Abstract
Limb-girdle muscular dystrophies (LGMD) are a heterogeneous group of disorders, which has led to certain investigators disputing its rationality. The mutual feature of LGMD is limb-girdle affection. Magnetic resonance imaging (MRI), perioral skin biopsies, blood-based assays, reverse-protein arrays, proteomic analyses, gene chips and next generation sequencing are the leading diagnostic techniques for LGMD and gene, cell and pharmaceutical treatments are the mainstay therapies for these genetic disorders. Recently, more highlights have been shed on disease biomarkers to follow up disease progression and to monitor therapeutic responsiveness in future trials. In this study, we review LGMD from a variety of aspects, paying specific attention to newly evolving research, with the purpose of bringing this information into the clinical setting to aid the development of novel therapeutic strategies for this hereditary disease. In conclusion, substantial progress in our ability to diagnose and treat LGMD has been made in recent decades, however enhancing our understanding of the detailed pathophysiology of LGMD may enhance our ability to improve disease outcome in subsequent years.
Keywords: LGMD, MRI, disease biomarkers
1. Introduction
Limb-girdle muscular dystrophies (LGMD) are a group of muscular dystrophies, that until the late 1980s were identified in patients by ‘diagnosis by exclusion’. Revolutionary advances in molecular biology in the last several decades have allowed the scientific community to understand and recognize this disease more clearly. Currently, there are >25 LGMD types that have been linked to specific gene loci, and they are now estimated to constitute one third of all Duchenne muscular dystrophy cases (1).
The review is constructed to cover LGMD from a variety of viewpoints and is established on the authors’ own experience and investigations, as well as inclusive MEDLINE searches on the topics of ‘limb-girdle muscular dystrophies’, ‘review’, ‘genotype-phenotype correlations’, ‘prevention’ and ‘surveillance’. In this review, we focused on peer-reviewed studies (in English) published in key scientific journals from 1995 to date, combined with several historical articles. All available articles were reviewed in depth. In consideration of the summary and usefulness for clinicians in the field of myology and for future citation, the information and references were summarized into tables.
2. Historical background
The term LGMD was, among hereditary muscle disorders, one of the most difficult to establish as a clinical entity. Early definitions were described by Erb where he designated a type of juvenile, scapulohumeral progressive muscular atrophy known as ‘a juvenile form of progressive muscular dystrophy’. In 1891, Erb (2) proposed to include with his cases the previous observations of Leyden (3) and Mobius (4). The theory was generally well accepted. Bell (5) was the first to differentiate this type of dystrophy from X-linked Duchenne muscular dystrophy and from autosomal dominant facioscapulohumeral muscular dystrophy. In their archetypal paper, Walton and Nattrass (6) first devised the name ‘limb-girdle muscular dystrophies’ to comprise cases of both sexes, beginning usually within the first three decades, with major involvement of scapular, pelvic girdle and trunk muscles, with sparing of facial muscles and infrequent pseudo hypertrophy, moderately severe progression and usually an autosomal recessive mode of inheritance.
With the development of physiological and histopathological means to assess muscular disorders, it rapidly seemed that a number of patients considered to be suffering from LGMD were, in fact, affected by other conditions, including spinal muscular atrophies, congenital myopathies or metabolic disorders. Hence, the clinicopathological consistency of this suggested entity was, nevertheless, again promptly disputed.
In the early development of immunostaining methods in the late 1980s, a precise distinction between LGMD and other conditions such as Becker’s dystrophy characterized by dystrophin abnormality was established (7).
In 1995, the European Neuromuscular Centre Workshop established more precise criteria for the diagnosis and classification of LGMD. More specifically, different subtypes of LGMD were grouped according to their genetic characteristics (8).
The abbreviation of the autosomal-dominant type is now LGMD1, whereas autosomal-recessive types are LGMD2. Each separate gene locus has a unique classification (Table I) (9–35).
Table I.
LGMD classification.
| Form | Locus | Gene | Proteinopathies | Key references |
|---|---|---|---|---|
| Autosomal dominant | ||||
| LGMD1A | 5q31 | MYOTM | Myotilinopathies | (9) |
| LGMD1B | 1q11-q21 | LMNA | Lamin A/C opathies | (10) |
| LGMD1C | 3p25 | CAV3 | Caveolinopathies | (11) |
| LGMD1D | 2q35 | DES | Desminopathies | (12) |
| LGMD1E | 7q36 | DNAJB6 | HSP40/DNAJ | (13,14) |
| LGMD1F | 7q32.1-q32.2 | - | - | (15) |
| LGMD1G | 4p21 | - | - | (16) |
| LGMD1H | 3p23-p25 | - | - | (17) |
| Autosomal recessive | ||||
| LGMD2A | 15q15.1 | CAPN3 | Calpainopathy | (18) |
| LGMD2B | 2p13 | DYSF | Dysferlinopathies | (19) |
| LGMD2Ca | 13q12 | SGCG | γ-sarcoglycanopathy | (20) |
| LGMD2Da | 17q12-q21.33 | SGCA | α-sarcoglycanopathy | (21) |
| LGMD2Ea | 4q12 | SGCB | β-sarcoglycanopathy | (22) |
| LGMD2Fa | 5q33 | SGCD | δ-sarcoglycanopathy | (23) |
| LGMD2G | 17q12 | TCAP | Telethoninopathy | (24) |
| LGMD2H | 9q31-q34 | TRIM32 | E3-ubiquitin ligase | (25) |
| LGMD2Ib | 19q13 | FKRP | Fukutin-related protein | (26) |
| LGMD2J | 2q31 | TTN | Titinopathies | (27) |
| LGMD2Kb | 9q34.1 | POMT1 | POMT1 | (28) |
| LGMD2L | 11p14.3 | ANO5 | Anoctaminopathies | (29) |
| LGMD2Mb | 9p3 | FKTN | Fukutinopathies | (30) |
| LGMD2Nb | 14q10-q24 | POMT2 | POMT2 | (31) |
| LGMD2Ob | 1p34-33 | POMGnT1 | POMGnT1 | (32) |
| LGMD2Pb | 3p21 | DAG1 | Dystroglycan | (33,34) |
| LGMD2Q | 8q24.3 | PLEC | Plectinopathies | (35) |
Nomenclature of LGMD1D/1E was according to OMIM.
Sarcoglycanopathies;
dystroglycanopathies.
LGMD, limb-girdle muscular dystrophies.
As is evident from its long nosological history, LGMD is not an homogeneous disease. LGMD can be considered an ‘umbrella’ term under which >24 gene defects have been recognized, where single gene defects encode numerous phenotypes and vice versa.
3. Mechanism of action
The mechanisms of action of LGMD-involved proteins are diverse. Emphasis is moving away from the identification of structural proteins and their implications in muscular dystrophies towards investigating proteins involved in muscle fiber maintenance in response to repeated injury (dysferlin, caveolin 3 and anoctamin 5), fiber remodeling under stressful conditions (calpain 3), and post-translational modifications of proteins and the enzymes involved in these processes (POMT1, POMT2, POMTGnT1). Despite the advances in modern biochemical techniques, certain proteins still exist without defined functions (Table II) (13,35–51).
Table II.
LGMD subtype: Proteins and putative functions.
| Form | Protein | Site | Anticipated function (ref.) |
|---|---|---|---|
| LGMD1A | Myotilin | Sarcomere | Z-disc structure protection, anchorage of thin filaments to the Z-disc (36) |
| LGMD1B | Lamin A/C | Nuclear membrane | Nuclear membrane stabilization, cell signaling, differentiation (37) |
| LGMD1C | Caveolin 3 | Sarcolemma | Membrane trafficking, signal transduction (38) |
| LGMD1D | Desmin | Sarcomere | Assembly and the formation of the extra-sarcomeric cytoskeleton (39) |
| LGMD1E | HSP40/DNAJ | Ubiquitous | Protecting client proteins from irreversible aggregation (13) |
| LGMD1F | - | - | |
| LGMD1G | - | - | |
| LGMD1H | - | - | |
| LGMD2A | Calpain3 | Cytosol, sarcomere | Sarcomeric remodeling; zygomatic and structural function (40,41) |
| LGMD2B | Dysferlin | Sarcolemma | Membrane repair and vesicle trafficking (42) |
| LGMD2C | γ-sarcoglycan | Sarcolemma | Part of DGC, involved in membrane integrity, cell signaling (43) |
| LGMD2D | α-sarcoglycan | Sarcolemma | Part of DGC, involved in membrane integrity, cell signaling |
| LGMD2E | β-sarcoglycan | Sarcolemma | Part of DGC, involved in membrane integrity, cell signaling |
| LGMD2F | δ-sarcoglycan | Sarcolemma | Part of DGC, involved in membrane integrity, cell signaling |
| LGMD2G | Telethonin | Sarcomere | Sarcomeric assembly, titin anchor (44) |
| LGMD2H | E3-ubiquitin ligase | Cytosol | Involved in ubiquitin-proteasome pathway (45) |
| LGMD2I | Fukutin-related protein | Extracellular | Unknown, glycosylation of α-dystroglycan (46) |
| LGMD2J | Titin | Sarcomere | Sarcomeric scaffold, elasticity, force bearing mechanism, cell signaling (47) |
| LGMD2K | POMT1 | Extracellular | Catalyze the first step in O-mannosylation of α-DG (48) |
| LGMD2L | Anoctamin 5 | Sarcolemma | Calcium-activated chloride channel function, reseal mechanism (29) |
| LGMD2M | Fukutin | Extracellular | Unknown, putative phospholigand transferase (49) |
| LGMD2N | POMT2 | Extracellular | Catalyze the first step in O-mannosylation of α-DG (48) |
| LGMD2O | POMGnT1 | Extracellular | Catalyze the second step in O-mannosylation of α-DG (50) |
| LGMD2P | Dystroglycan | Sarcolemma | Connect extracellular medium to intracellular scaffold (51) |
| LGMD2Q | Plectin | Sarcomere | Cytoskeleton system linker (35) |
LGMD, limb-girdle muscular dystrophies; ref, reference; DGC, dystrophin glycoprotein complex.
4. Pathophysiology
The majority of the developments in LGMD therapeutics have derived from insight gained in animal model investigations, created through the reversal of etiopathogenic agents prompting the disease or by disrupting certain steps believed to be downstream of the defect gene.
When reviewing all mutations in MYOT through the Leiden muscular dystrophy page (http://www.dmd.nl), it was evident that no null mutation has been reported to date in the MYOT gene, hence all mutations are of missense type. This evidence supports the theory that myotilin missense, but not nonsense, mutations are pathogenic in humans and there must be other proteins, such as palladin and myopalladin, which reimburse the structural and functional properties of myotilin (52). This hypothesis is further supported by the fact that the myotilin-null mouse demonstrates normal development, histology and performance, yet myotilin transgenic mice show dystrophic processes and double transgenic mice report a more severe phenotype (52,53). Authors hypothesize that genetic or pharmaceutical interference with mutant myotilin translation, may serve as promising therapeutic approaches in the treatment of LGMD. The LMNA gene encodes the lamin A/C protein, which is important in its function as a scaffold for nuclear lamina and as a vital component of cell signaling and differentiation. Currently, three pathways are implicated in the pathophysiology of LMNA-mediated cardiac and musculoskeletal dystrophies; retinoblastoma protein (pRb), mitogen activated protein kinases-extracellular signal-regulated kinases (MAPK-ERK) and transforming growth factor beta ligands (TGF-β). MAPK-ERK and pRb signaling mediate regulation of the cell cycle, while the TGF-β pathway is involved in the transduction of extracellular signals to trigger downstream TGF-β gene transcription in the nucleus (37). Several in vivo studies have obtained promising results by targeting these pathways. For example, Muchir et al despite identifying that inhibition of the ERK pathway had a minimal effect on cardiomyopathy, the impact of therapy on cardiac rhythm was not examined; the most common cause of mortality in humans (54). Further studies are essential to elucidate the precise mechanism involved in cardiac arrhythmias and skeletal muscle weakness. Myostatin or TGF-β receptor inhibition by various techniques is evolving therapeutic rationales for caveolin 3 mutations. Yet, these treatments were applied to a particular mutation (55). Whether these therapies are effective on other types of mutations or other models remains to be explored.
