Abstract
In newly diagnosed aggressive non-Hodgkin lymphoma (NHL), a positive midtreatment fluorine-18 fluorodeoxyglucose positron emission tomography (PET) scan often carries a poor prognosis, with reported 2-year event-free survival (EFS) rates of 0% to 30% after standard therapy. To determine the outcome of early treatment intensification for midtreatment PET-positive disease, a phase II trial of risk-adapted therapy was conducted. Fifty-nine newly diagnosed patients, 98% with B cell lymphoma, had PET/CT performed after 2 or 3 cycles of first-line chemotherapy. Those with negative PET on semiquantitative visual interpretation completed standard therapy. Those with positive PET received platinum-based salvage chemotherapy, high-dose therapy, and autologous stem cell transplantation (ASCT). Midtreatment PET was positive in 33 (56%); 28 received ASCT with an actuarial 2-year EFS of 75% (95% confidence interval, 60%–93%). On intention-to-treat analysis, 2-year EFS was 67% (53%–86%) in all PET-positive patients and 89% (77%–100%) in PET-negative patients. No association was found between the International Prognostic Index category and the midtreatment PET result. The favorable outcome achieved here in historically poor-risk patients warrants further, more definitive investigation of treatment modification based on early PET scanning.
Keywords: Lymphoma, Positron emission tomography (PET), Prognosis, Autologous stem cell transplantation
INTRODUCTION
Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET), when performed after only 2 to 3 cycles of first-line chemotherapy, is highly prognostic in aggressive non-Hodgkin lymphoma (NHL)[1–5]. A positive midtreatment PET during initial therapy has been retrospectively associated with 2-year progression-free or event-free survival (EFS) rates of 0% to 30%, compared with 72% to 93% if the midtreatment scan is negative [2,3,5]. The prognostic significance of midtreatment PET has been repeatedly recognized without the use of biopsy [1–7]. How to utilize the prognostic information provided by PET is not established. We report one of the first clinical trials of risk-adapted therapy for aggressive NHL based on early PET.
Study outcomes of autologous stem cell transplantation (ASCT) as part of initial therapy have been mixed [8,9]. Early ASCT is controversial, but appears to benefit the subset with poor-risk features [8,10,11], defined traditionally by the International Prognostic Index (IPI) [12]. For example, in a randomized study, overall survival (OS) was significantly greater after CHOP (cyclophosphamide [Cy], vincristine, doxorubicin, prednisone) plus ASCT than after CHOP alone in patients with high-intermediate risk disease [8]. Midtreatment PET may be a stronger and more individualized prognostic tool than pretreatment indices [2,3]. We hypothesized that a suboptimal metabolic response, as identified by midtreatment PET, is a reflection of chemoresistance and an indication for treatment intensification. Thus, we investigated whether early intensification with platinum-based chemotherapy then ASCT could change the natural history of midtreatment PET-positive disease, recognizing that comparison of phase II data with historic data cannot produce definitive results. We also felt it to be important to explore practical aspects of PET interpretation and management, and to evaluate this novel strategy in a phase II setting before considering a large-scale randomized trial.
METHODS
Study Design
A phase II study (J0348) was activated at Johns Hopkins in February 2004, and 59 patients accrued through April 2007. The study was approved by the institutional review board, and all participants gave informed consent. The study accrued patients aged ≥18 years with measurable, aggressive NHL who had received no more than 3 cycles of standard first-line chemotherapy. To maximize feasibility, patients were permitted to join after treatment had been initiated. The original protocol permitted diffuse large B cell, follicular grade 3, and peripheral T cell lymphomas. The study was later limited to diffuse large B cell lymphoma because the others were infrequent. Primary central nervous system, transformed, and human immunodeficiency virus (HIV)-associated lymphomas were excluded. Pathology materials in all cases were reviewed by members of the Johns Hopkins Division of Hematopathology.
