Abstract
Object
As the population ages, the incidence of glioblastoma multiforme (GBM) among older patients (age > 65 years) will increase. Older patients, unlike their younger counterparts, are not often offered aggressive surgery because of their age, comorbidities, and potential inability to tolerate surgery. The goal of this study was to identify preoperative factors associated with decreased survival for older patients who underwent resection of a GBM. The identification of these factors may provide insight into which patients would benefit most from aggressive surgery.
Methods
All patients older than 65 years who underwent nonbiopsy resection of an intracranial GBM at a single institution between 1997 and 2007 were retrospectively reviewed. Factors associated with overall survival were assessed using multivariate proportional hazards regression analysis after controlling for peri- and postoperative factors known to be associated with outcome (extent of resection, carmustine wafer implantation, temozolomide chemotherapy, and radiation therapy). Variables with p < 0.05 were considered statistically significant.
Results
A total of 129 patients with an average age of 73 ± 5 years met the inclusion/exclusion criteria. At last follow-up, all 129 patients had died, with a median survival of 7.9 months. The preoperative factors that were independently associated with decreased survival were Karnofsky Performance Scale (KPS) score less than 80 (p = 0.001), chronic obstructive pulmonary disease (p = 0.01), motor deficit (p = 0.01), language deficit (p = 0.005), cognitive deficit (p = 0.02), and tumor size larger than 4 cm (p = 0.002). Patients with 0–1 (Group 1), 2–3 (Group 2), and 4–6 (Group 3) of these factors had statistically different survival times, where the median survival was 9.2, 5.5, and 4.4 months, respectively. In log-rank analysis, the median survival for Group 1 was significantly longer than that for Group 2 (p = 0.004) and Group 3 (p < 0.0001), while Group 2 had longer survival than Group 3 (p = 0.02).
Conclusions
Older patients with an increasing number of these factors may not benefit as much from aggressive surgery as patients with fewer factors. This may provide insight into identifying which patients older than 65 years of age may benefit from aggressive surgery.
Keywords: elderly patient, function, glioblastoma multiforme, glioblastoma, survival
Glioblastoma multiforme is the most common and most malignant primary brain tumor in adults.11 There are more than 3 newly diagnosed cases per 100,000 people every year in the US, and the peak incidence occurs in individuals 65 years of age and older.13,43 As this subset of the population is expected to increase, the incidence of GBM among older patients will continue to increase.19,20 Older patients, however, tend to have significantly worse survival times than younger patients.6,29 As a result, older patients are often underrepresented in clinical trials, and studies devoted to these patients with GBM are few and limited.15,18–20
The standard procedure for patients younger than 65 years with GBM is to attempt maximal safe resection for diagnosis and tumor debulking before initiating radiation therapy and chemotherapy.9,34,39 Older patients, however, are rarely offered aggressive surgery.27,32 Aggressive surgery is not commonly offered because these patients are assumed to have poor prognoses, an inability to tolerate long surgery times, poor physiological reserves, and an increased risk of complications, among others.27,32 Recent studies have shown that older patients can indeed tolerate surgery, and some may actually benefit from aggressive resection.27,31 The ability to identify preoperatively those patients older than 65 years who will benefit from nonbiopsy resection may therefore help guide treatment strategies aimed at prolonging survival and minimizing surgical morbidity for those with GBM.
The goal of this study is to identify preoperative factors that are associated with decreased survival among patients older than 65 years who underwent aggressive GBM resection. A more thorough understanding of these factors may provide greater insight into which patients within this subpopulation would benefit most from aggressive therapy. This could potentially optimize treatment for older patients with GBM by helping select those who would benefit most from surgery.
Methods
Patient Selection
A total of 133 patients (age > 65 years) underwent surgery for a primary (de novo) intracranial GBM at a single academic tertiary care institution between 1997 and 2007. The pathology was determined by a senior neuropathologist in all cases, and the grading criteria were based on the WHO classification system.23,30 Patients older than 65 years with a tissue-proven diagnosis of a supratentorial GBM (WHO Grade IV) were included in the study. Patients who underwent needle biopsies, prior resections, and/or prior adjuvant therapy (radiation therapy or chemotherapy) were excluded from the analysis. Additionally, patients who were not confirmed as having died were excluded from the analysis. This was done to create a more uniform patient population with similar ages, tumor types and locations, treatments, and follow-up.
Recorded Variables
The clinical, operative, and hospital course records of the patients who met the inclusion/exclusion criteria were retrospectively reviewed. The information collected from clinical and operative notes included patient demographics, comorbidities, presenting symptoms, neurological function, neuroimaging, perioperative course including hospital LOS and rehabilitation needs, and adjuvant therapy. The KPS score was used to classify patients’ preoperative functional status.12 The KPS scores were assigned by a clinician, blinded to patient outcomes, at the clinic visit prior to surgery. A preoperative sensory deficit was defined as decreased sensation to any modality, and a preoperative motor deficit was defined as decreased strength as identified by a clinician during a physical examination. A preoperative language deficit was defined as any combination of receptive and/or expressive aphasia. Cognitive deficits were defined as confusion and/or memory loss. Deficits were recorded from the clinic visit prior to surgery.
