Abstract
OBJECTIVES
It is unclear whether postoperative salvage radiation therapy (SRT) and early adjuvant radiotherapy (ART) after radical prostatectomy lead to equivalent long-term tumor control. We studied a group of patients undergoing ART by comparing them with a matched control group undergoing SRT after biochemical failure.
METHODS
Using a multi-institutional database of 2299 patients, 449 patients with pT3-4N0 disease were eligible for inclusion, including 211 patients receiving ART and 238 patients receiving SRT. Patients were matched in a 1:1 ratio according to preoperative prostate-specific antigen Gleason score, seminal vesicle invasion, surgical margin status, and follow-up from date of surgery.
RESULTS
A total of 192 patients were matched (96:96). The median follow-up was 94 months from surgery and 73 months from RT completion. There was a significant reduction in biochemical failure with ART compared with SRT. The 5-year freedom from biochemical failure (FFBF) from surgery was 75% after ART, compared with 66% for SRT (hazard ratio [HR] [H11005] 1.6, P [H11005] .049). The 5-year FFBF from the end of RT was 73% after ART, compared with 50% after SRT (HR [H11005] 2.3, log rank [LR] P [H11005] .0007). From the end of RT, SRT and Gleason score ≥8 were independent predictors of diminished FFBF. From the date of surgery, Gleason score ≥8 was a significant predictor of FFBF.
CONCLUSIONS
Early ART for pT3-4N0 prostate cancer significantly reduces the risk of long-term biochemical progression after radical prostatectomy compared with SRT. Gleason score ≥8 was the only factor on multivariate analysis associated with metastasic progression.
Postoperative radiotherapy (RT) after radical pros-tatectomy (RP) for clinically localized prostate cancer remains controversial. Adverse pathology after surgery, such as positive surgical margins, extracapsular extension of disease, or seminal vesicle invasion, increases the likelihood of disease recurrence and micro scopic metastatic disease. Patient selection and accurate disease staging remains difficult, with concerns about overtreatment of patients never destined to fail, or undertreatment of patients with unrecognized distant metastatic disease. The appropriate timing of postoperative RT, either early in the adjuvant setting, or after prostate- specific antigen (PSA) recurrence in the salvage setting, remains unclear. It is assumed, but unproven, that timely salvage RT offers the same ultimate benefit in long-term freedom from biochemical failure as RT given in the early adjuvant setting. Randomized studies addressing this assumption have not been performed.
To determine the appropriate use of postoperative RT after RP, we created a multi-institutional database of postoperative patients receiving RT. Our hypothesis was that early adjuvant RT (ART) offered improved freedom from biochemical failure compared with delayed salvage RT (SRT) after RP. We performed a matched pair analysis comparing ART and SRT, derived from the pooled multi-institutional database, to investigate whether early ART improved freedom from biochemical failure.
MATERIAL AND METHODS
We collected a multi-institutional patient database of RP patients receiving postoperative RT from 17 centers in North America. We obtained institutional review board approval at all centers. Patients at high risk for disease recurrence (pT3-T4N0) were selected for this study before any analysis of the database. Patients with complete data, including presurgical tumor parameters, pathologic and surgical information, postoperative PSA measurements, RT information, and post-RT PSA measurements were eligible for inclusion. Eligibility for inclusion was restricted to patients with an undetectable PSA level (PSA <0.2 ng/mL) in the immediate postoperative period. Patients with a PSA after RP that did not nadir to an undetectable level after surgery were excluded. We divided patients into those receiving early ART (within 12 months of surgery) and those receiving SRT after PSA recurrence (at least 1 year after surgery). Patients receiving SRT with a pre-RT PSA >2 ng/mL were excluded from analysis, as were patients receiving adjuvant or neoadjuvant hormonal therapy. Patients from both groups were matched in a 1:1 fashion, based on disease parameters and length of follow-up from date of surgery. Matching variables included presurgical PSA value, surgical Gleason score, patient age, surgical margin status, the presence of seminal vesicle invasion (SVI), and the postoperative RT dose administered. We compared the probability of freedom from biochemical failure (FFBF), metastasis-free progression, and overall survival between groups. We defined biochemical failure after postoperative RT as a PSA >0.2 ng/mL and rising.