All who joined had midtreatment PET/CT in addition to conventional restaging. A baseline PET scan was not required, but when available was used for comparison. Midtreatment PET/CT was performed between days 11 and 20 of cycle 2 or 3 using a dedicated fusion PET/CT scanner at Johns Hopkins, acquiring 3-dimensional images in 2-dimensional mode. After a minimum 4-hour fast, 18F-FDG (typically 0.22 mCi/kg) was injected intravenously provided that the glucose was <200 mg/dL. Following an approximately 60-minute uptake phase, CT for attenuation correction and lesion localization was performed [13]. Emission data were then acquired from the base of the skull to the mid-femurs (5 to 7 bed positions, 5 minutes per position). Both CT attenuation corrected and noncorrected images were available.
One of 2 nuclear medicine specialists evaluated all PET scans for study purposes. The PET was interpreted qualitatively and the result dichotomized as “negative” (no evidence of malignant disease) or “positive” (focal or diffuse uptake in an area suspicious for a residual or new focus of malignancy) [2]. Within this designation, tumor FDG uptake relative to mediastinal blood pool structures was graded on a 5-point scale: 0, no tumor activity (cold); 1+, minimal (less than background); 2+, equivocal (equal to background); 3+, moderate (greater than background); 4+, intense (much greater than background) [14]. Scores of 3+ or 4+ were considered positive.
Patients with negative midtreatment PET completed the remaining standard therapy, without early ASCT. In the absence of progression, those with positive midtreatment PET received 2 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, cisplatin) or ICE (ifosfamide, carboplatin, etoposide) with rituximab added for B cell tumors, then stem cell collection during the second cycle followed by high-dose therapy and ASCT. Two patients with positive midreatment PET received an extra cycle of R-CHOP for logistic reasons before changing therapies. Biopsies of abnormally FDG avid areas were not performed. Radiation therapy after ASCT was permissible. A PET/CT was repeated 4 to 6 weeks following chemotherapy completion, prior to any radiation.
High-Dose Therapy
Eligibility requirements for ASCT included absence of clinically evident disease progression; ECOG performance status ≤2; neutrophils >1000/mm3, platelets ≥75,000/mm3, creatinine ≤2.0 mg/dL, and bilirubin ≤2.0 mg/dL (unless because of Gilbert’s disease or lymphoma) prior to mobilization; and adequate cardiac and pulmonary function.
Autografts were derived from peripherally mobilized stem cells or, if the yield was <2 × 106 CD34+ cells/kg ideal body weight, from bone marrow harvest (n = 2). Stem cells were mobilized with filgrastim 10 mg/kg/day s.c. begun after the second cycle of (R)ESHAP or (R)ICE. A 2- to 6-hour leukapheresis was performed once the absolute CD34+ cell count was >10/µL. Products of ≥5 × 106 CD34+ cells/kg were positively selected for CD34 using the Isolex® system. All but 1 transplant utilized a preparative regimen consisting of busulfan (Bu; 1 mg/kg every 6 hours for 4 days, with dose adjustments based on pharmacokinetic calculations), followed by Cy (50 mg/kg/day for 4 days) [15]. Filgrastim was given from day 5 until neutrophil recovery.
Statistical Analysis
We estimated that a minimum of 19 transplants was required to achieve at least 85% power to detect an absolute 25% increase in 2-year EFS in midtreatment PET-positive patients, assuming a 2-year EFS of 20% historically in midtreatment PET-positive patients who did not receive early ASCT and a 1-sided type I error of 5%. EFS and OS were estimated using the Kaplan-Meier method [16]. Survival was measured from the date of first chemotherapy or from day 0 for transplant outcomes. An event was defined as relapse or progression, diagnosis of myelodysplastic syndrome (MDS) or acute leukemia, or death. Cumulative incidence of progression or relapse was estimated using a competing risks analysis, where nonrelapse deaths and secondary hematologic cancers were competing risks [17]. Prognostic factors for progression or relapse were analyzed using proportional hazards models for competing risks [18]. All P-values are 2-sided. Statistical analyses were performed with R, version 2.6.0 [19], and represent data through October 2, 2008.
RESULTS
Overall Outcomes
Baseline characteristics are shown in Table 1. Ninety-five percent of patients had large B cell lymphoma, and 97% of patients received (R)CHOP-21 with the remaining 3% receiving R-CHOP-14. Twenty-six of 59 patients (44%) were interpreted as having negative midtreatment PET and 33 (56%) as having positive midtreatment PET. Corresponding PET scan scale readings, ranging from 0 through 4+, are provided.