The MR imaging characteristics that were recorded included the lesion’s size (largest diameter based on Gd contrast), specific lobe location, and eloquent brain involvement. The tumor was categorized as involving eloquent cortex if it involved the motor/sensory cortex, language cortex, basal ganglia/internal capsule, or thalamus. This assignment was based on radiographic and not clinical criteria. Extent of resection was classified from dictations of MR images obtained less than 48 hours after resection as either GTR (> 99% resection), NTR (95%–99%), or STR (80%–95%) by an independent neuroradiologist blinded to patient outcomes. This was done by comparing pre- and postoperative T1-weighted MR images with Gd.
The date of death was also recorded for all patients, where survival data were obtained from the Social Security Index Database (http://ssdi.rootsweb.ancestry.com). Perioperative death was defined as death within 30 days of surgery. All patients were assessed using physical and occupational therapy criteria following surgery to determine whether the patient was safe to be discharged home or to a rehabilitation center.
Perioperative Treatment
The general aim of surgery was to achieve GTR/NTR of the tumor when possible. An STR primarily occurred when resection was limited by eloquent brain as confirmed by intraoperative mapping and/or monitoring (awake/speech language mapping, direct cortical motor stimulation, and motor or somatosensory evoked potentials). Motor and somatosensory evoked potentials were routinely used in the majority of cases, while surgical navigation (CT and/or MR imaging wand) was used in all cases after 2001. The use of motor or speech mapping largely depended upon the preference of the surgeon, and was primarily used when the tumor was near the speech or motor cortex.
The use of polifeprosan with carmustine implant therapy (Gliadel Wafer, Eisai, Inc.) was determined by the surgeon and the patient. These implantable wafers were typically not used when tumors were multifocal, extended across the corpus callosum, or required large opening of the ventricle. Likewise, the particular use of adjuvant radiation therapy and chemotherapy was determined by the surgeon, radiation oncologist, medical oncologist, and the patients themselves.
Statistical Analysis
Summary data are presented as the mean ± SD for parametric data and as the median with the IQR in parentheses for nonparametric data. For intergroup comparison, the Student t-test was used for parametric data and Mann-Whitney U-test for nonparametric data. Percentages were compared using the chi-square test or Fisher exact test where appropriate.
Survival as a function of time was plotted using the Kaplan-Meier method. Log-rank analysis was used to compare Kaplan-Meier plots. The multivariate proportional hazards regression analysis was used to identify preoperative factors associated with decreased survival for older patients with GBM. This was done after controlling for peri- and postoperative factors that have consistently been shown to be associated with survival including extent of resection,26,29,34 carmustine wafer implantation,5,42 temozolomide chemotherapy,16,40 and radiation therapy.14 In this analysis, all variables associated with survival in univariate analysis (p < 0.10) were included in a step-wise multivariate proportional hazards regression model. Values with p < 0.05 in all analyses were considered statistically significant.
Results
Preoperative Characteristics
Of the 133 patients older than 65 years who underwent nonbiopsy resection of a primary GBM, 129 met the inclusion/exclusion criteria. The preoperative characteristics of these 129 patients are summarized in Table 1. Sixty-seven patients (52%) were men. The mean age was 73 ± 5 years at the time of surgery; 52 patients (40%) were between 66 and 70 years of age, 37 (29%) were between 71 and 75 years, 27 (21%) were between 76 and 80 years, and 13 (10%) were older than 80 years of age. A large percentage of the patients had hypertension (46%), diabetes (17%), and coronary artery disease (15%).
TABLE 1.
Preoperative characteristics of patients older than 65 years undergoing resection of an intracranial GBM*
| Characteristic | No. of Patients (%) |
|---|---|
| male sex | 67 (52) |
| mean age (yrs) | 73 ± 5 |
| age group (yrs) | |
| 66–70 | 52 (40) |
| 71–75 | 37 (29) |
| 76–80 | 27 (21) |
| >80 | 13 (10) |
| median KPS score | 80 (80–80) |
| preop symptoms | |
| median symptom duration (mos) | 1 (0.5–2) |
| seizures | 19 (15) |
| headaches/nausea/vomiting | 25 (19) |
| sensory deficit | 7 (5) |
| motor deficit | 45 (35) |
| language deficit | 29 (22) |
| visual deficit | 21 (16) |
| confusion/memory loss | 41 (32) |
| comorbidities | |
| coronary artery disease | 19 (15) |
| hypertension | 59 (46) |
| COPD | 7 (5) |
| diabetes | 22 (17) |
| depression | 7 (5) |
| radiography findings | |
| mean tumor size (cm) | 4.2 ± 1.4 |
| hemorrhagic | 17 (13) |
| location | |
| frontal | 46 (36) |
| temporal | 42 (33) |
| parietal | 31 (24) |
| occipital | 10 (8) |
| cortical | 49 (38) |
| eloquent cortex | |
| language cortex | 6 (5) |
| motor/sensory cortex | 4 (3) |
| basal ganglia/internal capsule | 14 (11) |
| thalamus | 6 (6) |
Unless noted otherwise. Mean values are presented as the mean ± SD, and median values are presented as the median with the IQR in parentheses.