Accumulating lines of evidence have demonstrated that gene transfer of CAPN3 and mutated myostatin propeptide delivery were efficient therapies in investigations utilizing the calpainopathy murine animal model. However in this study, the duration for gene persistence in muscles was relatively short (9 days), the mode of gene delivery (intramuscular) was not sufficient to improve lifestyle, and the parameters used for assessing improvement were poor, such as the contractile force, which was measured ex vivo (56). Several novel therapeutic strategies have been piloted to treat DYSF gene (DYSF) deficiency. The most well characterized study was that performed by Han et al, who disrupted the complement component C3 (C3), that is considered to be a crucial mediator of inflammatory cascades in DYSF deficient mice (57). Nonetheless, several queries were raised against the proposed model, including the limitation that the contractile force was not examined. In addition, the authors hypothesized that it is complement activation rather than contraction-induced injuries that is responsible for muscle weakness in the DYSF deficient mouse, however some dysferlinopathic patients have no infiltrates on their biopsies (58). It is unlikely that membrane attack complexes (MAC) underlie muscle weakness, given that the same study identified that C5 ablation (a terminal component of the MAC pathway) had minimal effects. This issue should be addressed in future trials when utilizing inflammatory and non-inflammatory models. Whether complement C3 inhibitors (easily administered and more convenient for human trials) will have a similar effect as C3 ablation remains to be clarified. In sarcoglycanopthies, various therapeutic stratgies have been investigated in vitro and in vivo, including gene transfer, stem cell grafting, myostatin blockade and calcium channel blockers. Notably, sarcoglycanopathies conveyed more success of gene therapy than other LGMD subtypes due to small size genes. Lastly, calpain 3 inhibition provided a rationale for the treatment of titinopathy.
Though numerous therapies have proved efficacious and safe in several different murine models, caution should be exercised when considering the applicability of these treatments to humans, given the evident differences in size, life span, genetic variants and immunological complexity between the two species. Table III (54–57,59–82) summarizes the majority of murine models constructed and the interventions applied with their results.
Table III.
Murine models with assumed therapy.
| LGMD | Animal model | Description | Comment (ref.) | Intervention (ref.) | Result (ref.) |
|---|---|---|---|---|---|
| LGMD1A | Myo−/− | Knockout mouse | Normal | ||
| Myo+/T57I | Transgenic mouse | Phenotype similar to myotilinopathy | |||
| DTg | Double transgenic | More severe phenotype than TgT57I, Tg WT | |||
| LGMD1B | LmnaH222P/H222P | Knockin mouse | More relevant to human laminopathy | ERK inhibition (PD98059) (54) | Reverse DCM in mice |
| Lmna−/− | Knockout mouse | Less relevant to human laminopathy | |||
| LGMD1C | Cav-3−/− | Knockout mouse | Muscle disease, HCM, defect in T-tubules | ||
| Cav-3+/P104L | Transgenic mouse | Myostatin (M) inhibition (55) | Revere atrophy, weakness | ||
| TGF-β receptor inhibitor (59) | Revere atrophy, weakness | ||||
| LGMD2A | Capn3C129S/C129S | Knockin mouse | Proteolytically inactive, structurally intact | AAV delivery mutated (M) (56) | Revese atrophy, weakness |
| Capn3−/− | Knockout mouse | No calpain-3 | AAV delivery calpain 3 (60) | Revese atrophy, weakness | |
| Capn3 Tg | Transgenic mouse | Normal | |||
| LGMD2B | A/J | Spontaneous | Retrotransposon insertion in intron 4 | Diltiazem (61), pyridostigmine (62) | Improved contractile function |
| B6.A/J | By breeding | Retrotransposon insertion in intron 4 | Dual AAV gene transfer (63) | Clinical, biochemical imp. | |
| SJL/J | Spontaneous | Splice site mutation at exon 45 | HUCB i.v. administration (64) | Biochemical imp. | |
| Q10 and resveratrol (65) | Histological imp | ||||
| B10.SJL | By breeding | Splice site mutation at exon 45 | |||
| Dysf−/− | Knockout mouse | Deletion of exon 45 | Genetic disruption of C3 (57) | Improved muscle pathology | |
| LGMD2C | gsg−/− | Knockout mouse | Exon 2 disruption | AAV gene transfer (66) | Biochemical imp. |
| Myostatin blockade (67) | Improve function not histology | ||||
| gxi | Double knockout | Lacking both integrin α7 and γ-sarcoglycan | Integrin α7β1 (68) | Compensate γ-sarcoglycan | |
| LGMD2D | Sgca−/− | Knockout mouse | AAV gene transfer (69) | Biochemical imp. | |
| Mesoangioblasts i.a. (70) | Biochemical imp. | ||||
| Deacetylase inhibitors (71) | Reverse morphology, function | ||||
| SGCAH77C/H77C | Knockin mouse | Normal | |||
| LGMD2E | Sgcb−/− | Knockout mouse | Exon 2 disruption | AAV gene transfer (72) | Biochemical imp. |
| LGMD2F | BIO14.6 | Spontaneous | AAV gene transfer (73) | Reverse morphology, function | |
| Tranilast, diltiazem (74) | Biochemical imp. | ||||
| TO-2 | By breeding | AAV gene transfer (75) | Biochemical and functional | ||
| HUCB i.myo. administration (76) | Short-term imp. | ||||
| Sgcd−/− | Knockdown | Exon 2 targeted replacement | Hematopoietic stem cells (77) | No imp. | |
| Myosphere-derived progenitors (78) | Improved heart function | ||||
| Myostatin blockade (79) | Early-stage imp. | ||||
| AAV gene transfer (80) | Heart but not muscle imp. | ||||
| LGMD2G | TCap−/− | Knockout mouse | |||
| LGMD2H | Trim32−/− | Knockout mouse | |||
| T32KI | Knockin mouse | Carries c.1459G>A, p. D487N mutation | |||
| LGMD2J | TTN+/c.43628insAT | Knockin mouse (het) | Wild allele compensate mutated one | ||
| TTNhom | Knockin mouse (hom) | Lethal at E9.5 | |||
| PEVK−/− | Knockout mouse | PEVK part of TTN highly phosphorylated | |||
| N2B−/− | Knockout mouse | Calcium-sensitive area of TTN | |||
| FINmaj | Knockout mouse | C-terminal area of TTN (homo. Het.) | FINmaj (Het.) X capn3−/−mice (81) | Improve muscle, heart | |
| LGMD2L | No model yet | ||||
| Dystroglycan | |||||
| LARGEmyd | Myodystrophy | LARGE gene mutated | LARGE gene transfer (82) | Improve structure and function | |
| LARGE++/++ | Transgenic mouse | Late-onset loss of force | |||
| LGMD2I | FKRP-NeoTyr307Asn | Knockin mouse | Lethal soon after birth | ||
| FKRPTyr307Asn | Knockin mouse | Normal | |||
| FKRP−/− | Knockout mouse | Lethal at E12.5 | |||
| FKRPP448L/ P448L | Knockin mouse | Structural anomalies reminiscent to human | |||
| LGMD2K | POMT1−/− | Knockout | Lethal between E7.5 and E9.5 | ||
| LGMD2M | FKTN+ETN | Transgenic mouse | Retrotransposon insertion in FKTN gene | ||
| LGMD2N | POMT2−/− | Knockout mouse | Lethal at E9.5 | ||
| LGMD2O | POMGnT1−/− | Knockout mouse | Muscle-eye-brain model | ||
| LGMD2P | DGS654A | Transgenic mice | Inhibit dystroglycan cleavage | ||
| DAG1T192M/ T192M | Knockin mouse | Neuromuscular abnormalities | |||
| LGMD2Q | No model yet |
LGMD, limb-girdle muscular dystrophies; C3, complement component C3; HUCB, human umbilical cord blood; imp., improvement; ref, reference; i.v., intravenous; i.a., intra arterial; i.myo., intramuscular; AAV, adeno-associated virus; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy.
LGMD, Limb-girdle muscular dystrophies; hom, homozygous; het, heterozygous.
5. Disease markers
With recent advances in therapeutic trials, more light has been shed on the clinical outcome measures that can be utilized to predict disease activity, regression and treatment efficacy without requiring multiple muscle biopsies.
There are multiple disease markers for LGMD, some of which are well known, and others which have only been identified in recent years. For example, high creatine kinase (CK) levels indicate disease activity; while low levels may indicate fibrosis or therapeutic responsiveness. In cases of local delivery of target therapy, CK level is not helpful. Hand grip strength, Medical Research Council (MRC) scale, Gardner-Medwin and Walton scales or other clinical scales are beneficial for assessing clinical outcome, however inter and intra examiner variability and false-negative results in depressed patients reduces the efficacy and reliability of this method. Another measure utilizes contrast agent-enhanced MRI scanning. Typically, albumin-targeted contrast agent, (MS-325) does not enter into myocytes. In membranopathies, the dye is usually observed in the sarcoplasm. The technique is considered a robust noninvasive disease follow-up tool in the therapeutic trials of the sarcoglycanopathies (83). Measuring the secreted alkaline phosphatase levels (Se AP) in blood (succeeded in murine models), involves insertion of the secreted alkaline phosphatase gene with the gene of interest into a viral vector. Following this, simple blood-based assay of Se AP can predict gene expression in specific muscles. The assay overcomes limitations of CK level identification that is nonspecific and minimally affected in cases of local delivery of the gene (84). Another approach measures dysferlin and calpain 3 expression levels using circulating monocytes that reflect expression level sin muscles given that the target therapy is delivered systemically (61).
Luciferase assay
Luciferase assay is considered as ‘regeneration reporter’ and its level mirrors the number of centrally nucleated fibers and embryonic heavy chain myosin positive cells (61).
Neutralizing antibodies and interferon (INF)-γ to r AAV
Measuring antibody titer or mononuclear cell mediated INF-γ secretion, by enzyme linked immunosorbent assay (ELISA) and enzyme-linked immunsorboent spot (ELI Spot) assays, respectively, were used to detect degree of immune rejection to inserted vector gene (85).
6. Disease prevalence
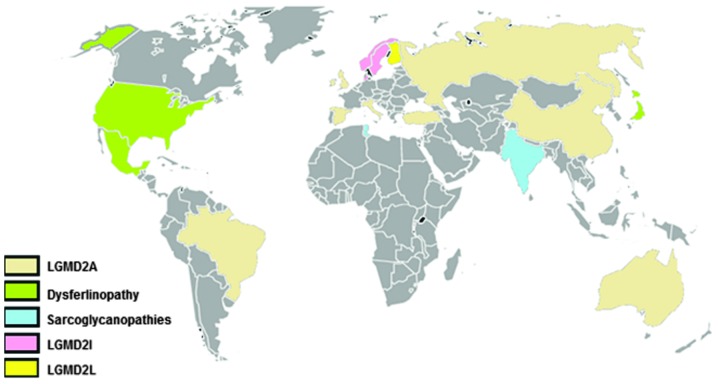
With the exception of countries such as Norway, Denmark and Finland, where the founder effect was determined, LGMD2A is the most frequent type of LGMD worldwide, followed either by dysferlinopathies in some areas or by sarcoglycanopthies in others (Fig. 1). The relative frequencies of the subtypes that exist among various ethnicities are described in Table IV (86,87,89–92,94–107). LGMD is considered the second most common muscular dystrophy in England, Mexico and Turkey, after dystrophinopathies, with a disease prevalence of up to 1/14,500 and a carrier frequency of up to 1/150 (86–88).
Figure 1.
World map with the most common LGMD forms represented in colors. Grey color indicates countries with no known cohort. In Spain, Italy, England, Turkey, Russia, China, Brazil and Australia, the most common type was calpainopathy. LGMD2I was more frequent than other forms in the Scandinavian Peninsula. However, dysferlinopathy was the most frequent in US, Japan and Mexico. In India, sarcoglycanopathies had the highest incidence, whereas in Finland, anoctaminopathy ranked the first (25%) amongst other forms.