Table 1.
Patient Characteristics
| Variable | All patients (n = 59) |
Transplanted patients (n = 28) |
|---|---|---|
| Median age at diagnosis (range) | 53 (20–78) | 46 (21–66) |
| Male sex | 39 | 17 |
| Histology | ||
| Diffuse large B cell or large B cell | 56 (95%) | 27 (96%) |
| Primary mediastinal | 10 | 8 |
| Follicular grade 3 | 2 | 0 |
| Peripheral T cell | 1 | 1 |
| ECOG performance status | ||
| 0–1 | 44 | 19 |
| 2–4 | 15 | 9 |
| Serum lactate dehydrogenase | ||
| Normal | 18 | 9 |
| Elevated | 39 | 19 |
| Undetermined | 2 | 0 |
| Clinical stage* | ||
| I | 1 | 0 |
| II | 19 | 10 |
| III | 13 | 7 |
| IV | 26 | 11 |
| 5-point IPI score† | ||
| Low or low-intermediate | 36 | 18 |
| Midtreatment PET positive | 21 (58%) | — |
| High-intermediate or high‡ | 20 | 9 |
| Midtreatment PET positive | 11 (55%) | — |
| First-line chemotherapy | ||
| R-CHOP 21 | 56 | 26 |
| R-CHOP 14 | 2 | 1 |
| CHOP 21 | 1 | 1 |
| Timing of midtreatment PET | ||
| Cycle 2 | 20 | 9 |
| Cycle 3§ | 39 (66%) | 19 (68%) |
| Midtreatment PET result | ||
| Negative | 26 (44%) | 0 |
| 0 | 10 | |
| 1+ | 3 | |
| 2+ | 13 | |
| Positive | 33 (56%) | 28 |
| 3+ | 18 | 16 |
| 4+ | 15 | 12 |
| Salvage regimen (if PET positive) | ||
| (R)ESHAP × 2 | — | 13 |
| R-ICE × 2 | — | 12 |
| R-ESHAP × 1, R-ICE × 1¶ | — | 3 |
| Preparative regimen | ||
| Bu-Cy | — | 27 |
| Cy-TBI | — | 1 |
| Stem cell source | ||
| Peripheral blood | — | 26 |
| Bone marrow | — | 2 |
| Posttransplant PET result | ||
| Negative | — | 19 |
| 0 | 3 | |
| 1+ | 5 | |
| 2+ | 11 | |
| Positive | — | 6 |
| 3+ | 6 | |
| 4+ | 0 | |
| Undetermined^ | — | 3 |
| Radiation after ASCT | — | 4 |
| Posttransplant PET negative | 3 | |
| Posttransplant PET positive | 1 |
ASCT indicates autologous stem cell transplantation; Bu-Cy, busulfan-cyclophosphamide; Cy-TBI, cyclophosphamide and total body irradiation; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, cisplatin; ICE, ifosfamide, carboplatin, etoposide; PET, positron emission tomography; R, rituximab; IPI, international prognostic index.
Baseline bone marrow biopsy was not performed in 4 patients diagnosed as stage II, 1 as stage III, and 3 as stage IV.
Excluding patients with follicular grade 3 or T cell lymphoma.
IPI scores in this group ranged from 3 to 4.
Includes 2 patients with PET during both cycle 2 and cycle 3, with reading changed in 1; results for cycle 3 reported. Another patient received cyclophosphamide initially because of hyperbilirubinemia, with PET during cycle 3 of R-CHOP.
Because of nephotoxicity (n = 2) or gastrointestinal symptoms (n = 1).
Because of death from early progression (n = 2) or from toxicity (n = 1).
Table 2 presents the actuarial survival outcomes for all patients and separately for the 56 patients with large B cell lymphoma, of whom 32 (57%) had positive midtreatment PET. The median follow-up for all patients is 33.6 months (range: 1.3–54.5 months) and 37.2 months for surviving patients. The estimated 2-year EFS of the entire cohort is 77% and 2-year OS is 82%.
Table 2.
