Abstract
Glial cells are critical players in every major aspect of nervous system development, function, and disease. Other than their traditional supportive role, glial cells perform a variety of important functions such as myelination, synapse formation and plasticity, and establishment of blood–brain and blood–nerve barriers in the nervous system. Recent studies highlight the striking functional similarities between Drosophila and vertebrate glia. In both systems, glial cells play an essential role in neural ensheathment thereby isolating the nervous system and help to create a local ionic microenvironment for conduction of nerve impulses. Here, we review the anatomical aspects and the molecular players that underlie ensheathment during different stages of nervous system development in Drosophila and how these processes lead to the organization of neuroglial junctions. We also discuss some key aspects of the invertebrate axonal ensheathment and junctional organization with that of vertebrate myelination and axon–glial interactions. Finally, we highlight the importance of intercellular junctions in barrier formation in various cellular contexts in Drosophila. We speculate that unraveling the genetic and molecular mechanisms of ensheathment across species might provide key insights into human myelin-related disorders and help in designing therapeutic interventions.
Keywords: Drosophila, Glia, Axonal ensheathment, Septate junctions, Blood–brain barrier, Blood–eye barrier
1. Introduction
Glia, originally thought to be nothing more than neuronal structural support, have come to be known as dynamic, multifunctional cells which are interdependent with neurons for proper nervous system development and function (Allen and Barres, 2009; Virchow, 1860). In the nineteenth century, anatomist Rudolf Virchow coined the lowly term neuroglia or “nervecement,” to characterize the supposed passive, secondary and largely structural nature of these cells (Allen and Barres, 2009; Virchow, 1860). Today the complex and critical nature of glia has exalted them to a pedestal close to that of the neurons—having even been suggested to play a direct role in information processing in the brain (Kettenmann and Verkhratsky, 2008). However, while glia are the predominant cell type in the vertebrate central nervous system (CNS) and have been shown to play critical roles in every aspect of nervous system development and function, the study of glia remains an exciting field due to the many unknowns regarding the genetic, molecular, and morphological characteristics of these cells.
Vertebrate glia have been extensively characterized, and their roles in nervous system function are critical and diverse. In Drosophila, glia guide axons to their appropriate targets during early development, aid in axonal pruning, provide and receive trophic support to and from neurons, permit undisturbed nervous system function by the maintenance of ion-selective barriers, and phagocytose dead and dying neurons (Banerjee and Bhat, 2007; Booth et al., 2000; Freeman et al., 2003; Hebbar and Fernandes, 2010; Sepp and Auld, 2003a). It is of note that the ratio of glia to neurons increases dramatically with overall nervous system complexity. In the Drosophila CNS, glia are outnumbered by neurons in a 10:1 ratio, while in mammals glia outnumber neurons 10:1 (Granderath and Klambt, 1999). This inversion of neuron to glia ratio underpins the concept that glia are not only critical for basic functions shared by simple and complex nervous systems, but play increasingly diverse and dynamic roles in higher level nervous systems as well.
Recently, Drosophila glia have come into focus because of their striking molecular, morphological, and functional similarities to vertebrate glia (Ebersole et al., 1996; Edenfeld et al., 2006; Freeman and Doherty, 2006; Pereanu et al., 2005; Sidman et al., 1964). Vertebrate astrocytes, microglia, oligodendrocytes, and Schwann cells have been recognized to perform specialized roles in nervous system development and function, and complementary subtypes of Drosophila glia have been identified which show a substantial overlap in function with each major category of vertebrate glia (Edwards and Meinertzhagen, 2010; Freeman and Doherty, 2006). Studies on Drosophila glia have provided insights into glial function that would be difficult or impossible to obtain from the study of vertebrate glia (Allen and Barres, 2009; Banerjee et al., 2006a, 2008; Jacobs and Goodman, 1989). In particular, the parallel roles of Drosophila ensheathing glia with vertebrate Schwann cells and oligodendrocytes suggest that the study of axonal ensheathment in Drosophila is directly relevant to vertebrate nervous system function. Furthermore, the roles of Drosophila glia in septate junction (SJ) assembly and blood–brain barrier (BBB) formation are critical for proper neuronal function (Banerjee and Bhat, 2007). The Drosophila nervous system is similar to the vertebrate nervous system, and it functions only within a narrow range of extracellular concentrations of sodium, potassium, and calcium ions. Therefore, to protect the Drosophila nervous system, the interface between the nervous system and circulatory system must regulate ion entry and exit, facilitate nutrient transport, and act as a barrier system to harmful molecules (Hawkins and Davis, 2005). The Drosophila BBB performs each of these functions to allow for proper nervous system activity.
Here, we will focus on the roles of glia in the ensheathment of individual axons and axon bundles (fascicles) throughout the development in embryonic (Figs. 3.1 and 3.3), larval (Fig. 3.2), and adult Drosophila. We will address the roles of glia in BBB, blood–nerve barrier (BNB), and glia-like cells in blood–eye barrier (BEB) formation (Fig. 3.4), and how these intercellular barriers are critical for proper neuronal function. We will present a detailed analysis of many of the molecular players that participate in Drosophila axonal ensheathment and intercellular barrier formation. Also, we will address the hallmarks of ensheathment which are similar to vertebrate myelination. The discovery of the morphological and molecular nature of ensheathment will likely continue to prove relevant to the study of myelination during vertebrate development. This in turn will aid in our understanding of demyelinating disorders and other myelin-related diseases of the human nervous system.
Figure 3.1.
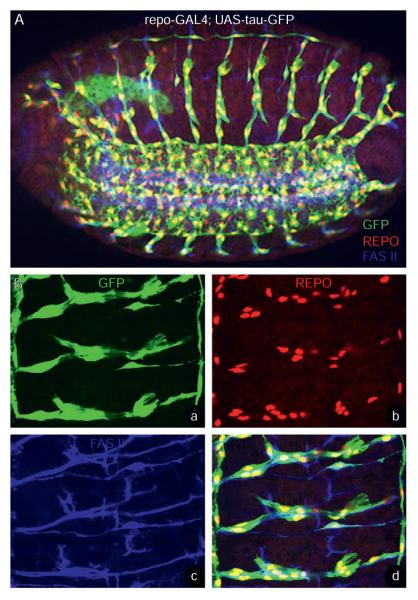
Axonal ensheathment in the Drosophila embryonic PNS. (A, B) Whole mount stage 16 repo-Gal4; UAS-tauGFP (A) and higher magnification (Ba–d) of a portion of the embryo shown in (A) is stained with anti-GFP (green; A, Ba, Bd), anti-Repo (red; A, Bb, Bd), and anti-Fas II (blue; A, Bc, Bd). The GFP staining reveals the glial processes that surround the Fas II labeled motor axons. The glial nuclei expressing Repo show the arrangement of glial cells along the length of the axon trajectories.
Figure 3.3.
Ensheathment of commissural axons in the Drosophila embryonic CNS. (A, B) sim-Gal4, UAS-tau-GFP embryo at a lower (Aa–d) and higher (Ba–d) magnifications show staining with anti-GFP (Aa, Ba, green), anti-Wrapper (Ab, Bb, red), and BP102 (Ac, Bc. blue). The GFP staining highlights the Sim-positive midline glia and neurons (Aa, Ba) while Wrapper expression is in the midline glia (Ab, Bb) and BP102 (Ac, Bc) labels the anterior commissure(AC) and posterior (PC) commissure. Note the midline glial processes (arrow, Aa, Ba) that ensheath the AC and PC (see merged panels, Ad, Bd). The midline glia express Wrapper (arrowheads, Bb).
Figure 3.2.
Septate junctions and axonal ensheathment in the larval peripheral nerve fibers. (A–C) A portion of the nrx IV::GFP third instar larval ventral nerve cord (VNC) with peripheral nerves stained with anti-GFP (green) and anti-Repo (red). nrx IV::GFP expresses GFP in endogenous Nrx IV pattern. The peripheral nerves (A, B) reveal glial membrane expression and SJ localization of Nrx IV (arrowheads, A, B) along the length of the axon, while VNC shows localization of Nrx IV in surface glia (arrows, A, C), which are known to have SJs. Under the surface glial layer, there are Repo-positive glial cells (C, red). A wild-type third instar larval peripheral nerve (D) in cross section shows the presence of SJs (arrowheads) between outer and inner glial membranes. A large number of axons (a) are tightly fasciculated and ensheathed by glial processes (m).
Figure 3.4.
Photoreceptor ensheathment and septate junctions in adult Drosophila eye. (A, B) A light microscopy image (A) and ultrastructural view in longitudinal section (B) of a single Drosophila ommatidium of the adult compound eye. Accessory cells (A), namely the cone cells (CC) and pigment cells (PC) express Nrx IV (green) while photoreceptors (PR) express the apical protein Crumbs (Crb). The ultrastructure at a lower magnification (B) reveals the anatomy of the ommatidium. On top of the pseudocone (PSC) is the lens (L) and at the bottom are the CC, PC, and PR. A higher magnification (C) reveals presence of extensive SJs (arrows) basal to the adherens junctions (arrowhead) that are formed between CC and PC. These SJs serve as protective barriers and seals the PR for proper phototransduction.
