for a short time (~13 seconds) and does not internalize with the budding vesicle. (D) In an sla2Δ mutant endocytosis is blocked and long actin tails are formed associated with endocytic proteins trapped at the plasma membrane as actin subunits continuously flux through the network. Actin binding proteins such as Sac6 decorate the tail while Ubp7ΔUCH-GFP is confined to the plasma membrane, similar to endocytic coat proteins. (E) Ubp7ΔUCH-GFP cortical localization is reduced in cells lacking SH3 domain proteins. Original and brightness/contrast enhanced images are shown. (F) GFP-Rsp5 primarily localizes to internal punctae, but in sla2Δ and ark1Δ prk1Δ mutants, which have strong endocytic blocks, GFP-Rsp5 also appears on the plasma membrane. Similar to the endocytic mutants, in cells grown in galactose, GFP-Rsp5 localizes to both internal punctae and to the plasma membrane. Line scans of wild-type cells show an even fluorescence punctuated by internal punctae, while line scans of sla2Δ, ark1Δ prk1Δ or cells grown in galactose show strong peaks at the edges of cells, consistent with a plasma membrane localization. Cortical GFP-Rsp5 localization can be observed in buds but not mother cells of some wild-type cells grown in glucose (far right frames, Nikon Elcipse TI). Scale bars are 1μm in A and D–F, 200nm in C. See also Figure S1.
Ubp7ΔUCH-GFP and GFP-Ubp7 punctae both colocalize with endocytic markers Sla1-mCherry, Ede1-RFP and Abp1-RFP (Figure 1B, Figure S1A). Abp1-RFP is generally detectable prior to the arrival of either GFP-labeled Ubp7. However, labeled Ubp7 is extremely dim due to very low protein levels [43], making it difficult to precisely determine which protein arrives first. In sla2Δ cells, which have a strong endocytic block and form long actin tails due a disruption of the link between the endocytic coat and actin filaments [44], Ubp7 localizes with the coat proteins at the plasma membrane, and not with the actin tails, where many actin-binding proteins localize (Figure 1D). In cells treated with the actin monomer sequestering drug latrunculin A, Ubp7ΔUCH-GFP localizes to cortical punctae even after actin has depolymerized (Figure S1C), a behavior shared with coat and adaptor proteins. The proline-rich region of Ubp7 interacts with the SH3 domains of endocytic proteins [41]. Surprisingly, single deletion mutants of any of several of these SH3-containing endocytic proteins greatly reduces Ubp7ΔUCH-GFP cortical localization (Figure 1E, S1D) suggesting that the SH3-containing endocytic proteins recruit Ubp7 to the plasma membrane. Neither N- nor C-terminal GFP tagged versions of Ubp2 revealed any specific localization, consistent with results of large-scale localization studies [21].
C-terminal tags fused to Rsp5 are lethal [45, 46], but N-terminal fusions as the sole source of Rsp5 allow for normal growth [35] (Figure S1E). N-terminal GFP fusions localize diffusely to the cytoplasm and to small, fast moving internal punctae and to larger slower moving punctae (Movie S1). Using an Olympus IX81 microscope, we did not observe plasma membrane localization under standard growth conditions for chromosomally integrated GFP-Rsp5 driven by either the glyceraldehyde-3-phosphate dehydrogenase or transcription elongation factor promoters (Figure 1F, far left panel). However, cortical localization was observed preferentially at the tips of medium and small budded cells using a Nikon Eclipse TI microscope equipped with a more sensitive camera (Figure 1F, far right panels). Galactose promoter driven, plasmid-borne, full-length Rsp5 or a protein fragment lacking the catalytic domain, were previously reported to localize to both the plasma membrane and internal punctae [27, 31, 34]. When cells were grown overnight in galactose, constitutively expressed GFP-Rsp5 was strongly localized to the plasma membrane of mother cells and buds as well as to internal punctae (Figure 1F). However, while partial colocalization between GFP-Rsp5 and endocytic patch proteins was observed in these situations, GFP-Rsp5 localized stably to large portions of the plasma membrane while endocytic patches are small, discreet and dynamic. Any colocalization appears to be coincidental rather than reflecting recruitment of GFP-Rsp5 to endocytic patches. There was no detectable colocalization of dynamic GFP-Rsp5 punctae with Abp1-RFP patches by epifluorescence or Sac6-RFP patches by epifluorescence or TIRFM. We therefore hypothesized that Rsp5's interactions with substrate endocytic proteins may be transient and thus difficult to visualize. Endocytic mutants sla2Δ and ark1Δ prk1Δ cause strong endocytic blocks, so we imaged GFP-Rsp5 in these mutants to test whether it gets trapped at the cell cortex. We observed a strong cortical fluorescent signal in these mutants on both mother cells and buds (Figure 1F) suggesting that GFP-Rsp5 is trapped at the plasma membrane when endocytosis is blocked.