Actuarial Survival and Competing-Risk Progression Analysis
| Cohort | n | Event-Free Survival (95% CI) | Overall Survival (95% CI) | Cumulative Incidence of Progression or Relapse (95% CI) |
|---|---|---|---|---|
| All | 59 | 2 y: 77% (67–89), 3 y: 69% (57–83) | 2 y and 3 y: 82% (73–93) | 2 y: 18% (8–28), 3 y: 23% (11–35) |
| Midtreatment PET negative | 26 | 2 y: 89% (77–100), 3 y: 82% (66–100) | 2 y and 3 y: 92% (83–100) | 2 y: 8% (0–18), 3 y: 14% (0–31) |
| Midtreatment PET positive (intention-to-treat) | 33 | 2 y: 67% (53–86), 3 y: 59% (43–80) | 2 y and 3 y: 74% (60–91) | 2 y: 26% (10–42), 3 y: 31% (13–48) |
| Midtreatment PET positive, after transplantation | 28 | 2 y: 75% (60–93), 3 y: 65% (49–87) | 2 y: 82% (69–98), 3 y: 76% (61−96) | 2 y: 18% (3–33), 3 y: 23% (6–40) |
| Large B cell lymphoma | 56 | 2 y: 76% (65–88), 3 y: 70% (58–84) | 2 y and 3 y: 81% (72–93) | 2 y: 19% (8–30), 3 y: 22% (10–33) |
| Midtreatment PET negative | 24 | 2 y: 88% (75–100), 3 y: 80% (64–100) | 2 y and 3 y: 92% (81–100) | 2 y: 8% (0–20), 3 y: 16% (0–33) |
| Midtreatment PET positive (intention-to-treat) | 32 | 2 y: 66% (51–86), 3 y: 62% (47-83) | 2 y and 3 y: 73% (59–91) | 2 y and 3 y: 27% (11–43) |
| Midtreatment PET positive, after transplantation | 27 | 2 y: 74% (59–93), 3 y: 69% (53–90) | 2 y: 81% (68–97), 3 y: 75% (59–95) | 2 y and 3 y: 19% (4–34) |
PET indicates positron emission tomography; CI, confidence interval; y, year (s).
Outcomes of the PET-Positive Cohort
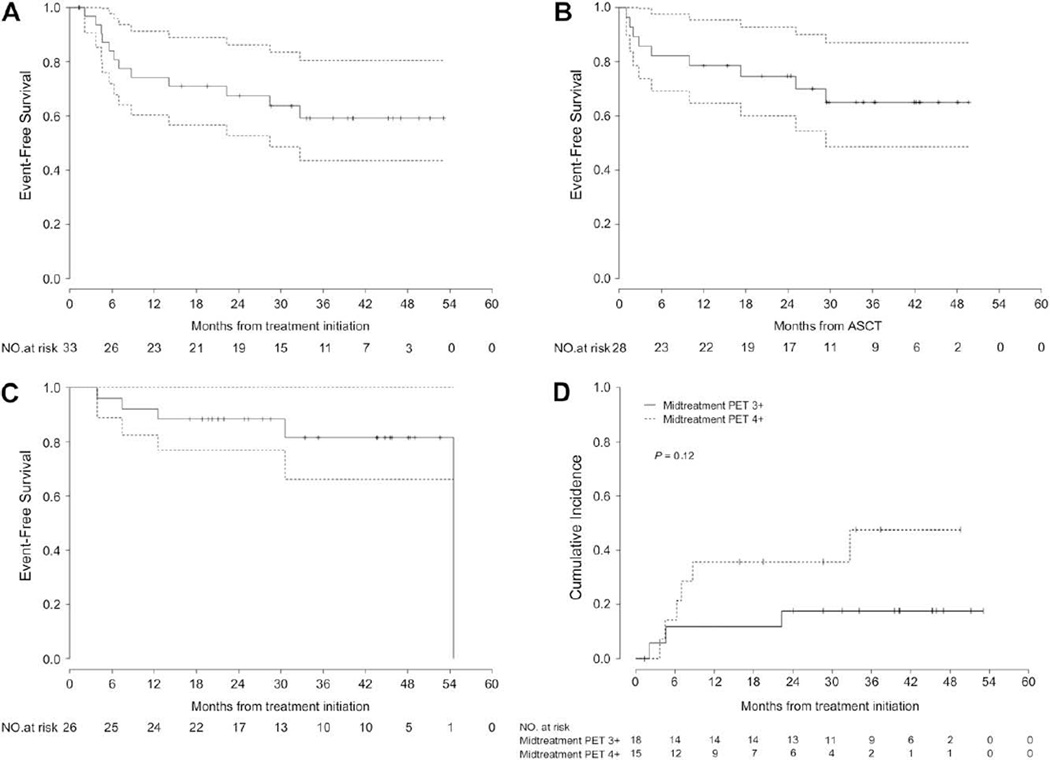
Of 33 patients with a positive midtreatment PET, 28 (85%) received ASCT. Two PET-positive patients withdrew consent and were censored on the midtreatment PET date. Three were ineligible because of disease progression prior to planned ASCT. Figure 1A presents an intention-to-treat survival analysis from the time of treatment initiation.