2. Drosophila Axonal Ensheathment and Vertebrate Myelination
Axonal ensheathment in Drosophila and myelination of axons in vertebrates in principle perform similar functions, forming a barrier between axons and an extracellular environment which would otherwise disallow the conductance of electrical signals along the axons. In both the systems the function of the barrier is to prevent ionic leakage (Hoyle, 1952; Sousa and Bhat, 2007). In vertebrates, myelination establishes distinct axonal domains which are critical for proper action potential propagation (Bhat et al., 2001; Poliak and Peles, 2003; Salzer, 2003; Thaxton and Bhat, 2009). A similar domain organization is not observed in Drosophila axons (Banerjee et al., 2006b).
The ensheathment of axons in Drosophila partitions the axons from hemolymph. This is important because hemolymph contains a high concentration of potassium as well as levels of other ions which fluctuate significantly after feeding, and which would inhibit electrical conductance by upsetting the resting membrane potential of axons upon passage into the Drosophila nervous system (Hoyle, 1952; Schofield and Treherne, 1985). In the vertebrate nervous system, the myelination of axons increases the conduction velocity of action potentials by insulating the internode to prevent the passive outflow of current (Hartline and Colman, 2007). In the Drosophila PNS, an inner glial cell layer and an outer layer of perineurial, or outer glia form around the nerve fiber to shield it from hemolymph. In the vertebrate nervous system the perineurium is composed of a layer of fibroblasts that surrounds the PNS nerve fasciculi to create ionic and molecular barriers (Jessen and Mirsky, 2005; Thaxton and Bhat, 2009). Various proteins that play a role in Drosophila axonal ensheathment and vertebrate axoglial junction formation is summarized in Table 3.1.
Table 3.1.
Proteins required for ensheathment and axoglial junction formation in Drosophila and vertebrates
2.1. Formation of SJs during axonal ensheathment and myelination
SJs were first identified in Hydra epithelia by electron microscopy, and were postulated correctly to play roles in cell adhesion and as permeability barriers (Wood, 1959). The presence of SJs has since been discovered in myriad invertebrate and vertebrate species (Banerjee et al., 2006a,b). Electron microscopy reveals that Drosophila SJs form rows of electron-dense inter-membranous structures between inner glia and outer glia. SJs function as physical barriers to diffusion, allow the regulation of solute/solvent exchange, and maintain cell adhesion (Banerjee and Bhat, 2007; Baumgartner et al., 1996). In vertebrates, paranodal junctions form between axons and myelinating glia (Bhat, 2003). The molecular composition and structural similarities between Drosophila SJs and paranodal axoglial SJs underscores profound functional similarities between the two structures (Bhat, 2003; Thaxton and Bhat, 2009).
SJs are formed between surface glial cells in the CNS (Banerjee and Bhat, 2007), between glial cells along the peripheral nerve, between the cap cells and scolopale cells of the chordotonal organs (COs; Banerjee et al., 2006a), and between accessory glial cells in the ommatidia (Banerjee et al., 2008). In the PNS and throughout the barrier systems protecting the CNS, SJs are critical for cell adhesion and barrier formation. In the absence of SJs, nervous system function is disabled due to BNB breakdown, leading to paralysis (Auld et al., 1995; Baumgartner et al., 1996; Bellen et al., 1998). While in vertebrates SJs are mostly found in the nervous system, in Drosophila SJs are present in all ectodermally derived epithelia (Banerjee et al., 2006b). Epithelial SJs form diffusion barriers to control the flow of solutes between the apical and basal regions. Some of the identified genes that encode SJ proteins are: neurexin IV (nrx IV), contactin (cont), neuroglian (nrg), coracle (cora), discs large (dlg), Na+K+ ATPase, gliotactin (gli), sinuous, (sinu), megatrachea (mega), lachesin (lac), and scribble (scrib; Banerjee et al., 2006a; Baumgartner et al., 1996; Behr et al., 2003; Bilder and Perrimon, 2000; Faivre-Sarrailh et al., 2004; Genova and Fehon, 2003; Llimargas et al., 2004; Paul et al., 2003; Woods and Bryant, 1991; Wu et al., 2004).
Similar to Drosophila ensheathment, vertebrate myelination results in the formation of specialized SJs called paranodal axoglial junctions for the proper organization of domains along developing axons (Salzer, 2003; Thaxton and Bhat, 2009). Paranodal axoglial junctions are orthologs of Drosophila SJs, they form a diffusion barrier at the paranodal region in myelinated axons (Banerjee and Bhat, 2007; Bhat, 2003; Salzer, 2003). The vertebrate axoglial paranodal junctions also display an electron-dense ladder-like ultrastructure between axons and glia similar to Drosophila glial–glial SJs (Banerjee et al., 2006b; Bhat et al., 2001). Paranodal SJs are formed at the interface of the paranodal axonal region and the noncompacted myelin loops. The vertebrate paranodal axoglial junctions separate voltage-gated sodium channels of the node of Ranvier from the juxtaparanodal voltage-gated potassium channels, thus allowing for proper saltatory conduction along myelinated axons, proper repolarization of the axonal membrane after an action potential, and the maintenance of the correct anterograde direction of action potential propagation (Bhat et al., 2001; Thaxton and Bhat, 2009).
The molecular components of Drosophila SJs are strikingly similar to vertebrate axoglial SJs. In particular, Drosophila SJ cell adhesion molecules Nrx IV, Cont, and Nrg are the orthologs of vertebrate paranodal SJ proteins Contactin-associated protein (Caspr), Cont, and Neurofascin 155 (NF155; Banerjee et al., 2006a; Bhat et al., 2001; Boyle et al., 2001; Faivre-Sarrailh et al., 2004; Peles et al., 1997). In Drosophila PNS, Nrx IV and Cont are expressed by glial cells; Nrg is expressed in both axons and glia. In contrast, vertebrates express Caspr and Cont on the axonal side and NF155 is expressed in the glial cells. Furthermore, Drosophila membrane-associated cytoskeleton protein Cora, known to bind to the C-terminus of Nrx IV, is homologous to mammalian protein 4.1B, which binds to Caspr C-terminus (Denisenko-Nehrbass et al., 2003; Gollan et al., 2002; Horresh et al., 2010; Peles et al., 1997; Tait et al., 2000; Ward et al., 1998). Loss of these Drosophila SJ proteins and their vertebrate orthologs, leads to junctional destabilization and nervous system dysfunction. The molecular, structural, and functional conservation between SJs and paranodal junctions is striking, and it is clear that a greater understanding of SJs in Drosophila will be very informative to provide a greater understanding of vertebrate paranodal SJs (Hortsch and Margolis, 2003).
3. Axonal Ensheathment in the Drosophila PNS
Drosophila PNS axonal ensheathment is a dynamic and highly coordinated process that begins during embryonic development and is completed by early larval development (Banerjee et al., 2006a; Sepp et al., 2000; Stork et al., 2008; Fig. 3.1). Because Drosophila is a holometabolous organism, it undergoes metamorphosis from which the adult body form emerges (Truman, 1990). This process requires a significant restructuring of the Drosophila nervous system, including retraction of the larval ensheathment apparatus and a second ensheathment of the adult nervous system (Fernandez and VijayRaghavan, 1993; Hebbar and Fernandes, 2010). In addition, PNS axonal ensheathment also takes place in the glomeruli of the olfactory system in the antennal lobes during late larval and pupal development ( Jhaveri and Rodrigues, 2002; Jhaveri et al., 2000; Sen et al., 2005). Here, we discuss each phase of PNS ensheathment in Drosophila.
3.1. Axonal ensheathment in the embryonic PNS
The critical events required for axonal ensheathment in the embryonic PNS are (1) proper differentiation of neural precursor cells into peripheral neurons and ensheathing glia, (2) correct spatiotemporal migration of neurons and glia to allow for the intricate molecular and cellular interactions which orchestrate axonal ensheathment, (3) the wrapping of individual motor and sensory axons as well as nerve tracts by PNS glia, and (4) the formation of SJs, which form an impregnable seal between glial processes to allow for the segregation of the PNS from the surrounding potassium-rich hemolymph. However, the undoubtedly intricate molecular events which allow for the ensheathment of individual PNS axons, as well as entire nerve fibers, have not been fully elucidated.
