Abstract
The blood–brain-barrier (BBB) limits the penetration of many systemic antineoplastic therapies. Consequently, many agents may be used in clinical studies and clinical practice though they may not achieve therapeutic levels within the tumor. We sought to compile the currently available human data on antineoplastic drug concentrations in brain and tumor tissue according to BBB status. A review of the literature was conducted for human studies providing concentrations of antineoplastic agents in blood and metastatic brain tumors or high-grade gliomas. Studies were considered optimal if they reported simultaneous tissue and blood concentration, multiple sampling times and locations, MRI localization, BBB status at sampling site, tumor histology, and individual subject data. Twenty-Four studies of 19 compounds were included. These examined 18 agents in contrast-enhancing regions of high-grade gliomas, with optimal data for 2. For metastatic brain tumors, adequate data was found for 9 agents. Considerable heterogeneity was found in the measurement value, tumor type, measurement timing, and sampling location within and among studies, limiting the applicability of the results. Tissue to blood ratios ranged from 0.054 for carboplatin to 34 for mitoxantrone in high-grade gliomas, and were lowest for temozolomide (0.118) and etoposide (0.116), and highest for mitoxantrone (32.02) in metastatic tumors. The available data examining the concentration of antineoplastic agents in brain and tumor tissue is sparse and limited by considerable heterogeneity. More studies with careful quantification of antineoplastic agents in brain and tumor tissue is required for the rational development of therapeutic regimens.
Keywords: Brain tumor, Tissue concentration, Antineoplastic agent, Microdialysis, Blood–brain-barrier
Introduction
The systemic administration of antineoplastic agents for malignancies of the central nervous system (CNS) assumes that these agents are able to reach the target tissue. For this to occur, they must move from the blood, through the blood–brain-barrier (BBB) and extracellular fluid to reach the malignant glial cells. However, many factors, including the BBB, interstitial pressure, and efflux pumps may limit the distribution of systemic therapies into brain and tumor tissue [1–4]. Cerebral spinal fluid (CSF) is often used as a surrogate marker of brain tissue or extra-cellular fluid (ECF) concentration, however, significant differences between the BBB and the blood-CSF barrier severely limit the accuracy of this approximation [5, 6]. Despite this knowledge, little data on the distribution of antineoplastic drugs within the human brain tissue have been published. Consequently, many agents may be studied in clinical trials and used in clinical practice without evidence of therapeutic CNS concentrations.
The intact BBB is thought to selectively inhibit agents of high molecular weight (MW), strong negative charge, and low lipid solubility [7]. In addition, efflux transporters actively extrude some agents from the brain [8–10]. Predictive models of BBB penetration have utilized these factors and others with variable success [3, 11–13]. Various authors have outlined the molecular factors that reliably predict penetration of the intact BBB. These include a small number of nitrogen and oxygen atoms (<5 is optimal), MW less than 400–500, a small number of hydrogen bond donors or acceptors (<8–10 is optimal), a log P (ratio of solute concentration in octanol relative to water) between 1.5 and 2.7, a log D (ratio of solute concentrations in octanol relative to water at a specified pH) between 1 and 3 (at physiological pH), and a small polar surface area (<60–70 Å2) [7, 11, 14]. Recent work has also identified physical size >11 nm as a potentially limiting factor in the diffusion of agents across the tumour BBB [15]. The ability of an agent to penetrate into brain tissue is therefore dependent on these inter-related factors.
In malignant glioma and other disease states, disruption of the BBB occurs via various mechanisms [16]. This can be measured qualitatively by contrast-enhanced computed tomography (CT) scan and magnetic resonance imaging (MRI) [17]. BBB disruption may allow for molecules with a wider range of properties to pass into the brain extra-cellular fluid (ECF) or alter the distribution of those known to cross [18]. Conversely, some agents, such as dexamethasone or bevacizumab, may restore the function of the BBB, also potentially altering concomitant drug pharmacokinetics [19, 20]. An understanding of the relative penetration of antineoplastic agents in various settings is therefore required for prudent selection of agents for further clinical investigation.
In recent years, several methods have been applied to measure drug levels in vivo (Table 1) [2, 3, 21–23]. In humans, the most common techniques are surgical tissue sampling and microdialysis, though the latter has been introduced to human studies relatively recently. Microdialysis (MD) involves the surgical implantation of a small catheter into specific regions of interest [24]. The catheter samples the ECF compartment, which is proposed to be the most predictive of active drug concentration and is not confounded with intravascular or intracellular concentrations [25]. In addition, it allows for repeated or continuous sampling of the brain ECF. The advantages of this technique are the definite in vivo localization (confirmed with imaging of the radio-opaque membrane tip) and the opportunity for multiple measurements, which enables detailed assessment of brain ECF pharmacokinetics. Brain and tumor tissue removed at the time of surgery (ST) provide the opportunity to measure drug concentrations directly. This technique is preferred for drugs with long half-lives that are at steady-state; however, it is limited by providing a single measurement time point, contamination of the measurement from the intravascular and intracellular compartments, and difficulty in determining a specific sample location in relation to BBB permeability. Finally, autopsy specimens have also been used to measure tissue concentration, however, significant variability may occur from time of drug administration to time of death, and simultaneous measurement of serum concentrations is not possible. Novel approaches to drug measurement in vivo take advantage of conventional imaging techniques, recently demonstrated in rodents with doxorubicin covalently linked to gadolinium and imaged with MRI, but do not allow for direct quantitative measurement [22]. There has thus far been little consideration about the ability of a drug to access the tissue of interest prior to advancing drugs to clinical trials in neuro-oncology. However, there is now increasing recognition of techniques that allow measurement of drug in human brain and there is increasing data about brain concentrations. We sought to compile the published data on the directly measured concentrations of antineoplastic agents in the human brain and brain tumor tissue.