Figure 1.
Outcomes according to midtreatment PET result. (A) Intention-to-treat analysis of EFS of patients with positive midtreatment PET. (B) EFS of patients with positive midtreatment PET who received early transplantation. (C) EFS of patients with negative midtreatment PET. (D) Cumulative incidence of progression or relapse in all patients with positive midtreatment PET, according to PET scale score of 3+ versus 4+.
The median follow-up after ASCT is 34.1 months overall (range: 1.3–49.7 months) and 36.0 months for surviving patients. Outcomes are shown in Figure 1B and Table 2, with an estimated 2-year EFS after ASCT of 75% and estimated 3-year EFS of 65%. In PET-positive patients having a score of 4+, the 2-year EFS after ASCT is 67%.
On last assessment, 19 of 28 transplant recipients were event free. Six progressed or relapsed, including the 1 patient with T cell lymphoma, at a median of 3.2 months (range: 1.0–29.4 months). Four of these had died. There were 3 nonrelapse deaths as described shortly.
Outcomes of the PET-Negative Cohort
The estimated 2-year and 3-year EFS of patients with negative midtreatment PET are 89% and 82%, respectively (Figure 1C and Table 2). In the absence of events, all completed full-course therapy, with none receiving more than 6 chemotherapy cycles. Of the 26 PET-negative patients, 4 (15%) have had documented relapse or progression to date. Formal comparison between the PET-positive and PET-negative cohorts is not intended because of the differences in therapy.
Predictors of Outcome
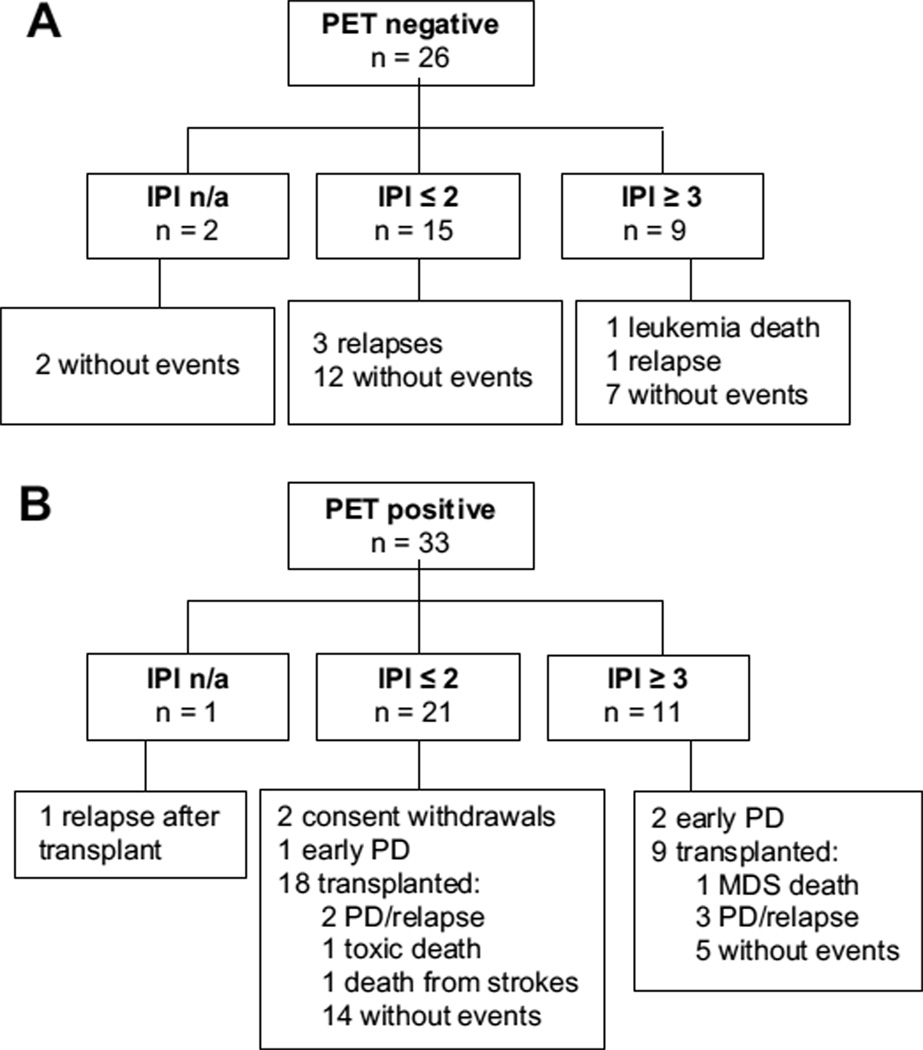
As a planned secondary analysis, we explored clinical variables at presentation in relation to the midtreatment PET result (positive versus negative). We found no association between the IPI, scored as low or low-intermediate versus high-intermediate or high, and the midtreatment PET result (P > .99). Of the 56 patients with large B cell lymphoma, 36 had an IPI ≤2, of whom 15 (42%) had negative midtreatment PET. Of 20 patients with IPI ≥3, 9 (45%) had negative midtreatment PET, of whom 7 were last event free (1 leukemia death, 1 relapse). The estimated 3-year EFS is 78% (65%–94%) with IPI ≤2 disease and 57% (37%–85%) with IPI ≥3 disease. Among midtreatment PET-positive patients, there tended to be a greater risk of progression or relapse in those with IPI ≥3 (hazard ratio [HR] 3.6; 95% confidence interval, 0.9–14.2; P = .07). Outcomes according to PET result and IPI are shown in Figure 2.