Statistical Analysis
We compared continuous and categorical demographic and treatment characteristics using two-sample t-tests and [H9273]2 statistics, respectively. We used nonparametric Wilcoxon statistics to validate parametric findings of the two-sample t-test. We calculated univariate estimates of biochemical failure using the Kaplan-Meier method and made comparisons in overall profiles on the basis of the log-rank statistic. We based stepwise multivariate analysis on the Cox proportional hazards model and the following covariates: age (continuous), treatment (SRT vs ART), preoperative PSA (continuous), margin status (positive vs negative), RT dose (continuous), SVI (positive vs negative), Gleason score (2-7 vs 8-10), and pathologic stage (T3 vs T4).
Patient Characteristics
We collected data on 2299 patients receiving postoperative RT after RP between 1987 and 2002. Of these patients, a total of 449 patients were eligible for inclusion. All eligible patients had complete data available for analysis, including preoperative tumor parameters, pathologic and surgical information, and postoperative and post-RT PSA determinations. All had an undetectable PSA in the immediate postoperative period (PSA <0.2 ng/mL), and had pT3-T4N0 disease on final surgical pathology. A total of 211 patients had early ART, and 238 had delayed SRT. The median PSA level before RT in the SRT cohort was 0.7 ng/mL, with a range of 0.2-2 ng/mL. The median postoperative RT dose administered was 64 Gy (range, 50-70 Gy).
A total of 192 patients were matched and included for analysis, in a 1:1 ratio of SRT to ART, with 96 receiving SRT and 96 receiving ART. The surgical Gleason scores of the patients included in the study were similar to the entire postoperative patient cohort, but there were slightly higher rates of adverse pathologic features in the study patients. Patients were matched for follow-up from date of surgery, with a median duration of 94 months from surgery, and 73 months from time of postoperative RT. Table 1 lists the characteristics of the matched patients. There were no significant differences between matched arms for patient age or tumor parameters (PSA, Gleason, SVI, and surgical margin status), but patients in the SRT arm received a slightly higher median RT dose (64.8 Gy vs 60.0 Gy, P < .0001), as might be expected.
Table 1.
Characteristics of matched patients
| Salvage (n = 96) No. (Range or %) | Adjuvant (n = 96) No. (Range or %) | |
|---|---|---|
| Age (y [median]) | 63.0 (47-75) | 62.0 (42-76) |
| Preop PSA (ng/dL) | ||
| Median | 9.0 (1.7-39) | 8.3 (1.1-65.9) |
| ≤10 | 55 (57%) | 56 (58%) |
| >10 | 41 (43%) | 40 (42%) |
| Gleason score | ||
| 2-6 | 22 (23%) | 22 (23%) |
| 7 | 57 (59%) | 57 (59%) |
| 8-10 | 17 (18%) | 17 (18%) |
| Seminal vesicle invasion | 23 (24%) | 23 (24%) |
| Surgical margin status | ||
| Positive | 80 (83%) | 80 (83%) |
| Negative | 16 (17%) | 16 (17%) |
| Length of follow-up (mon [median]) | ||
| From date of surgery | 94 (26-190) | 97 (30-207) |
| From date of RT | 58 (1-126) | 90 (24-200) |
| Radiation dose (Gy [median]) | 64.8 (59-70) | 60 (50-70)* |
Preop PSA = preoperative prostate-specific antigen; RT = radiation therapy.
P < .0001. No other statistical differences were found in the tumor or treatment parameters.
