Abstract
Progressive multifocal leukoencephalopathy (PML) is a rare, demyelinating disease of the central nervous system caused by JC virus. Fewer than 30 cases have been reported in HIV-infected and non-infected children. We report the case of a 15 year-old girl with PML and AIDS who presented with nystagmus, dysarthria, and ataxia. Following combined antiretroviral therapy, she developed immune reconstitution inflammatory syndrome, which proved fatal.
Keywords: Progressive multifocal leukoencephalopathy (PML), JC virus, HIV, granule cell neuron, pediatric
CASE DESCRIPTION
A 15-year-old girl with a history of recurrent otitis media and bilateral herpes simplex virus keratitis presented with a six-week history of fatigue, 12 kg weight loss, right hand and leg clumsiness, diplopia, dysarthria and gait instability. Although the patient’s biological mother was HIV infected, the patient’s HIV status was unknown to her adoptive family. Details regarding the biological mother’s medical history, including HIV management, were unknown. Physical examination revealed an acneiform rash of the face, thrush, sustained nystagmus with up-, left- and rightward gaze, diplopia with rightward gaze, right lower face weakness, dysarthria, truncal ataxia, and a wide-based gait.
Laboratory tests revealed a white blood cell count of 2,580/mm3 and absolute CD4 count of 5 cells/mm3. Additional laboratory testing demonstrated a positive HIV-1 antibody and plasma viral load of 82,000 copies/mL. Cerebrospinal fluid was notable for protein of 30.3 mg/dL, glucose 51 mg/dL, white blood cell count 2 cells/mL, HIV-1 viral load 99,400 copies/mL, and JCV PCR 4,200 copies/mL. Cerebrospinal fluid gram stain, culture, CMV PCR, EBV PCR, HSV PCR, KOH, fungal culture, cryptococcal antigen, acid fast bacillus (AFB) stain, AFB culture, and toxoplasma PCR were negative. A brain MRI demonstrated patchy regions of T2 signal hyperintensity within the right cerebellar hemisphere, middle cerebellar peduncle, and diffusely within the pons and medulla (Figure 1A). Vascular ectasia of the supraclinoid internal carotid arteries was present bilaterally and consistent with a history of perinatal HIV infection.
Figure 1. Radiographic, pathologic, and histologic characteristics of PML.
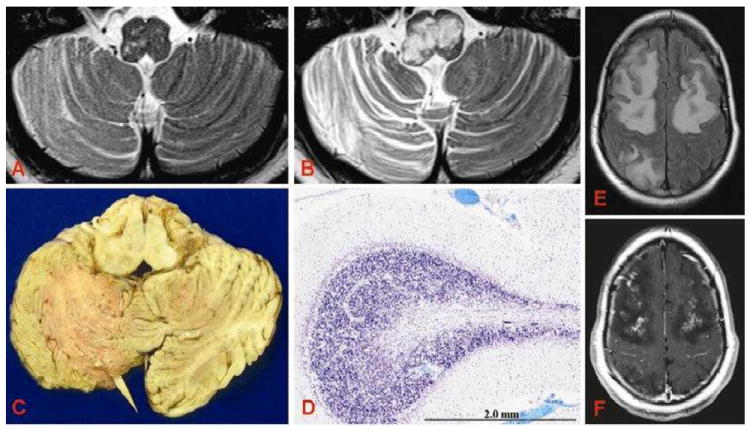
A, T2-weighted MRI images captured at the time of presentation and B, six weeks later (three days prior to death). C, Marked volume loss in the right cerebellar folia and distortion of the medulla are likewise seen at the gross level. D, Luxol fast blue (LFB) staining demonstrates marked destruction and demyelination of the cerebellar white matter. E, FLAIR and F, gadolinium-enhanced T1 MRI three days prior to death demonstrates extensive subcortical and deep white matter edema and contrast enhancement.
Based on the radiographic findings and positive JCV CSF PCR, the patient was diagnosed with AIDS and progressive multifocal leukoencephalopathy (PML). Combination antiretroviral therapy (cART) was initiated with nevirapine, tenofovir/emtricitabine and raltegravir. Her antiretrovirals were chosen based on their favorable penetration into the central nervous system. Shortly after cART initiation, the patient developed worsening dysarthria, dysphagia, and aspiration of liquids and a repeat MRI demonstrated new and increased bilateral medullary lesions concerning for immune reconstitution inflammatory syndrome (IRIS). Based on the interval radiographic changes and neurologic worsening, she was started on high-dose methylprednisolone followed by a prednisone taper. The patient’s neurologic status improved and she was discharged approximately one month following admission.
She was readmitted one week later for urinary incontinence, worsening dysarthria, new left hemiplegia, and inability to ambulate. A repeat MRI showed interval increase in T2-signal abnormalities of the brainstem and cerebellum. Given her significant clinical and radiographic progression, the patient was given a second course of methylprednisolone. Despite this, she had progressive weakness, inability to clear secretions, and developed generalized tonic-clonic seizures. Repeat MRI demonstrated new, diffuse supratentorial lesions and interval worsening of the infratentorial lesions (Figure 1B, E, F). She had worsening respiratory drive and a progressively diminished level of consciousness and died eight weeks following her initial presentation.