The median preoperative KPS was 80 (IQR 80–80, range 60–100), and the major presenting symptoms were motor deficit in 45 patients (35%), cognitive deficit (memory loss/confusion) in 41 (32%), language deficit in 29 (22%), and headaches/nausea/vomiting in 25 (19%). The median symptom duration was 1 month prior to surgery. The mean tumor size was 4.2 ± 1.4 cm, and 46 tumors (36%) involved the frontal lobe, 42 (33%) the temporal lobe, 31 (24%) the parietal lobe, and 10 (8%) the occipital lobe. The tumor primarily involved the cortex in 49 patients (38%). Among eloquent sites, 6 (5%) involved the language cortex, 4 (3%) the motor/sensory cortex, 14 (11%) the basal ganglia/internal capsule, and 6 (5%) the thalamus.
Perioperative and Postoperative Outcomes
The peri- and postoperative outcomes are summarized in Table 2; GTR, NTR, and STR were achieved in 39 (30%), 54 (42%), and 36 (28%) patients, respectively. There were no cases of perioperative mortality. Eleven (9%), 3 (2%), and 11 (9%) patients developed a new postoperative motor, language, or visual deficit, respectively. The median hospital LOS was 5 days (IQR 4–7 days). Eighty-four (65%) of the patients were discharged home, while the remaining 45 (35%) were discharged to a rehabilitation center. Fifty-eight patients (45%) had carmustine wafers placed at the time of surgery, while 20 (16%) underwent temozolomide chemotherapy. Radiotherapy was performed in 111 patients (86%), with a median dose of 6000 cGy (IQR 5940–6000 cGy).
TABLE 2.
Perioperative and postoperative characteristics of patients older than 65 years undergoing resection of an intracranial GBM*
| Characteristic | No. of Patients (%) |
|---|---|
| op variable | |
| GTR (>99%) | 39 (30) |
| NTR (>95%) | 54 (42) |
| STR (80–95%) | 36 (28) |
| perioperative variable | |
| mortality | 0 (0) |
| new motor deficit | 11 (9) |
| new language deficit | 3 (2) |
| new visual deficit | 11 (9) |
| median LOS | 5 (4–7) |
| discharge home | 84 (65) |
| adjuvant therapy | |
| carmustine wafers | 58 (45) |
| temozolomide | 20 (16) |
| radiation therapy | 111 (86) |
| survival | |
| dead at last follow-up | 129 (100) |
| median survival (mos) | 7.9 |
| survival rate (%) | |
| 3-mo | 81 |
| 6-mo | 57 |
| 9-mo | 41 |
| 12-mo | 23 |
| 18-mo | 12 |
| 24-mo | 7 |
Unless noted otherwise. Mean values are presented as the mean ± standard deviation, and median values are presented as the median with the IQR in parentheses.
At last follow-up, 129 (100%) of the patients had died. The median survival was 7.9 months, and the 3-, 6-, 9-, and 12-month survival rates were 81%, 57%, 41%, and 23%, respectively (Fig. 1).
Fig. 1.
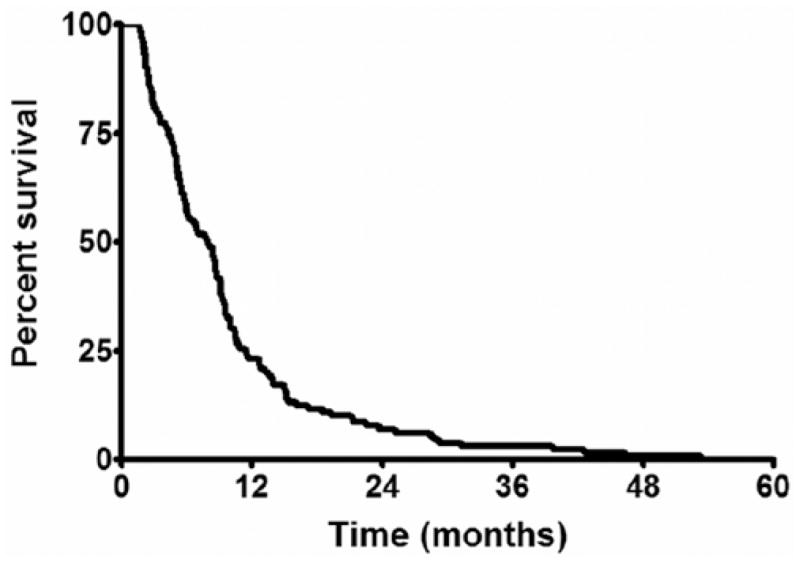
Survival of all patients older than 65 years with an intracranial GBM. The median survival was 7.9 months, where the 3-, 6-, and 12-month survival rates were 81%, 57%, and 23%, respectively.