Table 1.
Methods of drug concentration measurement in brain tissue
| Method | Pros | Cons |
|---|---|---|
| Microdialysis | Potentially very accurate and
reproducible Continuous/repeated measurement Allows simultaneous blood measurement Well localized |
Invasive Complex set-up Requires sensitive assays |
| Surgical tissue | Allows simultaneous blood
measurement Greater potential availability since surgical resection is standard practice |
Invasive Single measurement Poorly localized Vascular contamination |
| Imaging | Non-invasive Allows repeated measurement Allows simultaneous blood measurement Well localized |
Limited number of available
compounds Indirect measurement |
| Autopsy | No increased risk to patient | Not suitable for compounds with short
half-lives No simultaneous blood measurement Consent to autopsy |
Materials and methods
A review of the literature was performed to find all human studies reporting concentrations of antineoplastic agents in blood and high grade (grade III or IV) glioma (HGG) or tumor tissue metastatic to brain. Tumor concentration measurements were restricted to surgical tissue or microdialysis techniques, with contemporary analytical techniques of either mass spectrometry or liquid chromatograpy. Studies were identified by an electronic search of Medline from 1966 to the present. The following MeSH heading search strategy was used to search medline for relevant studies: (Antineoplastic Agents OR Antineoplastic Protocols OR Chemotherapy, Adjuvant OR Chemotherapy, Cancer, Regional Perfusion OR Drug Therapy OR Drug Therapy, Combination) AND (Brain OR Central Nervous System) AND Pharmacokinetics AND (Microdialysis OR Tissue). The New Approaches to Brain Tumor Therapy (NABTT) Consortium database was searched for studies of potential relevance. Online search of meeting proceedings from the American Society of Clinical Oncology, and the Society for Neuro-Oncology was also performed to identify data awaiting publication.
The abstracts and titles of the returned citations were searched to identify all publications reporting the concentration of an antineoplastic agent measured directly from human brain tissue or ECF. Two investigators (AD and MP) then reviewed the full texts of these studies and extracted data from those reporting a measure of brain tissue concentration. Studies reporting only cerebral spinal fluid concentrations were excluded. Where possible, studies reporting data on multiple histologic types were included if HGG or metastatic tumor data was separable from other histologies. All data included in the analysis were found in peer-reviewed publications.
Qualitative analysis was performed to determine the applicability of the study results. The following qualities were used to determine the suitability of the study for estimating the penetration of antineoplastic agents into the CNS: simultaneous brain and blood concentration measurements, multiple sampling times, multiple sampling sites, MRI localization of sampling site, tumor histology, individual subject data, multiple subjects, and BBB status at sampling site (contrast enhancing vs. no enhancement). Only studies meeting each of the above criteria were considered optimal, with data from these studies considered robust an easily interpretable. Studies not meeting the above criteria were included in the analysis but were considered qualitatively less robust, such that the lack of this data would make interpretation and comparison of the results more difficult.
Tissue to blood ratios were calculated as concentration of the agent in tumor or brain tissue divided by the simultaneous concentration of the agent in blood. Samples were considered simultaneous if they were taken within 10 min of one another or reported to be simultaneous. Where concentrations were not reported, area under the curve was used to calculate the ratio. When only a tissue to blood ratio was reported, this value was used for our analysis.
Results
Literature search retrieved 1441 studies that fit the criteria. Of these, 47 studies involving 27 antineoplastic compounds met our inclusion criteria. Adequate data was available for 17 agents in 22 studies for HGG, and 9 agents in 12 studies for metastatic tumors (Fig. 1); 10 studies contained data on both HGG and metastatic tumors [18, 26–48]. Two of these studies (Blakeley [18] and Portnow [26]) were regarded as optimal based on the criteria above.
Fig. 1.