Peripheral glia are born in the early embryonic ventral nerve cord (VNC). These glia arise from neuroglioblasts which express glial cells missing (gcm), a genetic switch which delineates glial cell fate from neuronal precursors (Hosoya et al., 1995). gcm is a master regulator gene for glial development and gcm mutant embryos feature differentiation of presumed glia into neurons, while overexpression of gcm switches the predominant cell fate from neurons to glia (Hosoya et al., 1995; Jones et al., 1995; Vincent et al., 1996). Furthermore, expression of gcm during embryonic development induces several mesodermal glial differentiation markers (Akiyama-Oda et al., 1998; Bernardoni et al., 1998). reversed polarity (repo) is one downstream target of gcm which has been utilized as a nuclear glial cell marker in both CNS and PNS glia, although it is not expressed in midline glia (MG; Banerjee and Bhat, 2008; Campbell et al., 1994; Halter et al., 1995; Jones, 2001). Interestingly, vertebrate gcm genes have not been shown to play any specific role in glial cell differentiation in vivo (Kim et al., 1998).
By stage 11, the glial markers Gcm and Repo are observed in all peripheral glial nuclei (Jones et al., 1995). At this stage the peripheral glia form a cone-shaped structure along each hemisegment in the Drosophila VNC, at the CNS/PNS transition zone (Banerjee and Bhat, 2008; Banerjee et al., 2006a; Sepp et al., 2000). At each hemisegment during stage 12, the anterior corner cell neuron migrates into the PNS after a “touch and pass” of the peripheral glia (Sepp and Auld, 2003a). This pioneer motor neuron establishes the intersegmental nerve (ISN) tract, and exhibits migration defects in the absence of peripheral glia (Jacobs and Goodman, 1989; Sepp and Auld, 2003a; Sepp et al., 2000). The segmental nerve (SN) tract is formed in an analogous manner (Sepp et al., 2000).
Ensheathment of the peripheral axon tracts begins immediately upon glial exit from the CNS (Banerjee et al., 2006a). Peripheral glia follow motor neurons across the CNS/PNS transition zone out of the VNC at stage 13 (Sepp et al., 2000). Just as peripheral glia are required for the proper migration of pioneer neurons into the PNS, likewise in the absence of ISN neurons, ensheathing glia cannot migrate into the periphery (Sepp and Auld, 2003a). At the onset of peripheral glial migration out of the CNS, the glial processes extend into the periphery along the axons tracts while the glial cell body follows behind. Notably, glia are interconnected as they migrate into the periphery along the pioneer neurons (Sepp et al., 2000). This migration out of the CNS by ensheathing glia requires a dramatic rearrangement of the glial actin cytoskeleton (Sepp and Auld, 2003b). The glial cell which leads this train of ensheathing glia out of the CNS along the ISN exhibits a distinct, spike-shaped, actin-rich filopodial protrusion. It has been suggested that the leading glial cell may be solely responsible for path finding duties along the ISN, and the following glia may simply adhere to the leading cell (Sepp and Auld, 2003b).
Sensory neuronal precursors are identified in the dorsolateral region of the embryo at stage 11 (Bodmer et al., 1989). Sensory neurons are born in the periphery in the dorsolateral region of the developing embryo at stage 12, and sensory axons begin extending toward the VNC in a stereotyped pattern in each hemisegment immediately (Banerjee and Bhat, 2008; Hartenstein, 1993). At stage 13, sensory and motor axons begin to connect, and by stage 14, all hemisegments show axon tracts which have formed a continuous motor/sensory axon fascicle (Sepp et al., 2001). These peripherally derived sensory neurons are dependent upon the already-present motor neurons and glia to guide their axons toward the CNS (Parker and Auld, 2006; Sepp et al., 2001). Interestingly, peripheral glia preferentially extend their cytoplasmic processes along sensory axons as opposed to motor axons, suggesting that the molecular profile of sensory axons is more favorable to interactions with ensheathing glial cells (Sepp et al., 2000). This extension of glial cytoplasmic processes is a critical aspect of axonal ensheathment, and is prevented by overexpression of a constitutively activated form of Rho1 GTPase. The PNS sensory axons are fully ensheathed by glia by the end of embryogenesis, while the motor neurons are not completely ensheathed until the larval stages (Sepp et al., 2000). Motor neuron ensheathment is completed when the ensheathing glial processes reach the larval neuromuscular junction (NMJ; Banerjee et al., 2006a). Further analysis of the ensheathment of sensory and motor axons has found that these distinct tracts are kept separate by ensheathing glia beginning with the ensheathment of sensory axons during embryogenesis and continuing with completion of larval ensheathment of the motor tracts (Stork et al., 2008). It would be interesting to determine whether the preference for glial interactions with sensory axons over motor axons is due to a differential chemotactic expression profile between the two neuronal subtypes, a differential cell adhesion molecule profile, or for some other reason.
Crooked Neck (Crn), a splicing factor, has been shown to be required for PNS axonal ensheathment because it is critical for migration and subsequent differentiation of ensheathing glia. In crn mutants, ensheathing glia assemble at the CNS/PNS transition zone, but they do not form the continuous sheath around ISN and SN tracts that is essential for partition from the hemolymph and proper conduction (Edenfeld et al., 2006). In crn mutants, while thin inner glial processes are observed around PNS nerve fibers, the glial processes fail to wrap the nerves. Further, perineurial glia appear to be absent in these fibers, and many axons appear to be free from contact with any glial cell membranes. Even in the rare instances of contact between axonal and glial membranes, a total lack of SJs is observed (Edenfeld et al., 2006).
The roles for Crn in migration, differentiation, and axonal ensheathment are mediated through its interactions with Held Out Wings cytoplasmic protein [HOW(S)]. how mutants exhibit similar, though less severe glial migration, differentiation, and axonal ensheathment defects as crn mutants. HOW(S), but not nuclear HOW[HOW(L)], directly interacts with Crn, and these proteins may form a cytoplasmic protein complex that regulates mRNA splicing components required for glial cell differentiation, glial ensheathment of axons, and formation of SJs (Edenfeld et al., 2006). Evidence suggests that Crn and HOW(S) may regulate splicing of nrx IV, whose protein product has been found to be essential for SJ assembly and proper BNB formation (Banerjee and Bhat, 2007; Banerjee et al., 2006a; Baumgartner et al., 1996; Edenfeld et al., 2006). Crn and HOW(S) may also regulate splicing of nervana 2, whose protein product is localized to SJs (Genova and Fehon, 2003).
Ultrastructural analysis of Drosophila peripheral nerves has revealed that individual sensory and motor axons are wrapped by inner glial cells which express nervana 2. These inner glial cells are then ensheathed by outer (perineurial) glia (Bellen et al., 1998). SJs are present between these outer and inner glial cells (Banerjee et al., 2006a,b; Fig. 3.2). One recent study also categorizes a thin glial cell layer between inner and outer glia termed the subperineurial glial cell layer (Stork et al., 2008). This cellular layer is composed of glia which express moody (Bainton et al., 2005). SJs form a seal around the PNS to preclude the disruption of signal transduction via potassium-rich hemolymph influx into the neural microenvironment, and the absence of SJs causes compromised BNB function (Auld et al., 1995; Banerjee and Bhat, 2007; Baumgartner et al., 1996).
The Drosophila late embryonic PNS offers an advantageous environment in which to study SJs. Intact glial SJs are critical for PNS function in Drosophila embryos due to their role in BNB formation and maintenance. This glial BNB protects the nascent PNS from the potassium-rich hemolymph, which would otherwise inhibit the action potential propagation if allowed to contact the peripheral neurons and therefore the BNB is an integral component of the Drosophila PNS (Hoyle, 1952).
Three of the proteins which comprise neuroglial SJs in the PNS are Nrx IV, Cont, and Nrg. These three proteins are interdependent for localization to PNS glial–glial SJs, and each is crucial for proper SJ formation and BNB function (Banerjee and Bhat, 2007; Banerjee et al., 2006a). Interestingly, the intermembrane distance between apposing glial cells is drastically increased in nrg null mutants, suggesting that Nrg may play a role in cell–cell interactions and cell adhesion between glia in junctional organization during early embryonic development (Banerjee et al., 2006a). Because Nrg is expressed in PNS axons as well as glia, and axonal defects are observed in nrg mutants, it is possible that Nrg is also critical for cell adhesion between axons and glia (Hall and Bieber, 1997). Interestingly in the PNS, Nrg is expressed in both neurons and glia, whereas Nrx IV and Cont are expressed in the glia. This raises interesting questions about the requirements for SJ organization by these two cell types across species. However, Nrx IV is expressed in neurons in the CNS. It is likely that the organizational mechanisms of SJs have switched during evolution as Caspr and Cont are expressed in the neurons and NF155 is expressed in the myelinating glia (Banerjee et al., 2010; Bhat et al., 2001; Stork et al., 2009; Thaxton and Bhat, 2009; Wheeler et al., 2009).