Factors Independently Associated With Survival
Univariate Analysis
In univariate analysis, after controlling for peri- and postoperative factors known to be associated with survival (extent of resection, carmustine wafer, temozolomide, and radiation therapy), the preoperative factors associated with decreased survival were age older than 75 years, male sex, COPD, depression, preoperative KPS score, motor deficit, sensory deficit, language deficit, cognitive deficit, frontal lobe location, and tumor size. No other clinical or imaging variables were found to be associated with decreased survival. These factors included older age, other comorbidities (coronary artery disease, diabetes, or hypertension), and eloquent cortex involvement.
Multivariate Analysis
In stepwise multivariate analysis, after controlling for peri- and postoperative factors known to be associated with survival (extent of resection, carmustine wafer, temozolomide, and radiation therapy), the factors that remained significantly associated with decreased survival were KPS score less than 80 (RR 1.756 [95% CI 1.431–2.754], p = 0.001), COPD (RR 3.762 [95% CI 1.350–9.061], p = 0.01), motor deficit (RR 3.480 [95% CI 1.279–7.993], p = 0.01), language deficit (RR 2.311 [95% CI 1.310–3.960], p = 0.005), cognitive deficit (RR 1.792 [95% CI 1.089–2.916], p = 0.02), and increasing tumor size (RR 1.982 [95% CI 1.264–3.142], p = 0.002). Of note, patients with tumors larger than 4 cm (RR 1.982 [95% CI 1.264–3.142], p = 0.002) had the greatest statistical association with decreased survival. Among patients who had COPD, 5 (71%) of the patients had a positive smoking history. Active smoking (p = 0.18) or a history of smoking (p = 0.23) was not correlated with decreased survival in this study.
Age and Survival
Age older than 75 years (RR 1.233 [95% CI 1.894–2.890], p = 0.004) was associated with decreased survival in univariate analysis after controlling for extent of resection, carmustine wafer, temozolomide, and radiation therapy. However, age older than 75 years was not associated with decreased survival in multivariate analysis (p = 0.27). In log-rank analysis, patients younger than 75 years had a median survival of 8.7 months compared with 5.1 months for individuals older than 75 years (p = 0.009, Fig. 2).
Fig. 2.
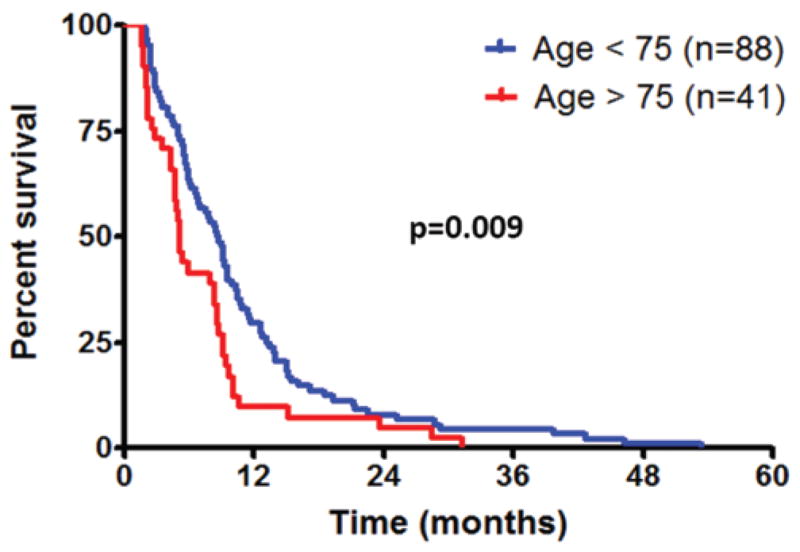
Survival of patients with intracranial GBM, older and younger than 75 years by age group. Patients younger than 75 years had a median survival of 8.7 months compared with 5.1 months for individuals older than 75 years (p = 0.009).
Number of Preoperative Factors and Survival
One point was assigned for each of the following independent factors associated with decreased survival for older patients with GBM: KPS score lower than 80, COPD, motor deficit, language deficit, cognitive deficit, and tumor size larger than 4 cm. Therefore, patients could have anywhere from 0 to 6 points. Patients were subdivided into 3 categories as follows: those with 0–1 point (Group 1), 2–3 points (Group 2), and 4–6 points (Group 3). Based on the subdivision, 72 patients (56%) were in Group 1, 41 (32%) in Group 2, and 16 (12%) in Group 3. For patients in Groups 1, 2, and 3, the median survival was 9.2, 5.5, and 4.4 months, respectively (Fig. 3). In log-rank analysis, the median survival for Group 1 was significantly longer than that for Groups 2 (p = 0.004) and 3 (p < 0.0001). Likewise, in log-rank analysis, the median survival for individuals in Group 2 was significantly longer than for individuals in Group 3 (p = 0.02).