REVIEW
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system that results from infection with JC virus (JCV), a neurotropic polyomavirus whose only known reservoir is humans.1 While well-described among HIV-infected adults, PML has been rarely reported in immunosuppressed pediatric patients who are HIV-infected or have a primary immunodeficiency, cancer, or solid organ transplant.
Epidemiology
Epidemiologic seroprevalence surveys estimate that up to 86% of healthy adults have been exposed to JCV. One recent study demonstrated that JCV age-specific seroprevalence rises from about 16% in children 1-5 years of age to 34% by age 21-50 years.1, 2 This increase in JCV seroprevalence over time may explain why PML is relatively uncommon among children. Once infected, latent virus persists within reservoirs including the bone marrow, tonsillar B-lymphocytes and proximal renal tubule cells.3 Factors that determine whether the virus remains quiescent, reactivates, or progresses to cause PML are unknown.
PML has been reported largely among patients infected with HIV.3 A retrospective review of 13 Pediatric AIDS Clinical Trials Group (PACTG) studies conducted before the advent of cART revealed a PML event rate of 0.06 per 100 person years, with affected children identified at a median age of 10.8 years and median CD4 count of 6 cells/mm.4, 5 The rarity of PML among HIV-infected children was initially thought to be secondary to perinatally-infected children dying before they experienced primary JCV acquisition.6 Concerns that improved outcomes and prolonged survival would result in an increased incidence of PML among HIV-infected children have not been borne out.7
We identified 19 reported cases of PML among HIV-infected children (Table 1).6, 8-23 Of the 19 published reports, 12 patients acquired HIV via vertical maternal-to-child transmission, 4 via blood product transfusions, 1 from a contaminated needle, and 1 from an orthotopic liver transplant. The source of infection for one child was not reported. Twelve (63%) of the identified patients were male and the median age at PML diagnosis was 12 years (range 6-22 years). Only 3/19 (16%) of patients were newly-diagnosed with HIV at the time of PML presentation.6, 12, 18 CD4 count was <500 cells/mm3 in all but one patient. Viral load was reported in 5 cases and ranged from 11,800-292,000 copies/mL.
Table 1.
Characteristics of Previously Reported Pediatric Progressive Multifocal Leukoencephalopathy (PML) Cases in HIV-Infected Children
| Case | Reference | Age at PML diagnosis, years | Sex | Initial presentation | CD4 count (cells/mm3) Viral load (copies/mL) | Initial neuroradiology | PML diagnosis | Treatment | IRIS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Index | 15 | F | Dysarthria, fatigue, dysphagia, hemiparesis | CD4=5 | MRI: T2 hyperintensities of right cerebellum, midbrain, pons, medulla; vascular ectasia supraclinoid carotid arteries | CSF JCV PCR + Typical histopathology Immunohistochemistry Granule cell neuron infection | cART, steroids | Yes | Died | |
| VL=82,000 | ||||||||||
|
| ||||||||||
| 1 | 6 | 7 | M | Left upper and lower extremity weakness, drooling, difficulty eating | CD4=390 | MRI: Bilateral white matter hyperintensities of right subcortical/ periventricular region | Typical histopathology In situ hybridization + | AZT | No | Died |
| VL=NR | ||||||||||
|
| ||||||||||
| 2 | 8 | 13 | F | Numbness of tongue and chin, sialorrhea, dysarthria, dysphagia, muteness | CD4=7 | MRI: Prominent basal ganglia lesions, scattered white matter lesions | CSF JCV PCR + Typical histopathology In situ hybridization + | AZT, interferon | No | Died |
| VL=NR | ||||||||||
|
| ||||||||||
| 3 | 8 | 10 | M | Right facial palsy, right hemiparesis, aphasia | NR | MRI: Left frontal lobe lesion | Typical histopathology In situ hybridization + | None | No | Died |
|
| ||||||||||
| 4 | 22 | 8 | M | Right-sided hemiparesis, aphasia | NR | CT: Tortuous, dilated internal carotid, left middle/anterior cerebral arteries | Typical histopathology | AZT | No | Died |