RESULTS
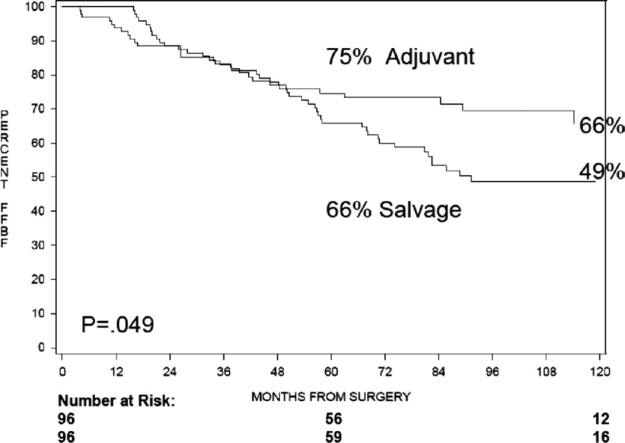
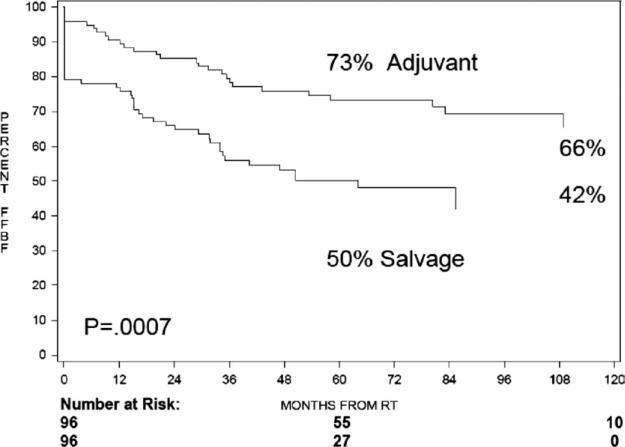
The actuarial 5-year Kaplan-Meier freedom from biochemical failure (FFBF), from the date of surgery, was 75% after ART compared with 66% for those undergoing SRT at time of biochemical failure (hazard ratio [HR] = 1.6, 95% confidence interval [CI] 1.0-2.6, log ratio [LR] P = .049), as shown in Figure 1. The 5-year Kaplan-Meier FFBF, from the end of RT, was 73% after ART compared with 50% after SRT (HR = 2.3, 95% CI = 1.4-3.8, LR P = .0007) (Fig. 2). The median FFBF after SRT was 89 months from time of surgery and 51 months from end of RT. The median FFBF for ART has not yet been reached in either cohort. On multivariate analysis, SRT (HR = 2.4, 95% CI = 1.5-3.8, P = .0005) and Gleason score ≥8 (HR = 2.5, 95% CI = 1.5-4.2, P = .0006) were independent predictors of increased biochemical failure after RT. On multivariate analysis from the end of surgery, Gleason score ≥8 was a significant predictor of BF (HR = 2.6, 95% CI = 1.5-4.4, P = .0003). Multivariate analysis for the development of distant metastatic disease, from both the end of RT and the date of surgery, revealed Gleason score ≥8 as a significant predictor. Other variables that were evaluated, including patient age, surgical margin status, preoperative PSA, Gleason score <, and timing of RT (ART vs SRT), were not independently predictive of biochemical failure or distant metastasis.
Figure 1.
Five-year actuarial freedom from biochemical failure, from date of surgery.
Figure 2.
Five-year actuarial freedom from biochemical failure, from end of RT.
COMMENT
Emerging evidence from the European Organization for Research and Treatment of Cancer (EORTC) Study 22911 and the Southwest Oncology Group (SWOG) 8794 trial examining early ART in postoperative patients with adverse pathologic features has shown significant improvements in freedom from biochemical failure at 5 and 10 years.1,2 Patients assigned to ART also appear less likely to develop metastatic disease with long-term follow-up.3 In addition, a secondary analysis of the SWOG 8794 trial demonstrated that disease recurrence was more likely to be within the prostatic fossa in patients assigned to initial observation.4 Other studies suggest that locally recurrent disease tends to undergo dedifferentiation to a more aggressive phenotype with the passage of time.5 Taken together, these observations suggest that there may be a benefit to achieving the highest possible local tumor control during the initial phase of treatment, in the form of postoperative radiotherapy. These studies confirm earlier retrospective observations of high-risk patients, for whom early ART showed benefit in decreasing biochemical failure.6-12 Randomized trials comparing ART and SRT after RP with adverse pathologic features have not been performed, but several single-institution retrospective studies have demonstrated improved biochemical control with ART.13-15
This study attempts to mimic a randomized trial through case matching in a retrospective multi-institutional cohort, and demonstrates a significant reduction in biochemical failure for ART compared with SRT. Using a different matching system, incorporating propensity scores to match 339 patients, similar results were found, with SRT patients significantly more likely to experience biochemical failure (unpublished).
Putatively, ART would benefit high-risk patients by giving additional local therapy to microscopic residual disease when the tumor burden is smallest. This is consistent with the finding that SRT is more effective when given earlier, with several studies indicating that pre-RT PSA levels <2 ng/mL,16 <0.6 ng/mL,17 or <0.5 ng/mL had the highest chance for FFBF.18 Presumably, earlier RT allows treatment of a smaller burden of disease, and may explain the improved outcomes noted when SRT is given with a low pre-RT PSA level. A nomogram predicting response to SRT after RP, recently published by our postprostatectomy radiotherapy consortium, demonstrated that pre-RT PSA was a significant predictor of FFBF, even with PSA levels as low as 0.2 ng/mL.18 The authors postulated that pre-RT PSA correlated with disease burden, with lower PSA values indicating lower tumor burden and higher chances for durable response. Carrying this postulate further, treatment in an adjuvant setting, with an undetectable PSA, presumably represents the lowest disease burden and best chance for durable treatment response.
