Abstract
High-dose interleukin-2 (HD IL-2) and interferon were the most commonly administered therapies before the recent introduction of targeted agents, including vascular endothelial growth factor and mammalian target of rapamycin pathway inhibitors. Although the new agents result in a progression-free survival benefit, high-dose IL-2 remains the only agent with proven efficacy in producing durable complete and partial responses in patients with metastatic renal cell carcinoma (RCC). Furthermore, although the use of single-agent interferon has decreased significantly since the introduction of targeted therapy, it remains in the frontline setting in combination with bevacizumab as a result of 2 large phase III trials. Lastly, improved understanding of immune regulation has led to the advancement of targeted immunotherapy using immune checkpoint inhibitors that have shown promising activity and are moving forward in clinical development. This article focuses on the current status of immunotherapy in the management of metastatic RCC.
Keywords: Renal cell cancer, immunotherapy, interleukin, tumor immunity
High-dose interleukin-2 (IL-2) and interferon were the most commonly administered therapies before the recent introduction of targeted agents, including vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathway inhibitors. Although the new agents result in a progression-free survival benefit, high-dose IL-2 remains the only agent with proven efficacy in producing durable complete and partial responses in patients with metastatic renal cell carcinoma (RCC). Furthermore, although the use of single-agent interferon has decreased significantly since the introduction of targeted therapy, it remains in the frontline setting in combination with bevacizumab as a result of 2 large phase III trials.1 Lastly, improved understanding of immune regulation has led to the advancement of targeted immunotherapy using immune checkpoint inhibitors that have shown promising activity and are moving forward in clinical development. This article focuses on the current status of immunotherapy in the management of metastatic RCC.
The incidence of RCC is on the rise, with an annual incidence of 58,000 cases as of 2010.2 RCC comprises a group of tumors arising from the epithelium of the kidney. The predominant types of kidney cancer include clear cell RCC (75%), papillary RCC (15%), chromophobe type (5%), and oncocytic type tumors. Furthermore, clear cell RCC, the most common and extensively studied histologic subtype, is characterized in most cases by distinctive features, including Von Hippel-Lindau (VHL) genetic and epigenetic silencing, resulting in accumulation of hypoxia-inducible factor, production of various proangiogenic growth factors, dependence on new blood vessel formation, and, finally, host immune dysfunction. In localized disease, surgery remains the preferred therapeutic modality for cure. In the metastatic setting, however, systemic treatment approaches are multifaceted and include immunotherapeutic approaches, such as IL-2, and signal transduction inhibitors, such as VEGF-targeted therapies and mTOR inhibitors.3–11
Role of Immunotherapy in RCC
The clinical application of immunotherapy became widely accepted after the significance of immune dysfunction that occurs in RCC became understood. Prior reports of spontaneous regression of small metastases after the removal of primary tumors have long suggested that immune-mediated approaches may be feasible. As documented by many studies thereafter, RCC is a unique malignancy in that it impairs tumor immunity through indirectly effecting the proliferation of T-regulatory (Treg) cells and myeloid-derived suppressor cells, and shifting the Th-1/Th-2 bias toward a proinflammatory status.12–14 Significant factors that lead to impaired immunity include the expression of B7-H1 and B7-H4 on the tumors, which, upon binding their receptors, act as negative regulators of T-cell–mediated immunity.15,16 The following section discusses the data on high-dose IL-2, interferon and other immunomodulatory approaches currently being studied.