|
| ||||||||||
| 5 | 9 | 10 | M | NR | NR | NR | NR | NR | No | NR |
|
| ||||||||||
| 6 | 9 | 12 | F | NR | NR | NR | NR | NR | No | NR |
|
| ||||||||||
| 7 | 19 | 13 | F | Blurred vision, headache, drooling, dysphagia, dysarthria | CD4=7 | MRI: Lesions of the corona radiata, centrum semiovale, basal ganglia, right periatrial white matter | CSF JCV PCR+ Typical histopathology In situ hybridization + | AZT, interferon | No | Died |
| VL=NR | ||||||||||
|
| ||||||||||
| 8 | 21 | 12 | M | NR | NR | NR | Typical histopathology | None | No | Died |
|
| ||||||||||
| 9 | 10 | 7 | M | Decreased activity, slurred speech, ataxia | CD4=0 | MRI: Prolonged T1/T2 relaxation in right cerebellar white matter, peduncle, pons | CSF JCV PCR+ Typical histopathology In situ hybridization+ | ddC, steroids, radiation therapy | No | Died |
| VL=NR | ||||||||||
|
| ||||||||||
| 10 | 23 | 11 | M | Fatigue, headache, unsteadiness, left- cerebellar symptoms | NR | MRI: Focal high T2 signal lesion in cerebellum/brainstem | Typical histopathology | AZT | No | Died |
|
| ||||||||||
| 11 | 11 | 12 | M | Left upper extremity weakness, left low extremity pain | CD4=9.5 | MRI: White matter lesions right frontal/parietal/occipital lobes | CSF JCV PCR + | cART | No | Hemiparesis |
| VL=76,000 | ||||||||||
|
| ||||||||||
| 12 | 12 | 13 | F | Headache, right hemiparesis | CD4=17 | MRI: Hyperintense T2 white matter lesions right cerebellar/pons | CSF JCV PCR + | cART, cidofovir | No | Died |
| VL=292,000 | ||||||||||
|
| ||||||||||
| 13 | 13 | 16.5 | M | Dysarthria, right facial palsy, drooling | CD4=36 | MRI: Diffuse confluent T2 areas of high signal in the bilateral corona radiata | CSF JCV PCR + | cART, cidofovir | No | Cerebellar dysfunction |
| VL=29,100 | ||||||||||
|
| ||||||||||
| 14 | 14 | 12 | M | Dizziness, headache, left arm weakness, left cerebellar dysfunction, left facial weakness | CD4=49 | MRI: Hyperintense lesions left cerebellar hemisphere, pons, medulla | CSF JCV PCR + | cART, steroids | Yes | Mild cerebellar dysfunction |
| VL=Undetectable | ||||||||||
|
| ||||||||||
| 15 | 15 | 8.5 | F | Change in mental status, spasticity of all four limbs, dystonia, seizures | CD4=320 | MRI: Asymmetrical subcortical, right frontoparietal, left occipitoparietal/basal ganglia lesions | Radiographic | cART | No | Dystonia |
| VL=NR | ||||||||||
|
| ||||||||||
| 16 | 20 | 6 | F | Short term memory loss, myoclonic and generalized seizures | NR | CT: Mineralization of left basal ganglia | CSF JCV PCR – Radiographic | None | No | LTFU |
|
| ||||||||||
| 17 | 16 | 22 | F | Speech difficulty, right facial drooping, right-sided weakness | CD4=10 | MRI: White matter lesions, left ventricular compression of left hemisphere | CSF JCV PCR + | cART, steroids | Yes | Progressive improvement |
| VL=11,800 | ||||||||||
|
| ||||||||||
| 18 | 17 | 15.5 | M | Dizziness, diplopia, fatigue, depression, ataxia | NR | MRI: Lesion of right cerebellar hemisphere with slight mass effect, right cerebellar peduncle | CSF JCV PCR+ Typical histopathology | cART | No | Died |
|
| ||||||||||
| 19 | 18 | 9 | M | Right-sided facial palsy, right-sided hemiplegia | CD4=4 | CT: Hypodense lesions left frontal lobe with lateral ventricle effacement | CSF JCV PCR+ | cART, steroids | Yes | Died |
| VL=185,976 | ||||||||||
PML, progressive multifocal leukoencephalopathy;
VL, viral load;
CSF, cerebrospinal fluid;
JCV, JC virus;
cART, combination antiretroviral therapy;
IRIS, immune reconstitution inflammatory syndrome;
NR, not reported;
AZT, zidovudine;
ddC, zalcitabine;
LTFU, lost to follow-up
PML has also been described in 10 children with a history of solid organ transplant, malignancy, or primary immunodeficiency, including hyperimmunoglobulin E, hyperimmunoglobulin M, Wiskott-Aldrich, and severe combined immunodeficiency (Table 2).24-33 PML presenting symptoms, radiographic findings, and histopathologic findings in these immunosuppressed children resembled those of HIV-infected children.
Table 2.