Fig. 3.
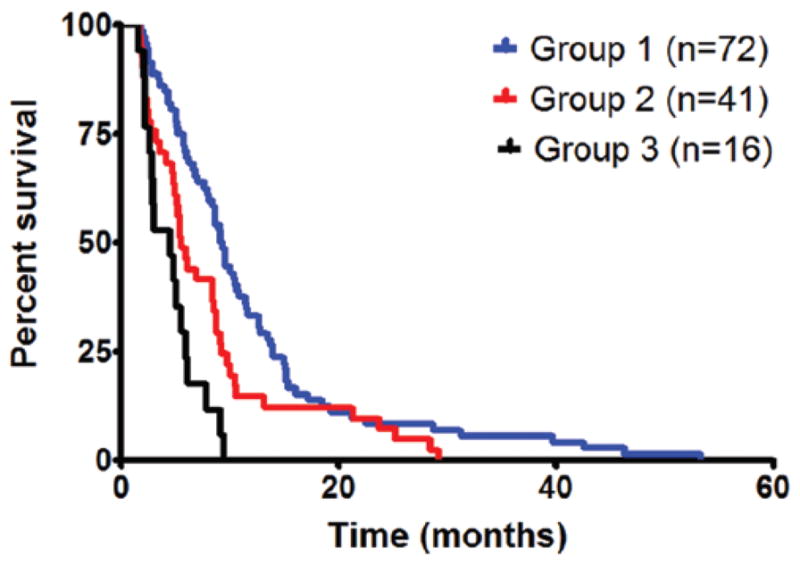
Survival of older patients by number of factors associated with survival. One point was given for each of the following: KPS score lower than 80, COPD, motor deficit, language deficit, cognitive deficit, and tumor size larger than 4 cm. The median survival for patients in Group 1 (0–1 points) was 9.2 months, Group 2 (2–3 points) was 5.5 months, and Group 3 (4–6 points) was 4.4 months. In log-rank analysis, the median survival for Group 1 was significantly longer than that for Groups 2 (p = 0.004) and 3 (p < 0.0001). Likewise, the median survival for individuals in Group 2 was significantly longer than for individuals in Group 3 (p = 0.02).
To minimize the chances that COPD and motor deficits were not dominating the survival curves, separate Kaplan-Meier and log-rank analyses were performed since these variables had the highest odds ratios (Table 3). Patients were assigned 1 point each if they presented with a KPS score lower than 80, tumor size larger than 4 cm, language deficit, and cognitive deficit. Patients with 0–1, 2, and 3–4 points had a median survival of 9.1, 5.7, and 4.6 months, respectively. Patients with 0–1 point had significantly longer survival times than patients with 2 points (p = 0.03) and 3–4 points (p = 0.0006). Likewise, patients with 2 points had significantly longer survival times than patients with 3–4 points (p = 0.04). These findings validate the use of tumor size, language deficit, and cognitive deficit in this prognostic classification scheme despite COPD and motor deficit having the largest odds ratios.
TABLE 3.
Multivariate associations with survival for patients older than 65 years undergoing resection of an intracranial GBM
| Multivariate Associations w/Survival
| ||
|---|---|---|
| Variables | OR (95% CI) | p Value* |
| factors associated w/decreased survival
| ||
| KPS score <80 | 1.756 (1.431–2.754) | 0.001 |
| COPD | 3.762 (1.350–9.061) | 0.01 |
| motor deficit | 3.480 (1.279–7.993) | 0.01 |
| language deficit | 2.311 (1.310–3.960) | 0.005 |
| cognitive deficit | 1.792 (1.089–2.916) | 0.02 |
| tumor size | 1.189 (1.018–1.358) | 0.01 |
| tumor size >4 cm | 1.982 (1.264–3.142) | 0.002 |
|
| ||
| factors notably not associated w/survival
| ||
| older age | 1.589 (0.704–3.507) | 0.26 |
| comorbidities | ||
| coronary artery disease | 0.760 (0.442–1.235) | 0.28 |
| diabetes | 1.333 (0.792–2.153) | 0.27 |
| hypertension | 1.155 (0.804–1.656) | 0.43 |
| atrial fibrillation | 0.737 (0.326–1.443) | 0.40 |
| symptom duration | 1.934 (0.509–5.810) | 0.31 |
| eloquent cortex involvement | 1.295 (0.611–2.478) | 0.48 |
The probability values for the factors associated with decreased survival were all significant.