Deletion of DUB genes extends endocytic coat lifetimes and causes recruitment of endocytic proteins to early endosomes
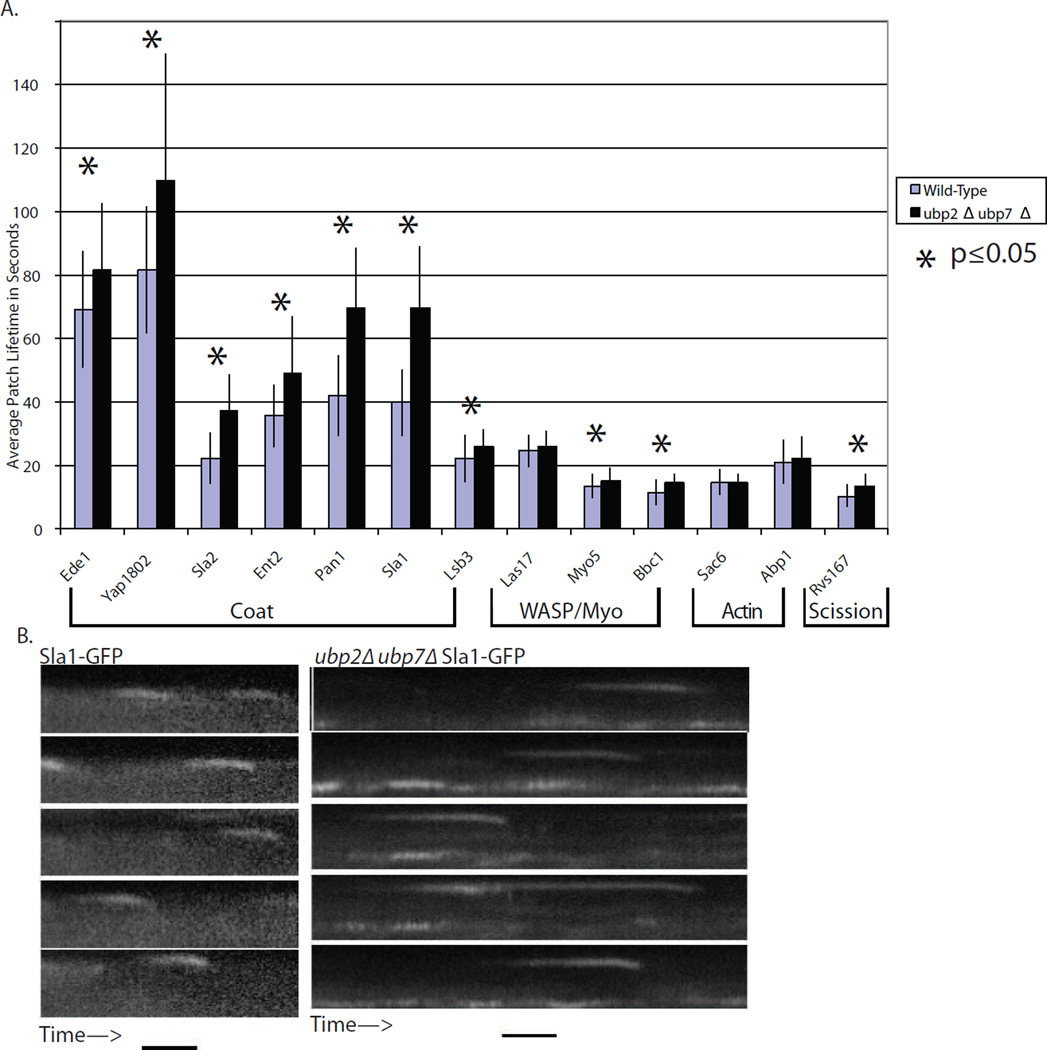
The cortical lifetimes of GFP or RFP labeled endocytic proteins were measured in wild-type and ubp2Δ ubp7Δ cells (Figure 2A). While the phenotypes described below were observed in both ubp2Δ and ubp7Δ single mutant cells (data not shown), the double mutants had stronger phenotypes than either single mutant. Lifetimes were extended for many endocytic proteins, especially those in the coat module, and to a lesser extent, for proteins in the WASP/Myo and scission modules, which arrive later (see Figure S5A). The fluorescently labeled coat proteins internalized normally (Figure 2B) even when their lifetimes were significantly extended (p ≤ 0.05).
Figure 2. ubp2Δ ubp7Δ double mutants have extended endocytic coat lifetimes.
(A) Cortical lifetimes of many endocytic proteins, especially proteins in the coat module, are extended significantly (p ≤ 0.05, T-test) in ubp2Δ ubp7Δ double mutants. Only modest effects were observed for proteins in later-arriving modules (n > 100 patches). Error bars are ± 1 standard deviation. (B) Example kymographs for Sla1-GFP in wild-type and double mutant cells. Mutant cells have longer patch lifetimes. Time bar is 20s.
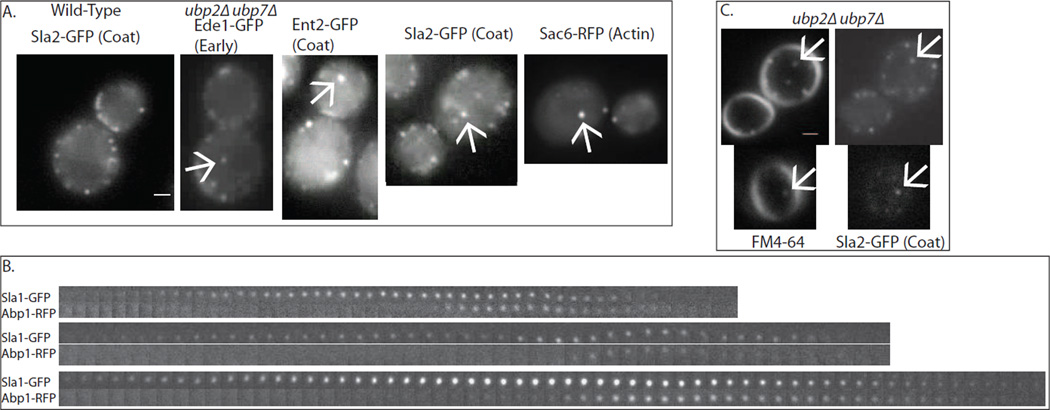
In addition to extended cortical lifetimes, endocytic proteins appeared on ectopic cytoplasmic patches in ubp2Δ ubp7Δ cells (Figure 3A, S2A). Under normal circumstances endocytic coat proteins appear at cortical patches and disappear upon uncoating after traveling a few hundred nanometers into the cell [1]. In ubp2Δ ubp7Δ cells, endocytic proteins localize to cytoplasmic punctae as well as to the plasma membrane. These punctae were stained by FM4-64 within three minutes of addition of the lipid dye to the media, suggesting that the endocytic proteins assemble on early endosomes (Figure 3C). All of the labeled endocytic proteins tested, including early, coat, and actin module proteins, appeared on the internal patches (Figure 3A). Internal patches generally move rapidly within the cytoplasm and then disappear, either by uncoating or by moving out of the plane of focus. In cells expressing both Sla1-GFP and Abp1-RFP, internal punctae are labeled by Sla1-GFP, which then is joined by Abp1-RFP. Then both proteins disappear (Figure 3B). These internal punctae may represent endocytic sites inappropriately initiated on early endosomes in response to an ubiquitin signal that has not been removed due to absence of Ubp2 and Ubp7.