Characteristics of Previously Reported Pediatric Progressive Multifocal Leukoencephalopathy (PML) Cases in non-HIV-Infected Children
| Case | Reference | Age at PML diagnosis, years | Sex | Initial presentation | Underlying Condition | Initial neuroradiology | PML diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Index | 15 | F | Dysarthria, fatigue, dysphagia, hemiparesis | HIV Infection | MRI: T2 hyperintensities of right cerebellum, midbrain, pons, medulla; vascular ectasia supraclinoid carotid arteries | CSF JCV PCR + Typical histopathology Immunohistochemistry Granule cell neuron infection | cART, steroids | Died | |
|
| |||||||||
| 1 | 24 | 15 | M | Nausea, headache, impaired ocular movements, anarthria, aphasia | 6mo post-BMT for WAS; immunosuppression not reported | MRI: High T2 signal in right occipital lobe, cerebellum, pons, right corona radiate, left thalamus, and basal ganglia | CSF JCV PCR + Typical histopathology | Acyclovir, ribavirin, IFN-α | Died |
|
| |||||||||
| 2 | 25 | 11 | M | Vomiting, fever, somnolence | 3.5y post-deceased donor renal transplant on MMF/prednisolone/rapamycin immunosuppression; CVID | MRI: Enhancing lesions of right thalamic region, brain stem, parietal medullary layer | Serum JCV PCR + Radiographic | Cidofovir MMF discontinued | Complete resolution |
|
| |||||||||
| 3 | 26 | 6 | M | Right-sided hemiparesis, memory deficits, decreased volume/fluency of speech, hyperreflexia | Hyper-IgM | MRI: Multifocal white matter lesions involving the left cerebral hemisphere and bilateral thalami | CSF JCV PCR + Brain biopsy JCV PCR + Typical histopathology | Cytarabine | Died |
|
| |||||||||
| 4 | 27 | 8 | M | Cognitive slowness, left facial muscle weakness | HIES | MRI: Hyperintense lesions of right frontal white matter, internal capsule, and right thalamus | CSF JCV PCR + Typical histopathology Immunohistochemistry | Cidofovir | Died |
|
| |||||||||
| 5 | 28 | 6 | M | Lethargy, left-sided hemiparesis, seizures | ALL (in remission for 2.5y) | MRI: Contrast enhancement with loss of cortical sulci due to cortical edema | Brain biopsy JCV PCR + Typical histopathology | Amantadine | Hemiparesis, pale optic disks, no light perception |
|
| |||||||||
| 6 | 29 | 7 | M | Left facial twitching and weakness, impaired vision | ALL (in remission for 2y) | MRI: Hyperintense lesions of gray-white interface involving the right frontal, parietal and occipital regions | Radiographic | NR | Died |
|
| |||||||||
| 7 | 30 | 15 | M | Right-sided weakness, behavior changes, decreased speech output | WAS | MRI: Multiple high-intensity lesions in brain stem and cerebrum, including left thalamus and basal ganglia | PBL JCV PCR + Typical histopathology Immunohistochemistry | Dexamethasone | Died |
|
| |||||||||
| 8 | 31 | 9 | M | Seizure, cognitive deficits, progressive spastic paraparesis | PNP Deficiency | MRI: T2-hyperintense lesions in the white matter of both hemispheres, particularly affecting the parietal regions | CSF JCV PCR+ | IVIG Exchange transfusion Trimethoprim-sulfamethoxazole | Died |
|
| |||||||||
| 9 | 32 | 16 | M | Mental status changes, decreased communication, nystagmus | 3y post cadaveric renal transplant on MMF/prednisone immunosuppression | MRI: T2-hyperintense lesions of midbrain, basal ganglia, cerebral white matter with more lesions on right | CSF JCV PCR + Brain biopsy JCV PCR + Typical histopathology Viral particles on EM | NR | Died |
|
| |||||||||
| 10 | 33 | 11 | M | Right-sided hemiparesis, irritability, aphasia, apraxia, inability to walk | SCID | NR | Typical histopathology Immunohistochemistry | NR | Died |
PML, progressive multifocal leukoencephalopathy;
CSF, cerebrospinal fluid;
JCV, JC virus;
cART, combination antiretroviral therapy;
BMT, bone marrow transplant;
IFN-α, interferon-alpha;
SCID, severe combined immunodeficiency;
NR, not reported;
WAS, Wiskott-Aldrich syndrome;
MMF, mycophenolate mofetil;
CVID, common variable immunodeficiency;
Hyper-IgM, hyperimmunoglobulin M syndrome;
HIES, hyperimmunoglobulin E recurrent infection syndrome;
ALL, acute lymphoblastic leukemia;
PBL, peripheral blood lymphocytes;
PNP, purine nucleoside phosphorylase;
IVIG, intravenous immunoglobulin;
EM, electron microscopy
Clinical Presentation
Clinical manifestations of PML are variable and depend on the region of the central nervous system affected. Symptoms are likely the result of demyelination that occurs due to lytic oligodendrocyte infection. The most commonly reported signs among adults include paresis, speech abnormalities, gait disturbances, ataxia and cranial nerve palsies.34, 35 Our review revealed similar findings in children, including hemiparesis, ataxia, and dysarthria. Seizures occur in 18% of PML adult patients and were noted in 4/29 (14%) of the pediatric cases.15, 20, 28, 31, 36
Neuroimaging
MRI is the most widely utilized radiographic modality for the evaluation of PML. Typical radiographic features include a single or multiple non-space-occupying, T2-hyperintense, T1-hypointense lesions without associated edema. These lesions commonly involve the frontal and parieto-occipital subcortical white matter.37 Involvement tends to be asymmetric with relative sparing of the periventricular white matter. PML lesions have also been described in gray matter structures and the spinal cord.38, 39 Enhancement of PML lesions in patients not undergoing immune reconstitution was once thought to be atypical, although this assumption has been challenged by more recent reports.9, 40 As in adults, lesions in the 25 pediatric PML cases with radiographic information predominantly involved white matter and most (84%) demonstrated no enhancement with gadolinium contrast. Posterior fossa and brainstem involvement was common. In the present case, areas of enhancement were noted in the right basal ganglia, medulla, and cerebellum; histopathology from these areas was consistent with PML. Cortical enhancement noted shortly before her death, however, was not consistent with PML on histopathology (Figure 1E, F).