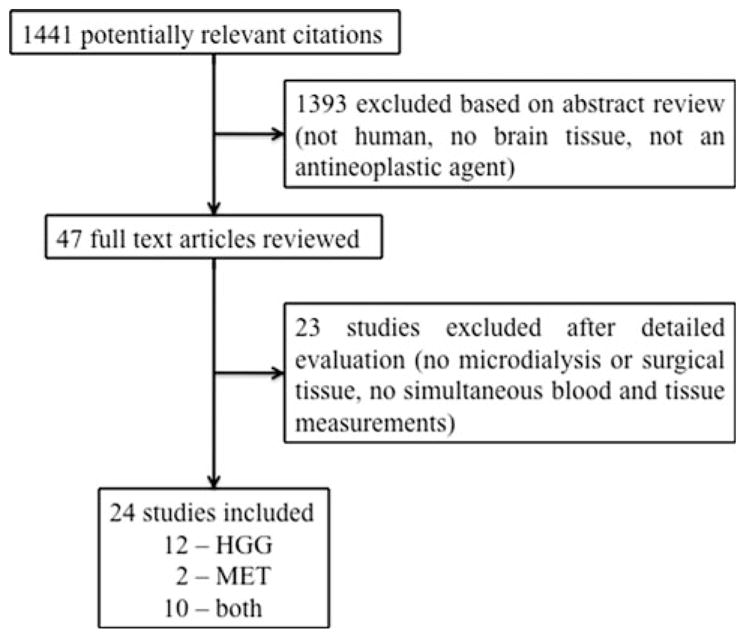
Flow diagram of study
Qualitative analysis of the included studies revealed considerable heterogeneity in the content and quality (Table 2). The measurement of tissue concentration was reported in various ways among studies, such as area under the concentration–time curve (AUC), peak concentration, simultaneous concentration, steady state concentration, and tissue to blood ratio. Blood values were reported with similar variability. The timing of blood and tumor samples relative to one another and relative to drug administration was variably reported. The dosing regimen of the agent often differed significantly within and among studies. Tissue sampling location was often reported vaguely, especially in studies of surgical tissue. This limited the ability of the investigators to classify the measurement according to location (contrast enhancing versus non-enhancing or tumor center versus tumor edge versus brain adjacent to tumor). Only microdialysis studies used precise MRI localization of tissue sampling, which enabled the determination of local BBB integrity. Finally, the patient population was often mixed within studies, including patients with low grade glioma, HGG, glioma of unspecified grade, and metastatic tumors of various histologies. Studies often reported results in this context without reporting individual patient data.
Table 2.
Qualitative summary of included studies
| Study | Drug | Simultaneous sampling | Measurement technique | Concentration parameter | Dosing | Localization method of BBB integrity | Tumor types | Multiple tumor sampling times | Individual patient data |
|---|---|---|---|---|---|---|---|---|---|
| Microdialysis | |||||||||
| Blakeley [18] | Methotrexate | Y | LC/MS | C, AUC | 12 g/m2 IV over 4 h | MRI | HGG | Y | Y |
| Portnow [26] | Temozolomide | Y | LC/MS | C, AUC | 150 mg/m2 | MRI | Mixed | Y | Y |
| Surgical tissue | |||||||||
| Shinohara [27] | Carboplatin | Y | U | C, R | U | U | HGG | Y | N |
| Whittle [28] | Carboplatin | Y | AAS | AUC | AUC 7 | Histology | HGG | Y | N |
| Gilbert [29] | Cilengitide | Y | U | C | 500 mg, 2,000 mg | U | GBM | N | N |
| Nakagawa [30] | Cisplatin | Y | AAS | C | 100 mg/m2 IV or IA immediately prior | U | Metastatic | N | Y |
| Albrecht [31] | Liposomal daunorubicin | Y | LC | C, R | 2 mg/kg, 12–50 h prior | Surgeon | Mixed | N | Y |
| Zucchetti [32] | Liposomal daunorubicin | Y | LC | C | 50 mg over 1 h 24–48 h preop | U | GBM | N | Y |
| Raizer [33] | Erlotinib | Y | LC | C, R | 150 mg OD | Surgeon | HGG | N | Y |
| Bergenheim [34] | Estramustine | Y | GC | C | 240 mg po, 12–14 h preop | U | Mixed | N | Y |
| Zucchetti [35] | Etoposide | Y | LC | C | 150 mg/m2, 1.5–12 h preop | Surgeon | Mixed | N | Y |
| Kiya [36] | Etoposide | Y | LC | C | 35–90 mg/m2, 1 h preop | U | Metastatic | N | Y |
| Stewart [37] | Etoposide | Y | LC | C | 100 mg/m2, 2–7.5 h preop | U | Mixed | N | Y |
| Hofer [38] | Gefitinib | U | LC | C | 500 mg OD for min 5 days | U | GBM | N | Y |
| Boogerd [39] | Idarubicin | Y | LC | C | 25–45 mg/m2, 15–24 h preop | U | Mixed | N | Y |