Figure 3. ubp2Δ ubp7Δ double mutants have internal patches.
(A) Double mutant cells have internal patches, to which multiple different endocytic proteins from several different modules get recruited in the normal sequence. These patches are dynamic and mobile, appearing and disappearing and moving around inside the cell. Scale bar is 1μm. (B) Dual-color microscopy of endocytic patches demonstrates that they behave similarly to cortical actin patches. Sla1p appears first, followed by Abp1, which marks actin, followed by the disappearance of the patch. Frames are 1 second apart. (C) Within 3 minutes of addition of FM4-64, colocalization is observed between FM4-64 punctae and cytoplasmic punctae marked by Sla2-GFP. Scale bar is 1μm. See also Figure S2, S5.
Ubiquitination of Ede1 is increased in ubp2Δ ubp7Δ cells
The early endocytic protein Ede1 is a strong candidate for regulation by ubiquitination. Ede1 is known to be ubiquitinated by Rsp5, and it may bind intramolecularly to its own ubiquitin modification [16]. Additionally, Ede1 arrives early in the endocytic pathway, and it is important for endocytic site initiation [47], a process that may be perturbed in ubp2Δ ubp7Δ cells, as evidenced by the formation of ectopic cytoplasmic punctae.
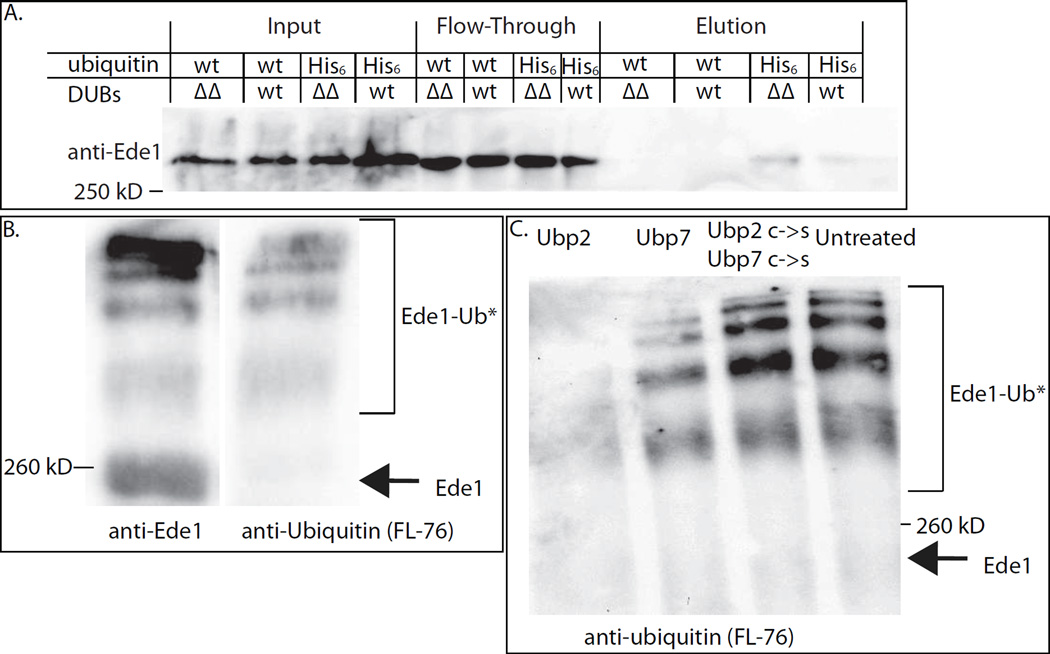
In order to test whether deletion of the two DUB genes changes the ubiquitination state of Ede1, we performed nickel affinity purifications of His6-Ub proteins from wild-type and DUB deleted strains that had all endogenous ubiquitin genes deleted and expressed His6-myc-ubiquitin as the sole source of ubiquitin, and probed immuno-blots for Ede1. More Ede1 was recovered from the ubp2Δ ubp7Δ strain than from the wild-type strain in four separate experiments, indicating that these deubiquitinases control Ede1 ubiquitination levels (Figure 4A, S3A, S3D).
Figure 4. Evidence that Ede1 is de-ubiquitinated by Ubp2 and Ubp7.
(A) His6-ubiquitinated proteins were purified from UBP2 UBP7 (wt) and ubp2Δ ubp7Δ (ΔΔ) cells and the proteins eluted from nickel columns were immune-blotted to detect Ede1. More Ede1 was purified in ubp2Δ ubp7Δ cells, suggesting that Ede1 is more highly ubiquitinated in mutant cells. This experiment was repeated four times with similar results. (B) Equal volumes of immuno-isolated Ede1 were run on a 7% polyacrylamide gel and probed with either anti-Ede1 or an anti-ubiquitin (FL-76) antibody. Higher molecular weight bands correspond to ubiquitinated Ede1. (C) Immuno-isolated Ede1 was incubated at room temperature with GST-Ubp2, GST-Ubp7 or both GST-Ubp2 C745S and GST-Ubp7 C618S, and then separated on a 7% gel and probed with an anti-ubiquitin antibody (FL-76). The amount of ubiquitinated Ede1 was greatly reduced upon incubation with GST-Ubp2, somewhat reduced following incubation with GST-Ubp7 (which had less activity against Ub-AMC, Figure S3B) and was not deubiquitinated by the catalytically dead C->S mutants. See also Figure S3.