Pathology
Characteristic histopathologic features of PML in children, as in adults, include foci of demyelination involving the white matter and gray-white junction, lesions with abundant lipid-laden macrophages and bizarre astrocytes, and enlarged oligodendrocytes with intranuclear inclusions. The case patient’s pathology included multiple foci of demyelination in the brainstem and cerebellum, featuring oligodendrocytes with large, ground-glass nuclei and large, bizarre astrocytes with prominent nucleoli (Figure 1D, Figure 2A, B). In addition, there were intraparenchymal infiltrates of CD163+ macrophages and CD8+ T lymphocytes.
Figure 2. Immunohistologic characteristics of PML.
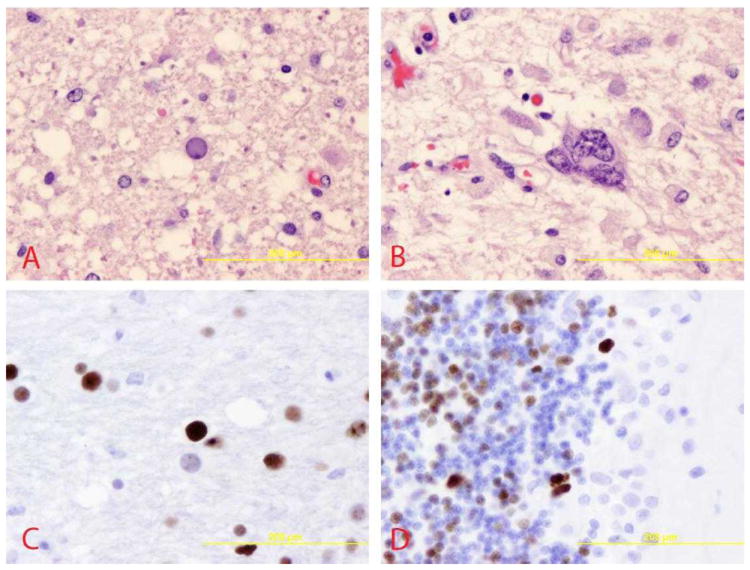
A, Infected and diseased oligodendrocytes showing enlarged, ground-glass appearing nuclei, mostly found at the edges of demyelination (pons, 400X). B, Bizarre, multi-nucleated astrocytes often found within demyelinated lesions (pons, 400X). C, Immunohistochemistry staining, highlighting JCV-infected oligodendrocytes (pons, 400X). D, and JCV-infected granule cell neurons of the cerebellum (400X).
In contrast to published pediatric cases, JCV immunoreactivity was visualized within both oligodendrocytes and cerebellar granule cell neurons in our case patient (SV40 antibody staining, Figure 2C, D). Further staining of the granule cell layer for JCV T Ag and VP1 protein expression was notable for a predominance of granule cell neurons expressing T Ag only, consistent with early or restricted infection, with rare VP1-expressing cells indicating productive infection. These findings are consistent with earlier observations that although JCV infection was initially thought to be limited to glial cells, restrictive granule cell neuron infection may also be important in pathogenesis.41
Laboratory Diagnostics
A definitive diagnosis of PML can be established by detection of JCV DNA in the CSF or viral proteins on brain biopsy. In adults with PML the reported sensitivity of JCV DNA CSF PCR is 58%, with an estimated specificity of 100%.42 Given the relatively low sensitivity, cases with characteristic clinical and neurologic manifestations can be categorized as having ‘possible PML’ even in the absence of positive molecular diagnostics.43 The majority of reported pediatric cases had a positive JCV CSF PCR (17/29, 59%).
CSF white cell count, protein, and glucose are usually normal to slightly elevated in patients with PML.34, 44 Among the reported cases in HIV-infected children, none had pleocytosis and all had a normal glucose (48-92 mg/dL) and a normal to slightly elevated protein (12-76 mg/dL).6, 10, 11, 13-16, 19, 20, 23, 34 Among case reports in children without HIV, the CSF profiles, including cell count, glucose, and protein, were also within normal limits.26, 28, 30-32
Treatment
Immune system restoration is the mainstay of PML management. Although cART does not have direct anti-JCV activity, survival among HIV-infected adults with PML has increased from 10% in the pre-cART era to 50% with cART.45, 46 The effects of antiretroviral drugs on PML outcomes in children are unknown; only 7/19 (37%) of published cases initiated cART at the time of their PML diagnosis.11-16, 18
Additional treatment modalities have been used for the management of PML. Inconsistent improvements in clinical progression have been reported in adults treated with cidofovir, cytarabine, or alpha-interferon therapy.47, 48 Among the HIV-infected pediatric patients, two were treated with cidofovir and had improvement in their neurologic status.12, 13 Two HIV-uninfected children were treated with cidofovir. One patient had complete resolution of neurologic symptoms, although this patient’s immunosuppression was also reduced as part of their PML management.25, 27
Immune Reconstitution Inflammatory Syndrome
In a subset of patients with PML, inflammation and an apparent increase in JCV-mediated tissue destruction follow the suppression of HIV infection with cART. This PML-associated IRIS is a severe, often fatal, complication. Histologically, the syndrome is marked by infiltration of brain parenchyma by CD8+ lymphocytes, as was noted in the case patient.40
PML-IRIS occurs in 19-31% of adult patients with PML, most often within 1 week to 26 months of cART initiation.49-51 Among the 19 reported cases of PML in HIV-infected children, 3 (16%) had clinical courses concerning for PML-IRIS, with the onset of neurologic symptoms 12-28 days after starting cART.14, 16, 18
Steroids have been used for PML-IRIS, especially in cases of rapid clinical deterioration or when neuroimaging demonstrates inflammation. In one series of adults who received steroids, 58% had good neurologic recovery; those who survived received steroids earlier in their course and had contrast enhancement of their PML lesions on MRI.51 All 4 reported cases of HIV-infected children with suspected PML-IRIS received steroids; two had a protracted neurologic course and two died.