Figure 2.
Outcomes according to midtreatment PET result and International Prognostic Index (IPI). (A) Midtreatment PET-negative cohort. (B) Midtreatment PET-positive cohort. Abbreviations: MDS, myelodysplastic syndrome; n/a, not applicable; PD, progressive disease.
There was no statistically significant association found between the midtreatment PET result and either stage (I–II versus III–IV) or histology (primary mediastinal versus other large B cell lymphoma), although a larger percentage of patients with primary mediastinal lymphoma had positive PET (80% versus 52%). Patients >50 years were significantly more likely to have negative midtreatment PET (P < .01), including those with nonmediastinal large B cell lymphomas.
A planned exploratory analysis of the impact of gradations of FDG uptake on outcome was performed. We hypothesized that, within the binary designation of a “positive” or “negative” scan, the intensity of remaining FDG uptake might be prognostic. In the PET-negative group, survival curves for a midtreatment PET score of 0 or 1+ versus 2+ were superimposable (not shown); however, there were too few events to discriminate differences. Within the PET-positive group, there tended to be greater risk of progression or relapse in patients having a score of 4+ versus 3+ (Figure 1D), although the result was not statistically significant (HR 2.9, P = .13 for all histologies; HR 2.5, P = .19 for large B cell lymphoma).
Major Toxicities
In the PET-negative group, 1 died of leukemia. Of the 28 transplanted PET-positive patients, 1 died at 1.4 months of hepatic veno-occlusive disease, and another developed self-limited veno-occlusive disease; 1 died at 9.9 months from multiple strokes and pneumonia, with negative evaluations for lymphoma; and 1 developed MDS and died after nonmyeloablative allogeneic transplantation.
DISCUSSION
We report encouraging phase II results with a novel, individualized, risk-adapted strategy for newly diagnosed aggressive NHL based on early metabolic imaging. The estimated 2-year EFS of 75% (67% on intention-to-treat analysis) in midtreatment PET-positive patients suggests that early treatment intensification, as carried out in our study, may improve the outcome of this historically poor-risk group. Our data also support prior observations of the favorable prognostic significance of a negative midtreatment PET scan, with excellent outcomes to date after R-CHOP alone.