Both DUBs are capable of deubiquitinating immuno-isolated Ede1
In order to directly test whether or not ubiquitinated Ede1 is a substrate for the DUBs, we performed in vitro deubiquitination experiments on immune-isolated Ede1. In order to increase the quantity of ubiquitinated Ede1, we used sla2Δ Ubp7ΔUCH-GFP cells, which have a strong endocytic block due to a disruption of the link between actin and the clathrin coat [44]. Our data suggest that Ede1 becomes deubiquitinated late in endocytosis, and we reasoned that ubiquitinated Ede1 may accumulate in cells blocked at an intermediate stage of endocytosis. Ede1 isolated from sla2Δ cells runs as a ladder with at least 6 different bands. Anti-ubiquitin antibodies only react with the upper bands, demonstrating that they represent ubiquitinated Ede1 (Figure 4B). Immuno-isolated Ede1 was incubated with purified GST-Ubp2 or GST-Ubp7 or with a combination of both of the active site C->S mutants. Either wild-type DUB was capable of deubiquitinating Ede1 (Figure 4C, S3C), although GST-Ubp2 was considerably more active than GST-Ubp7 as assayed by deubiquitination of Ede1 (Figure 4C) and Ub-AMC (Figure S3B, Ubp7 graph uses twice as much protein and Ub-AMC). While both wild-type DUBs were capable of deubiquitinating Ede1, the inactive C->S mutants were not (Figure 4C, S3C).
Expression of an Ede1-ubiquitin fusion protein phenocopies a ubp2Δ ubp7Δ double mutant
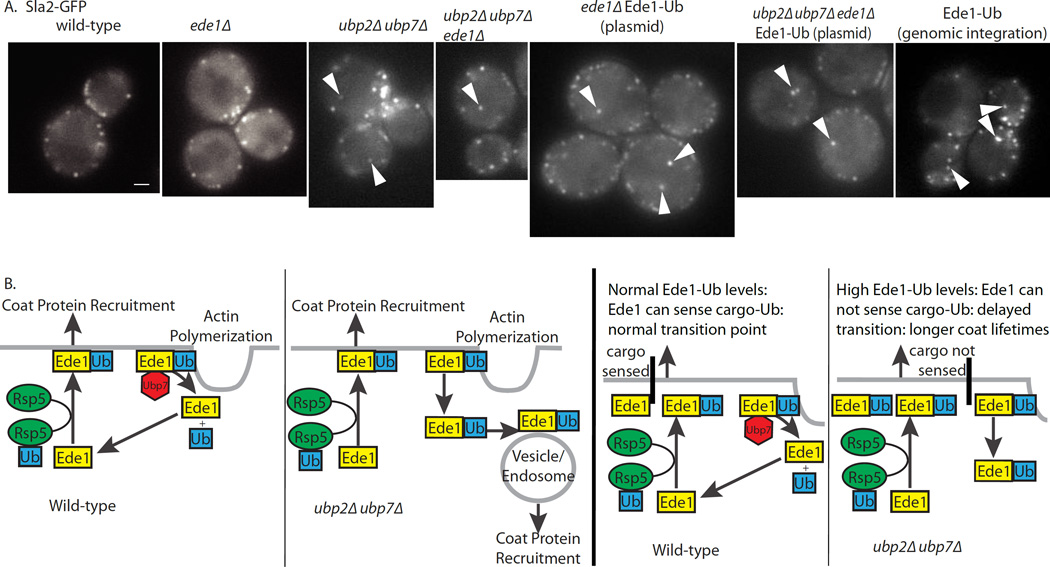
Similar to ubp2Δ ubp7Δ cells, expression of Ede1 carrying a C-terminal ubiquitin fusion, in the absence of endogenous Ede1, caused the appearance of cytoplasmic punctae marked by fluorescently tagged endocytic proteins, and extended the lifetimes of coat proteins at plasma membrane-associated endocytic sites (Figures 5A, S2A, S2B, S4). While expression of the Ede1-ubiquitin fusion was sufficient to cause both phenotypes, Ede1 was not necessary for the cytoplasmic punctae phenotype in ubp2Δ ubp7Δ cells since deletion of EDE1 did not suppress the appearance of internal punctae (Figures 5A, S2A). However, deletion of EDE1 did suppress the extended coat protein lifetime phenotype (Figure S2B). This result is consistent with the observation that deletion of EDE1 reduces the cortical lifetime of coat and WASP/myo proteins [47]. The coat protein lifetime increase seen in Ede1-Ub cells is not as strong as that seen in ubp2Δ ubp7Δ cells, further suggesting that Ede1 is not the only endocytic target of the DUBs. Nevertheless, expression of Pan1-Ub, another yeast protein with homology to mammalian Eps15 [48–50], did not replicate either phenotype (Figures S2A, S2C).
Figure 5. Permanently ubiquitinated Ede1 produces internal patches.
(A) Expression of Ede1-Ub results in internal patches similar to those found in ubp2Δ ubp7Δ double mutant cells. Scale bar is 1μm. (B) Model for regulation of endocytic patch dynamics by ubiquitination/deubiquitination. Ede1 is ubiquitinated by Rsp5, which facilitates endocytic site initiation and/or coat protein recruitment, and is deubiquitinated late in the process, just before scission, by Ubp2 or Ubp7, which facilitates disassembly of the coat. In ubp2Δ ubp7Δ cells or Ede1-Ub cells, Ede1 retains the ubiquitin and inappropriately initiates sites and promotes recruitment of endocytic coat proteins on early endosomes. See also Figure S4, S5.
To determine if internal patches are formed de novo or if they are formed through a failure in vesicle uncoating, 11–12 × 0.35 μm Z-slices were collected every second for 30 seconds in ubp2Δ ubp7Δ cells expressing Ede1-GFP or Sla1-GFP. Internal patches were tracked to determine their point of origin. For both Ede1-GFP and Sla1-GFP, internal patches appeared de novo rather than being associated with an endocytic internalization event at the plasma membrane (Figure S5B). These data support the conclusion that internal patches represent ectopic endocytic site initiation rather than an uncoating defect (Figure 5B).
DISCUSSION
The data presented here implicate dynamic ubiquitination and deubiquitination of the endocytic machinery in regulation of endocytic coat assembly and disassembly. The recruitment of a DUB during the late stages of clathrin-mediated endocytosis implicates ubiquitin removal in a late process like endocytic coat disassembly, while the appearance of endocytic proteins on internal membranes in ubp2Δ ubp7Δ and Ede1-Ub cells suggests that ubiquitin might be a signal for coat assembly or site initiation (Figure 5B).