Prognosis
The prognosis of PML in children is poor. Though the prognosis for HIV-infected patients with PML has improved with the introduction of cART, the lack of an agent with activity against JCV has hampered efforts to improve overall survival. Among pediatric PML cases, only 5/16 (31%) HIV-infected patients and 2/10 (20%) patients without HIV survived.11, 13-16, 25, 28 The remainder died within one year of PML diagnosis, most by 2-6 months. The majority of survivors had long-term neurologic deficits including residual hemiparesis and cerebellar dysfunction.
Several factors, including JCV CSF viral load, CD4 count >100 cells/mm3, contrast enhancement on radiographic imaging, evidence of neurologic function recovery, and the presence of JCV-specific cytotoxic T-cells, have been suggested to predict survival among HIV-infected adults with PML.52-57 Specific predictors of mortality in children remain to be elucidated.
Acknowledgments
source of funding: This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) T32 AI 007433 (L.R.); Agency for Healthcare Research and Quality (AHRQ) T32 HS 019485-01; National Institute of Child Health and Human Development (NICHD) T32 HD 055148-02 (L.R.), R56 NS 041198, R01 NS 047029, R01 NS 074995 and K24 NS 060950 (I.J.K).
Footnotes
Conflicts of interest
The authors have no additional financial relationships or conflicts of interest relevant to this article to disclose.
References
- 1.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger JR, Chauhan A, Galey D, Nath A. Epidemiological evidence and molecular basis of interactions between HIV and JC virus. J Neurovirol. 2001;7:329–338. doi: 10.1080/13550280152537193. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DM. HIV-associated PML: changing epidemiology and clinical approach. Cleveland Clinic journal of medicine. 2011;78(Suppl 2):S24–27. doi: 10.3949/ccjm.78.s2.06. [DOI] [PubMed] [Google Scholar]
- 5.Dankner WM, Lindsey JC, Levin MJ. Pediatric ACTGPT. Correlates of opportunistic infections in children infected with the human immunodeficiency virus managed before highly active antiretroviral therapy. The Pediatric infectious disease journal. 2001;20:40–48. doi: 10.1097/00006454-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Vandersteenhoven JJ, Dbaibo G, Boyko OB, et al. Progressive multifocal leukoencephalopathy in pediatric acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1992;11:232–237. doi: 10.1097/00006454-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Buchacz K, Baker RK, Palella FJ, Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994-2007: a cohort study. AIDS. 2010;24:1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 8.Berger JR, Scott G, Albrecht J, Belman AL, Tornatore C, Major EO. Progressive multifocal leukoencephalopathy in HIV-1-infected children. AIDS. 1992;6:837–841. doi: 10.1097/00002030-199208000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993;187:233–240. doi: 10.1148/radiology.187.1.8451420. [DOI] [PubMed] [Google Scholar]
- 10.Morriss MC, Rutstein RM, Rudy B, Desrochers C, Hunter JV, Zimmerman RA. Progressive multifocal leukoencephalopathy in an HIV-infected child. Neuroradiology. 1997;39:142–144. doi: 10.1007/s002340050383. [DOI] [PubMed] [Google Scholar]
- 11.Inui K, Miyagawa H, Sashihara J, et al. Remission of progressive multifocal leukoencephalopathy following highly active antiretroviral therapy in a patient with HIV infection. Brain Dev. 1999;21:416–419. doi: 10.1016/s0387-7604(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 12.Hugonenq C, Lethel V, Chambost H, Michel G, Chabrol B, Mancini J. Progressive multifocal leukoencephalopathy revealing AIDS in a 13-year-old girl. Arch Pediatr. 2002;9:32–35. doi: 10.1016/s0929-693x(01)00691-1. [DOI] [PubMed] [Google Scholar]
- 13.Robinson LG, Chiriboga CA, Champion SE, Ainyette I, DiGrado M, Abrams EJ. Progressive multifocal leukoencephalopathy successfully treated with highly active antiretroviral therapy and cidofovir in an adolescent infected with perinatal human immunodeficiency virus (HIV) J Child Neurol. 2004;19:35–38. doi: 10.1177/088307380401900107011. [DOI] [PubMed] [Google Scholar]
- 14.Nuttall JJ, Wilmshurst JM, Ndondo AP, et al. Progressive multifocal leukoencephalopathy after initiation of highly active antiretroviral therapy in a child with advanced human immunodeficiency virus infection: a case of immune reconstitution inflammatory syndrome. Pediatr Infect Dis J. 2004;23:683–685. doi: 10.1097/01.inf.0000130954.41818.07. [DOI] [PubMed] [Google Scholar]
- 15.Shah I, Chudgar P. Progressive multifocal leukoencephalopathy (PML) presenting as intractable dystonia in an HIV-infected child. J Trop Pediatr. 2005;51:380–382. doi: 10.1093/tropej/fmi034. [DOI] [PubMed] [Google Scholar]