Ideal patient selection for postoperative RT remains paramount, because the highest-risk patients also have a significant risk of microscopic metastatic disease, which would not be affected by additional local therapy. Conversely, RT for low-risk patients would likely be over-treatment, as they likely would never suffer biochemical failure. Patients with a pre-RT PSA > 2.0 were excluded from analysis, because this patient cohort has the highest likelihood of distant metastatic disease, for which additional local therapy would likely not offer benefit. Thus, this comparative analysis could be considered early ART compared with early SRT, which mimics current practice. Postoperative RT, whether ART or SRT, carries small but definite risks, including rectal and urinary toxicity,19 but it appears that both ART and SRT can be administered with relative safety after radical prostatectomy.20 As imaging modalities improve, the difficulty encountered with patient selection may be lessened.
Patients were matched for follow-up from the date of RP, which most accurately simulates the real-life decision making encountered postoperatively. Therefore, the study is biased toward improved outcomes in the SRT arm, which by definition had shorter follow-up from its completion than the ART, with necessarily shorter interval to demonstrate biochemical failure. In addition, the SRT cohort was treated with a significantly higher RT dose than the ART cohort (64.8 vs 60 Gy, respectively, P < .0001), and the median ART dose of 60 Gy in the postoperative setting has been demonstrated to be inferior than RT doses of ≥64.8 Gy.21 Despite these significant biases favoring the SRT arm, ART had a significant improvement in FFBF, highlighting the potential advantage of ART over SRT for high-risk patients.
Several potential weaknesses must be considered in an analysis of this type. The absence of a cohort of patients treated with surgery alone limits the analysis. All patients in the SRT arm had evidence of disease recurrence, whereas in the ART arm some of the patients may not have had residual disease and may not experience a biochemical failure, which alone may account for the improvement seen in the ART arm. This current analysis includes a complete group of patients treated with adjuvant therapy, but only the subset of patients who received surgery alone and subsequently developed biochemical failure. To estimate the overall biochemical relapse experience among surgery-alone patients, the Kattan postoperative nomogram22 was used to estimate the progression-free probability among patients with a similar demographic profile as our series, and this nomogram estimated a 50% progression-free probability at 84 months. This is similar to the SRT arm of our study, confirming a high risk of biochemical recurrence in this cohort.
The retrospective design of the study may allow significant bias or the introduction of unknown confounding variables. The case-matching strategy attempted to minimize these limitations, but subtle differences in practice pattern may not be evident or measurable in a retrospective series such as this one. The durable FFBF benefit was significant only on univariate analysis from date of surgery, and only just reached statistical significance. The Gleason score was an overwhelmingly strong predictor for long-term outcome, and we theorize that the Gleason score obscured other potential important covariates such as the timing of RT delivered (ART vs SRT). Other studies have demonstrated that patients with predominant Gleason pattern 4 (Gleason 4+3 = or higher) have poorer responses to ART or SRT.18 Also, the benefits observed were for biochemical failure only, and other clinically significant end points such as freedom from metastasis, and cancer-specific or overall survival were not significantly affected on multivariate analysis.
One of the strengths of this analysis rests on the centers that participated and whose patients comprise the multi-institutional postoperative RT database. In general, these treatment centers have strong multidisciplinary genitourinary oncology programs, with close collaboration between the surgeons and the radiation oncologists.23 Thus, although the study was retrospective and details of the reason for referral for SRT are not known, it is likely that patients were referred relatively promptly. Despite this, we observed an advantage for ART over SRT.
CONCLUSIONS
Postoperative RT after RP may benefit patients at high risk of local or delayed systemic relapse. The benefit appears strongest for those patients with Gleason scores < 8. The timing of postoperative RT remains controversial, because randomized trials to determine the incremental benefit of early ART compared with delayed SRT given for biochemical failure have not been performed. This multi-institutional, retrospective case matched comparison of early compared with delayed postoperative RT demonstrates a significant improvement in biochemical freedom of progression with early treatment. This study, combined with the recent SWOG and EORTC studies, highlights the need for a randomized clinical trial to confirm whether RP patients will benefit from ART, and whether there is any detriment to delayed SRT administered for postoperative biochemical failure.