Table IV.
Relative % of different LGMD forms in different countries.
| LGMD | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Country (ref.) | No. of patients | 2A | 2B | 2C-F | 2I | 2L | 1B | 1C |
| Italy (89) | 228 | 37% | 27% | 23% | 9% | 2% | - | 2% |
| Italy (90) | 181 | 28.4% | 18.7% | 18.1% | 6.4% | - | - | 1.3% |
| Italy (91) | 346 | 25.1% | 11.2% | 15% | 4.3% | - | 1.4% | 1.4% |
| Spain (92) | - | 80% | - | - | - | - | - | - |
| German (89) | 124 | - | - | - | 16% | - | - | - |
| UK (86,94) | 68 | 26.5% | 5.9% | 11.8% | 19.1% | 11.8% | 8.8% | - |
| Norway (95) | 326 | - | - | - | 27% | - | - | - |
| Denmark (96) | 118 | 10.2% | 1.7% | 19% | 32.2% | - | - | - |
| Finland (97) | 101 | - | - | - | - | 25% | - | - |
| Australia (98) | 76 | 8% | 5% | 2% | 3% | - | 1% | 3% |
| USA (99) | 226 | 12% | 18% | 15% | 15% | - | - | 1.5% |
| Mexico (87) | - | 25% | 40.6% | 31.2% | - | - | - | 3.1% |
| Turkey (100) | 20 | 50% | 5% | 40% | - | - | - | - |
| Russia (101) | 19 | 75% | - | - | - | - | - | - |
| Brazil (102) | - | 32% | 22% | 32% | 11% | - | - | - |
| China (In press) | 68 | 17% | 15% | 3% | - | - | - | 3% |
| Japan (103,104) | 80 | 26% | Most | 9% | - | - | - | - |
| India (105) | 26 | - | - | 53.8% | - | - | - | - |
| India (106) | 171 | 47% | - | - | - | - | - | |
| India (107) | 30 | 21% | - | - | - | - | - | - |
LGMD, limb-girdle muscular dystrophies; ref, reference.
7. Genotype-phenotype correlation
Clinical presentations of LGMD disorders vary among patients of the same subtype, or even within the same family (108). Genotype-phenotype correlational analysis is however, difficult to predict and it represents one of the most challenging obstacles in the field of genetic disorders. Trials to elucidate the specific clinical picture for individual genetic subtypes were futile (108).
Currently, two null mutations in the CAPN3 gene have been associated with severe phenotype, early onset and risk of being crippled (90). Natural exon 32 skipping of the DYSF gene notably reduced the severity of symptoms in a mother of two severely affected daughters by homozygous mutation (109). Mild features are most commonly reported in relation to the common Asian mutation c.2997G>T, p.W999C). However, the mutation has also been described in association with a variety of phenotypes (110–112). In cases of LGMD2H, it has been identified that mutations gathered in the NHL (named after the proteins NCL1, HT2A and LIN-41) domain result in LGMD2H/sarcotubular myopathy, whereas in Bardet-Biedl Syndrome, mutations are most commonly located in the B-box region of the gene. This may suggest that mutations in the NHL area render individuals more susceptible to muscular disorders (113). Late disease onset, mild phenotype, less susceptibility to loss of ambulation and more liability to myoglobinuria, are consistent features observed in patients homozygous to c.826C>A (p.L276I) of the FKRP gene compared with patients heterozygous to the same mutation (96). While several LGMD forms are phenotypically heterogeneous, it appears that ‘hot spot’ c.191dupA mutation in the ANO5 gene is associated with a more homogeneous phenotype (94). Of note, no LGMD2M patients to date have exhibited a 3 kb retrotransposal insertion in the FKTN gene, a founder mutation accounted for 87% of Fukuyama type congenital muscular dystrophy (114). Finally, it has been identified that mutations clustered in immunoglobulin-like fold and coil 2 of the LMNA gene are inconsistently correlated with the autosomal dominant form, LGMD1B (115).
Matching gene expression profiles of normal and affected muscles, identifying the crystalline structure of the protein of interest and recognizing the precise function of each protein domain, are approaches that will improve our understanding of the associations between various pathogenic mutations and disease presentation in LGMD (116).
8. Diagnostic strategy
Clinical, electrophysiological, imaging, biochemical and genetic testing techniques collectively should be utilized and tailored according to specific LGMD patient cases. Not only will this facilitate diagnosis and provide individualized genetic counseling to proband and relatives, but will also enhance the understanding of the underlying pathophysiology, to allow delivery of a therapeutic strategy that targets the precise pathways that are specific to that patient.
Clinical
Historical analysis and clinical examination are commonly used approaches to identify specific LGMD subtypes.
Age of onset
The vast majority of autosomal recessive LGMD cases have teenage onset-progressive muscle weakness, however, there are exceptions to this rule. In certain dystroglycanopathies, sporadic dysferlinopathy cases may start exhibiting perinatal ‘floppiness’ and mild weakness as late as 70 years-old (117,118). While calpainopathy and dysferlinopathies tend to manifest in late childhood to late teens, early childhood onset indicates the diagnosis will be a sarcoglycanopathy or dystroglycanopathy. The majority of, but not all, autosomal dominant LGMD patients begin experiencing symptoms following their twenties.
Pattern of distribution of muscles weakness
LGMD is associated with marked clinical disparity. As the rule of thumb, a defect in membrane scaffolds cause predominant proximal myopathies, whereas sarcomeric protein deficiencies typically result in initial distal myopathies. The exceptions to this include distal Miyoshi myopathies (MMs), which are associated with the membrane patch proteins dysferlinopathy and anoctaminopathy. Scapulohumeral muscle weakness or ‘Erb phenotype’ is most commonly observed in sarcoglycanopathies, calpainopathies and Duchene’s type of LGMD2I, while pelvic muscle weakness or ‘Leyden-Mobius phenotype’ (LM) is the leading indicator for the majority of LGMD subtypes. While calf, thigh and tongue hypertrophies are mostly encountered in sarcoglycanopthies and dystroglycanopathies, deltoid hypertrophy appears to be restricted to dysferlinopahies (119,120,121).
Skeletal manifestations
Contracture is reported in numerous types of LGMD, however predilection is given to laminopathy, calpainopathy, dystroglycanopathies and anoctminopathy. While kyphoscoliosis and lordosis manifest in calpainopathies, dystroglycanopathies and plectinopathies, features such as bent and rigid spines, appear to be limited to dysferlinopathies (112,122).
LGMD and heart
With the exception of myotilinopathy, laminopathies, telethoninopathy and LGMD2I, cardiac muscles are spared in the majority of LGMD subtypes or seldom involved in others (LGMD2A, 2B and 2C-2F). Patients with LGMD may present with wide range of cardiac abnormalities e.g., atrial fibrillations, flutters, atrio-ventricular conduction blocks, supraventricular, ventricular ectopic beats, ventricular tachycardia and sudden death commonly seen in the laminopathy patients; on the other side, dilated, hypertrophic and restrictive cardiomyopathy are noticed in some sarcoglycanopathies and a third of LGMD2I cases. Cardiac problems may precede, overlap with or follow skeletal muscle weakness. Periodical cardiac monitoring and pacemaker or defibrillator implantation are warranted in certain cases (123).
LGMD and pulmonary function
Respiratory muscle weakness is a rare manifestation and predominantly indicates diagnosis of LGMD2I, myotilinopathy and occasionally, LGMD2A and LGMD2C-2F. Patients with LGMD2I may also develop respiratory failure while ambulant. Pulmonary function tests should be part of routine examination in suspected LGMD subtypes.
Extra muscular features
Detailed neurological exams often provide an implication of the specific LGMD form. While neuropathy, paresthesia, nasal speech and dysarthria appear to commonly occur in myotilinopathies; opthalmoparesis, foot drop, and paresthesias have been sporadically reported in LGMD1C, 2G and 2H respectively (124,125). In dystroglycanopathies, with the exception of LGMD2I, muscle weakness is usually coupled with cognitive impairment, which has serious psychosocial consequences for the patient and their family. Patients with LGMD2I usually have normal intellect, nonetheless there may be some difficulties in visiospatial planning and memory function (126).
Clinical course
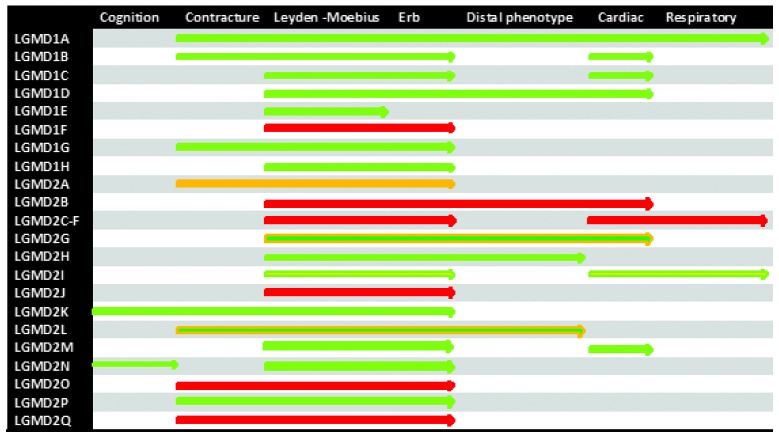
LGMD subtypes have a characteristically slow progression, with weakness most commonly beginning in the proximal lower limbs and sometimes the distal limbs. This is followed by weakness in all other limbs and then permanent disability is established two to three decades after disease onset. The subtypes LGMD1D, LGMD2L and LGMD2M represent slowly progressive diseases and patients are mildly affected and remain ambulant (94). Rapid progression is a classical feature of dysferlinopathies, sarcoglycanopathies and plectinopathies. In some LGMD forms there may be inconsistencies in the progression of the disease between male and female-affected patients. In female mild-onset patients, estrogen is considered to impact disease progression. LGMD2G and LGMD2L are examples of LGMD groups with inconsistent disease progression between male and female patients. The study discerned another type of LGMD that is also characterized by a two-peak onset; rapid progressive Duchene like childhood onset and milder Becker-like adolescent to adulthood onset. LGMD2I is representative of this set (96). Fig. 2 denotes disease spectrum for the rapid recall of clinical features of LGMD subtypes.
Figure 2.
Disease spectrum for rapid recall of LGMD subtypes. Note: green color represents slow progression, yellow color represents moderate progression, red color represents rapid progression and mixed color represents variable progression depending upon either type of the mutation or gender factor (see text).
Clinical constellations
LGMD remains an expanding group of diseases, and approximately one third of cases have not yet been associated with a specific subtype. Certain clinical constellations often favor specific LGMD subtypes, and eliminate a wide array of unnecessary genetic tests. In patients presenting with distal muscular weakness, contractures and cardiac problems in adulthood, myotilinopathy should be considered. Limb-girdle muscle weakness with a family history of sudden death and late onset contractures, most commonly indicate a laminopathy, whereas myalgia, rippling phenomenon, in association with proximal limb muscle weakness in the first four decades of life should raise suspicion of caveolinopathy. Proximal limb muscle weakness with contractures and calf atrophy in teens are features consistently associated with LGMD2A. Early childhood onset muscle weakness with cognitive impairment is a pathognomonic feature of dystroglycanopathies. In patients with cardiopulmonary involvement in association with myopathy and myoglobinuria, LGMD2I should be suspected.
Biochemical, imaging and electrophysiological studies
Muscle enzymes (CK)
CK levels are either normal or mildly elevated in the majority of autosomal dominant LGMDs, whereas in autosomal recessive types, they are highly elevated. While high CK levels in dysferlinopathies, dystroglycanopathies and sarcoglycanopathies are usually correlated with disease activity, calpain 3 deficient patients typically manifest mild to moderate CK level elevation. However, normal and high levels of CK have been sporadically described amongst the disease subtypes. Although CK levels are not specific measurements for the diagnosis of muscular diseases, they often act as a useful tool for guiding physicians to a specific disease process and exclude metabolic or acquired myopathic diseases (127).