High-Dose IL-2
For many investigators, high-dose IL-2 remains a valuable option for select patients with metastatic RCC because of its ability to produce durable responses. The FDA approved high-dose IL-2 in 1992 for use in metastatic RCC after several clinical trials showed durable complete responses in up to 7% of patients. No trial has been conducted to show a survival advantage of IL-2, but the durability of patient response has been recognized and has warranted continued use of high-dose IL-2, especially in patients with low-volume disease, good performance status (ECOG 0 or 1 or Karnofsky performance status 70–100), and lung/mediastinal-only disease.17 It is administered as infusion of 600,000 IU/kg every 8 hours over 15 minutes on days 1 through 5 and 15 through 19 every cycle; 2 to 3 cycles are usually considered for patients who experience response. One cycle allows no more than 28 doses (or 14 doses in 5 consecutive days). The challenges of therapy include expertise in administering the treatments and close monitoring of the cardiopulmonary status.18–20
Various other agents have been used for stimulating the tumor immunity. However, the most consistent results have been produced by high-dose IL-2, with the most recent reports noting response rates nearing 28%.4 Patients who had experienced response (complete or partial) to high-dose IL-2 had a median duration of response of approximately 19 months.3 Durable complete responses were noted in up to 7% of patients with metastatic RCC after the use of high-dose IL-2, and these patients were progression-free at a median of 30 months.3,21
Furthermore, the clinical behavior of RCC varies with histology and subtypes. Sarcomatoid dedifferentiation is identified as an aggressive phenotype that portends poorer outcome. A small retrospective study conducted by Cangiano et al.22 showed that treatment with high-dose IL-2 in sarcomatoid patients was associated with improved survival compared with surgery alone or other types of immunotherapy (P = .025). In contrast, a retrospective study by Upton et al.23 involving papillary RCC and granular features showed lack of benefit from high-dose IL-2 in these subsets of patients.
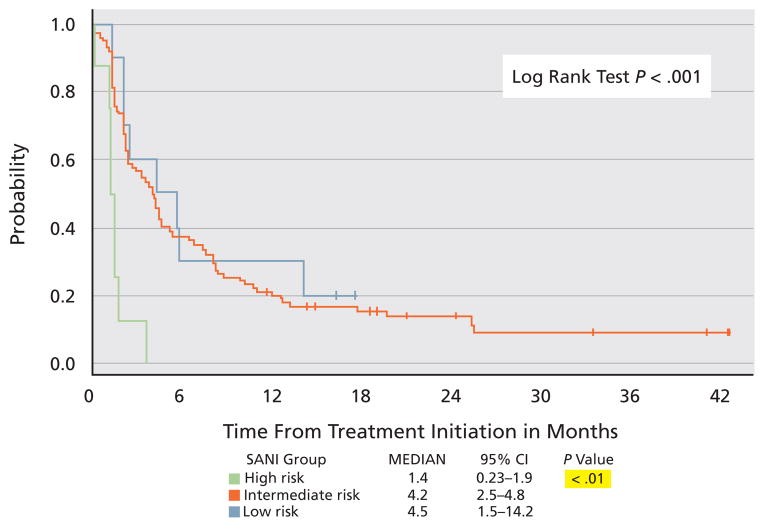
The most recent study involving high-density IL-2 was the SELECT trial, which was a multicenter, nonrandomized, phase II study that attempted to improve its therapeutic index. The study involved 120 patients with predominantly clear cell RCC (96%) and hypothesized that the response rate to high-dose IL-2 in a preselected population with good pathologic predictive features (> 50% alveolar features and no papillary or granular features) would be superior to that of a historical unselected population.23,24 Most patients had intermediate Memorial Sloan-Kettering Cancer Center (MSKCC) risk factors (94%) or an intermediate or good University of California, Los Angeles (UCLA) Survival After Nephrectomy and Immunotherapy (SANI) score, and 99% underwent prior nephrectomy.25 IL-2 was administered in a standard fashion. The toxicity profile was as expected, including 2 treatment-related deaths. This study reported a response rate of 28% (22% partial and 6% complete; Table 1), with a median progression-free survival of 4.2 months (Figure 1). Surprisingly, response to IL-2 was not associated with any pretreatment clinical factors nor was it seen in patients with non–clear cell histology and high UCLA SANI score. Additional analyses from this trial are ongoing in search of biomarker of response to IL-2 that can be validated in subsequent studies.
Table 1.
