Abstract
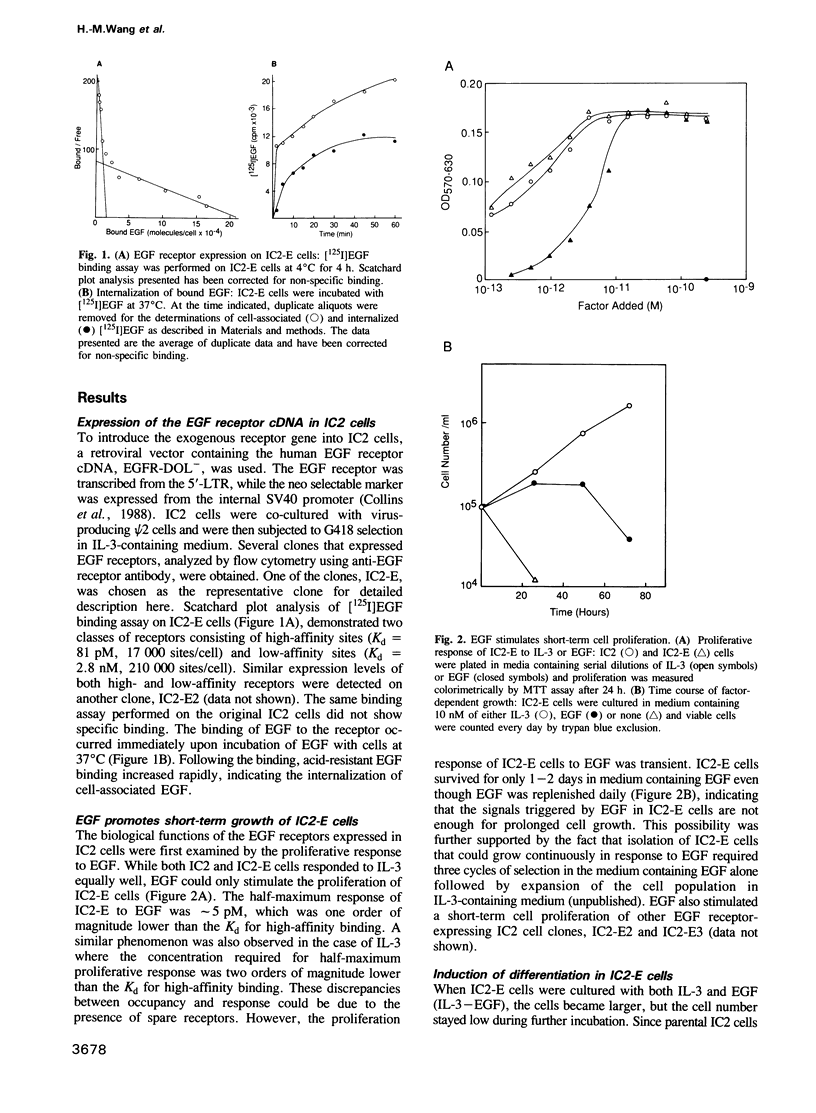
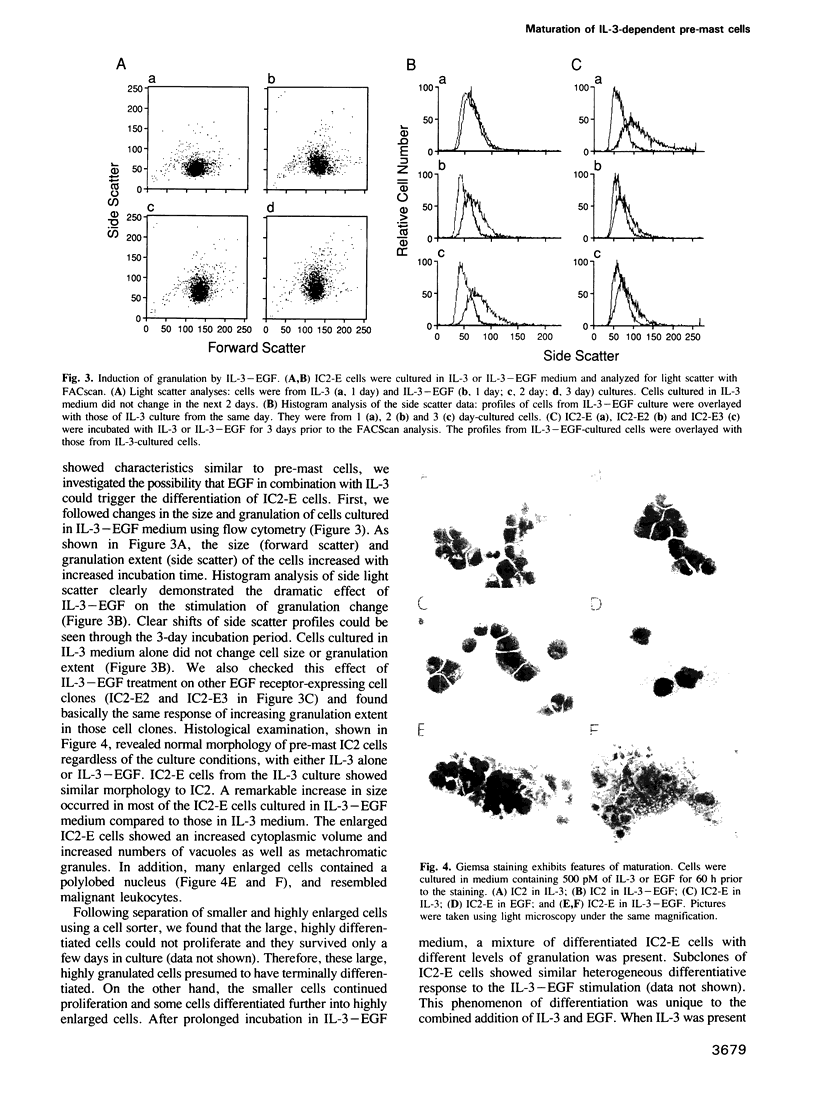
Interleukin 3 (IL-3) is a T cell-derived lymphokine that supports the growth and development of hematopoietic cells. Tyrosine phosphorylation has been suggested to play an important role in IL-3-dependent cell proliferation. To test whether a growth factor receptor carrying a tyrosine kinase can be functional in IL-3 dependent cells, we used a retroviral vector to introduce the human EGF receptor into a murine IL-3-dependent pre-mast cell line, IC2. The EGF receptors expressed on the infected clones bind EGF with both high and low affinities. EGF stimulates the infected cells for a short term growth response. In the presence of IL-3 and EGF, infected clones differentiate into more mature mast cells characterized by increases in intracellular granulation and histamine content. This differentiation is reversible when EGF is removed. EGF induces tyrosine phosphorylation of several cellular proteins and the expression of oncogenes c-fos and c-myc, in a manner analogous to IL-3 stimulation. These results indicate that the EGF receptor is functional in the pre-mast IC2 cells; EGF can support short-term proliferation and activates the signals that induce cell differentiation. Thus, EGF receptor-expressing IC2 cells provide a unique cellular system for in vitro study of mast cell differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai N., Nomura D., Yokota K., Wolf D., Brill E., Shohat O., Rotter V. Immunologically distinct p53 molecules generated by alternative splicing. Mol Cell Biol. 1986 Sep;6(9):3232–3239. doi: 10.1128/mcb.6.9.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Properties of the receptor for epidermal growth factor. Cell. 1984 Jun;37(2):357–358. doi: 10.1016/0092-8674(84)90365-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Downward J., Miyajima A., Maruyama K., Arai K., Mulligan R. C. Transfer of functional EGF receptors to an IL3-dependent cell line. J Cell Physiol. 1988 Nov;137(2):293–298. doi: 10.1002/jcp.1041370212. [DOI] [PubMed] [Google Scholar]
- Conscience J. F., Verrier B., Martin G. Interleukin-3-dependent expression of the c-myc and c-fos proto-oncogenes in hemopoietic cell lines. EMBO J. 1986 Feb;5(2):317–323. doi: 10.1002/j.1460-2075.1986.tb04215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Bernard O., Bowtell D., Schrader S., Schrader J. W. Murine c-myc retroviruses alter the growth requirements of myeloid cell lines. Oncogene Res. 1987 Jun;1(1):61–76. [PubMed] [Google Scholar]
- Dean M., Cleveland J. L., Rapp U. R., Ihle J. N. Role of myc in the abrogation of IL3 dependence of myeloid FDC-P1 cells. Oncogene Res. 1987 Aug;1(3):279–296. [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara H., Huang H. J., Arai N., Herzenberg L. A., Arai K., Zlotnik A. Interleukin 1 modulates messenger RNA levels of lymphokines and of other molecules associated with T cell activation in the T cell lymphoma LBRM33-1A5. J Immunol. 1987 Apr 15;138(8):2514–2519. [PubMed] [Google Scholar]