3.2. Axonal ensheathment in the larval and pupal PNS
3.2.1. fray mutants exhibit ensheathment defects
Peripheral glia complete the ensheathment of the motor axon tracts during the late larval instar stages. Mutations in fray, a member of the PASK Fray (PF) kinase family with a serine threonine kinase domain, uncovered a striking phenotype in the Drosophila larval PNS. Null fray mutants feature large swellings along the peripheral nerve fibers, and these mutants die during larval development (Leiserson et al., 2000). Analysis of the fray mutant larval peripheral nerve ultrastructure revealed that the inner glial processes fail to properly wrap individual axons. Interestingly, no defects are observed in the perineurial glial cells. The fray mutant phenotype can be rescued by the ectopic expression of Fray, and strikingly the fray phenotype can also be rescued with rat PASK, a mammalian homolog of Fray which is expressed in the nervous system, suggesting a high degree of functional conservation between these proteins. It has been speculated that the neuronal ultrastructural defects in fray mutants may underscore a role for Fray in the inner glial cell cytoskeleton, specifically affecting the ability of glial cell processes to ensheath axons (Leiserson et al., 2000).
3.2.2. Axonal ensheathment during morphogenesis and in the adult PNS
The Drosophila nervous system undergoes rapid and significant restructuring during metamorphosis to generate the adult nervous system (Consoulas et al., 2002; Fernandez and VijayRaghavan, 1993; Truman, 1990). Recently glial ensheathment of the pupal Posterior Dorsal Mesothoracic Nerves (PDMNs) was characterized from 6 to 38 h after puparium formation (APF; Hebbar and Fernandes, 2010). Since PDMNs innervate dorsal longitudinal flight muscles (DLMs) and are easy to identify, DLMs have served as an informative model for adult neural development (Hebbar and Fernandes, 2010; Ikeda and Koenig, 1988). Interestingly, from 10 to 18 h APF during metamorphosis axonal ensheathment of the PDMNs is remodeled: ensheathing glia retract during adult arbor formation and axonal outgrowth, leaving the developing PDMN axonal arbors without a protective barrier for about 10–12 h (Hebbar and Fernandes, 2010). During this time of axonal outgrowth glia remain relatively stable in their retracted positions. Maximum axonal outgrowth is followed by a period of migration by glia along the PDMN, beginning around 20–22 h APF. The ensheathing glia enwrap the PDMN nerve trunk during this period, corresponding to the timing of higher order branching of the axonal arbors (Hebbar and Fernandes, 2010). Ensheathing glia continue along the primary branches which extend to the main nerve trunk during the pruning of the second order branches. Ensheathment of the secondary branches appears completed by 38 h APF, resembling the adult ensheathment pattern of the PDMN (Hebbar and Fernandes, 2010). An elaborate, multistage arborization and pruning process enables the proper innervation of the DLMs by axonal synapses at the muscle surface. Thus the PDMNs can be used as a model to study axonal ensheathment and guidance during later stages of Drosophila development.
3.2.3. Axonal ensheathment in the olfactory system
The Drosophila olfactory system forms during pupation in a complex and interactive series of events which take place in the olfactory lobe of the brain and in the antenna in the periphery (Jhaveri and Rodrigues, 2002; Sen et al., 2005; Stocker, 1994). Similar to vertebrate olfaction, these connections between the olfactory sensory organs in the periphery and the CNS produce a spatial map in which specific external chemical stimuli are ultimately reflected by a unique pattern of neural activity in the glomeruli of the olfactory lobes of the brain (Galizia et al., 1999; Jhaveri et al., 2000). In short, three distinct types of sensory organs populate the third segment of the adult antenna, as well as the maxillary palps. These are the sensilla basiconica, the trichoidea, and the coeloconica (Stocker, 1994). Between one and four sensory neurons innervate each of these sensory organs and ultimately these project to the antennal lobe where they terminate in the glomeruli. A detailed description of olfactory development in Drosophila has been recently described (Jefferis et al., 2004). Here, we will briefly address olfactory development with a focus on ensheathment which occurs in the antenna during pupal development.
One interesting emerging area involving axonal ensheathment by Drosophila glia is the wrapping of sensory neurons and individual glomeruli of the olfactory system which takes place during morphogenesis (Jhaveri and Rodrigues, 2002; Jhaveri et al., 2000; Sen et al., 2005). About 20 h APF, developing sensory axons which originate from olfactory receptor neurons (ORNs) in the third segment of the antenna reach the brain. Repo-positive Mz-317 peripheral glia of the atonal lineage are already present in the third lobe of the antenna; these glia are found in close association with the developing axon tracts (Sen et al., 2005). Upon the arrival of the sensory axons in the brain, GH-146 glial cells begin to appear along the antennal nerve, and these cells seem to undergo mitosis as they travel along the antennal nerve out of the brain and toward the ORNs from which the axons originated in the periphery. GH-146 glia line the entire length of the axons by 30 h APF, from the olfactory lobe to the third segment of the antenna (Sen et al., 2005). During this same developmental timepoint, the Mz317-glial cells begin to associate more closely with the ORN cell bodies in preparation for the ensheathment of these neurons. By 36 h APF, glial ensheathment of the olfactory axonal tracts appears to be complete, with the brain-derived GH-146 positive glia associating closely with the axon tracts, similar to the inner glia which ensheath the peripheral nerves along the ISN, and the Mz317 glia form an outer layer around the axon tracts, perhaps in a similar fashion to the perineurial glia which wrap around the exterior of the ISN axon tracts (Banerjee et al., 2006a; Sen et al., 2005; Sepp et al., 2000). It would be interesting to know whether SJs are present between these glial cell types, and little is known about the molecular mechanisms that take place to allow for ensheathment in this organ. It would also be interesting to see if the molecular mechanisms responsible for ensheathment in the peripheral and central side of this dynamic neurological system are similar to those seen in the peripheral nerves PNS and/or the VNC.
4. Axonal Ensheathment in Drosophila CNS
4.1. Axonal ensheathment in the VNC
The two halves of the Drosophila VNC are connected by axonal commissures which span the distance between the longitudinal connectives which undergo ensheathment by glial cells during embryogenesis. This ensheathment is critical for neuron and glial survival as well as proper nervous system function. In the developing VNC, diverse glial subtypes play a host of roles in neural migration, axon guidance, separation of commissures, and axonal ensheathment (Banerjee et al., 2010; Klambt and Goodman, 1991; Noordermeer et al., 1998; Stork et al., 2009; Wheeler et al., 2009). VNC glia are critical during every stage of CNS development, and the ablation of glia leads to defects in neuronal differentiation, axon pathfinding, and cell survival (Banerjee et al., 2010; Hidalgo et al., 2001; Klambt and Goodman, 1991). Among the various glial subtypes in the CNS, the MG perform ensheathment of commissural axons while longitudinal glia (LG) ensheath the longitudinal axon trajectories ( Jacobs, 2000). The various glial subtypes and their developmental profiles have been discussed in detail elsewhere (Ito et al., 1995). Here, we will examine the role of LG and MG in the ensheathment of axons in the VNC.
4.1.1. LG ensheath longitudinal axon tracts
During early embryogenesis, LG are derived from lateral glioblasts which arise out of the neuroblast layer at the edge of the neuroectoderm (Doe et al., 1998; Jacobs and Goodman, 1989). These LG help forms the glial scaffold that help guide the axon tracts of the VNC (Jacobs and Goodman, 1989). LG are overproduced in wild-type embryos, and many undergo apoptosis during development. This apoptosis is prevented by LG interactions with pioneer neurons, and ablation of pioneer neurons results in the loss of LG (Kinrade et al., 2001). When pioneer neurons expressing the epidermal growth factor receptor (EGFR) ligand Vein come into contact with LG expressing Drosophila epidermal growth factor receptor (DER), it activates the Ras/MAPkinase survival pathway, preventing apoptosis of LG (Hidalgo et al., 2001). Thus, the surviving LG with Vein-activated Ras/MAPkinase pathway ensheath the longitudinal axon tracts during embryonic development (Jacobs and Goodman, 1989).
Axonal ensheathment of the longitudinal axon tracts by LG also requires the fibroblast growth factor receptor Heartless (Htl/DFR1; Shishodo et al., 1997). In htl mutants, LG exhibit reduced migration rates, inability to extend cytoplasmic processes, failure to properly position themselves for the ensheathment of longitudinal axons, and ultimately a failure to effectively wrap the developing longitudinal axon tracts (Shishodo et al., 1997). This suggests that FGF signaling is critical for the ensheathment of axons. Related experiments in the grasshopper CNS support this conclusion, indicating that FGF signaling induces glial morphogenesis in response to a localized FGF signal by extending membranous processes around the signal, ultimately leading to glial ensheathment of the source of the FGF signal (Condron, 1999).