| Holdoff [40] | Imatinib | Ya | LC | C | 600 mg OD ×7 days | Surgeon | GBM | N | Y |
| Kuhn [41] | Lapatanib | Y | LC/MS | C, R | 750 mg BID ×7 days | U | GBM | N | Y |
| Shinohara [27] | Ranimustine (MCNU) | Y | LC | C, R | U | U | HGG | Y | N |
| Green [42] | Mitoxantrone | Y | LC | C, R | 5–6 mg/m2, 0.2–25.8 h preop | U | Mixed | N | Y |
| Heimans [43] | Paclitaxel | Y | LC | C | 175 mg/m2, 3.5–4 h preop | Surgeon | HGG | N | Y |
| Fine [44] | Paclitaxel | U | LC | AUC | 175 mg/m2 over 3 h, 2–3 h preop | Surgeon | Mixed | N | N |
| Whittle [45] | Tauromustine (TCNU) | Ya | LC | C | 130 mg/m2 immediately preop/intraop | U | HGG | N | Y |
| Kuhn [46] | Temsirolimus | Y | LC/MS | C, R | 170 mg (non-EIAED)-250 mg (4)(EIAED) 2.7 –5.6 h pre-surgery | Surgeon | HGG | N | N |
| van Tellingen [47] | Teniposide | Y | LC | C, R | 150 mg/m2, 2–24 h preop | U | Mixed | N | Y |
| Zucchetti [35] | Teniposide | Y | LC | C | 100–150 mg/m2, 1.5–12 h preop | Surgeon | HGG | N | Y |
| Stewart [48] | Teniposide | Y | LC | C, R | 50–100 mg/m2, 7–96 h preop | U | Mixed | N | Y |
Studies sorted alphabetically by agent name. Y yes, N no, LC liquid chromatography, GC Gas Chromatography, MS Mass Spectrometry, AAS Atomic Absorption Spectrometry, C concentration, R ratio, AUC area under the concentration–time curve, U unknown, EIAED enzyme inducing anti-epileptic drug
Samples taken within minutes of each other
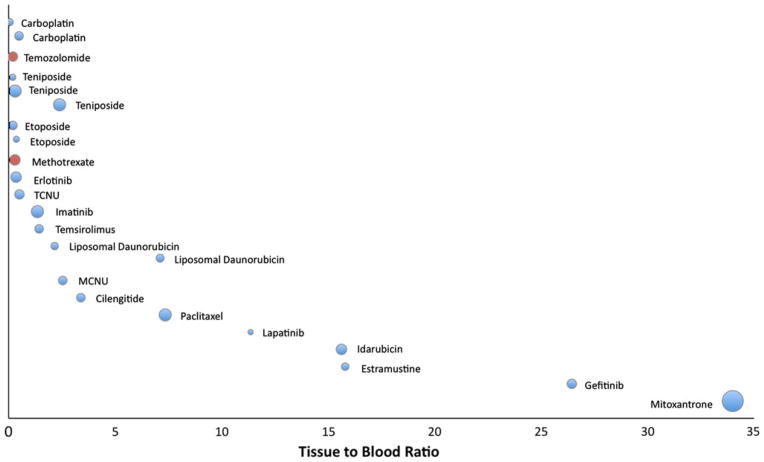
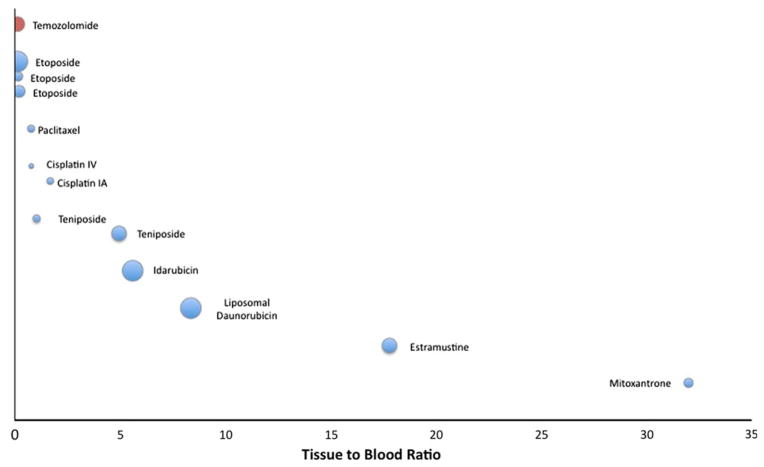
Summary data for the agents in these studies is found in Tables 3 and 4 for HGG and metastatic tumors, respectively. The MW, lipophilicity, number of nitrogen and oxygen atoms, are included as reference because of their potential importance in BBB translocation (as discussed above). Log P values were not found for the liposomal formulation of daunorubicin, therefore the Log P reported here is for the conventional parenteral daunorubicin formulation. Mean tissue to blood ratios are grouped according to sample location. Tissue to blood ratios from tumor center or contrast-enhancing areas were available from 20 studies on 18 compounds for HGG. The tissue to blood ratio from non-enhancing areas was reported in eight studies of six compounds, while four studies of three different compounds reported measurements from brain tissue distant to the tumor. A considerable range for tissue to blood ratios was found both within and among antineo-plastic agents, from a small percentage of plasma concentrations (such as 20% for temozolomide) to multiple times that found in blood (such as 34× for mitoxantrone) (Table 3). Generally, tissue to blood ratios were higher in contrast-enhancing than non-enhancing regions, and areas of brain distant to tumor. For tumors metastatic to brain, tissue to blood ratios were calculated from measurements of contrast-enhancing tumor in all 12 studies. Similar to HGG, tissue to blood ratios in the metastatic tumor group showed a wide range, from brain concentrations of 11% for etoposide to 32.02-fold that seen in blood for mitoxantrone (Table 4).
Table 3.