Response by Baseline Characteristics
| All Patients (n = 120) | RR (95% CI) 25% (18%–34%) |
P Value* .0014 |
|---|---|---|
| Tumor Type | ||
| Clear cell (n = 115) | 26% (18%–35%) | .33 |
| Non-clear cell (n = 5) | 0% (0%–52%) | |
| MSKCC Risk Group | ||
| Favorable (n = 21) | 23% (7%–44%) | .89 |
| Intermediate (n = 81) | 25% (16%–36%) | |
| Poor (n = 18) | 31% (9%–61%) | |
| UCLA Risk Group | ||
| Low (n = 10) | 20% (3%–56%) | .27 |
| Intermediate (n = 101) | 27% (19%–37%) | |
| High (n = 8) | 0% (0%–37%) | |
Abbreviations: MSKCC, Memorial Sloan-Kettering Cancer Center; RR, relative risk; UCLA, University of California, Los Angeles.
Courtesy of David McDermott, MD, Boston, MA. Presented at the 2010 ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois.
Figure 1.
Progression-free survival according to University of California, Los Angeles Survival After Nephrectomy and Immunotherapy risk group.
Abbreviation: Int, intermediate.
Courtesy of David McDermott, MD, Boston, MA. Presented at the 2010 ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois.
The therapeutic armamentarium for metastatic RCC considerably expanded in the past several years with the introduction of targeted agents.5,6,8–10,26,27 Currently, however, definitive recommendations on the optimal sequence of administering high-dose IL-2 and various signal transduction inhibitors remain elusive, mostly because of the lack of available data. Reports have shown that patients who receive IL-2 after anti-VEGF therapies experience increased toxicities, particularly cardiac-related.28 Alternatively, targeted agents after cytokine therapy have been shown to be safe. The TARGET trial of sorafenib, which was conducted in patients who experienced progression after first-line cytokines, including interferon and IL-2, reported no major increased toxicity in patients who had received prior IL-2 (42%).5 Furthermore, sunitinib was found to be similarly efficacious and safe in phase II studies conducted in patients with cytokine-refractory RCC.29,30 Similarly, pazopanib was also tested to safe in a large phase III trial that included patients with RCC who were treatment-naïve and those who were cytokine-refractory.27 Based on these results, and because increased toxicities have been reported in patients treated with IL-2 after exposure to VEGF tyrosine kinase inhibitors, it may be worthwhile to consider IL-2 as a first-line therapy in patients who meet the clinical and pathologic criteria for this treatment.
Lastly, preclinical and clinical studies are ongoing to enhance the immune response mediated by IL-2. For example, one combination strategy is to decrease the inhibitor effects of Tregs with the use of histone deacetylase inhibitors in conjunction with high-dose IL-2 (ClinicalTrials.gov identifier: NCT01038778).31–33
Interferon
Historically, interferon was the standard frontline option in the treatment of metastatic RCC. Interferon as a single agent had been associated with a small proportion of patients with RCC experiencing clinical benefit, with median survival reaching 13 months.34–37 In a retrospective analysis of 460 patients from various trials who received interferon α, Motzer et al.31 observed that the median time to progression was 4.7 months and the median overall survival was 13 months. This study was able to identify the risk factors associated with poor outcome (MSKCC risk factors), which are widely used today for prognostication purposes in patient counselling and clinical trial design.35 As the de facto standard frontline therapy, interferon logically became the default standard for comparison in first-line trials that led to the approval of 3 targeted agents in RCC,6,9,26 including the phase III trials of sunitinib and temsirolimus. Because of the improved progression-free survival seen in the experimental arms, the use of interferon has been largely replaced by targeted agents in the frontline setting. However, interferon remains part of the armamentarium in the frontline treatment of metastatic RCC in combination with bevacizumab, a regimen that has shown comparable efficacy to a single targeted agent in the frontline setting in 2 large phase III trials, and thus was approved as such in both the United States and the European community.5,6,8–10,26,27 However, the toxicity profile of the combination was significant, leading to higher drop-out rates in the combination arm.