It is intriguing that the recruitment of Ubp7ΔUCH-GFP to the plasma membrane can be reduced by deleting any of several SH3 domain containing endocytic proteins (Figure S1D), which arrive prior to Ubp7. Perhaps Ubp7 is recruited when other proteins with proline-rich regions, such as Gts1, Scd5 and Aim21, begin to leave the patch [41], or perhaps Ubp7 only associates with endocytic sites after a critical number of SH3 domain containing proteins have been recruited. Once recruited, late in the endocytic pathway, Ubp7 presumably removes ubiquitin from endocytic proteins, an activity that it can perform on ubiquitinated Ede1 (Figure 4C).
Increased lifetimes of coat proteins, but not actin, in endocytic ubiquitination and deubiquitination mutants suggests that the mechanisms regulating coat formation using ubiquitin and deubiquitination do not regulate endocytic actin polymerization or vesicle scission. The appearance on internal membranes of endocytic proteins that exhibit the normal ordered arrival and departure observed on the plasma membrane, suggests that deleting the two DUBs causes endocytic sites to be assembled inappropriately on early endosomes, and that these sites then proceed at least partially through the normal sequence of endocytic events. Our microscopy indicates that the internal punctae form de novo and are not a result of a failure to uncoat internalizing vesicles. We hypothesize that deletion of the DUBs leaves behind ubiquitin as a signal on some proteins, resulting in aberrant initiation of endocytosis on early endosomes. This signal could be ubiquitination of the endocytic machinery or transmembrane cargo molecules. However, the ability of the Ede1-Ub fusion protein to cause both extended coat lifetimes and recruitment of endocytic proteins to internal membranes, implicates Ede1 as a functional target of the DUBs for coat regulation (Figure 5B) rather than, or in addition to, transmembrane cargo molecules. This conclusion is consistent with previous reports that Ede1 is ubiquitinated by Rsp5 [16], with our detection of multiple Ede1 species under certain conditions, with our recovery of more Ede1 from ubp2Δ ubp7Δ cells than from wild-type cells during His6-Ub purifications, and with our demonstration that the DUBs are capable of deubiquitinating Ede1 in vitro.
The specific mechanism for the regulatory effect of ubiquitination on coat protein dynamics is not known. A proposed cargo transition point is thought to regulate the progression from site initiation to later stages [51–55]. Ede1 may bind ubiquitinated cargos [56] and is thought to be negatively regulated by its UBA domain [16]. We propose that under normal conditions, the UBA domain of Ede1 is capable of either directly binding to and sensing cargo or indirectly influencing the cargo checkpoint by stabilizing the network of endocytic proteins as previously proposed [16]. Ede1 may act similarly to its mammalian homolog, Eps15, and bind to its own ubiquitin modification via its C-terminal UBA domain [16, 18], and due to the intramolecular interaction be unable to perform its cargo-sensing function, extending the lifetime of endocytic coat proteins, but not the lifetimes of proteins arriving after the proposed transition point (Figure 2A, 5B for model). Ede1 is also extensively phosphorylated [57–59], raising the possibility that these modifications may function in a coordinated manner.
Evidence that Rsp5 performs an endocytic function comes from endocytic defects observed in rsp5 mutants [25, 28]. While definitive proof of an E3 ubiquitin ligase at endocytic sites remains elusive, the fact that Rsp5 accumulates at the plasma membrane when endocytosis is perturbed suggests that it normally spends time at the plasma membrane and is removed by endocytosis via an unknown mechanism. The observation that GFP-Rsp5 is enriched on the plasma membrane of buds is intriguing. Perhaps more Rsp5 is recruited because endocytosis occurs preferentially in buds.
While it had previously been shown that Rsp5 is responsible for the Ede1 ubiquitination [16], our data indicate that Ubp2 and Ubp7 are directly responsible for deubiquitination of Ede1 and likely other endocytic proteins. Moreover, the data presented here implicate dynamic ubiquitination and deubiquitination of the endocytic machinery, distinct from the previously documented ubiquitination and deubiquitination of cargo molecules, in regulation of endocytic coat assembly, maintenance and disassembly [11–14, 24, 38, 60]. As all of the implicated components are highly conserved, we expect that these results will apply broadly.
MATERIALS AND METHODS
Strains
Yeast strains used in this study are listed in Supplemental Table S1. Gene deletions were generated by replacing the gene open reading frame with the Candida glabrata LEU2 or URA3, hphNT1 or KanMX4 cassette. C-terminal GFP tags were integrated as previously described [61]. The N-terminal GFP tag for Rsp5 was integrated as previously described [62]. N-terminal tagging of Ubp7 was performed as previously described [63].
Microscopy
Yeast strains for imaging were grown to log phase at 30°C (25°C for sla2Δ and ark1Δ prk1Δ) in synthetic media lacking tryptophan and immobilized on concanavalin A-coated coverslips. For FM4-64 experiments a flow cell was constructed with double-stick tape allowing for addition of FM4-64 or DMSO while imaging.
Microscopy was performed on Olympus IX71 and IX81 microscopes with 100×/numerical aperture (NA) 1.4 objectives and Orca cameras (Hamamatsu, Hamamatsu, Japan) at 25°C. Simultaneous two-color imaging was performed using a argon-ion laser (CVI Melles Griot, Albuquerque, NM) to excite GFP and a 561-nm diode-pumped solid-state laser (CVI Melles Griot) to excite RFP. TIRFM was performed using an IX81 microscope equipped with a 100×/NA 1.65 objective with independently adjustable 488- and 561-nm lasers. Images were acquired with a frame rate of 1 frame per second using MetaMorph software (Molecular Devices, Sunnyvale, CA), patch lifetimes were calculated using ImageJ (National Institutes of Health, Bethesda, MD) and graphs and statistics were generated using Excel (Microsoft, Redmond, WA).