Discussion
In this study of 129 patients older than 65 years of age who underwent resection of an intracranial primary (de novo) GBM, the median survival was 7.9 months. The preoperative factors that were independently associated with decreased survival were KPS score lower than 80, COPD, motor deficit, language deficit, cognitive deficit, and tumor size larger than 4 cm. Patients with 0–1 (Group 1), 2–3 (Group 2), and 4–6 (Group 3) of these factors had statistically different survival times, and the median survival times were 9.2, 5.5, and 4.4 months, respectively.
Older patients who present with tumors consistent with GBM often undergo needle biopsy for diagnosis followed by potential adjuvant therapy.27,32 Older patients are not typically offered aggressive surgery, presumably because of their historically dismal survival times.4,20,22 Their median survival is typically 4 months, compared with 14 months for the general population.3,19,20,40 Additionally, older patients are believed to harbor tumors with more mutations, causing these tumors to behave more aggressively and infiltrate more widely.1,38,41 These patients also tend to have more medical comorbidities, making it more difficult for them to tolerate long surgical times and prolonged hospital courses.1,38,41 However, it is thought that extensive resection of malignant gliomas is associated with prolonged survival.29,34,39 Extensive resection reduces tumor burden, optimizes penetration of radiation therapy, enhances the effectiveness of chemotherapy, and allows for the possibility of implanting carmustine wafers or brachytherapy.5,33,45 Nonetheless, older patients are typically not offered aggressive surgery for fear of compromising remaining surviving time or causing new neurological deficits.27,32,35 The ability to preoperatively identify which patients may benefit from aggressive surgery may therefore aid treatment strategies and provide prognostic information for surgeons, oncologists, and the patients themselves.
This study demonstrates that among older patients, the influence of age is relative. It is well known that older age is associated with a poor prognosis.6,28,29 Most studies have found that the age associated with the poorest prognoses is approximately 60 years.6,28,29 However, among older patients, the correlation between age and survival is poorly understood. This present study found that no age was independently associated with survival, but age older than 75 years had the strongest association. This age, however, was not associated with survival when controlling for other preoperative factors, which suggests that age itself may not predict survival. A more important association than age with survival, however, is a patient’s preoperative neurological status. This finding has been previously documented primarily in younger patients with low- and high-grade gliomas.25,28,29 Patients with higher preoperative neurological function may be able to better tolerate neurological insults caused by the tumor, surgery, and/or adjuvant therapy.25,28,29 This toleration may lead to better survival times.
The presence of specific deficits, namely preoperative motor, language, and cognitive deficits, portends a worse prognosis. Patients with preoperative motor deficits had a 3.5-fold decreased survival compared with those without motor deficits, while patients with preoperative language deficits had a 2.3-fold decreased survival compared with patients without language deficits. Patients with preoperative cognitive deficits had a 1.8-fold or 80% decreased survival rate compared with patients without cognitive deficits. These were all independent of preoperative KPS. Interestingly, the location of the tumor, including motor/sensory and language cortex on preoperative MR imaging, was not significantly associated with survival. The presence of these deficits may therefore indicate a more infiltrative tumor, where the tumor extends beyond what can be identified on neuroimaging.
Medical comorbidities are more common in older individuals, but only patients with COPD in this study had significantly decreased survival times. Older patients with GBM in previous studies were shown to commonly have one or more comorbid conditions, which often affected treatment decisions. Older patients with COPD had a 3.8-fold decrease in survival after resection compared with those without COPD. This might actually be a function of smoking and its impact on decreasing survival since the majority of patients with COPD in this study had a history of smoking.37 No other comorbidities afflicting these patients were found to have a significant association with survival.
Patients with tumors larger than 4 cm had an approximately 2-fold decreased survival time than patients with smaller tumors. This association between tumor size and survival has been demonstrated primarily for nonelderly patients with low- and high-grade tumors.7,24 Larger tumors may have an increased proliferative ability leading to increased tumor burden, and subsequently worse survival times.7,24
This study identifies specific preoperative factors that independently predicted outcomes for older patients with GBM. Previous classification schemes have been limited primarily to younger patients with malignant gliomas (anaplastic gliomas and GBMs),6,10 which is not necessarily applicable to the patients in this series. In this series, patients presenting with more of these factors appeared to benefit less from surgery than those with fewer factors. In fact, patients with at most 1 factor have survival times that rival the general population.6,40 These factors can therefore be used as a preoperative classification system to identify which older patients will benefit most from surgery. This is especially important prognostic information because not all older patients will pursue resection.
Strengths and Limitations
We believe that this study provides several useful insights for older patients with GBM. First, studies in older patients and those attempting to identify preoperative factors associated with survival are few and limited. This study not only confirms the associations of preoperative neurological status and survival, but it also adds motor, language, and cognitive deficits as well as tumor size. It also shows that age is not necessarily associated with survival among older patients. Patients who were older than 75 years appeared to have worse survival than younger patients, but this was not independent of other preoperative factors. Second, studies applying preoperative risk factors in a manner that provides useful prognostic information have yet to be established. This study provides a potentially useful 3-class system that may prognosticate which elderly patients may benefit the most from surgery before any treatment is pursued. Lastly, this study may provide useful information that may help guide treatment strategies aimed at prolonging survival for older patients with GBM.