4.1.2. MG ensheath anterior and posterior commissures in the CNS
MG can be subdivided into anterior MG (AMG) which express high levels of Wrapper and posterior MG (PMG) which express Engrailed (En; Noordermeer et al., 1998; Wheeler et al., 2006). Throughout embryonic development, MG perform an impressive variety of functions, including production of chemotactic gradients to control axon guidance and sculpt the VNC, induction of the differentiation of neural subtypes, separation of anterior commissure (AC) and posterior commissure (PC), and ensheathment of commissural axons (Kidd et al., 1999; Menne et al., 1997; Wheeler et al., 2009). AMG, but not PMG, send out elaborate membranous processes that completely enwrap the commissures and penetrate the commissural axon bundles to extensively ensheath these axons and divide the commissures into subdomains (Stollewerk et al., 1996). A role for PMG during VNC development has not been determined, and all PMG undergo apoptosis by the end of embryonic development. Here, we will focus on the mechanisms of the ensheathment of commissural axons by AMG.
Ten MG derive from mesectodermal cells in each segment at the midline of the neuroepithelium in the VNC. After four MG undergo reaper- and grim-mediated apoptosis six glia remain per segment (Zhou et al., 1997). These six MG compete for survival; only three ultimately avoid apoptosis (Bergmann et al., 2002; Sonnenfeld and Jacobs, 1995). MG compete for access to axons, and the surviving glia escape apoptosis via activation of the EGFR/RAS/MAPK pathway by direct interaction with axons and neuronally secreted SPITZ, and ultimately suppression of proapoptotic protein HID by activated MAPK (Bergmann et al., 2002). Early stage 12 of embryonic development marks the beginning of CNS axonogenesis, and during this stage MG are positioned in close contact with the pioneering axons which are together in a single axon bundle (Wheeler et al., 2009). During early stage 12, three AMG contact the anterior of the commissure, and a fourth undergoes apoptosis (Bergmann et al., 2002). In late stage 12 of development, membranous processes begin to extend from the AMG across the dorsal and ventral surfaces of the commissure and in between the dividing AC and PC (Jacobs, 2000; Wheeler et al., 2009). The AC is completely ensheathed by a glial process by stage 13 and by stage 14 of embryonic development, an AMG moves to a position between the AC and PC (Klambt and Goodman, 1991; Wheeler et al., 2009). During stage 15 one single AMG migrates across the dorsal surface of the PC, and extends elaborate processes posteriorly across the dorsal side of the PC which cover the entire dorsal surface commissural tract from one longitudinal connective to the other (Klambt and Goodman, 1991; Noordermeer et al., 1998; Wheeler et al., 2009). At this timepoint, long intricate membranous processes begin to extend from the MG across the ventral surface as well. At stage 16 the glial processes completely cover the dorsal surface of the commissures, extend into the commissural axon bundles for the purpose of axonal ensheathment, and enwrap the ventral surface of the commissures (Fig. 3.3; Jacobs, 2000; Noordermeer et al., 1998). Throughout stages 15–17, AMG extend elaborate cytoplasmic projections into the AC and PC which divide each commissure into subdomains and ensheath individual axon fascicles (Stollewerk et al., 1996; Stork et al., 2009). Detailed molecular mechanisms of axonal ensheathment in the CNS involving MG and LG remain to be further investigated to identify adhesion molecules that underlie intercellular neuron–glial interactions.
4.1.2.1. Wrapper and Nrx IV mediate axonal ensheathment at the embryonic midline
AMG-expressing Wrapper interacts with midline neurons expressing Nrx IV in trans to promote ensheathment of axon commissures (Noordermeer et al., 1998; Stork et al., 2009; Wheeler et al., 2009). Wrapper is a member of the Ig-superfamily and contains a signal peptide followed by three Ig domains, a fibronectin domain and a GPI-linkage (Noordermeer et al., 1998). Wrapper has been a known player in MG-neuron adhesion for over a decade, as wrapper mutants showed a failure of MG to ensheath the commissural axons (Noordermeer et al., 1998). More recent studies have explored the role of wrapper in MG migration and axonal ensheathment (Stork et al., 2009; Wheeler et al. 2009). Embryonic stage 12 wrapper mutants show normal AMG and PMG migration in the VNC and appear to be positioned properly between the AC and PC. By stage 15, as the AMG comes into contact with the AC, wrapper mutants begin to show MG ensheathment defects. During this stage, while wild-type MG processes have wrapped the entire dorsal surface of the AC, wrapper mutant MG show a lack of membranous process extension around the dorsal surface, and this defect is especially apparent at the lateral side of the commissures near the area where the commissures meet the longitudinal tracts (Noordermeer et al., 1998; Wheeler et al., 2009). Furthermore, the AMG does not become properly positioned between the AC and PC during this time (Wheeler et al., 2009). By stage 16, some MG in wrapper mutants die prematurely, likely due to lack of contact with and ensheathment of the PC, and those remaining MG fail to enwrap the commissural axon bundles or extend processes to penetrate and ensheath axons throughout the commissures (Bergmann et al., 2002; Noordermeer et al., 1998).
Interestingly nrx IV mutant embryos also revealed a failure of VNC commissure separation (Wheeler et al. 2009). Although Nrx IV is known as a component of SJs, ultrastructural analysis has not revealed the presence of any septate-like junctions in the embryonic midline so far (Stollewerk and Klämbt, 1997; Stollewerk et al., 1996). Furthermore, many of the molecular components of SJs are not expressed in the embryonic midline (Stork et al., 2009). Recent studies showed Nrx IV and Wrapper bind to mediate the MG migration, and subsequent ensheathment and subdivision of the axonal commissures at the midline (Stork et al., 2009; Wheeler et al., 2009). Wild-type Nrx IV expression is sharply concentrated at the MG-commissural axon contact points, adjacent to Wrapper expressed by MG. Furthermore, nrx IV mutants show striking similarities in the MG migration and ensheathment defects with wrapper mutants (Banerjee et al., 2010; Stork et al., 2009; Wheeler et al., 2009). In nrx IV null mutants, AMG migrate normally to the commissure at stage 12, but instead of beginning commissural ensheathment, the AMG migrate past the AC toward the PC (Wheeler et al., 2009). Furthermore, AMG fail to elaborate cytoplasmic projections to divide the commissures into subdomains (Wheeler et al., 2009). When Nrx IV expression was driven in a subset of commissural axons in the nrx IV null mutant background, glial wrapping defects were often rescued specifically in the Nrx IV-expressing fascicles (Stork et al., 2009). Furthermore, expression of Nrx IV with a strong neuronal driver caused the expansion of the Wrapper expression domain within the commissures, and the expression of Nrx IV in only a few ipsilaterally-projecting neurons, Wrapper localization was redistributed and long MG protrusions along the Nrx IV-positive axons (Stork et al., 2009). Conversely, ectopic expression of Wrapper redirected Nrx IV expression in the midline and in other tissues as well (Stork et al., 2009). Nrx IV and Wrapper bind in co-immunoprecipitation experiments (Wheeler et al., 2009). Adhesion experiments in S2 cell culture confirmed that Nrx IV and Wrapper bind in trans and mediate cell adhesion (Stork et al., 2009; Wheeler et al., 2009). Interestingly, Wrapper exhibited a stronger affinity for the Nrx IV splice variant that is present in neurons more so than the one specific to epithelial cells (Stork et al., 2009). These studies established for the first time that Nrx IV and Wrapper trans interactions underlie the establishment of the midline neuron–glial scaffold for proper neuronal development.
4.2. Axonal ensheathment in the Drosophila brain
An interesting but largely unexplored area is to understand how the process of ensheathment works in the Drosophila brain. During embryonic brain development, neuroblasts generate primary neurons and glial lineages which later develop into the functional larval brain. The neurons extend neurites which are directed and nourished by glia and are assembled into neuropile compartments (Pereanu et al., 2005; Younossi-Hartenstein et al., 2003). Secondary neurons are produced in the brain during larval development. These neurons cross the cortex in large bundles and join the primary neurons in the neuropile (Dumstrei et al., 2003). The adult Drosophila brain is formed during pupal development, when axonal and dendritic branching from secondary neurons form connections with the primary neurons in the adult brain neuropile (Pereanu et al., 2005). Glial cells in the Drosophila brain have been classified by various criteria, and this has led to some confusion about the nomenclature for brain glia (Awasaki et al., 2008; Freeman and Doherty, 2006; Hoyle, 1986). The most basic classification of glia divides them into three categories: surface glia, which form the BBB and will be examined in Section 5, cortex glia, which form a scaffold around neuronal cell bodies in the cortex but also share a BBB role with surface glia (Fig. 3.2) and have some ensheathing properties, and neuropile glia, which play a role in the ensheathment of axons and axon fascicles. These three basic categories of glia can be divided into subcategories based on criteria such as location, ultrastructure, function, or gene expression patterns (Edwards and Meinertzhagen, 2010). The role of cortex glia and neuropile glia is discussed below.