- Hume C. R., Nocka K. H., Sorrentino V., Lee J. S., Fleissner E. Constitutive c-myc expression enhances the response of murine mast cells to IL-3, but does not eliminate their requirement for growth factors. Oncogene. 1988 Mar;2(3):223–226. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Ihle J. N., Weinstein Y. Immunological regulation of hematopoietic/lymphoid stem cell differentiation by interleukin 3. Adv Immunol. 1986;39:1–50. doi: 10.1016/s0065-2776(08)60347-8. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Stevens D., May W. S., Ihle J. N. Interleukin 3 binds to a 140-kDa phosphotyrosine-containing cell surface protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7982–7986. doi: 10.1073/pnas.85.21.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R., Huhn R. D., Frackelton A. R., Jr, Ihle J. N. Stimulation of factor-dependent myeloid cell lines with interleukin 3 induces tyrosine phosphorylation of several cellular substrates. J Biol Chem. 1988 Dec 15;263(35):19203–19209. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Reversible dependence on growth factor interleukin-3 in myeloid cells expressing temperature sensitive v-abl oncogene. Oncogene Res. 1988 Feb;2(3):277–284. [PubMed] [Google Scholar]
- Koyasu S., Nakauchi H., Kitamura K., Yonehara S., Okumura K., Tada T., Yahara I. Production of interleukin 3 and gamma-interferon by an antigen-specific mouse suppressor T cell clone. J Immunol. 1985 May;134(5):3130–3136. [PubMed] [Google Scholar]
- Koyasu S., Tojo A., Miyajima A., Akiyama T., Kasuga M., Urabe A., Schreurs J., Arai K., Takaku F., Yahara I. Interleukin 3-specific tyrosine phosphorylation of a membrane glycoprotein of Mr 150,000 in multi-factor-dependent myeloid cell lines. EMBO J. 1987 Dec 20;6(13):3979–3984. doi: 10.1002/j.1460-2075.1987.tb02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livneh E., Prywes R., Kashles O., Reiss N., Sasson I., Mory Y., Ullrich A., Schlessinger J. Reconstitution of human epidermal growth factor receptors and its deletion mutants in cultured hamster cells. J Biol Chem. 1986 Sep 25;261(27):12490–12497. [PubMed] [Google Scholar]
- Miyajima A., Miyatake S., Schreurs J., De Vries J., Arai N., Yokota T., Arai K. Coordinate regulation of immune and inflammatory responses by T cell-derived lymphokines. FASEB J. 1988 Jun;2(9):2462–2473. doi: 10.1096/fasebj.2.9.2836253. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Schreurs J., Otsu K., Kondo A., Arai K., Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58(2-3):273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Curran T., Müller D., Guilbert L. Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature. 1985 Apr 11;314(6011):546–548. doi: 10.1038/314546a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Peterson L. Identification of distinct receptors for two hemopoietic growth factors (granulocyte colony-stimulating factor and multipotential colony-stimulating factor) by chemical cross-linking. J Biol Chem. 1986 Sep 15;261(26):12384–12389. [PubMed] [Google Scholar]