Magnetic resonance imaging (MRI)
Novel diagnostic tools have been recently introduced to the field of neuromuscular disorders. Studies have utilized modern imaging facilities to facilitate in the diagnosis of highly complicated genetic diseases and to allow physicians to direct patients for further protein and gene testing. The distinction between the most common subtypes (LGMD2A and LGMD2I) that account for up to 80% of recessive LGMD disorders (in some ethnic groups) is now possible using imaging techniques (128). LGMD2A is characterized by the involvement of gluteus maximus, the posteromedial thigh area and the selective involvement of medial calf muscles. This is in marked contrast to LGMD2I where calf muscles are non-selectively involved. In addition, winging of scapulae and calf atrophy are more evident in calpainopathies than in LGMD2I. The anterior thigh muscles are more affected than the posterior ones in α-sarcoglycanopathy, where the calf muscles are relatively spared. This is in contrast to dystrophinopathies, where early and striking changes in the gastrocnemii muscles are prominent (128,129). The specific pattern of affected muscles was eventually delineated in anoctaminopathy, as demonstrated by high signal intensities in posterior thigh muscles, partial atrophy of quadriceps and posteromedial calf affection (89). In myotilinopathy, distal muscle groups represented by the calves are more affected than proximal peroneal muscles and medial pelvic, thigh and lower leg muscles are more involved than lateral sets. The opposite is true for desminopathy (130).
Electrophysiological studies; nerve conduction velocity (NCV) study and electromyography (EMG)
Electrophysiological studies are highly important in differentiating myopathic diseases from neurogenic ones, particularly if the patient presents with distal myopathy or contracture deformity where other differential diagnoses, including Charcot-Marie-Tooth syndrome, may be considered. However, neurogenic damages have been sporadically reported in calpainopathy (131) and dysferlinopathy (unpublished observations).
Muscle biopsy: optical and electron microscopy (EM)
Muscle biopsy is a transitional step in the diagnostic algorithm of muscle disorders. Several studies are ongoing to simplify the diagnosis of muscular disorders using more superficial tissues like skin (132).
A wide spectrum of optical microscopic alterations, ranging from mild to severe degenerative muscle changes, has been described in LGMD biopsy specimens. Aside from the myopathic features (fiber size variation, internal myonuclei and fiber splitting), inflammatory components appear frequently in biopsy analysis of specific LGMD subtypes like dysferlinopathies (133), anoctaminopathies (94) and dystroglycanopathies and rarely in others, including LGMD2A (134), LGMD2C (135) and LGMD2D (manuscript in press). While rimmed and non-rimmed vacuoles are non-classical features in LGMD disorders (observed mainly in autosomal dominant patients), amyloid deposits are frequently encountered in dysferlinopathies and anoctaminopathies. Features of chronicity, including lobulated fibers, cytochrome C oxidase (COX) negative fibers and fibers with focal areas of reduced or absent nicotinamide adenine dinucleotide tetrazolium reductase (NADH-TR) are frequently detected in calpainopathy. Desminopathy should be considered in cases with menadione-linked nitro blue tetrazolium (M-NTB) positive cytoplasmic inclusions (12). In cases with only trivial muscle pathology, biopsy analyses are usually non-specific or even normal. However, many genetically confirmed LGMD cases have been mistakably diagnosed as acquired myopathies (136). Hence, EM and immunohistochemistry are essential to establish the diagnosis of LGMD.
EM has a crucial role in diagnostic analyses of certain LGMD subtypes particularly those associataed with myofibrillar proteinopathies. Cytoplasmic filamentous inclusions, spheroid bodies, myofibrillar protein aggregates and Z-disc streaming are features that are commonly diagnosed as myotilinopathies, whereas excess subsarcolemmal granulofilamentous material is in keeping with the characteristics of desminopathy (137). With the exception of laminopathy and myotilinopathies, myonuclei are usually spared in LGMD, which is a feature that facilitates its distinction from sporadic and hereditary inclusion body myopathy. Recently, EMs has been used to explore etiopathogenic mechanism underlying some dystrophic processes. These studies identified plasma membrane defects, basal lamina duplications and submembranous flocculations detected in LGMD2B and LGMD2L as the key indicators of a membrane reseal defect. Of note, some features e.g., vacuolar structures, dilated T tubules and myelin bodies, are non-specific and are shared with a variety of muscular disorders.
Immunohistochemistry (IHC) and western blot (WB) assays
With respect to their diagnostic significance, WB and immunostaining of muscle sections with antibodies against dysferlin (138), sarcoglycans (139), caveolin 3 (140) and telethonin (141) are now the ‘gold standards’ owing to their high specificities and cost-effectiveness.
In LGMD2A, this approach is often hindered by incomplete sensitivity and specificity. The process of staining muscle sections with available antibodies against calpain 3 is generally disputed as different staining patterns have been detected in abnormal LGMD2A biopsies; however, the specificity of western blotting has been improved by assessing calpain 3 autolytic activity (142,143). In addition, quantitative analyses of calpain 3 bands offer high diagnostic yield ranging from 84% in certain studies to 100% in others (144–147).
Other specific antibodies are those raised against the C-terminus of the giant titin protein and against truncated TRIM32 protein due to compound heterozygous mutations (148,149). A putative broken linkage between the sarcolemma and sarcomere, due to plectin 1f isoform mutation, most commonly results in absent sarcolemma immunostaining that is highly suggestive of plectinopathy. Whereas, reduced expression of dystroglycan-α and laminin-α2 overlay is a sensitive diagnostic indicator for dystroglycanopathies. However, this approach should be interpreted in association with clinical data, to facilitate selecting a specific gene testing method for the patient.
Proper sample handling, freezing and homogenization usually solve the limitation of denatured proteins, when certain antibodies cannot identify and may not be appropriate for biochemical assays. On the other hand, the masking effect of the epitope of an antibody may provide an inaccurate signal and some cases can be easily be overlooked (150).
High expression of calpain 3 and dysferlin in monocytes and the skin has reduced the necessity for muscle biopsies (132,151). Furthermore, multiplex blot analyses technique allows the investigator to envisage protein interaction and secondary reductions more clearly. Also, ‘reverse protein array’ confers high sensitivity to minimal protein changes that make it suitable to follow-up markers and predict drug responsiveness in upcoming trials (152).
Genetic diagnosis
Careful analysis of clinical and pathological findings and physiological and biochemical data, often provides crucial clues for the diagnosis of a distinct LGMD form. Currently, the documentation of a pathogenic mutation is currently warranted as a tool for identifying the diagnosis of a hereditary muscular disorder.
Genetic analyses are presumed to offer diagnosis for ~99% of cases with known gene loci. However in some types, such as LGMD 2A, virtually 25% of cases have no defects in the CAPN3 gene and ~22% have only one affected allele (153). It was estimated ~10–15 % of mutations are either intronic or subtle exonic splice sites. Therefore, muscle flesh, skin or in special cases (LGMD2A, 2B) blood monocytes are necessary to obtain mRNA (153,154). With the exception of selected centers in USA and certain European countries that offer genetic analyses for LGMD patients, genetic diagnosis is only affordable on a research basis. Nonetheless, the common hot spot mutations in certain ethnicities can be targeted prior to running whole gene sequencing (Table V) (13,14,25,27,35,90,92, 94,95,96,101,104,141,148,155–162).
Table V.
LGMD: Common mutations with founder effects.
| Type | Gene | Exon | Hot-spot mutations (exon no.) | Populations that express mutations (ref.) | Predicted phenotype |
|---|---|---|---|---|---|
| LGMD1A | MYOT | 10 | Exon 2 | - | |
| LGMD1B | LMNA | 12 | - | - | |
| LGMD1C | CAV3 | 2 | - | - | |
| LGMD1D | DES | 9 | - | - | |
| LGMD1E | DNAJB6 | 10 | c.279C>G (E5) | Finland, Americans (13,14) | |
| LGMD2A | CAPN3 | 24 | c.550delA (E4) | Russia, Czech, Turkey (40%), Italy, UK (101,155,156) | |
| c.2362_2363delinsTCATCT (E22) | Spain (30%), Brazil (Hispanics) (92) | ||||
| c.1469G4A, p.R490Q (E11) | Italy, Turkey (10%) (155,156) | ||||
| LGMD2B | DYSF | 55 | c.937+1G>A (E10) | Japan (104) | |
| c.1566C>G, p.Y522X (E18) | Japan (104,157) | ||||
| c.2997G>T, p.W999C (E28) | Japan, China, S. Korea (104) | Homozygous, mild | |||
| c.3373delG, p.E1125KfsX1134 (E1) | Japan (104) | ||||
| c.2494C>T, p.Q832X (E24) | S. Korea (158) | ||||
| c.663+1G>C, splicing defect (E6) | S. Korea (158) | ||||
| c.2372C>G, p.p791R (E24) | Canada (natives) (159) | ||||
| c.2875C>T, p.R959W (E27) | Italy (159) | ||||
| c.5713C>T, p.R1905X (E51) | Spain (159) | ||||
| c.2779delG., p.A927LfsX21 (E26) | Caucasian Jewish population (159) | ||||
| c.4872_4876delinsCCCC (E44) | Libyan Jewish population (159) | ||||
| LGMD2C | SGCG | 8 | - | - | |
| LGMD2D | SGCA | 10 | c.229C>T, p.R77C(E3) | Europe, Finland, Brazil (160) | Homozygous, mild |
| LGMD2E | SGCB | 6 | - | - | |
| LGMD2F | SGCD | 9 | - | - | |
| LGMD2G | TCAP | 2 | c.172C>T, p.Q53X (E2) | Brazil (141) | |
| LGMD2H | TRIM32 | 2 | c.1459G>A, p.D487N (E2) | Hutterites (USA, Canada, Germany) (25,161) | |
| LGMD2I | FKRP | 4 | c.826C>A, p.L276I (E4) | Europe, American (90,95,96,162) | Homozygous, Becker like Heterozygous, Duchenne-like |
| LGMD2J | TTN | 363 | Mex6( 11-bp change) | Finland (148) | |
| LGMD2K | POMT1 | 20 | c.598G>C, p.A200P (E7) | Turkey (27) | |
| LGMD2L | ANO5 | 22 | c.191dupA, p.Asn64Lysfs*15 (E5) | Northern europeans (94) | Homogeneous phenotype |
| LGMD2M | FKTN | 11 | - | - | |
| LGMD2N | POMT2 | 21 | - | - | |
| LGMD2O | POMGnT1 | 23 | - | - | |
| LGMD2P | DAG1 | 6 | - | - | |
| LGMD2Q | PLEC | 33 | c.1_9del, p.0(E2i) | Turkey (35) |
LGMD, limb-girdle muscular dystrophies.
As delineated in this review, LGMD is still an underdiagnosed entity and families with no identifiable gene locus may benefit from approaches like linkage analysis (34). Furthermore, gene chips, exome and wide genome sequencing are promising diagnostic tools for the diagnosis of de novo mutations, yet due to the high cost and extensive number of sequence variants, they are limited by challenging monetary and interpretation tasks (163).
9. Prevention and surveillance
The optimum time for determination of genetic risk, elucidation of carrier status, and discussion of the availability of prenatal testing, is prior to pregnancy. It is appropriate and necessary to offer genetic counseling to young adults who are affected, are carriers, or are at risk of being carriers. The identification of certain LGMD subtypes has led to changed advice e.g., certain sarcoglycanopathies (autosomal recessive trait) had previously been diagnosed as Becker muscular dystrophy (X-linked recessive trait that mainly transmitted to males) (164).