- 16.Ch’ng TW, Dieudonne A. Immune reconstitution inflammatory syndrome associated with progressive multifocal leukoencephalopathy in a perinatally acquired human immunodeficiency virus-infected young adult. Pediatr Infect Dis J. 2007;26:1068–1070. doi: 10.1097/INF.0b013e31812e62fa. [DOI] [PubMed] [Google Scholar]
- 17.Liptai Z, Papp E, Barsi P, et al. Progressive multifocal leukoencephalopathy in an HIV-infected child. Neuropediatrics. 2007;38:32–35. doi: 10.1055/s-2007-981482. [DOI] [PubMed] [Google Scholar]
- 18.Oberdorfer P, Washington CH, Katanyuwong K, Jittamala P. Progressive Multifocal Leukoencephalopathy in HIV-Infected Children: A Case Report and Literature Review. Int J Pediatr. 2009;2009:348507. doi: 10.1155/2009/348507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer C, Berger JR, Bowen BC, Bruce JH, Weiner WJ. Akinetic-rigid syndrome in a 13-year-old girl with HIV-related progressive multifocal leukoencephalopathy. Mov Disord. 1993;8:113–116. doi: 10.1002/mds.870080120. [DOI] [PubMed] [Google Scholar]
- 20.Wilmshurst JM, Burgess J, Hartley P, Eley B. Specific neurologic complications of human immunodeficiency virus type 1 (HIV-1) infection in children. J Child Neurol. 2006;21:788–794. doi: 10.1177/08830738060210091901. [DOI] [PubMed] [Google Scholar]
- 21.Wrzolek MA, Brudkowska J, Kozlowski PB, et al. Opportunistic infections of the central nervous system in children with HIV infection: report of 9 autopsy cases and review of literature. Clin Neuropathol. 1995;14:187–196. [PubMed] [Google Scholar]
- 22.Lang C, Jacobi G, Kreuz W, et al. Rapid development of giant aneurysm at the base of the brain in an 8-year-old boy with perinatal HIV infection. Acta Histochem Suppl. 1992;42:83–90. [PubMed] [Google Scholar]
- 23.Araujo AP, Pereira HS, Oliveira RH, Frota AC, Esperanca JC, Duarte F. Progressive multifocal leukoencephalopathy in a child with acquired immunodeficiency syndrome (AIDS) Arq Neuropsiquiatr. 1997;55:122–125. doi: 10.1590/s0004-282x1997000100019. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda Y, Yabe H, Inoue H, et al. Progressive multifocal leukoencephalopathy after allogeneic bone marrow transplantation for Wiskott-Aldrich syndrome. Pediatr Int. 2008;50:238–240. doi: 10.1111/j.1442-200X.2008.02549.x. [DOI] [PubMed] [Google Scholar]
- 25.Weber SC, Uhlenberg B, Raile K, Querfeld U, Muller D. Polyoma virus-associated progressive multifocal leukoencephalopathy after renal transplantation: regression following withdrawal of mycophenolate mofetil. Pediatr Transplant. 2011;15:E19–24. doi: 10.1111/j.1399-3046.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 26.Redfearn A, Pennie RA, Mahony JB, Dent PB. Progressive multifocal leukoencephalopathy in a child with immunodeficiency and hyperimmunoglobulinemia M. The Pediatric infectious disease journal. 1993;12:399–401. doi: 10.1097/00006454-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Angelini L, Pietrogrande MC, Delle Piane MR, et al. Progressive multifocal leukoencephalopathy in a child with hyperimmunoglobulin E recurrent infection syndrome and review of the literature. Neuropediatrics. 2001;32:250–255. doi: 10.1055/s-2001-19119. [DOI] [PubMed] [Google Scholar]
- 28.Demir E, Liebert UG, Soylemezoglu F, Yalaz K, Kose G, Anlar B. Childhood case of progressive multifocal leukoencephalopathy with improved clinical outcome. J Child Neurol. 2005;20:241–244. doi: 10.1177/08830738050200031301. [DOI] [PubMed] [Google Scholar]
- 29.Ganguly S, Ganguly SB, Biswas K. Progressive multifocal leukoencephalopathy in a case of acute lymphocytic leukemia. Indian Pediatr. 1995;32:684–686. [PubMed] [Google Scholar]
- 30.Katz DA, Berger JR, Hamilton B, Major EO, Post MJ. Progressive multifocal leukoencephalopathy complicating Wiskott-Aldrich syndrome. Report of a case and review of the literature of progressive multifocal leukoencephalopathy with other inherited immunodeficiency states. Arch Neurol. 1994;51:422–426. doi: 10.1001/archneur.1994.00540160128016. [DOI] [PubMed] [Google Scholar]
- 31.Parvaneh N, Ashrafi MR, Yeganeh M, Pouladi N, Sayarifar F, Parvaneh L. Progressive multifocal leukoencephalopathy in purine nucleoside phosphorylase deficiency. Brain Dev. 2007;29:124–126. doi: 10.1016/j.braindev.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Phillips T, Jacobs R, Ellis EN. Polyoma nephropathy and progressive multifocal leukoencephalopathy in a renal transplant recipient. J Child Neurol. 2004;19:301–304. doi: 10.1177/088307380401900412. [DOI] [PubMed] [Google Scholar]