Experimental Approaches to RCC Immunology
Vaccines and Stem Cell Transplant
The role of reduced-intensity allogeneic stem cell transplant has been explored as an adoptive immunotherapeutic approach in patients with cytokine-refractory metastatic RCC. Most benefit was observed in patients in favorable or intermediate MSKCC risk groups. Various studies showed that transplantation induced a long-term disease-free interval in a small fraction of patients.38,39 The development of a graft-versus-tumor effect is thought to be the reason for response among the patients who showed clinical benefit.40–42 Various vaccine-based approaches are being explored in RCC in the adjuvant and meta-static settings. The mechanisms explored include vaccine strategies against tumor-associated antigens of RCC and tumor cell lysate along with activated dendritic cells.43–46 Several studies are ongoing and results are awaited (ClinicalTrials.gov identifiers: NCT01265368, NCT00862303, NCT00004880).
Other Cytokines and Immunomodulatory Approaches
Other cytokines used include IL-4, IL-6, IL-12, and pegylated forms of interferon, but none has been able to show any clinically meaningful benefit from their application.47,48 Two separate trials combining interferon with sorafenib in patients with RCC showed modest improvement in progression-free survival and response rates compared with single agents (ClinicalTrials.gov identifiers: NCT00098618, NCT00101114).40 Even though sunitinib is a signal transduction inhibitor that predominantly targets VEGF signaling, some studies have shown the immunomodulatory effects of this agent in terms of suppressing myeloid-derived suppressor cells and Tregs49,50 and warrant further investigation. However, results have been disappointing, with a phase I study in combination with an immune checkpoint inhibitor tremelimumab resulting in significant renal toxicities, which led investigators to recommend against this combination.51
Immune Checkpoint Inhibitors
A new area of investigation in cancer immunotherapy involves the specific targeting of regulatory elements of the adaptive immune response. In the past decade, studies have shown that T cells recognize antigens associated with the major histocompatibility complex as the first signal, but that additional signals via coreceptors are required for optimal T-cell recognition and generation of a potent and long-lasting T-cell immune response (Figure 2). These additional signals for optimal T-cell priming involve agonist coreceptors, such as CD28, 4-1BB, and OX40, and inhibitory coreceptors, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1). Antibodies to these immunomodulatory elements (both the receptors and their ligands) have been developed, and some are moving forward in clinical testing with promising activity, especially in immunogenic tumor models such as melanoma, RCC, breast cancer, and prostate cancer. Ipilimumab is the prototypical antibody directed against CTLA-4, the most extensively studied inhibitory T-cell coreceptor. Ipilimumab has recently been approved for the treatment of patients with metastatic melanoma and studies are ongoing in RCC. PD-1, along with its ligand PD-L1, constitutes another immune inhibitory axis against which abrogating human antibodies (MDX-1106 and MDX-1105, respectively) are undergoing clinical testing.
Figure 2.
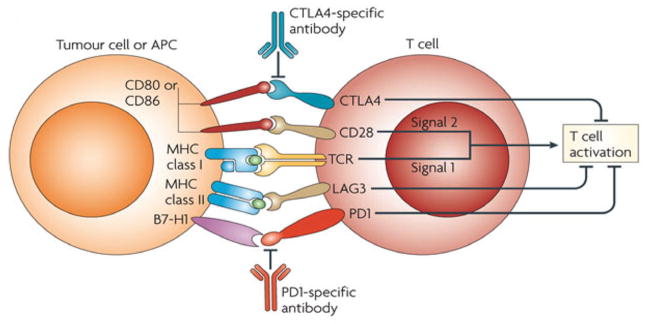
Schematic illustration of antigen-presenting cell/tumor cell and T-cell receptor interaction in T cell activation.
Abbreviation: APC, antigen-presenting cell.
From Nature Reviews: Immunology; with permission.