In certain LGMD disorders, joint contracture, cardiac or respiratory muscle dysfunctions arise earlier and later in the disease course. Regular surveillance with early physiotherapy, orthotics and stretching exercises facilitate joint deformities and delays disabilities for approximately two years (165). Consistent monitoring of cardiac function with early pacemaker or improved implantable cardioverter-defibrillator (ICD) instruments, may rescue the lives of certain laminopathy patients. Consistent monitoring of respiratory function, using of annual influenza vaccines, early physiotherapy, nocturnal ventilation with use of mucolytic and antibiotics if necessary, are all strategies that will reduce the rate of hospitalization and delay the need for tracheostomy and mechanical ventilation for several years. The majority of LGMD patients suffer from depression, social isolation, low self-esteem and culpability (166), so often pyschiatric therapy is of much benefit too.
The patient may be monitored for cardiac and respiratory function in the outpatient neurology clinic, nonetheless, a multi-disciplinary team is recommended to improve outcome and to confirm the optimal timing for intervention. Finally, in cases with unidentified gene defects, should they develop cardiopulmonary or skeletal complications then the above mentioned principles will apply.
10. Management
Like other hereditary disorders, despite extensive research, there are currently no therapeutic strategies to treat LGMD. The existing management techniques include emotion and physical support, such as in the use of canes, walkers, splinters, surgical intervention in case of contracture deformities, and support of cardiac and respiratory functions in cases of myotilinopathy, laminopathy and dystroglycanopathy.
Several clinical trials have been completed in humans and others are actively recruiting. These trials in humans have been initiated due to the trials in murine models which successfully demonstrated gene, cell transfer and pharmaceutical therapy in prinicipal (54–82).
While ‘replacement therapy’ of the defect using gene- or cell-based therapy is the gold standard, pharmaceutical therapy seems to be a more favorable approach, due to the fact the medicine can be evenly distributed to the whole body and is suitable for both modes of inheritance. The proposed pharmaceutical approaches act by targeting specific pathophysiological pathways in the disease. Increasing muscle mass by enhancing positive regulators or by inhibition of negative regulators of muscle growth, such as neutralizing myostatin antibodies, have been utilized in clincal trials, however unfortunately, despite proving safe and tolerable, demonstrated negative results at the endpoints (167). Another therapeutic target is calcium channels, which are more permeable in sarcoglycanopthies, caveolinopathies and other LGMD forms. These observations have been confirmed by reports that anecdotal improvement of CK level was demonstrated in an MM Japanese patient treated with dantrolene to reduce muscle pain, which is a drug that can block calcium channels. Furthermore, a combination of lisinopril (a calcium channel blocker agent) and Co Q10 are included in the upcoming trials in the treatment of LGMD. In dysferlinopathies, treatment startegies differ, because inflammatory mechanisms are often active in the DYSF mutant muscles. These approaches include, the use monoclonal antibodies like rituximab to block B cell activation or the use of intravenous immunoglobulin to prevent complement attack complex activations. Recently, vitamin D3 has been shown to possess DYSF promoter properties and has improved dysferlin expression in muscles and monocytes of DYSF mutation carriers (Table VI) (30, 85,167–174). The beneficial effect of steroids in the treatment of sarcoglycanopthies and dystroglycanopathies is a matter of interest, but the mechanism underlying how they improve muscle strength remains elusive. Similarly, this also applies to creatine monohydrate and Co Q10. Another encouraging potential strategy involves the use of calpain inhibitors to stop ubiquitous degradation of misfolded proteins in the Golgi apparatus, however there is no evidence of their effect in humans. Lastly, identifying drugs that upregulate surrogate proteins like ɛ-sarcoglycan in cases of α-sarcoglycanopthies, Integrin α7β1 in cases of γ-sarcoglycanopathies, or LARGE in cases of dystroglycanopathies, may also be of potential interest.
Table VI.
Update of therapeutic trials in humans.
| Therapeutic option | Mechanism | LGMD form (ref.) | Comment |
|---|---|---|---|
| Gene therapy (rAAV) | I.M. Intact gene transfer | LGMD2D (85) | Application on larger and more functional muscle is required Intra-arterial delivery to whole-body muscles is warranted The study represents histological but not functional improvement |
| Rituximab (I.V) (monoclonal AB) | Against CD20-positive B cells 375 mg/m2/week (4 doses) | Miyoshi M (168) | Small number of patients, female responsiveness is requested Muscle adaptation to specific exercise should be considered Effect of treatment on quality of life is doubtful |
| Dantrolene (25 mg/day) | Ca2+ ion blocker in ER (ryanodine receptor binding) | Miyoshi M (169) | Query effect on weakness Hepatopathy side effect in up to 91% |
| Vitamin D3 (1/week for 1 year) | MEK/ERK pathway D3 receptor to DYSF promoter |
DYSF carriers (170) | The study represents cohort of asymptomatic carriers |
| Deflazacort | Steroids (1 mg/kg/day) | DYSF-opathy (171) | Worsening of muscle strength |
| LGMD2D (172) | Mildly symptomatic female patient | ||
| Prednisone | Steroids (1–2 mg/kg/day) (0.35 mg/kg/day) | LGMD2M (30) | Partial responsiveness, multiple fractures and susceptibility to infections |
| LGMD2I (173) | Growth arrest, vertebral fractures and susceptibility to infection | ||
| Creatine MH | Helps to supply energy | Sarcoglycans (174) | Mild improvement (3%) |
| MYO-029 | Neutralizing AB to myostatin | MD (167) | No improvements at end point |
| CoQ10 + lisinopril | Vitamin-like+ Ca2+ blocker | LGMD | Recruiting |
LGMD, limb-girdle muscular dystrophies; AB, antibodies; MD, muscular dystrophies; I.M., intramuscular; I.V., intravenous; rAAV, recombinant adeno-associated virus.
11. Conclusion
In recent years, our understanding of LGMD has advanced, with regards to disease occurrence, founder effect in some locations, certain aspects of pathophysiology and phenotype-genotype correlations. In addition, elucidation of hot spot mutations, disease biomarkers, general strategies of the diagnosis and treatments would point toward efficient and safe follow up, and intervention. However, more emphasis should be placed on pathogenesis, updating of diagnostic guidelines, with regular assessment and close follow up of disease progression to better elucidate the history of the disease and to enrich translational research. Universal patient registry and collaborated multicenter LGMD disease projects should be encouraged, to provide invaluable insight into its common and unique features and to settle standardized patient care.
12. Future perspectives
In the past six decades, advances in the field of molecular biology has opened new avenues to understand the LGMD clinical diagnosis, classification, pathogenesis and treatment possibilities. However, our understanding of the pathophysiology of the majority of LGMD forms is still in its infancy.
The majority of of autosomal dominant disorder mutations behave in a ‘dominant negative fashion’. It remains unresolved why single amino acid substitution results in negative adverse function. What is equally intriguing, is whether all mutations act by same mechanism and whether protein interaction with other known and yet undisclosed proteins affect the phenotypes of these diseases. Multiple strategies have been devised to overcome dominant negative cytotoxicity in animal models, including RNA interference, however their safety and efficacy needs to be proved in forthcoming clinical trials.
While the pathogenicity of most autosomal recessive disorders remains to be elucidated, a combination of biochemical tests and the availability of variable animal models have provided invaluable clues for physiological functions of key proteins involved in LGMD. However, it remains unclear how protein deficiencies result in muscle fiber degeneration. A parallel question is why some ubiquitous proteins are involved specifically in muscle diseases. What is equally unclear, is how congenital and adult muscular diseases are produced by the same mutation. The use of a variety of animal models with different types of mutations within the same gene and observing their effects may feasibly solve the ‘paradox of single gene and multiple phenotypes’.
Acknowledgements
This study was supported by the Jilin University Award. The authors would like to thank Mr. Ming Chang and Mr. Yu G. Ma for their technical support.
References
- 1.Danièle N, Richard I, Bartoli M. Ins and outs of therapy in limb girdle muscular dystrophies. Int J Biochem Cell Biol. 2007;39:1608–1624. doi: 10.1016/j.biocel.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Erb W. Dystrophia muscularis progressiva. Dtsch Z Nervenheilkd. 1891;1:13–94. 173–261. (In German) [Google Scholar]
- 3.Leyden E. Klinik Der Rückenmarks-Krankheiten. Vol. 2. Hirschwald; Berlin: 1875. pp. 531–540. (In German) [Google Scholar]
- 4.Möbius PJ. Samml Klin Votr 171. Breitkopf und Härtel; Leipzig: 1879. Ueber die hereditären nervenkrankheiten; pp. 1505–1531. (In German) [Google Scholar]
- 5.Bell J. On pseudohypertrophic and allied types of progressive Muscular dystrophy. In: Fischer RA, editor. The Treasury of Human Inheritance. Part 4. Vol. 4. Cambridge University Press; London: 1943. pp. 283–342. [Google Scholar]
- 6.Walton JN, Nattrass FJ. On the classification, natural history and treatment of the myopathies. Brain. 1954;77:169–231. doi: 10.1093/brain/77.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Bushby KM, Gardner-Medwin D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I. Natural history. J Neurol. 1993;240:98–104. doi: 10.1007/BF00858725. [DOI] [PubMed] [Google Scholar]
- 8.Bushby KM. Diagnostic criteria for the limb-girdle muscular dystrophies: report of the ENMC Consortium on Limb-Girdle Dystrophies. Neuromuscul Disord. 1995;5:71–74. doi: 10.1016/0960-8966(93)e0006-g. [DOI] [PubMed] [Google Scholar]
- 9.Hauser MA, Horrigan SK, Salmikangas P, et al. Myotilin is mutated in limb girdle muscular dystrophy 1A. Hum Mol Genet. 2000;9:2141–2147. doi: 10.1093/hmg/9.14.2141. [DOI] [PubMed] [Google Scholar]
- 10.Muchir A, Bonne G, van der Kooi AJ, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 11.Minetti C, Sotgia F, Bruno C, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet. 1998;18:365–368. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SA, Salajegheh M, Judge DP, et al. Etiology of limb girdle muscular dystrophy 1D/1E determined by laser capture microdissection proteomics. Ann Neurol. 2012;71:141–145. doi: 10.1002/ana.22649. [DOI] [PubMed] [Google Scholar]
- 13.Harms MB, Sommerville RB, Allred P, et al. Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann Neurol. 2012;71:407–416. doi: 10.1002/ana.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarparanta J, Jonson PH, Golzio C, et al. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat Genet. 2012;44:450–455. doi: 10.1038/ng.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palenzuela L, Andreu AL, Gàmez J, et al. A novel autosomal dominant limb-girdle muscular dystrophy (LGMD 1F) maps to 7q32.1-32.2. Neurology. 2003;61:404–406. doi: 10.1212/01.wnl.0000073984.46546.4f. [DOI] [PubMed] [Google Scholar]