High grade glioma tissue concentration of agents
| Drug | MW | Lipophilicitya | N | O | Enhancing TBR | Non-enhancing TBR | Distant brain TBR | n |
|---|---|---|---|---|---|---|---|---|
| Methotrexate [18] | 454 | −2.2 | 8 | 5 | 0.295 | 0.063 | 4 | |
| Temozolomide [26] | 194 | −2.8 | 6 | 2 | 0.202 | 5 | ||
| Carboplatin [27, 28] | 371 | −2.3 | 2 | 4 | 0.488 0.054 |
0.172 | 6 9 |
|
| Cilengitide [29] | 589 | −1.4 | 8 | 7 | 3.39 | 6 | ||
| Liposomal Daunorubicin [31, 32] | 564 | 0.1b | 1 | 10 | 7.11 2.16 |
7.88 2.02 |
4.55 1.1 |
7 8 |
| Erlotinib [33] | 393 | 3.3 | 3 | 4 | 0.348 | 4 | ||
| Estramustine [34] | 440 | 5.7 | 1 | 3 | 15.8 | 8 | ||
| Etoposide [35–37] | 589 | 1 | 0 | 13 | 0.362 0.198 |
0.127 | 12 6 |
|
| Gefitinib [38] | 447 | 3.2 | 4 | 3 | 26.44 | 5 | ||
| Idarubicin [39] | 497 | 0.2 | 1 | 9 | 15.625 | 3.75 | 4 | |
| Imatinib [40] | 589 | 2.5 | 7 | 4 | 1.35 | 3 | ||
| Lapatinib [41] | 581 | 5.4 | 4 | 4 | 11.36 | 16 | ||
| Ranimustine (MCNU) [27] | 328 | −0.8 | 3 | 7 | 2.54 | 0.159 | 6 | |
| Mitoxantrone [42] | 444 | −3.1 | 4 | 6 | 34 | 1 | ||
| Paclitaxel [43, 44] | 854 | 3 | 1 | 14 | 7.35 | 2 | ||
| Tauromustine (TCNU) [45] | 287 | 0.1 | 4 | 4 | 0.506 | 0.565 | 5 | |
| Temsirolimus [46] | 1030 | 3.5 | 1 | 16 | 1.43 | 6 | ||
| Teniposide [35, 47, 48] | 657 | 1.5 | 0 | 13 | 2.39 | 3 | ||
| 0.187 0.304 |
0.029 0.194 |
11 3 |
Studies sorted alphabetically by agent name; agents with optimal studies grouped separately. TBR tissue to blood ratio, MW molecular weight rounded to nearest g mol−1, N number of nitrogen atoms, O number of oxygen atoms, n sample size; MW, N, O, log(p) data from http://pubchem.ncbi.nlm.nih.gov
Lipophilicity measured as log(p)
Chemical data shown is for daunorubicin hydrochloride, with no available data on liposomal formulation
Table 4.
Metastatic brain tumor tissue concentration of agents
| Drug | MW | Lipophilicitya | N | O | TBR | n | Primary cancer |
|---|---|---|---|---|---|---|---|
| Cisplatin [30] | 298 | −2.1939 | 2 | 0 | 0.78 1.68b |
18 9 |
Lung Lung |
| Liposomal Daunorubicin [31, 32] | 564 | 0.1c | 1 | 10 | 8.36 | 1 | Adenocarcinoma NOS |
| Estramustine [34] | 440 | 5.7 | 1 | 3 | 17.8 | 2 | Melanoma, Thyroid |
| Etoposide [35–37] | 589 | 1 | 0 | 13 | 0.116 0.155 0.199 |
1 5 3 |
Adenocarcinoma NOS Not stated Lung, Melanoma |
| Idarubicin [39] | 497 | 0.2 | 1 | 9 | 5.6 | 1 | Breast |
| Mitoxantrone [42] | 444 | −3.1 | 4 | 6 | 32.02 | 5 | Multipled |
| Paclitaxel [43, 44] | 854 | 3 | 1 | 14 | 0.77 | 8 | Lung, Melanoma |
| Teniposide [33, 45, 46] | 657 | 1.5 | 0 | 13 | 1.03 4.95 |
8 2 |
Lung, melanoma, colon Breast, Melanoma |
| Temozolomide [25] | 194 | −2.8 | 6 | 2 | 0.118 | 5 | NSCLC |
Studies sorted alphabetically by agent name. TBR tissue to blood ratio, MW molecular weight rounded to nearest g mol−1, N number of nitrogen atoms, O number of oxygen atoms, n sample size; MW, N, O, log(p) data from http://pubchem.ncbi.nlm.nih.gov unless otherwise referenced
Lipophilicity measured as log(p)
Cisplatin administered intra-arterially
Chemical data shown is for daunorubicin hydrochloride
Breast, lung, paraganglioma, teratocarcinoma
Compounds where data was available from multiple studies were grouped together, and plotted according to their contrast-enhancing tissue to blood ratio for HGG (Fig. 2) and metastatic tumors (Fig. 3). Compounds were ranked according to mean tissue to blood ratio, with sample size inversely proportional to the size of the bubbles. The antineoplastic agents with the lowest tissue to blood ratios included carboplatin, temozolomide, teniposide, etoposide, and methotrexate. Those with the highest tissue to blood ratios included mitoxantrone, gefitinib, and estramustine. Idarubicin and paclitaxel were found to have a much higher tissue to blood ratio in HGG compared to metastatic brain tumors.
Fig. 2.
Contrast-enhancing tissue to blood ratio shown graphically by individual study for HGG. Bubble size is inversely proportional to study sample size. Optimal studies represented by red bubbles. Agents ranked along vertical axis according to tissue to blood ratio; agents with multiple studies shown individually but grouped together. (Color figure online)
Fig. 3.
Contrast-enhancing tissue to blood ratio shown graphically by individual study for metastatic brain tumors. Bubble size is inversely proportional to study sample size. Optimal studies represented by red bubbles. Agents ranked along vertical axis according to tissue to blood ratio; agents with multiple studies shown individually but grouped together. IV Intravenous, IA Intra-arterial. (Color figure online)
Discussion
The literature surrounding the tissue concentrations of systemically administered antineoplastic agents in human HGG and metastatic brain tumors is sparse and heterogeneous. Few agents have published data on tissue concentration despite their routine use in clinical practice. Those with published data are studied and reported with significant variation, severely limiting the applicability of the data. There is a need for a more systematic approach to the collection and reporting of this data so that agents are chosen for clinical efficacy studies in a rational scientific manner.