GFP-Rsp5 imaging and high time resolution stacks were collected using a Nikon Eclipse TI equipped with a 100× NA1.4 objective, Ti-ND6-PFS Ti-E Perfect Focus Unit, Andor Neo sCMOS camera, ASI PZ-2000 piezo stage, Lumencor Spectra-X Light Engine and an In Vivo Scientific Incubator using MetaMorph software (Molecular Devices, Sunnyvale, CA) and were analyzed using ImageJ (National Institutes of Health, Bethesda, MD).
His6-Ubiquitin Purification
His6-Ubiquitin purifications were adapted from Ziv et al [23]. Yeast were grown to log phase in 500mL of YPD at 30°C and collected via centrifugation at 3500 RPM in a Sorvall SLA-3000 rotor. Cells were resuspended in a small volume of water and frozen in liquid nitrogen. TCA (Fisher) was added to 20% and cells were lysed by bead beating in a vortex (4×30s with 30s on ice between). Supernatants were collected by centrifugation (1 minute 1000g), beads were washed with 12% TCA and the wash was collected by centrifugation (1 minute 1000g). Supernatants were incubated on ice for 30 minutes and pellets were collected by centrifugation (15 minutes, 14,000 RPM 4°C) and the pH was adjusted using NH4OH. Pellets were resuspended in urea buffer (8M urea, 20mM Tris pH 8.0, 100mM K2HPO4, 10mM imidazole, 100mM NaCl, 0.1% Triton X-100) and incubated on ice for 30 minutes. Supernatants were collected by centrifugation and incubated with Ni-NTA beads (Qiagen) overnight at 4°C. Beads were collected by centrifugation and washed 2× with urea buffer, the flow-through was reapplied to beads and rotated at room temperature for 3 hours. Beads were collected by centrifugation and washed twice with wash buffer 1 (20mM Tris pH 8.0, 100mM K2HPO4, 20mM imidazole, 100mM NaCl, 0.1% Triton X-100), twice with wash buffer 2 (20mM Tris pH 8.0, 100mM K2HPO4, 20mM imidazole, 100mM NaCl) and eluted with low pH buffer (20mM Tris pH 4.5, 100mM K2HPO4, 150mM imidazole, 100mM NaCl) and analyzed by SDS-PAGE. Rabbit anti-Ede1 and was a generous gift from Linda Hicke. Anti-rabbit HRP was purchased from GE Healthcare and blots were read using a BioRad ChemiDoc XRS+ system.
GST-DUB purification
UBP2 and UBP7 ORFs were cloned into pGEX-2T (GE Healthcare Life Sciences) and expressed in BL21 cells as previously described [64]. Following induction, the bacteria were frozen in liquid nitrogen, resuspended in HEN (50mM HEPES pH 7.5, 1mM EDTA, 200mM NaCl) supplemented with 2mM PMSF, 4mM NEM (Sigma), and 2× Protease Inhibitor Cocktail Set II (Calbiochem) and lysed by six ten-second pulses of sonication with 30 seconds on ice between pulses. Cellular debris was pelleted twice by centrifugation at 14,000 RPM in a microcentrifuge at 4°C for 15 minutes. Following addition of Triton X-100 to 1%, supernatants were rocked with glutathione beads (GE Healthcare) overnight. Beads were washed twice with HEN + 1% Triton, three times with HEN and eluted three times for 30 min with HEN + 50 mM Tris pH 8.0 + 20 mM glutathione (Sigma-Aldrich) with a final overnight elution. Catalytically dead mutants were purified similarly following mutagenesis of the pGEX-2T plasmids using a QuikChange Lightning kit (Agilent technologies). DUBs were assayed in 100μl reactions with final buffer concentrations of 50mM Tris pH 7.5, 1mM dithiothreitol, 100μg/ml BSA (NEB) and 100nM or 200mM (for Ubp7) Ub-AMC (Enzo Life Sciences) using a Perkin Elmer Victor3 1420 Multilabel Counter.
Ede1 Immuno-isolation
Immuno-isolations were performed as previously described [52] using 100 OD600s of cells and anti-Ede1 antibodies (a kind gift from Linda Hicke). Lysis buffer was supplemented with 10 mM NEM and Protease Inhibitor Cocktail Set IV at 1:200. During the final wash step, the beads were divided into equal volumes for the subsequent in vitro deubiquitination reactions.
In vitro deubiquitination reactions
Reactions were prepared with 10μl protein-G beads from an Ede1 immuno-isolation, 10ng purified DUB, buffer to final concentrations of: 50mM Tris pH 7.5, 1mM dithiothreitol, 100μg/ml BSA (NEB) and rotated at room temperature for 60 minutes. Reactions were stopped by addition of 10μl of 8M urea (final concentration 1.25M) and 12.5μl of 5X SDS sample buffer (final concentration 1X). Samples were analyzed by SDS-PAGE and immuno blotting with anti-Ede1 (Linda Hicke) and anti-ubiquitin (FL-76 Santa Cruz Biotech) sera.
Supplementary Material
Highlights.
Ubp7 localizes to endocytic sites and purified DUBs deubiquitinate Ede1 in vitro
Deleting UBP2 and UBP7 causes recruitment of endocytic proteins to internal punctae
Ede1 is more highly ubiquitinated in ubp2Δ ubp7Δ cells
Permanently ubiquitinated Ede1 phenocopies deletion of UBP2 and UBP7
Acknowledgements
We are grateful to Dr. Georjana Barnes and the members of the Drubin/Barnes laboratory for helpful discussions, especially comments on this manuscript from Yidi Sun and Christa Cortesio. We thank Dr. Linda Hicke for plasmids LHP561 and LPH2798 and anti-Rsp5 and anti-Rvs167 antibodies and Dr. Daniel Finley for strains SUB280 and SUB592. This work was supported by research grant R01 GM50399 from the National Institutes of Health to D.G.D.