- 16.Starling A, Kok F, Passos-Bueno MR, Vainzof M, Zatz M. A new form of autosomal dominant limb-girdle muscular dystrophy (LGMD1G) with progressive fingers and toes flexion limitation maps to chromosome 4p21. Eur J Hum Genet. 2004;12:1033–1040. doi: 10.1038/sj.ejhg.5201289. [DOI] [PubMed] [Google Scholar]
- 17.Bisceglia L, Zoccolella S, Torraco A, et al. A new locus on 3p23-p25 for an autosomal-dominant limb-girdle muscular dystrophy, LGMD1H. Eur J Hum Genet. 2010;18:636–641. doi: 10.1038/ejhg.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard I, Broux O, Allamand V, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi S, McNally EM, Ben Othmane K, et al. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science. 1995;270:819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
- 21.Roberds SL, Leturcq F, Allamand V, et al. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell. 1994;78:625–633. doi: 10.1016/0092-8674(94)90527-4. [DOI] [PubMed] [Google Scholar]
- 22.Lim LE, Duclos F, Broux O, et al. Beta-sarcoglycan: characterization and role in limb-girdle muscular dystrophy linked to 4q12. Nat Genet. 1995;1:257–265. doi: 10.1038/ng1195-257. [DOI] [PubMed] [Google Scholar]
- 23.Nigro V, de Sá Moreira E, Piluso G, et al. Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the delta-sarcoglycan gene. Nat Genet. 1996;14:195–198. doi: 10.1038/ng1096-195. [DOI] [PubMed] [Google Scholar]
- 24.Moreira ES, Wiltshire TJ, Faulkner G, et al. Limb-girdle muscular dystrophy type 2G is caused by mutations in the gene encoding the sarcomeric protein telethonin. Nat Genet. 2000;24:163–166. doi: 10.1038/72822. [DOI] [PubMed] [Google Scholar]
- 25.Frosk P, Weiler T, Nylen E, et al. Limb-girdle muscular dystrophy type 2H associated with mutation in TRIM32, a putative E3-ubiquitin-ligase gene. Am J Hum Genet. 2002;70:663–672. doi: 10.1086/339083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brockington M, Yuva Y, Prandini P, et al. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001;10:2851–2859. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- 27.Haravuori H, Vihola A, Straub V, et al. Secondary calpain3 deficiency in 2q-linked muscular dystrophy: titin is the candidate gene. Neurology. 2001;56:869–877. doi: 10.1212/wnl.56.7.869. [DOI] [PubMed] [Google Scholar]
- 28.Balci B, Uyanik G, Dincer P, et al. An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord. 2005;15:271–275. doi: 10.1016/j.nmd.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Bolduc V, Marlow G, Boycott KM, et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet. 2010;86:213–221. doi: 10.1016/j.ajhg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey C, Escolar D, Brockington M, et al. Fukutin gene mutations in steroid-responsive limb girdle muscular dystrophy. Ann Neurol. 2006;60:603–610. doi: 10.1002/ana.21006. [DOI] [PubMed] [Google Scholar]
- 31.Biancheri R, Falace A, Tessa A, et al. POMT2 gene mutation in limb-girdle muscular dystrophy with inflammatory changes. Biochem Biophys Res Commun. 2007;363:1033–1037. doi: 10.1016/j.bbrc.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 32.Clement EM, Godfrey C, Tan J, et al. Mild POMGnT1 mutations underlie a novel limb-girdle muscular dystrophy variant. Arch Neurol. 2008;65:137–141. doi: 10.1001/archneurol.2007.2. [DOI] [PubMed] [Google Scholar]
- 33.Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, et al. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 2011;364:939–946. doi: 10.1056/NEJMoa1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nigro V, Aurino S, Piluso G. Limb girdle muscular dystrophies: update on genetic diagnosis and therapeutic approaches. Curr Opin Neurol. 2011;24:429–436. doi: 10.1097/WCO.0b013e32834aa38d. [DOI] [PubMed] [Google Scholar]
- 35.Gundesli H, Talim B, Korkusuz P, et al. Mutation in exon 1f of PLEC, leading to disruption of plectin isoform 1f, causes autosomal-recessive limb-girdle muscular dystrophy. Am J Hum Genet. 2010;87:834–841. doi: 10.1016/j.ajhg.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Nandelstadh P, Grönholm M, Moza M, Lamberg A, Savilahti H, Carpén O. Actin-organising properties of the muscular dystrophy protein myotilin. Exp Cell Res. 2005;310:131–139. doi: 10.1016/j.yexcr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Maraldi NM, Capanni C, Cenni V, Fini M, Lattanzi G. Laminopathies and lamin-associated signaling pathways. J Cell Biochem. 2011;112:979–992. doi: 10.1002/jcb.22992. [DOI] [PubMed] [Google Scholar]
- 38.Gazzerro E, Sotgia F, Bruno C, Lisanti MP, Minetti C. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet. 2010;18:137–145. doi: 10.1038/ejhg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schröder R, Schoser B. Myofibrillar myopathies: a clinical and myopathological guide. Brain Pathol. 2009;19:483–492. doi: 10.1111/j.1750-3639.2009.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ojima K, Ono Y, Ottenheijm C, et al. Non-proteolytic functions of calpain-3 in sarcoplasmic reticulum in skeletal muscles. J Mol Biol. 2011;407:439–449. doi: 10.1016/j.jmb.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ojima K, Kawabata Y, Nakao H, et al. Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J Clin Invest. 2010;120:2672–2683. doi: 10.1172/JCI40658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bansal D, Miyake K, Vogel SS, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 43.Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou P, Pinotsis N, Lange S, et al. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006;439:229–233. doi: 10.1038/nature04343. [DOI] [PubMed] [Google Scholar]
- 45.Shieh PB, Kudryashova E, Spencer MJ. Limb-girdle muscular dystrophy 2H and the role of TRIM32. Handb Clin Neurol. 2011;101:125–133. doi: 10.1016/B978-0-08-045031-5.00009-8. [DOI] [PubMed] [Google Scholar]
- 46.Brockington M, Blake DJ, Prandini P, et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isralewitz B, Gao M, Schulten K. Steered molecular dynamics and mechanical functions of proteins. Curr Opin Struct Biol. 2001;11:224–230. doi: 10.1016/s0959-440x(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 48.Akasaka-Manya K, Manya H, Nakajima A, Kawakita M, Endo T. Physical and functional association of human protein O-mannosyltransferases 1 and 2. J Biol Chem. 2006;281:19339–19345. doi: 10.1074/jbc.M601091200. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto T, Shibata N, Saito Y, Osawa M, Kobayashi M. Functions of fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy, in neuromuscular system and other somatic organs. Cent Nerv Syst Agents Med Chem. 2010;10:169–179. doi: 10.2174/187152410791196369. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida A, Kobayashi K, Manya H, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 51.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 52.Moza M, Mologni L, Trokovic R, Faulkner G, Partanen J, Carpén O. Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice. Mol Cell Biol. 2007;27:244–252. doi: 10.1128/MCB.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garvey SM, Liu Y, Miller SE, Hauser MA. Myotilin overexpression enhances myopathology in the LGMD1A mouse model. Muscle Nerve. 2008;37:663–667. doi: 10.1002/mus.20994. [DOI] [PubMed] [Google Scholar]
- 54.Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2009;18:241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawakami E, Kinouchi N, Adachi T, et al. Atelocollagen-mediated systemic administration of myostatin-targeting siRNA improves muscular atrophy in caveolin-3-deficient mice. Dev Growth Differ. 2011;53:48–54. doi: 10.1111/j.1440-169X.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 56.Bartoli M, Poupiot J, Vulin A, et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther. 2007;14:733–740. doi: 10.1038/sj.gt.3302928. [DOI] [PubMed] [Google Scholar]
- 57.Han R, Frett EM, Levy JR, et al. Genetic ablation of complement C3 attenuates muscle pathology in dysferlin-deficient mice. J Clin Invest. 2010;120:4366–4374. doi: 10.1172/JCI42390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallardo E, Rojas-García R, de Luna N, Pou A, Brown RH, Jr, Illa I. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology. 2001;57:2136–2138. doi: 10.1212/wnl.57.11.2136. [DOI] [PubMed] [Google Scholar]
- 59.Ohsawa Y, Okada T, Nishimatsu S, et al. An inhibitor of transforming growth factor beta type I receptor ameliorates muscle atrophy in a mouse model of caveolin 3-deficient muscular dystrophy. Lab Invest. 2012;92:1100–1114. doi: 10.1038/labinvest.2012.78. [DOI] [PubMed] [Google Scholar]
- 60.Bartoli M, Roudaut C, Martin S, et al. Safety and efficacy of AAV-mediated calpain 3 gene transfer in a mouse model of limb-girdle muscular dystrophy type 2A. Mol Ther. 2006;13:250–259. doi: 10.1016/j.ymthe.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 61.Albrecht DE, Rufibach LE, Williams BA, Monnier N, Hwang E, Mittal P. Neuromuscul Disord; 5th Annual Dysferlin Conference; 11–14, July 2011; Chicago, Illinois, USA. 2012. pp. 471–477. [DOI] [PubMed] [Google Scholar]
- 62.Albrecht DE, Garg N, Rufibach LE, et al. Neuromuscul Disord; 3rd Annual Dysferlin Conference; 2–5 June, 2009; Boston, Massachusetts, USA. 2009. pp. 867–873. [DOI] [PubMed] [Google Scholar]
- 63.Lostal W, Bartoli M, Bourg N, et al. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum Mol Genet. 2010;19:1897–1907. doi: 10.1093/hmg/ddq065. [DOI] [PubMed] [Google Scholar]
- 64.Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., Jr Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem Cells. 2004;22:981–993. doi: 10.1634/stemcells.22-6-981. [DOI] [PubMed] [Google Scholar]
- 65.Potgieter M, Pretorius E, Van der Merwe CF, et al. Histological assessment of SJL/J mice treated with the antioxidants coenzyme Q10 and resveratrol. Micron. 2011;42:275–282. doi: 10.1016/j.micron.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Cordier L, Hack AA, Scott MO, et al. Rescue of skeletal muscles of gamma-sarcoglycan-deficient mice with adeno-associated virus-mediated gene transfer. Mol Ther. 2000;1:119–129. doi: 10.1006/mthe.1999.0019. [DOI] [PubMed] [Google Scholar]
- 67.Bogdanovich S, McNally EM, Khurana TS. Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve. 2008;37:308–316. doi: 10.1002/mus.20920. [DOI] [PubMed] [Google Scholar]
- 68.Allikian MJ, Hack AA, Mewborn S, Mayer U, McNally EM. Genetic compensation for sarcoglycan loss by integrin alpha7beta1 in muscle. J Cell Sci. 2004;117:3821–3830. doi: 10.1242/jcs.01234. [DOI] [PubMed] [Google Scholar]
- 69.Allamand V, Donahue KM, Straub V, Davisson RL, Davidson BL, Campbell KP. Early adenovirus-mediated gene transfer effectively prevents muscular dystrophy in alpha-sarcoglycan-deficient mice. Gene Ther. 2000;7:1385–1391. doi: 10.1038/sj.gt.3301247. [DOI] [PubMed] [Google Scholar]
- 70.Galvez BG, Sampaolesi M, Brunelli S, et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minetti GC, Colussi C, Adami R, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–1150. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- 72.Dressman D, Araishi K, Imamura M, et al. Delivery of alpha- and beta-sarcoglycan by recombinant adeno-associated virus: efficient rescue of muscle, but differential toxicity. Hum Gene Ther. 2002;13:1631–1646. doi: 10.1089/10430340260201725. [DOI] [PubMed] [Google Scholar]