The study of brain tissue concentration of antineoplastic agents is vitally important to neuro-oncology. Drugs cannot be expected to provide benefit without first reaching the target tissue, yet brain tissue concentration is only one factor determining drug efficacy. For example, the tissue to blood ratio for temozolomide was found to be quite low despite its apparent clinical utility in HGG while other drugs have very high tissue to blood ratios, such as gefitinib, and yet no clear evidence of efficacy [49, 50]. Thus, although tissue to blood ratio provides a measure of tissue concentration of these agents, the therapeutic effect also relies on other properties of the drug and its target. Furthermore, one would not expect brain tumor tissue concentrations to be homogeneous, since factors such as BBB permeability are not homogeneous. Thus measuring drug concentration at a single well-defined point may be expected to generate different results than a slightly different area of tumor or over multiple time points. Failure to achieve adequate penetration into heterogeneous portions of tumor may explain why many conventional and novel therapies with in vitro efficacy have had disappointing clinical results. This tumor heterogeneity may also help to explain the observation that idarubicin and paclitaxel had much higher tissue to blood ratios in HGG than metastatic tumors. This may difference may also indicate qualitative or quantitative differences in the BBB disruption caused by HGG and metastatic tumors. Further work evaluating these potential differences may provide many insights into the BBB, the differences between metastatic and primary brain tumors, and their clinical behavior.
Studies such as the ones reviewed here are clearly a small step towards understanding the penetration of drugs into the CNS. As novel techniques allow for more precise measurement of drug concentrations in vivo, we will begin to better understand which agents are reaching the target tissue in adequate concentrations. Future studies should include a clear statement about the location of tissue concentration sampling in relation to contrast-enhancing areas on MRI. Sampling from contrast enhancing and non-enhancing areas, and in the setting of BBB-active agents will greatly improve the understanding of tissue penetration in relation to BBB integrity. Such data may ultimately inform the most effective timing and use of combination therapy, particularly when considering the potentially mixed effects of radiotherapy, angiogenesis inhibitors and other agents on BBB integrity [19, 51].
Although we designed our search strategy to be comprehensive, it should be noted that it did not include grey literature, and as such may have missed some pharmaceutical industry studies. The impact of this is likely to be small given that neurosurgical tissue specimens or MD catheter implantation in patients would have likely occurred at academic institutions and therefore be published in peer-reviewed journals. We also specifically excluded animal studies to reduce the heterogeneity of the results, although the importance of animal studies in this area cannot be overstated, particularly for agents not currently approved for human use. We are not aware of in vivo data to suggest that animal and human models are directly comparable, and of the studies retrieved by our search strategy, no such comparison was found. In addition, controversy exists as to the appropriate measure of tissue concentration. Our analysis found many different methods are used to report this single measure. In order to attempt a standardized comparison, we calculated or used the reported tissue to blood ratio, however, the tissue to blood ratio itself provides a global picture of many processes, and its relationship to therapeutic efficacy is not clear [52]. Furthermore, the vast majority of studies only reported tissue concentrations on which to base a tissue to blood ratio calculation. The use of area under the concentration–time curve to calculate tissue to blood ratios may be more informative as a single measure by accounting for the exposure time course of a particular drug, both those reaching steady-state and those given intermittently, but was rarely reported. Our requirement of multiple sampling times for optimal studies was meant be inclusive of studies where AUC was not directly reported but where an attempt had been made to consider changes in drug concentration over time. The heterogeneity in the reporting of all study details also greatly limited the current analysis, and thus clearly hinders the application of the results to clinical practice. Ideally, all studies of this nature would report on the details of drug administration, dose, and route, the timing with respect to sampling, the tissue type, a precise location of the sample in relation to BBB integrity, and use a standardized unit of measure such as the area under the concentration–time curve in both tumor tissue and blood. This requires sampling at multiple time points but would provide significantly more detail. As more studies of this nature are published, reporting more of these details with individual patient data and sampling from multiple tumor locations would provide meaningful data on which to base rational decisions for clinical trials and clinical practice, thereby allowing neuro-oncologists to plan treatment strategies in an evidence-based manner.
We have evaluated the existing literature on brain tumor and tissue concentrations of antineoplastic agents in humans. Further work to characterize the penetration of antineoplastic agents in the human CNS is clearly required. Techniques that allow for accurate localization, multiple sampling times, and that facilitate the study of tissue concentrations in various clinical settings are preferred. This may include microdialysis, surgical tissue, and novel methods of direct and indirect measurement of drug concentration. As additional data becomes available, it may be beneficial to develop an online public database to provide a forum for freely accessibly and searchable data. Such an effort would minimize duplication, guide new studies towards agents without data, improve reporting standards, and allow investigators to extract information on potential compounds of interest to ultimately design rational clinical efficacy studies.
Contributor Information
Marshall W. Pitz, Email: pitz@cc.umanitoba.ca, University of Manitoba, 675 McDermot Avenue, Winnipeg, MB R3E 0V9, Canada. CancerCare Manitoba, 675 McDermot Avenue, Winnipeg, MB R3E 0V9, Canada
Arati Desai, University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19104, USA.