This study, however, has some limitations. First, there is a need for external validation of this classification scale and applicability in a prospectively followed cohort. Second, this study does not necessarily apply to older patients undergoing needle biopsy, as all the patients in this study underwent aggressive resection. Furthermore, the majority of patients in this study did not undergo GTR and/or receive triple combinatorial adjuvant therapy (carmustine wafer, temozolomide, and radiation therapy). As a result, the relevance of this prognostic model may be altered in the context of those patients receiving the most aggressive of treatment regimens. This study also does not account for the potential implication of molecular markers and genotypes, which may be associated with survival. Recent studies on nonelderly patients with GBM and O6-methylguanine–DNA methyltransferase (MGMT) promoter methylation reported prolonged survival after temozolomide and radiation therapy compared with patients without this molecular marker.17 Additionally, Parsons et al.36 found that patients with isocitrate dehydrogenase 1 (IDH1) mutations had prolonged survival times. These molecular markers and others may also be associated with survival in this older cohort, but they were not analyzed in this study. Additionally, this study was unable to evaluate other potential prognostic factors that have been found to be associated with survival in other studies including marital status,21,44 presence of a caregiver,8 and ethnicity2 because these were not consistently recorded in patient records. This study also did not assess recurrence and thus progression-free survival as well as functional outcomes or quality of life. These outcome measures were also not consistently recorded in this cohort. Finally, this study is inherently limited by its retrospective design, and, as a result, it is not appropriate to infer direct causal relationships. However, we tried to create a uniform patient population by using strict inclusion and exclusion criteria, thus providing more relevant information for older patients with primary intracranial GBM. We included only patients older than 65 years who underwent aggressive resection of a primary GBM. In addition, we excluded patients with incomplete medical records and those who had undergone prior resections, previous adjuvant therapies, and needle biopsies, and those with infratentorial tumors. Furthermore, we performed multivariate analyses and controlled for potential peri- and postoperative confounding variables. Given these statistical controls and a relatively precise outcome measure, we believe that our findings offer useful insights for the treatment of older patients with primary GBM. Prospective studies are needed to provide better data to guide clinical decision making.
Conclusions
Older patients with GBM are considered to have poor prognoses and are therefore rarely offered aggressive resection. However, previous studies have shown that aggressive surgery may prolong survival for some older patients with GBM. Among older patients undergoing aggressive resection, patients with preoperative KPS scores lower than 80, COPD, motor deficit, language deficit, cognitive deficit, and tumor size larger than 4 cm have worse prognoses. Older patients with an increasing number of these factors may not benefit as much from aggressive surgery as patients with a fewer number of these factors. This may provide insight into identifying which older patients may benefit from aggressive surgery.
Abbreviations used in this paper
- COPD
chronic obstructive pulmonary disease
- GBM
glioblastoma multiforme
- GTR
gross-total resection
- IQR
interquartile range
- KPS
Karnofsky Performance Scale
- LOS
length of stay
- NTR
near-total resection
- STR
subtotal resection
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: KL Chaichana. Acquisition of data: KL Chaichana, KK Chaichana. Analysis and interpretation of data: Quiñones-Hinojosa, KL Chaichana, Olivi, Weingart. Drafting the article: KL Chaichana, KK Chaichana, Olivi, Bennett, Brem. Critically revising the article: Quiñones-Hinojosa, KL Chaichana, Olivi, Weingart, Bennett, Brem. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: KL Chaichana. Administrative/technical/material support: Quiñones-Hinojosa, KL Chaichana. Study supervision: Quiñones-Hinojosa, KL Chaichana.