- 73.Hoshijima M, Hayashi T, Jeon YE, et al. Delta-sarcoglycan gene therapy halts progression of cardiac dysfunction, improves respiratory failure, and prolongs life in myopathic hamsters. Circ Heart Fail. 2011;4:89–97. doi: 10.1161/CIRCHEARTFAILURE.110.957258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwata Y, Katanosaka Y, Shijun Z, et al. Protective effects of Ca2+ handling drugs against abnormal Ca2+ homeostasis and cell damage in myopathic skeletal muscle cells. Biochem Pharmacol. 2005;70:740–751. doi: 10.1016/j.bcp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 75.Zhu T, Zhou L, Mori S, et al. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005;112:2650–2659. doi: 10.1161/CIRCULATIONAHA.105.565598. [DOI] [PubMed] [Google Scholar]
- 76.Henning RJ, Aufman J, Shariff M, et al. Human umbilical cord blood mononuclear cells decrease fibrosis and increase cardiac function in cardiomyopathy. Regen Med. 2010;5:45–54. doi: 10.2217/rme.09.71. [DOI] [PubMed] [Google Scholar]
- 77.Lapidos KA, Chen YE, Earley JU, et al. Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle. J Clin Invest. 2004;114:1577–1585. doi: 10.1172/JCI23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nomura T, Ashihara E, Tateishi K, et al. Skeletal myosphere-derived progenitor cell transplantation promotes neovascularization in delta-sarcoglycan knockdown cardiomyopathy. Biochem Biophys Res Commun. 2007;352:668–674. doi: 10.1016/j.bbrc.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 79.Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–1985. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goehringer C, Rutschow D, Bauer R, et al. Prevention of cardiomyopathy in delta-sarcoglycan knockout mice after systemic transfer of targeted adeno-associated viral vectors. Cardiovasc Res. 2009;82:404–410. doi: 10.1093/cvr/cvp061. [DOI] [PubMed] [Google Scholar]
- 81.Charton K, Danièle N, Vihola A, et al. Removal of the calpain 3 protease reverses the myopathology in a mouse model for titinopathies. Hum Mol Genet. 2010;19:4608–4624. doi: 10.1093/hmg/ddq388. [DOI] [PubMed] [Google Scholar]
- 82.Barresi R, Michele DE, Kanagawa M, et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 83.Straub V, Donahue KM, Allamand V, Davisson RL, Kim YR, Campbell KP. Contrast agent-enhanced magnetic resonance imaging of skeletal muscle damage in animal models of muscular dystrophy. Magn Reson Med. 2000;44:655–659. doi: 10.1002/1522-2594(200010)44:4<655::aid-mrm22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 84.Bartoli M, Poupiot J, Goyenvalle A, et al. Noninvasive monitoring of therapeutic gene transfer in animal models of muscular dystrophies. Gene Ther. 2006;13:20–28. doi: 10.1038/sj.gt.3302594. [DOI] [PubMed] [Google Scholar]
- 85.Mendell JR, Rodino-Klapac LR, Rosales XQ, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68:629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132:3175–3186. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gómez-Díaz B, Rosas-Vargas H, Roque-Ramírez B, et al. Immunodetection analysis of muscular dystrophies in Mexico. Muscle Nerve. 2012;45:338–345. doi: 10.1002/mus.22314. [DOI] [PubMed] [Google Scholar]
- 88.Diniz G, Eryaşar G, Türe S, et al. A regional panorama of dysferlinopathies. Turk Patoloji Derg. 2012;28:259–265. doi: 10.5146/tjpath.2012.01133. [DOI] [PubMed] [Google Scholar]
- 89.Magri F, Bo RD, D’Angelo MG, et al. Frequency and characterisation of anoctamin 5 mutations in a cohort of Italian limb-girdle muscular dystrophy patients. Neuromuscul Disord. 2012;22:934–943. doi: 10.1016/j.nmd.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guglieri M, Magri F, D’Angelo MG, et al. Clinical, molecular, and protein correlations in a large sample of genetically diagnosed Italian limb girdle muscular dystrophy patients. Hum Mutat. 2008;29:258–266. doi: 10.1002/humu.20642. [DOI] [PubMed] [Google Scholar]
- 91.Fanin M, Nascimbeni AC, Aurino S, et al. Frequency of LGMD gene mutations in Italian patients with distinct clinical phenotypes. Neurology. 2009;72:1432–1435. doi: 10.1212/WNL.0b013e3181a1885e. [DOI] [PubMed] [Google Scholar]
- 92.Urtasun M, Sáenz A, Roudaut C, et al. Limb-girdle muscular dystrophy in Guipúzcoa (Basque Country, Spain) Brain. 1998;121:1735–1747. doi: 10.1093/brain/121.9.1735. [DOI] [PubMed] [Google Scholar]
- 93.Walter MC, Petersen JA, Stucka R, et al. FKRP (826C>A) frequently causes limb-girdle muscular dystrophy in German patients. J Med Genet. 2004;41:e50. doi: 10.1136/jmg.2003.013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hicks D, Sarkozy A, Muelas N, et al. A founder mutation in Anoctamin 5 is a major cause of limb-girdle muscular dystrophy. Brain. 2011;134:171–182. doi: 10.1093/brain/awq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stensland E, Lindal S, Jonsrud C, et al. Prevalence, mutation spectrum and phenotypic variability in Norwegian patients with Limb Girdle Muscular Dystrophy 2I. Neuromuscul Disord. 2011;21:41–46. doi: 10.1016/j.nmd.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Sveen ML, Schwartz M, Vissing J. High prevalence and phenotype-genotype correlations of limb girdle muscular dystrophy type 2I in Denmark. Ann Neurol. 2006;59:808–815. doi: 10.1002/ana.20824. [DOI] [PubMed] [Google Scholar]
- 97.Penttila S, Palmio J, Suominen T, et al. Eight new mutations and the expanding phenotype variability in muscular dystrophy caused by ANO5. Neurology. 2012;78:897–903. doi: 10.1212/WNL.0b013e31824c4682. [DOI] [PubMed] [Google Scholar]
- 98.Lo HP, Cooper ST, Evesson FJ, et al. Limb-girdle muscular dystrophy: diagnostic evaluation, frequency and clues to pathogenesis. Neuromuscul Disord. 2008;18:34–44. doi: 10.1016/j.nmd.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 99.Moore SA, Shilling CJ, Westra S, et al. Limb-girdle muscular dystrophy in the United States. J Neuropathol Exp Neurol. 2006;65:995–1003. doi: 10.1097/01.jnen.0000235854.77716.6c. [DOI] [PubMed] [Google Scholar]
- 100.Dinçer PP, Leturcq F, Richard I, et al. A biochemical, genetic, and clinical survey of autosomal recessive limb girdle muscular dystrophies in Turkey. Ann Neurol. 1997;42:222–229. doi: 10.1002/ana.410420214. [DOI] [PubMed] [Google Scholar]
- 101.Pogoda TV, Krakhmaleva IN, Lipatova NA, Shakhovskaya NI, Shishkin SS, Limborska SA. High incidence of 550delA mutation of CAPN3 in LGMD2 patients from Russia. Hum Mutat. 2000;15:295. doi: 10.1002/(SICI)1098-1004(200003)15:3<295::AID-HUMU15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 102.Zatz M, de Paula F, Starling A, Vainzof M. The 10 autosomal recessive limb-girdle muscular dystrophies. Neuromuscul Disord. 2003;13:532–544. doi: 10.1016/s0960-8966(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 103.Chae J, Minami N, Jin Y, et al. Calpain 3 gene mutations: genetic and clinico-pathologic findings in limb-girdle muscular dystrophy. Neuromuscul Disord. 2001;11:547–555. doi: 10.1016/s0960-8966(01)00197-3. [DOI] [PubMed] [Google Scholar]
- 104.Hayashi S, Ohsawa Y, Takahashi T, et al. Rapid screening for Japanese dysferlinopathy by fluorescent primer extension. Intern Med. 2010;49:2693–2696. doi: 10.2169/internalmedicine.49.3771. [DOI] [PubMed] [Google Scholar]
- 105.Meena AK, Sreenivas D, Sundaram C, et al. Sarcoglycanopathies: a clinico-pathological study. Neurol India. 2007;55:117–121. doi: 10.4103/0028-3886.32781. [DOI] [PubMed] [Google Scholar]
- 106.Pathak P, Sharma MC, Sarkar C, et al. Limb girdle muscular dystrophy type 2A in India: a study based on semi-quantitative protein analysis, with clinical and histopathological correlation. Neurol India. 2010;58:549–554. doi: 10.4103/0028-3886.68675. [DOI] [PubMed] [Google Scholar]
- 107.Gayathri N, Alefia R, Nalini A, et al. Dysferlinopathy: spectrum of pathological changes in skeletal muscle tissue. Indian J Pathol Microbiol. 2011;54:350–354. doi: 10.4103/0377-4929.81636. [DOI] [PubMed] [Google Scholar]
- 108.Hadj Salem I, Kamoun F, Louhichi N, Trigui M, Triki C, Fakhfakh F. Impact of single-nucleotide polymorphisms at the TP53-binding and responsive promoter region of BCL2 gene in modulating the phenotypic variability of LGMD2C patients. Mol Biol Rep. 2012;39:7479–7486. doi: 10.1007/s11033-012-1581-4. [DOI] [PubMed] [Google Scholar]
- 109.Sinnreich M, Therrien C, Karpati G. Lariat branch point mutation in the dysferlin gene with mild limb-girdle muscular dystrophy. Neurology. 2006;66:1114–1116. doi: 10.1212/01.wnl.0000204358.89303.81. [DOI] [PubMed] [Google Scholar]
- 110.Tagawa K, Ogawa M, Kawabe K, et al. Protein and gene analyses of dysferlinopathy in a large group of Japanese muscular dystrophy patients. J Neurol Sci. 2003;211:23–28. doi: 10.1016/s0022-510x(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi T, Aoki M, Tateyama M, et al. Dysferlin mutations in Japanese Miyoshi myopathy: relationship to phenotype. Neurology. 2003;60:1799–1804. doi: 10.1212/01.wnl.0000068333.43005.12. [DOI] [PubMed] [Google Scholar]
- 112.Nagashima T, Chuma T, Mano Y, et al. Dysferlinopathy associated with rigid spine syndrome. Neuropathology. 2004;24:341–346. doi: 10.1111/j.1440-1789.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 113.Saccone V, Palmieri M, Passamano L, et al. Mutations that impair interaction properties of TRIM32 associated with limb-girdle muscular dystrophy 2H. Hum Mutat. 2008;29:240–247. doi: 10.1002/humu.20633. [DOI] [PubMed] [Google Scholar]
- 114.Kondo-lida E, Kobayashi K, Watanabe M, et al. Novel mutations and genotype-phenotype relationships in 107 families with Fukuyama-type congenital muscular dystrophy (FCMD) Hum Mol Genet. 1999;8:2303–2309. doi: 10.1093/hmg/8.12.2303. [DOI] [PubMed] [Google Scholar]
- 115.Scharner J, Gnocchi VF, Ellis JA, Zammit PS. Genotype-phenotype correlations in laminopathies: how does fate translate? Biochem Soc Trans. 2010;38:257–262. doi: 10.1042/BST0380257. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y, Ye J, Chen D, et al. Differential expression profiling between the relative normal and dystrophic muscle tissues from the same LGMD patient. J Transl Med. 2006;4:53. doi: 10.1186/1479-5876-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godfrey C, Clement E, Mein R, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130:2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 118.Klinge L, Dean AF, Kress W, et al. Late onset in dysferlinopathy widens the clinical spectrum. Neuromuscul Disord. 2008;18:288–290. doi: 10.1016/j.nmd.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 119.Rosales XQ, Gastier-Foster JM, Lewis S, et al. Novel diagnostic features of dysferlinopathies. Muscle Nerve. 2010;42:14–21. doi: 10.1002/mus.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Broglio L, Tentorio M, Cotelli MS, et al. Limb-girdle muscular dystrophy-associated protein diseases. Neurologist. 2010;16:340–352. doi: 10.1097/NRL.0b013e3181d35b39. [DOI] [PubMed] [Google Scholar]
- 121.Mercuri E, Brockington M, Straub V, et al. Phenotypic spectrum associated with mutations in the fukutin-related protein gene. Ann Neurol. 2003;53:537–542. doi: 10.1002/ana.10559. [DOI] [PubMed] [Google Scholar]
- 122.Gáti I, Danielsson O, Gunnarsson C, et al. Bent spine syndrome: a phenotype of dysferlinopathy or a symptomatic DYSF gene mutation carrier. Eur Neurol. 2012;67:300–302. doi: 10.1159/000336265. [DOI] [PubMed] [Google Scholar]