References
- 1.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radio-therapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Tangen CM. Advanced prostate cancer and postoperative radiotherapy: reply. JAMA. 2007;297:951. doi: 10.1001/jama.297.9.950-a. [DOI] [PubMed] [Google Scholar]
- 4.Swanson GP, Hussey MA, Tangen CM, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–2229. doi: 10.1200/JCO.2006.09.6495. [DOI] [PubMed] [Google Scholar]
- 5.Grossfeld GD, Olumi AF, Connolly JA, et al. Locally recurrent prostate tumors following either radiation therapy or radical pros-tatectomy have changes in Ki-67 labeling index, p53 and bcl-2 immunoreactivity. J Urol. 1998;159:1437–1443. doi: 10.1097/00005392-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Valicenti RK, Gomella LG, Ismail M, et al. Pathologic seminal vesicle invasion after radical prostatectomy for patients with prostate carcinoma: effect of early adjuvant radiation therapy on biochemical control. Cancer. 1998;82:1909–1914, 1998. doi: 10.1002/(sici)1097-0142(19980515)82:10<1909::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Valicenti RK, Gomella LG, Ismail M, et al. The efficacy of early adjuvant radiation therapy for pT3N0 prostate cancer: a matched-pair analysis. Int J Radiat Oncol Biol Phys. 1999;45:53–58. doi: 10.1016/s0360-3016(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 8.Anscher MS, Prosnitz LR. Postoperative radiotherapy for patients with carcinoma of the prostate undergoing radical prostatectomy with positive surgical margins, seminal vesicle involvement and/or penetration through the capsule. J Urol. 1987;138:1407–1412. doi: 10.1016/s0022-5347(17)43656-1. [DOI] [PubMed] [Google Scholar]
- 9.Zietman AL, Coen JJ, Shipley WU, et al. Adjuvant irradiation after radical prostatectomy for adenocarcinoma of prostate: analysis of freedom from PSA failure. Urology. 1993;42:292–298. doi: 10.1016/0090-4295(93)90618-k. discussion 298-299. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RP, Cole BS, Richardson RG, et al. Adjuvant radiotherapy following radical prostatectomy: results and complications. J Urol. 1986;135:65–68. doi: 10.1016/s0022-5347(17)45519-4. [DOI] [PubMed] [Google Scholar]
- 11.Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52:1034–1040. doi: 10.1016/s0090-4295(98)00405-1. [DOI] [PubMed] [Google Scholar]
- 12.Anscher MS, Clough R, Robertson CN, et al. Timing and patterns of recurrences and deaths from prostate cancer following adjuvant pelvic radiotherapy for pathologic stage T3/4 adenocarcinoma of the prostate. Prostate Cancer Prostatic Dis. 2006;9:254–260. doi: 10.1038/sj.pcan.4500903. [DOI] [PubMed] [Google Scholar]
- 13.Morris MM, Dallow KC, Zietman AL, et al. Adjuvant and salvage irradiation following radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 1997;38:731–736. doi: 10.1016/s0360-3016(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy JF, Catalona WJ, Hudson MA. Effect of radiation therapy on detectable serum prostate specific antigen levels following radical prostatectomy: early versus delayed treatment. J Urol. 1994;151:1575–1578. doi: 10.1016/s0022-5347(17)35305-3. [DOI] [PubMed] [Google Scholar]
- 15.Taylor N, Kelly JF, Kuban DA, et al. Adjuvant and salvage radio-therapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56:755–763. doi: 10.1016/s0360-3016(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 17.Katz MS, Zelefsky MJ, Venkatraman ES, et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical pros-tatectomy for prostate cancer. J Clin Oncol. 2003;21:483–489. doi: 10.1200/JCO.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung C, Cookson MS, Chang SS, et al. Toxicity following high-dose salvage radiotherapy after radical prostatectomy. BJU Int. 2006;99:529–533. doi: 10.1111/j.1464-410X.2006.06661.x. [DOI] [PubMed] [Google Scholar]
- 20.Feng M, Hanlon AL, Pisansky TM, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1417–1423. doi: 10.1016/j.ijrobp.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Valicenti RK, Gomella LG, Ismail M, et al. Effect of higher radiation dose on biochemical control after radical prostatectomy for PT3N0 prostate cancer. Int J Radiat Oncol Biol Phys. 1998;42:501–506. doi: 10.1016/s0360-3016(98)00270-3. [DOI] [PubMed] [Google Scholar]
- 22.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–1507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 23.Valicenti RK, Gomella LG, El-Gabry EA, et al. The multidisciplinary clinic approach to prostate cancer counseling and treatment. Semin Urol Oncol. 2000;18:188–191. [PubMed] [Google Scholar]