Abbreviations List
- DUB
deubiquitinase
- NEM
n-ethylmaleimide
- RFP
red fluorescent protein
- SH3 domain
SRC homology 3 domain
- TIRFM
total internal reflection microscopy
- UCH domain
ubiquitin carboxyl-terminal hydrolase
- WASP
Wiskott-Aldrich Syndrome Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Materials
Figure S1 shows that GFP-Ubp7 and Ubp7ΔUCH-GFP have similar dynamics, and that Sla1-mCherry dynamics are the same in cells expressing either tagged form of Ubp7 described in the text in support of Figure 1A, Ubp7ΔUCH-GFP localization in strains lacking endocytic SH3 domain proteins in support of Figure 1E, and GFP-Rsp5 expression levels in support of Figure 1F. Figure S2 shows the quantification of phenotypes of Ede1-Ub and Pan1-Ub expressing cells in support of Figure 2. Figure S3 shows quantification of His6-Ub purifications in support of Figure 4A, GST-Ubp2 and GST-Ubp7 characterization in support of Figure 4B, C, the blot from 4C reprobed with anti-Ede1 antisera in support of Figure 4C, and Ede1 immuno-isolations from wild-type and DUBΔ cells in support of Figure 4. Figure S4 shows Ede1-Ub expression levels in support of Figures 3 and 5. Figure S5 shows the organization of endocytic proteins into functionally and temporally linked modules in support of Figure 5B, Ede1-GFP internal puncta appear de novo in support of Figure 5A. Table S1 shows yeast strains used in this study. Table S2 shows plasmids used in this study. Movie S1 shows GFP-Rsp5 dynamics in support of Figure 1F.
References
- 1.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123(2):305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115(4):475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9(3):e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng G, Yu X, Cai M. Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol Biol Cell. 2001;12(12):3759–3772. doi: 10.1091/mbc.12.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toret CP, et al. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic. 2008;9(5):848–859. doi: 10.1111/j.1600-0854.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 6.Sekiya-Kawasaki M, et al. Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis. J Cell Biol. 2003;162(5):765–772. doi: 10.1083/jcb.200305077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope MJ. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J Cell Biol. 1999;144(6):1203–1218. doi: 10.1083/jcb.144.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi RJ, et al. Role of Scd5, a protein phosphatase-1 targeting protein, in phosphoregulation of Sla1 during endocytosis. J Cell Sci. 2012 doi: 10.1242/jcs.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84(2):277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 10.Egner R, Kuchler K. The yeast multidrug transporter Pdr5 of the plasma membrane is ubiquitinated prior to endocytosis and degradation in the vacuole. FEBS Lett. 1996;378(2):177–181. doi: 10.1016/0014-5793(95)01450-0. [DOI] [PubMed] [Google Scholar]
- 11.Kolling R, Hollenberg CP. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J. 1994;13(14):3261–3271. doi: 10.1002/j.1460-2075.1994.tb06627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galan JM, et al. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. Journal of Biological Chemistry. 1996;271(18):10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- 13.Meijer IMJ, et al. Recycling of EGFR and ErbB2 is associated with impaired Hrs tyrosine phosphorylation and decreased deubiquitination by AMSH. Cellular Signalling. 2012;24(11):1981–1988. doi: 10.1016/j.cellsig.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Alwan HAJ, van Leeuwen JEM. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. Journal of Biological Chemistry. 2007;282(3):1658–1669. doi: 10.1074/jbc.M604711200. [DOI] [PubMed] [Google Scholar]
- 15.Lin CH, et al. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135(4):714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Dores MR, et al. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic. 2010;11(1):151–160. doi: 10.1111/j.1600-0854.2009.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamenova SD, et al. The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J Biol Chem. 2004;279(16):16017–16025. doi: 10.1074/jbc.M313479200. [DOI] [PubMed] [Google Scholar]
- 18.Hoeller D, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8(2):163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- 19.Woelk T, et al. Molecular mechanisms of coupled monoubiquitination. Nat Cell Biol. 2006;8(11):1246–1254. doi: 10.1038/ncb1484. [DOI] [PubMed] [Google Scholar]
- 20.Fallon L, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3) K-Akt signalling. Nature Cell Biology. 2006;8(8) doi: 10.1038/ncb1441. 834-U87. [DOI] [PubMed] [Google Scholar]
- 21.Cherry JM, et al. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Research. 2012;40(D1):D700–D705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R, et al. Ubiquitination screen using protein microarrays for comprehensive identification of Rsp5 substrates in yeast. Mol Syst Biol. 2007;3:116. doi: 10.1038/msb4100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziv I, et al. A Perturbed Ubiquitin Landscape Distinguishes Between Ubiquitin in Trafficking and in Proteolysis. Molecular & Cellular Proteomics. 2011;10(5) doi: 10.1074/mcp.M111.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: Role of Nedd4/Rsp5p family of ubiquitin-protein ligases. Journal of Membrane Biology. 2000;176(1):1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 25.Gajewska B, et al. WW domains of Rsp5p define different functions: determination of roles in fluid phase and uracil permease endocytosis in Saccharomyces cerevisiae. Genetics. 2001;157(1):91–101. doi: 10.1093/genetics/157.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn R, et al. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J Cell Biol. 2004;165(1):135–144. doi: 10.1083/jcb.200309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminska J, et al. Yeast Rsp5 ubiquitin ligase affects the actin cytoskeleton in vivo and in vitro. European Journal of Cell Biology. 2011;90(12):1016–1028. doi: 10.1016/j.ejcb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Kaminska J, et al. Rsp5p, a new link between the actin cytoskeleton and endocytosis in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 2002;22(20):6946–6948. doi: 10.1128/MCB.22.20.6946-6958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih SC, et al. A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. Embo Journal. 2003;22(6):1273–1281. doi: 10.1093/emboj/cdg140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisk HA, Yaffe MP. A role for ubiquitination in mitochondrial inheritance in Saccharomyces cerevisiae. Journal of Cell Biology. 1999;145(6):1199–1208. doi: 10.1083/jcb.145.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann S, et al. Formation and nuclear export of tRNA, rRNA and mRNA is regulated by the ubiquitin ligase Rsp5p. Embo Reports. 2003;4(12):1156–1162. doi: 10.1038/sj.embor.7400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong SJ, et al. Role of RNA polymerase II carboxy terminal domain phosphorylation in DNA damage response. Journal of Microbiology. 2005;43(6):516–522. [PubMed] [Google Scholar]