CTLA-4
CTLA-4 is a member of the CD28:B7 immunoglobulin superfamily and is normally expressed at low levels on the surface of T cells.52 CTLA-4 competes with CD28 (immune stimulatory coreceptor) for CD80/CD86 on antigen-presenting cells or tumors, effectively shutting off T-cell receptor signaling.53 In vitro and in vivo, T cells lacking CTLA-4 have been shown to exhibit high rates of proliferation and an activated phenotype, providing a therapeutic rationale in tumor models such as melanoma and RCC that result in host immune dysfunction partly by inhibition of T-cell activation and proliferation.54
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 that showed promising activity in several phase II studies in patients who underwent pretreatment for melanoma. This success led to a large phase III study, in which ipilimumab became the first agent to show overall survival benefit in patients with metastatic melanoma, with median survival of 10.1 versus 6.4 months against gp100 vaccine (hazard ratio, 0.66; P = .003),55 leading to FDA approval on March 25, 2011. Based on tumor regression seen in patients with melanoma in earlier studies, a phase II study was performed in patients with RCC, which showed a response rate of 13% when given at 3 mg/kg every 3 weeks.56 As suspected from preclinical studies in which CTLA-4 knockout mice developed a profound lymphoproliferative disorder,57 treatment-related toxicities were immune-mediated and occasionally serious in earlier studies, with colitis and hypophysitis being the most common. However, with diligent patient monitoring and appropriate and early institution of corticosteroids and/or immune suppressive agents, such as tumor necrosis factor–blocking agents or mycophenolate, these side effects have proven to be manageable without tempering the therapeutic response.58 Anti-CTLA4 antibody continues to be investigated in RCC in both adults and the pediatric population (ClinicalTrials.gov identifiers: NCT00378482, NCT00556881).
PD-1
PD-1 is another immune checkpoint molecule that is expressed on activated T cells and is involved in regulating the balance between immune activation and tolerance.59 It shares homology with CTLA-4 but with distinct immune-inhibitory signals. Engagement of PD-1 by its ligands PD-L1 (B7-H1) or PD-L2 (B7-H2) transduces a signal that inhibits T-cell proliferation, cytokine production, and cytolytic function.60 PD-L1, the main ligand of PD-1, was found to be aberrantly expressed on tumors, and its expression of PD-L1 on tumors correlated with the presence of tumor-infiltrating lymphocytes, and with poor clinical outcome for several cancers, including RCC.61–64 In preclinical studies, unlike CTLA-4 knockout mice, which showed significant lymphoproliferative disorder and early lethality, PD-1 knockouts showed modest late-onset strain- and organ-specific autoimmunity.65,66 A phase I clinical trial of PD-1 blockade conducted with the fully human monoclonal antibody MDX-1106 in 39 patients with advanced treatment-refractory solid tumors included 1 patient with RCC who experienced a partial response that lasted more than 16 months after a single dose of 10 mg/kg without further therapy.67 Furthermore, MDX-1106 was well tolerated, with only 1 patient among the 39 enrolled in the study experiencing a serious adverse event (inflammatory colitis). An additional phase I study evaluating the safety and efficacy of extended biweekly dosing of MDX-1106 at 1, 3, and 10 mg in patients with advanced solid tumors was conducted and results reported at the 2011 ASCO Genitourinary Cancers Symposium,68 with a special focus on RCC. Maximum tolerated dose was not reached. The most frequent drug-related adverse events were fatigue, rash, pruritus, and diarrhea. No relationship was seen between dose and frequency of adverse events. Of the 18 patients on the study, 16 were treated with 10 mg/kg. Median duration of treatment was longer than 7.6 months. The overall response rate was 31% (5/16) and 6 of the 16 patients experienced stable disease that lasted longer than 4 months (37.5%). The median duration of response was greater than 4 months. Of the 2 patients with RCC treated with 1 mg/kg, 1 obtained a complete response for longer than 12 months and 1 had stable disease for longer than 21 months.68 However, further studies involving multiple doses will need to be completed to definitively evaluate its safety and efficacy profile. Based on these encouraging results, a phase II dose-ranging study is planned. Lastly, further targeting of the PD-1/PD-L1 axis is under investigation, with anti-PD-L1 antibody (MDX1105-01) currently in the phase I stage in multiple advanced tumors, including RCC (ClinicalTrials.gov identifier: NCT00729664).