In the rituximab era, outcomes of midtreatment PET-positive patients are expected to be better than those reported historically. Midtreatment PET is, however, prognostic whether or not the regimen includes rituximab [4].
PET performed after 2 or 3 cycles of first-line chemotherapy, as done in this study, appears to be optimal for prognostication [3,4]. Most studies of the prognostic significance of PET have been based on visual (qualitative) assessments. Our criteria for a positive or negative scan are similar to the recently proposed International Harmonization Project criteria [20], which this study predated. However, FDG uptake on a PET scan is a continuous variable, and criteria for a “positive” or “negative” result have varied in the literature [3,20,21]. In this regard, the high rate of PET positivity in our patients with primary mediastinal lymphoma is notable; additional study is required to define whether the prognostic significance of PET differs in this histologic subtype. The meaning of the association between older age and having a negative midtreatment PET result is unclear in this limited dataset. We took a conservative approach to PET interpretation, with the many cases that might be regarded as “borderline” (score 2+) treated as negative. A prospective trial of PET in lymphoma response assessment is evaluating a cutoff of 1.5 times blood pool activity for differentiating between positive and negative results [22]. Our imaging analysis suggests that the intensity of residual FDG uptake may have further prognostic significance.
Biopsy of FDG avid lesions was not performed in this study. Importantly, however, midtreatment PET does not require corroboration with biopsy to be prognostic, as the consistently strong prognostic value of PET has been recognized without biopsy data [1–5,23]. Biopsy is subject to sampling error, even if guided by PET. Although false positive PET results are possible, the role of biopsy as a potential solution to that problem is not established.
Moskowitz et al. [24] recently conducted a promising phase II study of advanced diffuse large B cell lymphoma, involving dose-dense R-CHOP, midtreatment PET, then ICE, with ASCT reserved for PET-positive patients having biopsy confirmation [24]. Only 4 of 31 PET-positive patients had an abnormal biopsy. In contrast to what has been found by other groups, EFS did not significantly differ by PET result; however, all patients received treatment intensification through a change to ICE. The use of R-ICE as intensification for midtreatment PET-positive disease is being investigated in ECOG. It has additionally been suggested that the false positive rate of midtreatment PET increases after rituximab-containing therapy because of inflammation [25]. Prospective observational studies will address this question.
Despite the reasonable follow-up in this study (median 3 years after ASCT), additional follow-up will be needed to determine whether the apparent improvement in the PET-positive group is sustained. Although our results are encouraging, our single-institution study has several limitations including potential referral bias, lack of requirement for a baseline PET (which may reduce the accuracy of response assessment), and recruitment of some patients after treatment initiation. Permitting registration after treatment initiation could, however, select either for or against better-risk disease. Of note, the distribution of patients by age, stage, and IPI risk category is very similar to larger published series of patients with aggressive lymphoma [12,26]. We further examined outcome stratified by a good-risk versus poor-risk revised IPI and found similar outcomes to those reported by Sehn et al. [26] with R-CHOP. The number of patients in our study does not permit a more rigorous examination of this question.
Preemptive treatment intensification remains controversial. Results of a phase III U.S. Intergroup study comparing ASCT versus observation after full-course R-CHOP in IPI ≥2 disease should inform future approaches in this regard. In our study, 9 patients had both a high IPI and negative midtreatment PET, with generally good overall outcomes to date without treatment intensification. Although analysis is limited, this might suggest that on an individual basis, a negative midtreatment PET is reliable in high IPI disease. It may be that risk-assessment strategies incorporating both the IPI and early PET scanning will ultimately prove to be the most informative.
Our results suggest that midtreatment PET scanning is useful in guiding therapy, and that such individualized therapy is feasible. The relative contribution of ASCT compared with a platinum- and etoposide-containing salvage regimen, and to what degree early ASCT affects survival in midtreatment PET-positive patients, are ultimately phase III questions. Further investigations of individualized, risk-adapted strategies based on early metabolic imaging are warranted.
ACKNOWLEDGMENTS
Dr. Louis Diehl was involved in the study’s inception and Dr. Eric Seifter in manuscript review.