4.2.1. Neuropile glia partition axons of the brain
Axonal ensheathment in the Drosophila brain has not been examined in detail compared to the VNC or the PNS. However, evidence exists that glia in the Drosophila brain play roles in the ensheathment of axons. Neuropile glia proliferate in the larval brain while secondary neurons trek across the cortex and infiltrate the neuropile (Awasaki et al., 2008; Pereanu et al., 2005). This distinct class of glia are partly defined by morphology and location: most neuropile glia are irregular in shape, and their cell bodies are either located at the interface of the cortex and the neuropile, or deep within the neuropile itself (Pereanu et al., 2005). Neuropile glia are divided into two categories: ensheathing glia and astrocyte-like glia (Awasaki et al., 2008). Similar to oligodendrocytes, Drosophila ensheathing glia enclose axons. The ensheathing glia extend processes around the neuropile and form SJs within the neuropile to partition the neuropile compartments (Pereanu et al., 2005). The boundary created by the membranous processes extended by the ensheathing glia may insulate neuropiles from lateral miscommunication between the neighboring neuropiles, allowing for independent neural activity within each neuropile (Awasaki et al., 2008). Furthermore, the glial process extended within the neuropiles may further divide subcompartments to allow for independent activity within each neuropile subcompartment, aided by glial–glial SJs between the ensheathing glial membranes (Awasaki et al., 2008; Pereanu et al., 2005). These SJs formed by neuropile glia do not form a comprehensive diffusion barrier which could be likened to the barrier systems which partition the nervous system from hemolymph throughout Drosophila. Instead these SJs are found in short stretches between adjacent compartments which create nearly independent environments within the compartments, but allow neurons to pass into the compartments through neuropile portals during development and between the neighboring compartments in selective areas (Pereanu et al., 2005). A large glial sheath is present between the deep cortex and the neuropile to separate the neurons of these domains, and in the basal and medial regions of the brain this sheath is formed primarily by the ensheathing neuropile glia (Pereanu et al., 2005). Due to the complex nature of the neuronal terrain in the brain, detailed mechanistic insights are still lacking regarding any junctional organization and intercellular adhesion that take place between glial and neuronal cells.
4.2.2. Cortex glia and ensheathment
Cortex glia exhibit properties which are most often compared to vertebrate astrocytes (Awasaki et al., 2008; Freeman and Doherty, 2006). These glia are highly branched, lamellated cells which form close associations with primary and secondary neurons during development. Cortex glia differentiate during stage 16 of embryogenesis, and proliferate rapidly in the brain during the larval stages, where like astrocytes they play roles in trophic support of neurons and synapse modification (Freeman and Doherty, 2006). Cortex glial processes also play roles in stabilizing the position of neurons during secondary neuronal migration in the larval brain, and extension of axons from the neurons during development (Dumstrei et al., 2003). Like astrocytes, cortex glia also play a role in the BBB, and this will be addressed in Section 5 (Pereanu et al., 2005). While neuropile glia are responsible for the ensheathment of the neuropile in the basal and medial regions of the brain, it is the cortex glia which are responsible for this ensheathment in the dorsal and lateral regions (Pereanu et al., 2005). Cortex glia enclose each primary and secondary neuron during development. However, the role that cortex glia play appears to be trophic in nature as opposed to one of axonal ensheathment, and the cortex glial processes associate thoroughly with neuronal cell bodies as opposed to neurites (Awasaki et al., 2008). Much remains to be characterized regarding the mechanisms that underlie neuronal and axonal ensheathment in the Drosophila brain. New emerging methodologies of in vivo imaging combined with fluorescently tagged-glial and -neuronal markers could provide more detailed insights into the complexities of neuron–glial interactions and brain function.
5. BBB Formation in Drosophila
In Drosophila and other invertebrates, the neuron–glial BBB preserves the molecular microenvironment necessary for the generation and propagation of action potentials. In the Drosophila nervous system as in vertebrates, concentrations of sodium, potassium, and calcium ions must be strictly regulated to allow for proper nervous system function. The BBB takes the form of SJs between neighboring glia throughout the developing organism to partition the nervous system from direct contact with the openly circulating hemolymph. This is distinct from the vertebrate BBB, in which TJs between capillary endothelia of the closed circulatory system serve as the primary barrier system of the brain. However, despite these differences, the study of the Drosophila barrier systems has proven informative to our understanding of the vertebrate BBB. Moreover, the vertebrate BBB has proven to be both an obstacle for the successful therapeutic treatment of CNS disorders due to its selectively permeable nature, as well as a pathological focus due to its breakdown in CNS disorders such as multiple sclerosis (MS). Studies in the Drosophila BBB may help us find new methods to circumvent the BBB to deliver therapeutic agents, as well as to explore therapies to prevent or repair its damage in disease state.
In the Drosophila PNS, this barrier first forms in the CO during early embryogenesis (Carlson et al., 1997). Here, proprioreceptors are protected by SJs which form an impermeable seal between the cells, creating a barrier so that ion passage into and out of the PNS is regulated by passage through glia. By late embryonic stages, the CNS is also protected by a functional BBB, and in early pupal life the BEB is formed as well. These barrier systems are critical for Drosophila nervous system function and ultimately for survival into adulthood (Auld et al., 1995; Banerjee et al., 2006a, 2008; Baumgartner et al., 1996). Interestingly, while much of what comprises the BBB is unique between the CNS and PNS, the neural lamella is an extra cellular matrix which extends across the entire exterior of the CNS and PNS forming a comprehensive layer around the Drosophila nervous system from embryonic stage 16 onwards (Stork et al., 2008). In this section, we will describe the unique functions of vertebrate and invertebrate barrier systems. We will also address cellular and molecular profiles of these barrier systems, as well as their relationship to vertebrate barriers and roles in health and disease.
5.1. BNB in the Drosophila PNS
5.1.1. BNB between inner and perineurial glia
In many species, where the nervous system requires complex sensory and motor processing, there is a particular physiological need for a BBB to provide ionic homeostasis around central integrating synapses to allow for the proper communication of signals (Abbott et al., 1986). Because of the strikingly high, often broadly fluctuating levels of potassium and other ions in hemolymph, many insects depend upon additional components for an effective barrier (Hoyle, 1952). In insects, higher arachnids, and decapod Crustacea, a BBB is a critical part of the ensheathment of the CNS (Abbott et al., 1986). The types of junctions which form the BBB vary by species. For instance, TJs are responsible for the cockroach, locust, and moth barrier, SJs are barrier components in flies, linker junctions play a role in the centipede and millipede barrier, and novel restricting junctions are an integral component of squid Sepia (Abbott et al., 1985; Lane, 1989; Lane and Swales, 1979). In Drosophila, the barrier resides between various glial subtypes and relies on SJs for proper function (Banerjee et al., 2006a; Edwards and Meinertzhagen, 2010; Swales and Lane, 1983).
As described in Section 3, in the Drosophila PNS SJs form between inner glia and perineurial glia to allow for ensheathment of individual axons as well as axon fascicles (Banerjee and Bhat, 2008; Banerjee et al., 2006a; Stork et al., 2008). Therefore, the BNB is established in the Drosophila PNS between ensheathing glia (Banerjee et al., 2006a). For proper BNB formation, inner and outer glial processes must form a seal to prevent hemolymph entry into the nervous system (Banerjee et al., 2006b). SJs are critical to this barrier function in the developing Drosophila PNS. The first mutants identified to display severe BNB defects due to loss of SJs are gli and nrx IV mutants (Auld et al., 1995; Baumgartner et al., 1996). In gli mutants, glial cell development, differentiation, and morphology do not appear to be affected. However, ultrastructural analysis of gli mutants reveals that the peripheral glia do not completely ensheath axons and exhibit defects in glial SJs, leading to a failure to form a sealed BNB (Auld et al., 1995; Baumgartner et al., 1996). Because of neural exposure to the hemolymph, action potentials do not properly propagate leading to paralysis (Auld et al., 1995). In nrx IV mutants, ensheathing glial membranes lack SJs entirely; therefore, although the glial membranes are in close proximity to the peripheral nerves, they cannot properly complete peripheral axonal ensheathment and BNB formation (Banerjee and Bhat, 2007; Banerjee et al., 2006b). Other proteins which have been shown to associate with Drosophila PNS SJs include Cont, Nrg, Lachesin, Moody, and Loco (Bainton et al., 2005; Banerjee et al., 2006a; Schwabe et al., 2005; Strigini et al., 2006).