Stuart A. Grossman, Johns Hopkins University School of Medicine, Suite 1M-16, 1550 Orleans Street, Baltimore, MD 21232, USA
Jaishri O. Blakeley, Johns Hopkins University School of Medicine, Suite 1M-16, 1550 Orleans Street, Baltimore, MD 21232, USA
References
- 1.Motl S, Zhuang Y, Waters CM, Stewart CF. Pharmacokinetic considerations in the treatment of CNS tumours. Clin Pharmacokinet. 2006;45:871. doi: 10.2165/00003088-200645090-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cecchelli R, Berezowski V, Lundquist S, et al. Modelling of the blood-brain barrier in drug discovery, development. Nat Rev Drug Discov. 2007;6:650. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 3.Abbott N. Prediction of blood-brain barrier permeation in drug discovery from in vivo, in vitro, in silico models. Drug Discov Today Technol. 2004;1:407. doi: 10.1016/j.ddtec.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 5.Collins J, Dedrick R. Distributed model for drug deliver to CSF and brain tissue. Am J Physiol Regul Integr Comp Physiol. 1983;245:R303. doi: 10.1152/ajpregu.1983.245.3.R303. [DOI] [PubMed] [Google Scholar]
- 6.de Lange E, Danhof M. Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood, brain. Clin Pharmacokinet. 2002;41:691. doi: 10.2165/00003088-200241100-00001. [DOI] [PubMed] [Google Scholar]
- 7.Pardridge W. The blood-brain barrier: bottleneck in brain drug development. NeuroRX J Am Soc Exp NeuroTher. 2005;2:3. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller DS, Bauer B, Hartz AMS. Modulation of P-glyco-protein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nies A. The role of membrane transporters in drug delivery to brain tumors. Cancer Lett. 2007;254:11. doi: 10.1016/j.canlet.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression, function in the central nervous system. Pharmacol Rev. 2006;58:140. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- 11.Norinder U, Haeberlein M. Computational approaches to the prediction of the blood-brain distribution. Adv Drug Deliv Rev. 2002;54:291. doi: 10.1016/s0169-409x(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 12.Winkler D, Burden F. Modelling blood-brain barrier partitioning using Bayesian neural nets. J Mol Graph Model. 2004;22:499. doi: 10.1016/j.jmgm.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Basak S, Gute B, Drewes L. Predicting blood-brain transport of drugs: a computational approach. Pharm Res. 1996;13 doi: 10.1023/a:1016064003554. [DOI] [PubMed] [Google Scholar]
- 14.Clark DE. In silico prediction of blood-brain barrier permeation. Drug Discov Today. 2003;8:927. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 15.Sarin H. Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors. J Transl Med. 2009;7 doi: 10.1186/1479-5876-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groothuis D, Vick N. Brain tumors and the blood-brain barrier. Trends Neurosci. 1982;5 [Google Scholar]
- 17.Essig M, Weber M, Von Tengg-Kobligk H, et al. Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging. 2006;17 doi: 10.1097/01.rmr.0000245464.36148.dc. [DOI] [PubMed] [Google Scholar]
- 18.Blakeley JO, Olson J, Grossman SA, et al. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91:51. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Pulfer S, Li S, et al. Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angio-genesis inhibitor TNP-470. Cancer Res. 2001;61:5491. [PubMed] [Google Scholar]
- 20.Claes A, Wesseling P, Jeuken J, et al. Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization. Mol Cancer Ther. 2008;7:71. doi: 10.1158/1535-7163.MCT-07-0552. [DOI] [PubMed] [Google Scholar]
- 21.Bickel U. How to measure drug transport across the blood-brain-barrier. NeuroRX J Am Soc Exp NeuroTher. 2005;2:15. doi: 10.1602/neurorx.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarin H, Kanevsky A, Wu H, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80. doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alavijeh M, Palmer A. Measurement of the pharmacokinetics, pharmacodynamics of neuroactive compounds. Neurobiol Dis. 2009;37:38. doi: 10.1016/j.nbd.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Q, Gallo J. In vivo microdialysis for PK, PD studies of anticancer drugs. AAPS J. 2005;7:E659. doi: 10.1208/aapsj070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blakeley J. Drug delivery to brain tumors. Curr Neurol Neurosci Rep. 2008;8:235. doi: 10.1007/s11910-008-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portnow J, Badie B, Chen M, et al. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinohara C, Matsumoto K, Kuriyama M, et al. Clinical pharmacokinetics of carboplatin, MCNU. Gan to kagaku ryoho. Cancer Chemother. 1994;21:1163. [PubMed] [Google Scholar]
- 28.Whittle IR, Malcolm G, Jodrell DI, Reid M. Platinum distribution in malignant glioma following intraoperative intravenous infusion of carboplatin. Br J Neurosurg. 1999;13:132. doi: 10.1080/02688699943871. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert M. Tumor tissue delivery of celingitide after intravenous administration to patients with recurrent glioblastoma (GBM): preliminary data from NABTC protocol 03-02. Neurooncol. 2007;9:525. [Google Scholar]