Financial disclosure: The study was supported by institutional funds from Johns Hopkins.
Footnotes
Presented in part at the 49th Annual Meeting of the American Society of Hematology, December 8–11, 2007, Atlanta, GA, and the 42nd American Society of Clinical Oncology Annual Meeting, June 2–6, 2006, Atlanta, GA.
REFERENCES
- 1.Jerusalem G, Beguin Y, Fassotte MF, et al. Persistent tumor 18F-FDG uptake after a few cycles of polychemotherapy is predictive of treatment failure in non-Hodgkin’s lymphoma. Haematologica. 2000;85:613–618. [PubMed] [Google Scholar]
- 2.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18) F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–1363. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 3.Mikhaeel NG, Hutchings M, Fields PA, et al. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–1523. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 4.Haioun C, Itti E, Rahmouni A, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–1381. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Qiao W, Wang C, et al. Therapeutic evaluation and prognostic value of interim hybrid PET/CT with (18) F-FDG after three to four cycles of chemotherapy in non-Hodgkin’s lymphoma. Hematology. 2007;12:423–430. doi: 10.1080/10245330701393840. [DOI] [PubMed] [Google Scholar]
- 6.Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood. 2007;109:486–491. doi: 10.1182/blood-2005-11-006957. [DOI] [PubMed] [Google Scholar]
- 7.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–59. doi: 10.1182/blood-2002-12-3842. [DOI] [PubMed] [Google Scholar]
- 8.Milpied N, Deconinck E, Gaillard F, et al. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004;350:1287–1295. doi: 10.1056/NEJMoa031770. [DOI] [PubMed] [Google Scholar]
- 9.Gisselbrecht C, Lepage E, Molina T, et al. Shortened first-line high-dose chemotherapy for patients with poor-prognosis aggressive lymphoma. J Clin Oncol. 2002;20:2472–2479. doi: 10.1200/JCO.2002.02.125. [DOI] [PubMed] [Google Scholar]
- 10.Greb A, Bohlius J, Trelle S, et al. High-dose chemotherapy with autologous stem cell support in first-line treatment of aggressive non-Hodgkin lymphoma—results of a comprehensive meta-analysis. Cancer Treat Rev. 2007;33:338–346. doi: 10.1016/j.ctrv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Strehl J, Mey U, Glasmacher A, et al. High-dose chemotherapy followed by autologous stem cell transplantation as first-line therapy in aggressive non-Hodgkin’s lymphoma: a meta-analysis. Haematologica. 2003;88:1304–1315. [PubMed] [Google Scholar]
- 12.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 13.Tatsumi M, Cohade C, Nakamoto Y, et al. Direct comparison of FDG PET and CT findings in patients with lymphoma: initial experience. Radiology. 2005;237:1038–1045. doi: 10.1148/radiol.2373040555. [DOI] [PubMed] [Google Scholar]
- 14.Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22:277–285. doi: 10.1200/JCO.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 15.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 20.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 21.Zijlstra JM, Comans EF, van Lingen A, et al. FDG PET in lymphoma: the need for standardization of interpretation. An observer variation study. Nucl Med Commun. 2007;28:798–803. doi: 10.1097/MNM.0b013e3282eff2d5. [DOI] [PubMed] [Google Scholar]
- 22.Kelloff GJ, Sullivan DM, Wilson W, et al. FDG-PET Lymphoma Demonstration Project Invitational Workshop. Acad Radiol. 2007;14:330–339. doi: 10.1016/j.acra.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Kostakoglu L, Goldsmith SJ, Leonard JP, et al. FDG-PET after1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer. 2006;107:2678–2687. doi: 10.1002/cncr.22276. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz C, Hamlin PA, Horwitz SM, et al. Phase II trial of dose-dense R-CHOP followed by risk-adapted consolidation with either ICE or ICE and ASCT, based upon the results of biopsy confirmed abnormal interim restaging PET scan, improves outcome in patients with advanced stage DLBCL. Blood. 2006;108 abstract 532. [Google Scholar]
- 25.Han HS, Escalon MP, Hsiao B, et al. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2008 Oct 7; doi: 10.1093/annonc/mdn629. Prepublished on-line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]