5.1.2. BNB of the COs
The Drosophila COs are proprioreceptors which contain neurons and glia and serve as an informative model to study the mechanisms involved in BNB formation (Banerjee et al., 2006a; Carlson et al., 2000). The glial cell types of the CO are the cap cell, the scolopale cell, and the ligament cell. Of these, cap cells and scolopale cells participate in BNB formation, as SJs at the interface between these two cell types create the seal to protect the developing nervous system from the hemolymph (Carlson et al., 1997). In addition to their role in peripheral nerves, Nrx IV, Cont, and Nrg have been found to play critical, interdependent roles as molecular components of CO BNB (Banerjee et al., 2006a). In the COs, Nrx IV, Cont, and Nrg localize to the scolopale and cap cells, and Nrg is also localized to neurons (Banerjee et al., 2006a). nrx IV, cont, and nrg mutant embryos show altered CO morphology and a breakdown of the functional BNB, highlighting the importance of each of these SJ proteins for proper CO function (Banerjee and Bhat, 2007; Banerjee et al., 2006a).
5.2. BBB in the Drosophila CNS
For proper CNS function, the BBB exists at the surface of the Drosophila brain (Swales and Lane, 1985). Beginning in the larval stages, the BBB is formed by two distinct glial cell layers which are speculated to play complementary roles in barrier function (Awasaki et al., 2008). The outer layer of the BBB is formed by surface glia. Surface glia are flattened cells with no processes which grow radially from the surface into the cortex (Fig. 3.2; Pereanu et al., 2005). These glia come into direct contact with hemolymph, and are therefore responsible for the controlled flow of nutrients from the hemolymph into the brain. Surface glia are generally classified into two subgroups based upon localization in the brain and cell morphology: perineurial glia and subperineurial glia (Pereanu et al., 2005). These subgroups form the external and internal sublayers of the outer BBB layer. Perineurial glia form the external layer, and develop during the larval stages and are not dependent upon GCM for proper differentiation. This GCM-independent development has led to postulation that perineurial glia are in fact not a glial subclass but hemocytes; however, recently perineurial glia were shown to express Repo, a direct target for GCM which is present in most glia, but is never expressed in hemocytes, supporting the notion that these cells are in fact glia (Awasaki et al., 2008; Lee and Jones, 2005). Specific perineurial BBB function has not been addressed, but this layer appears to lack extensive SJs which would classify it as a part of traditional BBB function. Subperineurial glia can be identified by their expression of Moody (Bainton et al., 2005; Schwabe et al., 2005). These glia form the internal sublayer of the outer glial layer, and are large, thin glia which are thought to be indispensible components of the larval BBB (Awasaki et al., 2008; Bainton et al., 2005). This is due to extensive SJs between the subperineurial glia, as well as data from functional assays which indicates that dyes cannot penetrate past the subperineurial layer of the Drosophila BBB (Baumgartner et al., 1996; Fehon et al., 1994; Stork et al., 2008). Subperineurial glia do not come into direct contact with CNS neurons, as they are sandwiched between the perineurial glia and are blocked from direct neuronal contact in the CNS by the neuropile glia and cortex glia (Pereanu et al., 2005; Stork et al., 2008).
The inner layer of the BBB is composed of cortex glia. Cortex glia are found throughout the cortex, and unlike surface glia, cortex glia extend lamelliform processes throughout the cortex. Cortex glia form a dense meshwork throughout the cortex in which one cortex glial cell can enwrap several neurons, similar to vertebrate oligodendrocytes (Awasaki et al., 2008). Notably, a layer of these processes are found in apposition to the inner side of the surface glia forming the inner layer of the BBB. The glial processes of cortex glia also ensheath the neuronal cell bodies, but this is thought to perform trophic as opposed to barrier functions (Edwards and Meinertzhagen, 2010). However, extensive SJs exist between cortex glia as well as cortex glia and neurons which perform BBB and neuron stabilization functions throughout the Drosophila brain (Carlson et al., 2000; Pereanu et al., 2005).
5.2.1. SJs in BBB formation
The presence of SJs has long been known to confer selective permeability upon tissues which require a partitioning of microenvironments and controlled entry and exit of substrates between the apposing domains. A common test of this barrier function is the dye injection assay, in which extracellular tracers such as dextran are injected into body cavity, and then permeability across the barrier is assessed. Drosophila CNS which lack glial cells or have severely impaired glial function exhibit reduced or disabled barrier function (Auld et al., 1995; Bainton et al., 2005; Baumgartner et al., 1996). Nrx IV, Cont, Nrg, and Gli are some of the well known molecular players expressed in SJs which compose the Drosophila CNS BBB, and mutations in each of these and many other SJ proteins result in breakdown of the BBB and subsequent entry of dye into the normally protected space (Auld et al., 1995; Banerjee et al., 2006a; Baumgartner et al., 1996; Faivre-Sarrailh et al., 2004). Interestingly, while the loss of Nrx IV causes a severe breakdown of the BBB observed by the immediate entry of 10 kDa dextran dye into the CNS due to complete absence of SJs, the loss of Moody in the surface glia causes a more subtle breakdown of the BBB, and less complete entry of 10 kDa dextran into the CNS, which correlates with the diminished, but not entirely ablated SJs between the subperineurial glia in moody mutants (Bainton et al., 2005; Baumgartner et al., 1996; Stork et al., 2008). These results highlight the importance of SJs in BBB function and axonal ensheathment.
5.2.2. Non-SJ-related BBB
While SJs are critical for the proper function of Drosophila barrier systems, recently the Drosophila BBB has been shown to be a dynamic series of barriers in which multiple components inhibit the passage of small and large substances. Mdr65, an ATP-binding cassette (ABC) transporter gene expressed in subperineurial glia, plays a role in BBB blockage of toxic pharmaceuticals from the brain (Mayer et al., 2009). mdr65 mutations caused the passage of ABC transporter substrates into the brain, but did not affect the successful blockage of small dextrans (3 kDa and 10 kDa) into the brain, a process known to be mediated by SJs (Mayer et al., 2009). This result indicates that Mdr65 plays a non-SJ-related role in BBB function in the Drosophila CNS. In a related study, dextran dyes of various sizes were injected into gcm mutant embryos in which virtually no glia were present, nrx IV mutant embryos in which glia are present but SJs do not form, and moody mutant embryos in which proper SJ formation is impaired. The injection of a large 70 kDa dye into the hemolymph resulted in immediate influx of the dye across the BBB of gcm mutants, while in nrx IV mutants comparable levels of dye did not infiltrate the CNS until 20 min after injection (Stork et al., 2008). This contrasts the finding that 10 kDa dextran dyes enter the CNS of gcm and nrx IV mutants at comparable rates and suggests that another barrier mechanism exists independent of the SJs (Stork et al., 2008). An injection of a 500-kDa dye into the hemolymph of these mutants confirmed the findings from the 70 kDa dye injections, as gcm mutants showed immediate CNS uptake of the dye, nrx IV mutants showed significantly less dye uptake, and moody mutant embryos exhibited very little CNS uptake of the dextran dye (Stork et al., 2008). So while the SJs are critical for BBB integrity, other structures may also play important roles in the exclusion of large molecules from the Drosophila CNS. Future studies aimed at defining the molecular nature of these barriers should help us understand how barriers are established which is relevant to vertebrate BBB formation and function.
5.3. BEB in Drosophila ommatidia
5.3.1. BEB in Drosophila and BRB in vertebrates
In the vertebrate eye, the retina houses the photoreceptors rods, cones, and photosensitive ganglion cells. Rod cells are sensitized to detect low levels of white light, cones detect colors and are less sensitive, thereby functioning well in bright light, and photosensitive ganglion cells play a role in circadian rhythm maintenance and hormonal regulation. In the neural retina, photoreceptors (PRs) are excluded from contact with blood by the blood–retinal barrier (BRB) between retinal pigment epithelia. This barrier is similar to the vertebrate BBB, in that it utilizes TJs to seal the extracellular space between neighboring epithelia (Williams and Rizzolo, 1997). Furthermore, breakdown of the vertebrate BRB epithelial TJs has been shown to be a cause of BRB dysfunction in vertebrates (Peng et al., 2003). The BRB has been shown to play an important role in human ocular disorders such as macular edema and diabetic retinopathy (Vinores et al., 1999).
Adult Drosophila have compound eyes, which develop from the eye imaginal disc, a single layer of proliferative epithelia. These cells begin to differentiate during larval development. Differentiation is controlled by eyeless, dacshund, sine oculis, and eyes absent (Chen et al., 1997; Halder et al., 1998; Mardon et al., 1994; Wolff, 2003). During third instar larval development, an epithelial indentation called the morphogenetic furrow develops, forming the eye imaginal disc. While cell division occurs asynchronously in the region anterior to the morphogenetic furrow, cells posterior to the morphogenetic furrow develop into ommatidial precursor clusters and begin to differentiate into PR (Cagan and Ready, 1989; Tomlinson and Ready, 1987; Wolff and Ready, 1991). The Drosophila compound eye features about 800 units, called ommatidia. Each ommatidium features eight PRs, four cone cells (CCs), and three types of pigment cells (PCs; Tomlinson and Ready, 1987; Wolff and Ready, 1993). In each ommatidium, PRs are specified during late larval development, followed by the CCs. The ommatidia are completed during the pupal stages when PCs surround the CCs and sensory units form (Cagan and Ready, 1989; Wolff and Ready, 1993). Each ommatidium contains a BEB, which is essential for proper phototransduction.