- 30.Nakagawa H, Fujita T, Izumoto S, et al. Cis-diamminedi-chloroplatinum (CDDP) therapy for brain metastasis of lung cancer. I. Distribution within the central nervous system after intravenous, intracarotid infusion. J Neurooncol. 1993;16:61. doi: 10.1007/BF01324836. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht KW, Hamer PCdW, Leenstra S, et al. High concentration of Daunorubicin and Daunorubicinol in human malignant astrocytomas after systemic administration of liposomal Daunorubicin. Journal of Neuro-Oncology. 2001;53 doi: 10.1023/a:1012287212388. [DOI] [PubMed] [Google Scholar]
- 32.Zucchetti M, Boiardi A, Silvani A, et al. Distribution of daunorubicin, daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother Pharmacol. 1999;44:173. doi: 10.1007/s002800050964. [DOI] [PubMed] [Google Scholar]
- 33.Raizer JJ, Abrey L, Lassman AB, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas, nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12:95. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergenheim AT, Gunnarsson PO, Edman K, et al. Uptake, retention of estramustine, the presence of estratmustine binding protein in malignant brain tumours in humans. Br J Cancer. 1993;67:358. doi: 10.1038/bjc.1993.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zucchetti M, Rossi C, Knerich R, et al. Concentrations of VP16, VM26 in human brain tumors. Ann Oncol. 1991;2:63. doi: 10.1093/oxfordjournals.annonc.a057826. [DOI] [PubMed] [Google Scholar]
- 36.Kiya K, Uozumi T, Ogasawara H, et al. Penetration of etoposide into human malignant brain tumors after intravenous, oral administration. Cancer Chemother Pharmacol. 1992;29:339. doi: 10.1007/BF00686001. [DOI] [PubMed] [Google Scholar]
- 37.Stewart DJ, Richard MT, Hugenholtz H, et al. Penetration of VP-16 (etoposide) into human intracerebral, extracerebral tumors. J Neurooncol. 1984;2:133. doi: 10.1007/BF00177899. [DOI] [PubMed] [Google Scholar]
- 38.Hofer S, Frei K. Gefitinib concentrations in human glioblastoma tissue. J Neurooncol. 2007;82:175. doi: 10.1007/s11060-006-9257-3. [DOI] [PubMed] [Google Scholar]
- 39.Boogerd W, Tjahja IS, Sandt MMvd, Beijnen JH. Penetration of idarubicin into malignant brain tumor tissue. J Neurooncol. 1999;44:65. doi: 10.1023/a:1006335517191. [DOI] [PubMed] [Google Scholar]
- 40.Holdoff M, Supko J, Gallia GL, et al. Intratumoral concentrations of imatinib after oral administration in patients with glioblastoma multiforme. J Neurooncol. 2009;97:241. doi: 10.1007/s11060-009-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn JG. Tumor sequestration of lapatinib. Neuro-oncol. 2008;10:783. [Google Scholar]
- 42.Green RM, Stewart DJ, Hugenholtz H, et al. Human central nervous system, plasma pharmacology of mitoxantrone. J Neurooncol. 1988;6:75. doi: 10.1007/BF00163544. [DOI] [PubMed] [Google Scholar]
- 43.Heimans JJ, Vermorken JB, Wolbers JB, et al. Paclitaxel (TAXOL) concentrations in brain tumor tissue. Ann Oncol. 1994;5:951. doi: 10.1093/oxfordjournals.annonc.a058736. [DOI] [PubMed] [Google Scholar]
- 44.Fine RL, Chen J, Balmaceda C, et al. Randomized study of paclitaxel, tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res. 2006;12:5770. doi: 10.1158/1078-0432.CCR-05-2356. [DOI] [PubMed] [Google Scholar]
- 45.Whittle IR, MacPherson JS, Miller JD, Smyth JF. The disposition of TCNU (tauromustine) in human malignant glioma: phamacokinetic studies, clinical implications. J Neurosurg. 1990;72:721. doi: 10.3171/jns.1990.72.5.0721. [DOI] [PubMed] [Google Scholar]
- 46.Kuhn JG, Chang SM, Wen PY, et al. Pharmocokinetic and tumor distribution characteristics of temsirolimus. Clin Cancer Res. 2007;13:7401. doi: 10.1158/1078-0432.CCR-07-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Tellingen O, Boogerd W, Nooijen WJ, Beijnen JH. The vascular compartment hampers accurate determination of teniposide penetration into brain tumor tissue. Cancer Chemother Pharmacol. 1997;40:330. doi: 10.1007/s002800050665. [DOI] [PubMed] [Google Scholar]
- 48.Stewart DJ, Richard MT, Hugenholtz H, et al. Penetration of teniposide (VM-26) into human intracerebral tumors. J Neurooncol. 1984;2:315. doi: 10.1007/BF00178114. [DOI] [PubMed] [Google Scholar]
- 49.Stupp R, Mason WP, Bent MJ, et al. Radiotherapy plus concomitant, adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 50.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96:1047. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vulpen Mv, Kal HB, Taphoorn MJ, Sharouni SYE. Changes in blood-brain barrier permeability induced by radio-therapy: implications for timing of chemotherapy? Oncol Rep. 2002;9:683. [PubMed] [Google Scholar]
- 52.Martin I. Prediction of blood-brain barrier penetration: are we missing the point? Drug Discov Today. 2004;9:161. doi: 10.1016/S1359-6446(03)02961-1. [DOI] [PubMed] [Google Scholar]