- 33.Bledsoe T. Progressive multifocal leukoencephalopathy in a child with severe combined immunodeficiency. N Engl J Med. 1978;299:257–258. doi: 10.1056/NEJM197808032990516. [DOI] [PubMed] [Google Scholar]
- 34.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 35.Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2003;36:1047–1052. doi: 10.1086/374048. [DOI] [PubMed] [Google Scholar]
- 36.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 37.Weber T. Progressive multifocal leukoencephalopathy. Neurol Clin. 2008;26:833–854. x–xi. doi: 10.1016/j.ncl.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Bernal-Cano F, Joseph JT, Koralnik IJ. Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J Neurovirol. 2007;13:474–476. doi: 10.1080/13550280701469178. [DOI] [PubMed] [Google Scholar]
- 39.Mark AS, Atlas SW. Progressive multifocal leukoencephalopathy in patients with AIDS: appearance on MR images. Radiology. 1989;173:517–520. doi: 10.1148/radiology.173.2.2798883. [DOI] [PubMed] [Google Scholar]
- 40.Huang D, Cossoy M, Li M, et al. Inflammatory progressive multifocal leukoencephalopathy in human immunodeficiency virus-negative patients. Ann Neurol. 2007;62:34–39. doi: 10.1002/ana.21085. [DOI] [PubMed] [Google Scholar]
- 41.Wuthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;68:15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43:4175–4177. doi: 10.1128/JCM.43.8.4175-4177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol. 2003;9(Suppl 1):88–92. doi: 10.1080/13550280390195298. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Kirby JE, Qian Q. Effective use of JC virus PCR for diagnosis of progressive multifocal leukoencephalopathy. J Med Microbiol. 2009;58:253–255. doi: 10.1099/jmm.0.004432-0. [DOI] [PubMed] [Google Scholar]
- 45.Falco V, Olmo M, del Saz SV, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr. 2008;49:26–31. doi: 10.1097/QAI.0b013e31817bec64. [DOI] [PubMed] [Google Scholar]
- 46.Giancola ML, Rizzi EB, Lorenzini P, et al. Progressive multifocal leukoencephalopathy in HIV-infected patients in the era of HAART: radiological features at diagnosis and follow-up and correlation with clinical variables. AIDS Res Hum Retroviruses. 2008;24:155–162. doi: 10.1089/aid.2006.0252. [DOI] [PubMed] [Google Scholar]
- 47.De Luca A, Giancola ML, Ammassari A, et al. Cidofovir added to HAART improves virological and clinical outcome in AIDS-associated progressive multifocal leukoencephalopathy. AIDS. 2000;14:F117–121. doi: 10.1097/00002030-200009290-00001. [DOI] [PubMed] [Google Scholar]
- 48.Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338:1345–1351. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- 49.Cinque P, Bossolasco S, Brambilla AM, et al. The effect of highly active antiretroviral therapy-induced immune reconstitution on development and outcome of progressive multifocal leukoencephalopathy: study of 43 cases with review of the literature. J Neurovirol. 2003;9(Suppl 1):73–80. doi: 10.1080/13550280390195351. [DOI] [PubMed] [Google Scholar]
- 50.Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9:625–636. doi: 10.1016/S1473-3099(09)70226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72:1458–1464. doi: 10.1212/01.wnl.0000343510.08643.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Luca A, Giancola ML, Ammassari A, et al. The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. J Infect Dis. 2000;182:1077–1083. doi: 10.1086/315817. [DOI] [PubMed] [Google Scholar]
- 53.Garcia De Viedma D, Diaz Infantes M, Miralles P, et al. JC virus load in progressive multifocal leukoencephalopathy: analysis of the correlation between the viral burden in cerebrospinal fluid, patient survival, and the volume of neurological lesions. Clin Infect Dis. 2002;34:1568–1575. doi: 10.1086/340535. [DOI] [PubMed] [Google Scholar]
- 54.Bossolasco S, Calori G, Moretti F, et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis. 2005;40:738–744. doi: 10.1086/427698. [DOI] [PubMed] [Google Scholar]
- 55.Lima MA, Bernal-Cano F, Clifford DB, Gandhi RT, Koralnik IJ. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. Journal of neurology, neurosurgery, and psychiatry. 2010;81:1288–1291. doi: 10.1136/jnnp.2009.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yiannoutsos CT, Major EO, Curfman B, et al. Relation of JC virus DNA in the cerebrospinal fluid to survival in acquired immunodeficiency syndrome patients with biopsy-proven progressive multifocal leukoencephalopathy. Annals of neurology. 1999;45:816–821. doi: 10.1002/1531-8249(199906)45:6<816::aid-ana21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 57.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–1558. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]