- 33.Kaliszewski P, et al. Rsp5p ubiquitin ligase and the transcriptional activators Spt23p and Mga2p are involved in co-regulation of biosynthesis of end products of the mevalonate pathway and triacylglycerol in yeast Saccharomyces cerevisiae. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2008;1781(10):627–634. doi: 10.1016/j.bbalip.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Wang GL, et al. Localization of the Rsp5p ubiquitin-protein ligase at multiple sites within the endocytic pathway. Molecular and Cellular Biology. 2001;21(10):3564–3575. doi: 10.1128/MCB.21.10.3564-3575.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kee Y, Lyon N, Huibregtse JM. The Rsp5 ubiquitin ligase is coupled to and antagonized by the Ubp2 deubiquitinating enzyme. EMBO J. 2005;24(13):2414–2424. doi: 10.1038/sj.emboj.7600710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kee Y, et al. The deubiquitinating enzyme Ubp2 modulates Rsp5-dependent Lys63-linked polyubiquitin conjugates in Saccharomyces cerevisiae. J Biol Chem. 2006;281(48):36724–36731. doi: 10.1074/jbc.M608756200. [DOI] [PubMed] [Google Scholar]
- 37.Springael JY, et al. NH4+-induced down-regulation of the Saccharomyces cerevisiae Gap1p permease involves its ubiquitination with lysine-63-linked chains. Journal of Cell Science. 1999;112(9):1375–1383. doi: 10.1242/jcs.112.9.1375. [DOI] [PubMed] [Google Scholar]
- 38.Galan JM, HaguenauerTsapis R. Ubiquitin Lys63 is involved in ubiquitination of a yeast plasma membrane protein. Embo Journal. 1997;16(19):5847–5854. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paiva S, et al. Glucose-induced Ubiquitylation and Endocytosis of the Yeast Jen1 Transporter ROLE OF LYSINE 63-LINKED UBIQUITIN CHAINS. Journal of Biological Chemistry. 2009;284(29):19228–19236. doi: 10.1074/jbc.M109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncan LM, et al. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. Embo Journal. 2006;25(8):1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tonikian R, et al. Bayesian modeling of the yeast SH3 domain interactome predicts spatiotemporal dynamics of endocytosis proteins. PLoS Biol. 2009;7(10):e1000218. doi: 10.1371/journal.pbio.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michelot A, et al. Reconstitution and protein composition analysis of endocytic actin patches. Curr Biol. 2010;20(21):1890–1899. doi: 10.1016/j.cub.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 44.Skruzny M, et al. Molecular basis for coupling the plasma membrane to the actin cytoskeleton during clathrin-mediated endocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):E2533–E2542. doi: 10.1073/pnas.1207011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvat C, et al. The-4 phenylalanine is required for substrate ubiquitination catalyzed by HECT ubiquitin ligases. Journal of Biological Chemistry. 2004;279(18):18935–18943. doi: 10.1074/jbc.M312201200. [DOI] [PubMed] [Google Scholar]
- 46.Lam MHY, et al. Interaction of the Deubiquitinating Enzyme Ubp2 and the E3 Ligase Rsp5 Is Required for Transporter/Receptor Sorting in the Multivesicular Body Pathway. Plos One. 2009;4(1) doi: 10.1371/journal.pone.0004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stimpson HE, et al. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol Biol Cell. 2009;20(22):4640–4651. doi: 10.1091/mbc.E09-05-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toshima J, et al. Negative regulation of yeast Eps15-like Arp2/3 complex activator, Pan1p, by the Hip1R-related protein, Sla2p, during endocytosis. Mol Biol Cell. 2007;18(2):658–668. doi: 10.1091/mbc.E06-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan MC, et al. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat Cell Biol. 2001;3(7):687–690. doi: 10.1038/35083087. [DOI] [PubMed] [Google Scholar]
- 50.Wendland B, et al. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol. 1996;135(6 Pt 1):1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7(3):e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carroll SY, et al. Analysis of yeast endocytic site formation and maturation through a regulatory transition point. Molecular Biology of the Cell. 2012;23(4):657–668. doi: 10.1091/mbc.E11-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mettlen M, et al. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. Journal of Cell Biology. 2010;188(6):919–933. doi: 10.1083/jcb.200908078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mettlen M, et al. Endocytic Accessory Proteins Are Functionally Distinguished by Their Differential Effects on the Maturation of Clathrin-coated Pits. Molecular Biology of the Cell. 2009;20(14):3251–3260. doi: 10.1091/mbc.E09-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henry AG, et al. Regulation of Endocytic Clathrin Dynamics by Cargo Ubiquitination. Developmental Cell. 2012;23(3):519–532. doi: 10.1016/j.devcel.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shih SC, et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nature Cell Biology. 2002;4(5):389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 57.Li X, et al. Large-scale phosphorylation analysis of alpha-factor-arrested Saccharomyces cerevisiae. Journal of Proteome Research. 2007;6(3):1190–1197. doi: 10.1021/pr060559j. [DOI] [PubMed] [Google Scholar]
- 58.Smolka MB, et al. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10364–10369. doi: 10.1073/pnas.0701622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chi A, et al. Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2193–2198. doi: 10.1073/pnas.0607084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenness DD, Spatrick P. Down regulation of the alpha-factor pheromone receptor in S. cerevisiae. Cell. 1986;46(3):345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- 61.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 62.Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21(11):947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 63.Prein B, Natter K, Kohlwein SD. A novel strategy for constructing N-terminal chromosomal fusions to green fluorescent protein in the yeast Saccharomyces cerevisiae. Febs Letters. 2000;485(1):29–34. doi: 10.1016/s0014-5793(00)02179-7. [DOI] [PubMed] [Google Scholar]
- 64.Russell NS, Wilkinson KD. Deubiquitinating enzyme purification, assay inhibitors, and characterization. Methods Mol Biol. 2005;301:207–219. doi: 10.1385/1-59259-895-1:207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.