Conclusions
Immunogenicity of RCC has allowed unique treatment modalities in the past, including high-dose IL-2 and interferon, which remained the mainstay of systemic management for patients with metastatic RCC for several decades. The recent past has seen reduced use of interferon after the introduction of targeted agents, which have shown progression-free survival benefit compared with interferon in large clinical trial settings. Nevertheless, IL-2 remains the only therapeutic modality to have shown proven durable responses replicated in several large clinical trials. For high-dose IL-2, a high degree of expertise is required in patient selection and administration, which has probably limited its widespread use historically. The appropriate patient population includes individuals with excellent organ function, minimal comorbidity, and good performance status, and IL-2 should be administered in an intensive care type of setting with close evaluation before every dose. This therapy is still recommended and should be performed only at high-volume centers with expertise in choosing the appropriate patient population and managing the untoward toxicities in a systematic fashion.
The future of immunotherapy is exciting and will be determined by the outcome of trials that use newer approaches of immunotherapy, such as MDX-1106 (ClinicalTrials.gov identifiers: NCT00441337, NCT01354431). Other efforts, such as vaccine-based and IL-2 combination approaches, are ongoing and results are anxiously awaited.
Footnotes
Dr. Pili has disclosed that he is a consultant, advisory board member, and/or speakers’ bureau member for Genetech and Pfizer. Drs. George, Carducci, and Kim have disclosed that they have no financial interests, arrangements, or affiliations with the manufacturers of any products discussed in this article or their competitors.
References
- 1. [Accessed July 18, 2011.];NCCN Trends Survey - Chronic Myelogenous Leukemia, Kidney cancer, and KRAS testing. 2009 Feb; Available at: https://subscriptions.nccn.org/c-6-nccn-trends-surveys-amp-data.aspx.
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 4.McDermott DF, Ghebremichael MS, Signoretti S, et al. The high-dose aldesleukin (HD IL-2) “SELECT” trial in patients with metastatic renal cell carcinoma (mRCC) [abstract] J Clin Oncol. 2010;28(Suppl 1):Abstract 4514. [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178:1883–1887. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternberg CN, David ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 12.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatsumi T, Herrem CJ, Olson WC, et al. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–4489. [PubMed] [Google Scholar]
- 15.Krambeck AE, Thompson RH, Dong H, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of b7-h1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huland E, Heinzer H, Mir TS, Huland H. Inhaled interleukin-2 therapy in pulmonary metastatic renal cell carcinoma: six years of experience. Cancer J Sci Am. 1997;3 (Suppl 1):S98–105. [PubMed] [Google Scholar]
- 18.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24:287–293. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 22.Cangiano T, Liao J, Naitoh J, et al. Sarcomatoid renal cell carcinoma: biologic behavior, prognosis, and response to combined surgical resection and immunotherapy. J Clin Oncol. 1999;17:523–528. doi: 10.1200/JCO.1999.17.2.523. [DOI] [PubMed] [Google Scholar]
- 23.Upton MP, Parker RA, Youmans A, et al. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28:488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 24.Atkins MB. Treatment selection for patients with metastatic renal cell carcinoma: identification of features favoring upfront IL-2-based immunotherapy. Med Oncol. 2009;26(Suppl 1):18–22. doi: 10.1007/s12032-008-9148-x. [DOI] [PubMed] [Google Scholar]
- 25.Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;98:2566–2575. doi: 10.1002/cncr.11851. [DOI] [PubMed] [Google Scholar]
- 26.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzberg T, Regan MM, Liu V, et al. Retrospective analysis of interleukin-2 therapy in patients with metastatic renal cell carcinoma who had received prior antiangiogenic therapy [abstract] J Clin Oncol. 2008;26(Suppl 1):Abstract 5044. [Google Scholar]
- 29.Escudier B, Roigas J, Gillessen S, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4068–4075. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 31.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 33.Kato Y, Yoshimura K, Shin T, et al. Synergistic in vivo antitumor effect of the histone deacetylase inhibitor MS-275 in combination with interleukin 2 in a murine model of renal cell carcinoma. Clin Cancer Res. 2007;13:4538–4546. doi: 10.1158/1078-0432.CCR-07-0014. [DOI] [PubMed] [Google Scholar]