- Pandiella A., Beguinot L., Velu T. J., Meldolesi J. Transmembrane signalling at epidermal growth factor receptors overexpressed in NIH 3T3 cells. Phosphoinositide hydrolysis, cytosolic Ca2+ increase and alkalinization correlate with epidermal-growth-factor-induced cell proliferation. Biochem J. 1988 Aug 15;254(1):223–228. doi: 10.1042/bj2540223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for a multi-lineage colony-stimulating factor (CSF-2 alpha). J Biol Chem. 1986 Jan 5;261(1):205–210. [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Prywes R., Livneh E., Ullrich A., Schlessinger J. Mutations in the cytoplasmic domain of EGF receptor affect EGF binding and receptor internalization. EMBO J. 1986 Sep;5(9):2179–2190. doi: 10.1002/j.1460-2075.1986.tb04482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Salzman G. C., Crowell J. M., Martin J. C., Trujillo T. T., Romero A., Mullaney P. F., LaBauve P. M. Cell classification by laser light scattering: identification and separation of unstained leukocytes. Acta Cytol. 1975 Jul-Aug;19(4):374–377. [PubMed] [Google Scholar]
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988 May 3;27(9):3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- Schreurs J., Sugawara M., Arai K., Ohta Y., Miyajima A. A monoclonal antibody with IL-3-like activity blocks IL-3 binding and stimulates tyrosine phosphorylation. J Immunol. 1989 Feb 1;142(3):819–825. [PubMed] [Google Scholar]
- Watson J. D., Eszes M., Overell R., Conlon P., Widmer M., Gillis S. Effect of infection with murine recombinant retroviruses containing the v-src oncogene on interleukin 2- and interleukin 3-dependent growth states. J Immunol. 1987 Jul 1;139(1):123–129. [PubMed] [Google Scholar]
- Wheeler E. F., Askew D., May S., Ihle J. N., Sherr C. J. The v-fms oncogene induces factor-independent growth and transformation of the interleukin-3-dependent myeloid cell line FDC-P1. Mol Cell Biol. 1987 May;7(5):1673–1680. doi: 10.1128/mcb.7.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Huang S. J., Dexter T. M., Downes C. P. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proc Natl Acad Sci U S A. 1988 May;85(10):3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Molecular analysis of signal transduction by growth factors. Biochemistry. 1988 May 3;27(9):3113–3119. doi: 10.1021/bi00409a001. [DOI] [PubMed] [Google Scholar]
- von Rüden T., Wagner E. F. Expression of functional human EGF receptor on murine bone marrow cells. EMBO J. 1988 Sep;7(9):2749–2756. doi: 10.1002/j.1460-2075.1988.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]







