Abstract
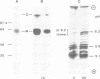
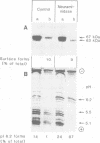
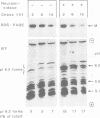
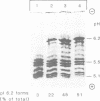
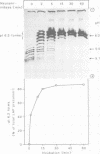
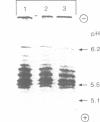
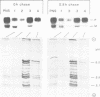
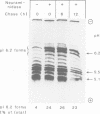
Lysosomal acid phosphatase (LAP) is transported as a transmembrane protein to dense lysosomes. The pathway of LAP to lysosomes includes the passage through the plasma membrane. LAP is transported from the trans-Golgi to the cell surface with a half-time of less than 10 min. Cell surface LAP is rapidly internalized. Most of the internalized LAP is transported back to the cell surface. On average, each LAP molecule cycles greater than 15 times between the cell surface and the endosomes before it is transferred to dense lysosomes. At equilibrium approximately 4 times more LAP precursor is present in endosomes than at the cell surface. Exposing cells to reduced temperature or weak bases such as NH4Cl, chloroquine and primaquine decreases the steady-state concentration of LAP at the cell surface. The recycling pathway is operative at greater than or equal to 20 degrees C and does not include passage of the Golgi/trans-Golgi network. LAP is transferred with a half-time of 5-6 h from the plasma membrane/endosome pool to dense lysosomes, from where it does not recycle to the endosome/plasma membrane pool at a measurable rate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chen J. W., Cha Y., Yuksel K. U., Gracy R. W., August J. T. Isolation and sequencing of a cDNA clone encoding lysosomal membrane glycoprotein mouse LAMP-1. Sequence similarity to proteins bearing onco-differentiation antigens. J Biol Chem. 1988 Jun 25;263(18):8754–8758. [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze E., Ivanov I. E., Kreibich G., Adesnik M., Sabatini D. D., Rosenfeld M. G. Endolyn-78, a membrane glycoprotein present in morphologically diverse components of the endosomal and lysosomal compartments: implications for lysosome biogenesis. J Cell Biol. 1989 May;108(5):1597–1613. doi: 10.1083/jcb.108.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draye J. P., Courtoy P. J., Quintart J., Baudhuin P. A quantitative model of traffic between plasma membrane and secondary lysosomes: evaluation of inflow, lateral diffusion, and degradation. J Cell Biol. 1988 Dec;107(6 Pt 1):2109–2115. doi: 10.1083/jcb.107.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M., Takeyasu K., Lippincott-Schwarz J., Siegel N. R. Structure of LEP100, a glycoprotein that shuttles between lysosomes and the plasma membrane, deduced from the nucleotide sequence of the encoding cDNA. J Cell Biol. 1988 Jan;106(1):61–67. doi: 10.1083/jcb.106.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Viitala J., Matteson J., Carlsson S. R. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988 Dec 15;263(35):18920–18928. [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gottschalk S., Waheed A., Schmidt B., Laidler P., von Figura K. Sequential processing of lysosomal acid phosphatase by a cytoplasmic thiol proteinase and a lysosomal aspartyl proteinase. EMBO J. 1989 Nov;8(11):3215–3219. doi: 10.1002/j.1460-2075.1989.tb08480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S., Waheed A., von Figura K. Targeting of lysosomal acid phosphatase with altered carbohydrate. Biol Chem Hoppe Seyler. 1989 Jan;370(1):75–80. doi: 10.1515/bchm3.1989.370.1.75. [DOI] [PubMed] [Google Scholar]
- Gregorou M., Rees A. R. Properties of a monoclonal antibody to epidermal growth factor receptor with implications for the mechanism of action of EGF. EMBO J. 1984 May;3(5):929–937. doi: 10.1002/j.1460-2075.1984.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C. Antibody against the insulin receptor causes disappearance of insulin receptors in 3T3-L1 cells: a possible explanation of antibody-induced insulin resistance. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2508–2511. doi: 10.1073/pnas.81.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. L., Granger B. L., Hull M., Green S. A., Gabel C. A., Helenius A., Mellman I. Derived protein sequence, oligosaccharides, and membrane insertion of the 120-kDa lysosomal membrane glycoprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7577–7581. doi: 10.1073/pnas.85.20.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Cathepsin D and beta-hexosaminidase synthesized in the presence of 1-deoxynojirimycin accumulate in the endoplasmic reticulum. J Biol Chem. 1984 Aug 25;259(16):10129–10135. [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Synthesis and transport of lysosomal acid phosphatase in normal and I-cell fibroblasts. J Biol Chem. 1985 Jul 25;260(15):9023–9030. [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Tulsiani D. R., Touster O., Yam A., Novikoff A. B. Immunocytochemical localization of alpha-D-mannosidase II in the Golgi apparatus of rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Pearse B. M. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988 Nov;7(11):3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Krentler C., Schmidt B., Schröder W., Lorkowski G., Culley J., Mersmann G., Geier C., Waheed A., Gottschalk S. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J. 1988 Aug;7(8):2343–2350. doi: 10.1002/j.1460-2075.1988.tb03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Rup D. Biosynthesis of the human asialoglycoprotein receptor. J Biol Chem. 1983 Sep 25;258(18):11249–11255. [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Viitala J., Carlsson S. R., Siebert P. D., Fukuda M. Molecular cloning of cDNAs encoding lamp A, a human lysosomal membrane glycoprotein with apparent Mr approximately equal to 120,000. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3743–3747. doi: 10.1073/pnas.85.11.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Gottschalk S., Hille A., Krentler C., Pohlmann R., Braulke T., Hauser H., Geuze H., von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J. 1988 Aug;7(8):2351–2358. doi: 10.1002/j.1460-2075.1988.tb03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Klausner R. D., Rao K., Harford J. B. Exposure of K562 cells to anti-receptor monoclonal antibody OKT9 results in rapid redistribution and enhanced degradation of the transferrin receptor. J Cell Biol. 1986 Mar;102(3):951–958. doi: 10.1083/jcb.102.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]