- 34.Minasian LM, Motzer RJ, Gluck L, et al. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol. 1993;11:1368–1375. doi: 10.1200/JCO.1993.11.7.1368. [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 36.Pyrhonen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999;17:2859–2867. doi: 10.1200/JCO.1999.17.9.2859. [DOI] [PubMed] [Google Scholar]
- 37.Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet. 1999;353:14–17. [PubMed] [Google Scholar]
- 38.Bregni M, Bernardi M, Servida P, et al. Long-term follow-up of metastatic renal cancer patients undergoing reduced-intensity allografting. Bone Marrow Transplant. 2009;44:237–242. doi: 10.1038/bmt.2009.9. [DOI] [PubMed] [Google Scholar]
- 39.Barkholt L, Bregni M, Remberger M, et al. Allogeneic haematopoietic stem cell transplantation for metastatic renal carcinoma in Europe. Ann Oncol. 2006;17:1134–1140. doi: 10.1093/annonc/mdl086. [DOI] [PubMed] [Google Scholar]
- 40.Rzepecki P, Zolnierek J, Sarosiek T, et al. Allogeneic non-myeloablative hematopoietic stem cell transplantation for treatment of metastatic renal cell carcinoma — single center experience. Neoplasma. 2005;52:238–242. [PubMed] [Google Scholar]
- 41.Tykodi SS, Warren EH, Thompson JA, et al. Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens. Clin Cancer Res. 2004;10:7799–7811. doi: 10.1158/1078-0432.CCR-04-0072. [DOI] [PubMed] [Google Scholar]
- 42.Artz AS, Van Besien K, Zimmerman T, et al. Long-term follow-up of nonmyeloablative allogeneic stem cell transplantation for renal cell carcinoma: The University of Chicago Experience. Bone Marrow Transplant. 2005;35:253–260. doi: 10.1038/sj.bmt.1704760. [DOI] [PubMed] [Google Scholar]
- 43.Schwaab T, Schwarzer A, Wolf B, et al. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and IFN-{alpha}2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res. 2009;15:4986–4992. doi: 10.1158/1078-0432.CCR-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amato RJ, Shingler W, Goonewardena M, et al. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother. 2009;32:765–772. doi: 10.1097/CJI.0b013e3181ace876. [DOI] [PubMed] [Google Scholar]
- 45.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 46.Tatsumi T, Kierstead LS, Ranieri E, et al. MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin Cancer Res. 2003;9:947–954. [PubMed] [Google Scholar]
- 47.McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer. 2009;115(10 Suppl):2298–2305. doi: 10.1002/cncr.24236. [DOI] [PubMed] [Google Scholar]
- 48.Dutcher J, Atkins MB, Margolin K, et al. Kidney cancer: the Cytokine Working Group experience (1986-2001): part II. Management of IL-2 toxicity and studies with other cytokines. Med Oncol. 2001;18:209–219. doi: 10.1385/MO:18:3:209. [DOI] [PubMed] [Google Scholar]
- 49.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 50.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 51.Rini BI, Stein M, Shannon P, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117:758–767. doi: 10.1002/cncr.25639. [DOI] [PubMed] [Google Scholar]
- 52.Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 53.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 54.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 55.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang JC, Hughes M, Kammla U, et al. Ipilimumab (Anti-CTLA4 Antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 58.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitisi in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and tis ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from the host immune system and tumor immunotherpay by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 62.Krambeck AE, Dong H, Thompson RH, et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749–1756. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
- 63.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death ligand 1 and tumor infiltrating CD8+ T lymphocytes are prognostic factors in human ovarian cancer. Proc Natl Acad Sci USA. 2007;194:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hino R, Kabashinma K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 65.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 67.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDermott DF, Drake CG, Sznol M, Sosman JA. A phase I study to evaluate safety and antitumor activity of biweekly BMS-936558 (Anti-PD-1, MDX-1106/ONO-4538) in patients with RCC and other advanced refractory malignancies [abstract] J Clin Oncol. 2011;29(Suppl 7):Abstract 331. [Google Scholar]