References
- 1.Alonso M, Hamelin R, Kim M, Porwancher K, Sung T, Parhar P, et al. Microsatellite instability occurs in distinct subtypes of pediatric but not adult central nervous system tumors. Cancer Res. 2001;61:2124–2128. [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Maldonado JL, Williams VL, Curry WT, Rodkey EA, Barker FG, II, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85:171–180. doi: 10.1007/s11060-007-9405-4. [DOI] [PubMed] [Google Scholar]
- 3.Brandes AA, Compostella A, Blatt V, Tosoni A. Glioblastoma in the elderly: current and future trends. Crit Rev Oncol Hematol. 2006;60:256–266. doi: 10.1016/j.critrevonc.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 5.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 6.Chaichana K, Parker S, Olivi A, Quiñones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. Clinical article. J Neurosurg. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 7.Chaichana KL, McGirt MJ, Laterra J, Olivi A, Quiñones-Hinojosa A. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. Clinical article. J Neurosurg. 2010;112:10–17. doi: 10.3171/2008.10.JNS08608. [DOI] [PubMed] [Google Scholar]
- 8.Chang SM, Barker FG., II Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975–1984. doi: 10.1002/cncr.21399. [DOI] [PubMed] [Google Scholar]
- 9.Chang SM, Parney IF, Huang W, Anderson FA, Jr, Asher AL, Bernstein M, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557–564. doi: 10.1001/jama.293.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 11.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 12.Dutta D, Vanere P, Gupta T, Munshi A, Jalali R. Factors influencing activities of daily living using FIM-FAM scoring system before starting adjuvant treatment in patients with brain tumors: results from a prospective study. J Neurooncol. 2009;94:103–110. doi: 10.1007/s11060-009-9810-y. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25:867–890. vii. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Genc M, Zorlu AF, Atahan IL. Accelerated hyperfractionated radiotherapy in supratentorial malignant astrocytomas. Radiother Oncol. 2000;56:233–238. doi: 10.1016/s0167-8140(00)00198-5. [DOI] [PubMed] [Google Scholar]
- 15.Gross CP, Herrin J, Wong N, Krumholz HM. Enrolling older persons in cancer trials: the effect of sociodemographic, protocol, and recruitment center characteristics. J Clin Oncol. 2005;23:4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 16.Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2008;(4):CD007415. doi: 10.1002/14651858.CD007415. [DOI] [PubMed] [Google Scholar]
- 17.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 18.Hutterer M. Elderly patients with glioblastoma multiforme—an underestimated subpopulation? Neuroepidemiology. 2009;33:23–24. doi: 10.1159/000210018. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997–2007) Cancer. 2009;115:3758–3766. doi: 10.1002/cncr.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwamoto FM, Reiner AS, Nayak L, Panageas KS, Elkin EB, Abrey LE. Prognosis and patterns of care in elderly patients with glioma. Cancer. 2009;115:5534–5540. doi: 10.1002/cncr.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64:628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 22.Kimple RJ, Grabowski S, Papez M, Collichio F, Ewend MG, Morris DE. Concurrent temozolomide and radiation, a reasonable option for elderly patients with glioblastoma multiforme? Am J Clin Oncol. 2010;33:265–270. doi: 10.1097/COC.0b013e3181a76a24. [DOI] [PubMed] [Google Scholar]
- 23.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–229. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 24.Kreth FW, Faist M, Rossner R, Volk B, Ostertag CB. Supratentorial World Health Organization Grade 2 astrocytomas and oligoastrocytomas. A new pattern of prognostic factors. Cancer. 1997;79:370–379. doi: 10.1002/(sici)1097-0142(19970115)79:2<370::aid-cncr21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 27.Laigle-Donadey F, Delattre JY. Glioma in the elderly. Curr Opin Oncol. 2006;18:644–647. doi: 10.1097/01.cco.0000245324.19411.19. [DOI] [PubMed] [Google Scholar]
- 28.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 30.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangiola A, Maira G, De Bonis P, Porso M, Pettorini B, Sabatino G, et al. Glioblastoma multiforme in the elderly: a therapeutic challenge. J Neurooncol. 2006;76:159–163. doi: 10.1007/s11060-005-4711-1. [DOI] [PubMed] [Google Scholar]
- 32.Marijnen CA, van den Berg SM, van Duinen SG, Voormolen JH, Noordijk EM. Radiotherapy is effective in patients with glioblastoma multiforme with a limited prognosis and in patients above 70 years of age: a retrospective single institution analysis. Radiother Oncol. 2005;75:210–216. doi: 10.1016/j.radonc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Matheus MG, Castillo M, Ewend M, Smith JK, Knock L, Cush S, et al. CT and MR imaging after placement of the GliaSite radiation therapy system to treat brain tumor: initial experience. AJNR Am J Neuroradiol. 2004;25:1211–1217. [PMC free article] [PubMed] [Google Scholar]
- 34.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. Clinical article. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 35.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quiñones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–470. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 36.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. Int J Epidemiol. 2010;39:430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rickert CH, Sträter R, Kaatsch P, Wassmann H, Jürgens H, Dockhorn-Dworniczak B, et al. Pediatric high-grade astrocytomas show chromosomal imbalances distinct from adult cases. Am J Pathol. 2001;158:1525–1532. doi: 10.1016/S0002-9440(10)64103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–766. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 40.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 41.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148:269–275. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 43.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrensch M, Rice T, Miike R, McMillan A, Lamborn KR, Aldape K, et al. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro Oncol. 2006;8:12–26. doi: 10.1215/S1522851705000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu GN, Ford JM, Alger JR. MRI measurement of the uptake and retention of motexafin gadolinium in glioblastoma multiforme and uninvolved normal human brain. J Neurooncol. 2006;77:95–103. doi: 10.1007/s11060-005-9101-1. [DOI] [PubMed] [Google Scholar]