- 123.Hermans MC, Pinto YM, Merkies IS, de Die-Smulders CE, Crijns HJ, Faber CG. Hereditary muscular dystrophies and the heart. Neuromuscul Disord. 2010;20:479–492. doi: 10.1016/j.nmd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Filosto M, Tonin P, Vattemi G, et al. Chronic ophthalmoparesis in limb girdle muscular dystrophy 1C. J Neurol Neurosurg Psychiatry. 2009;80:448–449. doi: 10.1136/jnnp.2008.150540. [DOI] [PubMed] [Google Scholar]
- 125.Selcen D. Myofibrillar myopathies. Neuromuscul Disord. 2011;21:161–171. doi: 10.1016/j.nmd.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Palmieri A, Manara R, Bello L, et al. Cognitive profile and MRI findings in limb-girdle muscular dystrophy 2I. J Neurol. 2011;258:1312–1320. doi: 10.1007/s00415-011-5930-3. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Huang JJ, Wang ZQ, Wang N, Wu ZY. Value of muscle enzyme measurement in evaluating different neuromuscular diseases. Clin Chim Acta. 2012;413:520–524. doi: 10.1016/j.cca.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 128.Wattjes MP, Kley RA, Fischer D. Neuromuscular imaging in inherited muscle diseases. Eur Radiol. 2010;20:2447–2460. doi: 10.1007/s00330-010-1799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fischer D, Walter MC, Kesper K, et al. Diagnostic value of muscle MRI in differentiating LGMD2I from other LGMDs. J Neurol. 2005;252:538–547. doi: 10.1007/s00415-005-0684-4. [DOI] [PubMed] [Google Scholar]
- 130.Fischer D, Kley RA, Strach K, et al. Distinct muscle imaging patterns in myofibrillar myopathies. Neurology. 2008;71:758–765. doi: 10.1212/01.wnl.0000324927.28817.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Starling A, de Paula F, Silva H, Vainzof M, Zatz M. Calpainopathy: how broad is the spectrum of clinical variability? J Mol Neurosci. 2003;21:233–236. doi: 10.1385/jmn:21:3:233. [DOI] [PubMed] [Google Scholar]
- 132.Santoro L, Nolano M, Faraso S, et al. Perioral skin biopsy to study skeletal muscle protein expression. Muscle Nerve. 2010;41:392–398. doi: 10.1002/mus.21506. [DOI] [PubMed] [Google Scholar]
- 133.Nagaraju K, Rawat R, Veszelovszky E, et al. Dysferlin deficiency enhances monocyte phagocytosis: a model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol. 2008;172:774–785. doi: 10.2353/ajpath.2008.070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown RH, Jr, Amato A. Calpainopathy and eosinophilic myositis. Ann Neurol. 2006;59:875–877. doi: 10.1002/ana.20900. [DOI] [PubMed] [Google Scholar]
- 135.Baumeister SK, Todorovic S, Milić-Rasić V, Dekomien G, Lochmüller H, Walter MC. Eosinophilic myositis as presenting symptom in gamma-sarcoglycanopathy. Neuromuscul Disord. 2009;19:167–171. doi: 10.1016/j.nmd.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 136.Vinit J, Samson M, Jr, Gaultier JB, et al. Dysferlin deficiency treated like refractory polymyositis. Clin Rheumatol. 2010;29:103–106. doi: 10.1007/s10067-009-1273-1. [DOI] [PubMed] [Google Scholar]
- 137.Claeys KG, Fardeau M, Schröder R, et al. Electron microscopy in myofibrillar myopathies reveals clues to the mutated gene. Neuromuscul Disord. 2008;18:656–666. doi: 10.1016/j.nmd.2008.06.367. [DOI] [PubMed] [Google Scholar]
- 138.Cacciottolo M, Numitone G, Aurino S, et al. Muscular dystrophy with marked Dysferlin deficiency is consistently caused by primary dysferlin gene mutations. Eur J Hum Genet. 2011;19:974–980. doi: 10.1038/ejhg.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trabelsi M, Kavian N, Daoud F, et al. Revised spectrum of mutations in sarcoglycanopathies. Eur J Hum Genet. 2008;16:793–803. doi: 10.1038/ejhg.2008.9. [DOI] [PubMed] [Google Scholar]
- 140.Herrmann R, Straub V, Blank M, et al. Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum Mol Genet. 2000;9:2335–2340. doi: 10.1093/oxfordjournals.hmg.a018926. [DOI] [PubMed] [Google Scholar]
- 141.Vainzof M, Moreira ES, Suzuki OT, et al. Telethonin protein expression in neuromuscular disorders. Biochim Biophys Acta. 2002;1588:33–40. doi: 10.1016/s0925-4439(02)00113-8. [DOI] [PubMed] [Google Scholar]
- 142.Charlton R, Henderson M, Richards J, et al. Immunohistochemical analysis of calpain 3: advantages and limitations in diagnosing LGMD2A. Neuromuscul Disord. 2009;19:449–457. doi: 10.1016/j.nmd.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 143.Fanin M, Nascimbeni AC, Fulizio L, Trevisan CP, Meznaric-Petrusa M, Angelini C. Loss of calpain-3 autocatalytic activity in LGMD2A patients with normal protein expression. Am J Pathol. 2003;163:1929–1936. doi: 10.1016/S0002-9440(10)63551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fanin M, Nascimbeni AC, Tasca E, Angelini C. How to tackle the diagnosis of limb-girdle muscular dystrophy 2A. Eur J Hum Genet. 2009;17:598–603. doi: 10.1038/ejhg.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sáenz A, Leturcq F, Cobo AM, et al. LGMD2A: genotype-phenotype correlations based on a large mutational survey on the calpain 3 gene. Brain. 2005;128:732–742. doi: 10.1093/brain/awh408. [DOI] [PubMed] [Google Scholar]
- 146.Groen EJ, Charlton R, Barresi R, et al. Analysis of the UK diagnostic strategy for limb girdle muscular dystrophy 2A. Brain. 2007;130:3237–3249. doi: 10.1093/brain/awm259. [DOI] [PubMed] [Google Scholar]
- 147.Fanin M, Fulizio L, Nascimbeni AC, et al. Molecular diagnosis in LGMD2A: mutation analysis or protein testing? Hum Mutat. 2004;24:52–62. doi: 10.1002/humu.20058. [DOI] [PubMed] [Google Scholar]
- 148.Hackman P, Vihola A, Haravuori H, et al. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet. 2002;71:492–500. doi: 10.1086/342380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borg K, Stucka R, Locke M, et al. Intragenic deletion of TRIM32 in compound heterozygotes with sarcotubular myopathy/LGMD2H. Hum Mutat. 2009;30:E831–844. doi: 10.1002/humu.21063. [DOI] [PubMed] [Google Scholar]
- 150.Sewry CA. Muscular dystrophies: an update on pathology and diagnosis. Acta Neuropathol. 2010;120:343–358. doi: 10.1007/s00401-010-0727-5. [DOI] [PubMed] [Google Scholar]
- 151.Ho M, Gallardo E, McKenna-Yasek D, De Luna N, Illa I, Brown RH., Jr A novel, blood-based diagnostic assay for limb girdle muscular dystrophy 2B and Miyoshi myopathy. Ann Neurol. 2002;51:129–133. doi: 10.1002/ana.10080. [DOI] [PubMed] [Google Scholar]
- 152.Escher C, Lochmüller H, Fischer D, et al. Reverse protein arrays as novel approach for protein quantification in muscular dystrophies. Neuromuscul Disord. 2010;20:302–309. doi: 10.1016/j.nmd.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 153.Blázquez L, Azpitarte M, Sáenz A, et al. Characterization of novel CAPN3 isoforms in white blood cells: an alternative approach for limb-girdle muscular dystrophy 2A diagnosis. Neurogenetics. 2008;9:173–182. doi: 10.1007/s10048-008-0129-1. [DOI] [PubMed] [Google Scholar]
- 154.De Luna N, Freixas A, Gallano P, et al. Dysferlin expression in monocytes: a source of mRNA for mutation analysis. Neuromuscul Disord. 2007;17:69–76. doi: 10.1016/j.nmd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 155.Teer JK, Mullikin JC. Exome sequencing: the sweet spot before whole genomes. Hum Mol Genet. 2010;19:R145–151. doi: 10.1093/hmg/ddq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balci B, Aurino S, Haliloglu G, et al. Calpain-3 mutations in Turkey. Eur J Pediatr. 2006;165:293–298. doi: 10.1007/s00431-005-0046-3. [DOI] [PubMed] [Google Scholar]
- 157.Fanin M, Nascimbeni AC, Fulizio L, Angelini C. The frequency of limb girdle muscular dystrophy 2A in northeastern Italy. Neuromuscul Disord. 2005;15:218–224. doi: 10.1016/j.nmd.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 158.Takahashi T, Aoki M, Suzuki N, et al. Clinical features and a mutation with late onset of limb girdle muscular dystrophy 2B. J Neurol Neurosurg Psychiatry. 2012;84:433–440. doi: 10.1136/jnnp-2011-301339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park YE, Kim HS, Lee CH, Nam TS, Choi YC, Kim DS. Two common mutations (p.Gln832X and c.663+1G>C) account for about a third of the DYSF mutations in Korean patients with dysferlinopathy. Neuromuscul Disord. 2012;22:505–510. doi: 10.1016/j.nmd.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 160.Barthélémy F, Wein N, Krahn M, Lévy N, Bartoli M. Translational research and therapeutic perspectives in dysferlinopathies. Mol Med. 2011;17:875–882. doi: 10.2119/molmed.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hackman P, Juvonen V, Sarparanta J, et al. Enrichment of the R77C alpha-sarcoglycan gene mutation in Finnish LGMD2D patients. Muscle Nerve. 2005;31:199–204. doi: 10.1002/mus.20267. [DOI] [PubMed] [Google Scholar]
- 162.Schoser BG, Frosk P, Engel AG, Klutzny U, Lochmüller H, Wrogemann K. Commonality of TRIM32 mutation in causing sarcotubular myopathy and LGMD2H. Ann Neurol. 2005;57:591–595. doi: 10.1002/ana.20441. [DOI] [PubMed] [Google Scholar]
- 163.Kang PB, Feener CA, Estrella E, et al. LGMD2I in a North American population. BMC Musculoskelet Disord. 2007;8:115. doi: 10.1186/1471-2474-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Norwood F, de Visser M, Eymard B, Lochmüller H, Bushby K. EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. Eur J Neurol. 2007;14:1305–1312. doi: 10.1111/j.1468-1331.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- 165.Eagle M. Neuromuscul Disord; Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases; Newcastle. January 2002; 2002. pp. 975–983. [DOI] [PubMed] [Google Scholar]
- 166.Miladi N, Bourguignon JP, Hentati F. Cognitive and psychological profile of a Tunisian population of limb girdle muscular dystrophy. Neuromuscul Disord. 1999;9:352–354. doi: 10.1016/s0960-8966(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 167.Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 168.Lerario A, Cogiamanian F, Marchesi C, et al. Effects of rituximab in two patients with dysferlin-deficient muscular dystrophy. BMC Musculoskelet Disord. 2010;11:157. doi: 10.1186/1471-2474-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hattori H, Nagata E, Oya Y, et al. A novel compound heterozygous dysferlin mutation in Miyoshi myopathy siblings responding to dantrolene. Eur J Neurol. 2007;14:1288–1291. doi: 10.1111/j.1468-1331.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 170.Luna ND, Díaz-Manera J, Paradas C, et al. 1α,25(OH)(2)-Vitamin D3 increases dysferlin expression in vitro and in a human clinical trial. Mol Ther. 2012;20:1988–1997. doi: 10.1038/mt.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Walter MC, Reilich P, Thiele S, et al. Treatment of dysferlinopathy with deflazacort: a double-blind, placebo-controlled clinical trial. Orphanet J Rare Dis. 2013;8:26. doi: 10.1186/1750-1172-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Angelini C, Fanin M, Menegazzo E, Freda MP, Duggan DJ, Hoffman EP. Homozygous alpha-sarcoglycan mutation in two siblings: one asymptomatic and one steroid-responsive mild limb-girdle muscular dystrophy patient. Muscle Nerve. 1998;21:769–775. doi: 10.1002/(sici)1097-4598(199806)21:6<769::aid-mus9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 173.Darin N, Kroksmark AK, Ahlander AC, Moslemi AR, Oldfors A, Tulinius M. Inflammation and response to steroid treatment in limb-girdle muscular dystrophy 2I. Eur J Paediatr Neurol. 2007;11:353–357. doi: 10.1016/j.ejpn.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 174.Walter MC, Lochmüller H, Reilich P, et al. Creatine monohydrate in muscular dystrophies: A double-blind, placebo-controlled clinical study. Neurology. 2000;54:1848–1850. doi: 10.1212/wnl.54.9.1848. [DOI] [PubMed] [Google Scholar]