5.3.2. SJs in BEB formation
Drosophila BEB formation requires SJs for its proper assembly and function. Studies of the insect BEB in other organism such as adult locust and houseflies show the presence of a barrier protecting the optic lobes impenetrable to dyes injected into the circulating hemolymph; the discovery of SJs and tight junctions in insects provide an anatomical structure to perform this function of hemolymph exclusion from the insect eye (Chi and Carlson, 1981; Lane, 1981; Saint Marie and Carlson, 1983a,b; Shaw, 1984). In the housefly, fenestrated glial cells form a barrier between the retina and brain (Carlson et al., 2000; Saint Marie and Carlson, 1983b). Invaginations in the distal membranes of these glia allow for increased surface to volume ratio in these cells, providing the necessarily large amount of surface area for vesicular trafficking to allow for the adequate influx of nutrients into the ommatidia (Kretzschmar et al., 2000). Expression of adaptin, a vesicle-specific protein, also supports the large amount of vesicular trafficking required at the distal surface of the glial cells between the retina and brain (Kretzschmar et al., 2000). Lamina glia also partition the lamina from the retina and other visual ganglia. Glia of the laminar cortex are extensively coupled with neurons as well as other glia (Kretzschmar et al., 2000).
In the developing third instar larval ommatidia, SJs are present in the basolateral regions of the differentiating retinal disc epithelial cells (Banerjee et al., 2008). In pupal ommatidial development, SJs are found in the differentiating retinal epithelial cells. During pupation, BEB formation occurs in time to protect the developing photoreceptor neurons, which are bathed in distinct extraneuronal fluid distinct from the hemolymph, to be shielded from the environment outside of the visual system. Conduction in the visual system begins during the pupal stage, and the series of partitions which comprise the BEB develop temporally to accommodate this activity through the entire adult life of the organism. In the adult Drosophila ommatidia, long ladder-like stretches of SJs are present basal to adherens junctions (AJs) in the apical region between neighboring CCs, between CCs, and PCs (Fig. 3.4) below the level of the lens and pseudocone. This localization of SJs basal to AJs but in the apical region between neighboring cells is similar to SJ location in embryonic epithelia (Banerjee et al., 2008; Faivre-Sarrailh et al., 2004). Furthermore, SJs are found in the CC basal region as well, near the CC endfeet at the bottom of the adult ommatidia.
Similar to observations in epithelial and PNS SJs, Nrx IV expression occurs synchronously with the development of SJs in ommatidia. Nrx IV is expressed in CCs in the eye imaginal disc during late third instar larval development. Nrx IV is also found at the basal regions of PCs, and is not expressed in the neuronal cells of the larval ommatidia. During pupal development Nrx IV is expressed in the CCs and PCs, specifically in the SJ-expressing regions between these two cell types. In the adult eye, Nrx IV is expressed in the apical-most region of the ommatidia in the membranes of CCs and PCs (Fig. 3.4A), in the basal regions of the CCs near the endfeet (Banerjee et al., 2008). In adult ommatidia lacking Nrx IV, ommatidial collapse occurs and SJs between the CC are completely absent (Banerjee et al., 2008). The loss of Nrx IV leads to altered ommatidial cell morphological and functional defects, including degeneration of PRs (Banerjee et al., 2008). This PR degeneration in the absence of CC SJs suggests that the accessory cells of the ommatidia serve an important role in PR development and maintenance, and the SJs may have a particularly important role in ensuring proper PR function (Banerjee et al., 2008). In several nrx IV hypomorphic alleles, as well as in nrx IV null mutants, proper BEB function is compromised when subjected to in vivo diffusion barrier assays (Banerjee et al., 2006a, 2008). This is due to a defective SJ structure between CCs, suggesting that the ommatidial CC SJs are required for proper BEB function (Banerjee et al., 2008). In conclusion, adult ommatidial SJs function to both provide cell adhesion between CCs and PCs, as well as to act as the BEB, protecting the PRs from hemolymph to allow for proper phototransduction (Banerjee et al., 2008).
6. Concluding Remarks
Drosophila ensheathment research continues to be a field which yields interesting and relevant findings that provide insights into nervous system function, and are often highly translational to studies in vertebrates. One area of Drosophila research which remains to be explored in depth is axonal ensheathment by glia in the brain. While CNS glial subtypes have been classified based on morphology, location, and molecular profile, much work regarding the diverse functions of brain glia remains to be accomplished. One interesting finding was the discovery that swiss cheese (sws) mutants exhibit an excess of axonal wrapping by glia in the pupal and adult Drosophila brain, suggesting a yet-to-be defined role for sws in axonal ensheathment (Kretzschmar et al., 1997). Ultimately sws mutants experience neurodegeneration and shortened lifespan. This may be relevant to human disorders such as Charcot-Marie-Tooth neuropathy, a heterogeneous group of demyelinating diseases (Kretzschmar et al., 1997). In contrast to sws mutants, drop-dead mutants exhibit incomplete wrapping of axons in the adult brain, and like sws mutants also ultimately leads to neurodegeneration (Buchanan and Benzer, 1993). These results point to an interesting potential field of inquiry in Drosophila: does improper ensheathment lead to neurodegeneration? Answering this question could have ramifications for vertebrate neurobiology, as the most common human demyelinating disorder, MS, becomes a largely neurodegenerative disorder in its later stages (Bjartmar and Trapp, 2003).
Several aspects of Drosophila axonal ensheathment resemble vertebrate myelination, and ensheathment research has already provided critical insights into vertebrate neuron–glial interactions. One aspect of Drosophila PNS ensheathment in particular that is highly relevant to vertebrate myelination is the formation of neuron–glial SJs between peripheral glia, which exhibits a high degree of molecular homology to vertebrate paranodal SJs. As mechanisms of ensheathment are in many ways highly homologous to mammalian myelination, the study of ensheathment is particularly pertinent to our understanding of human demyelinating disorders, and in the years ahead this research should yield results which will help us better understand and treat human nervous system disorders.
ACKNOWLEDGMENTS
Research in our laboratory is supported by grants from the National Institute of General Medical Sciences (GM063074) and National Institute of Neurological Disorders and Stroke (NS050356) of the National Institutes of Health; National Multiple Sclerosis Society and the State of North Carolina.
REFERENCES
- Abbott NJ, Bundgaard M, Cserr HF. Tightness of the blood–brain barrier and evidence for brain interstitial fluid flow in the cuttlefish, Sepia officinalis. J. Physiol. 1985;368:213–226. doi: 10.1113/jphysiol.1985.sp015854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Lane NJ, Bundgaard M. The blood–brain interface in invertebrates. Ann. N. Y. Acad. Sci. 1986;481:20–42. doi: 10.1111/j.1749-6632.1986.tb27136.x. [DOI] [PubMed] [Google Scholar]
- Akiyama-Oda Y, Hosoya T, Hotta Y. Alteration of cell fate by ectopic expression of Drosophila glial cells missing in non-neural cells. Dev. Genes Evol. 1998;208:578–585. doi: 10.1007/s004270050217. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Neuroscience: glia—more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood–nerve barrier in Drosophila. Cell. 1995;81:757–767. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood–brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron–glial interactions in blood–brain barrier formation. Annu. Rev. Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Glial ensheathment of peripheral axons in Drosophila. J. Neurosci. Res. 2008;86:1189–1198. doi: 10.1002/jnr.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J. Neurosci. 2006a;26:3319–3329. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem. Biophys. 2006b;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bainton RJ, Mayer N, Beckstead R, Bhat MA. Septate junctions are required for ommatidial integrity and blood–eye barrier function in Drosophila. Dev. Biol. 2008;317:585–599. doi: 10.1016/j.ydbio.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Blauth K, Peters K, Rogers S, Fanning A, Bhat M. Drosophila neurexin IV interacts with roundabout and is required for repulsive midline axon guidance. J. Neurosci. 2010;30:5653–5667. doi: 10.1523/JNEUROSCI.6187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, et al. A Drosophila neurexin is required for septate junction and blood–nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev. Cell. 2003;5:611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin—novel members of theneurexinfamily:encountersofaxonsandglia. Trends Neurosci. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Tugentman M, Shilo BZ, Steller H. Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev. Cell. 2002;2:159–170. doi: 10.1016/s1534-5807(02)00116-8. [DOI] [PubMed] [Google Scholar]
- Bernardoni R, Miller AA, Giangrande A. Glial differentiation does not require a neural ground state. Development. 1998;125:3189–3200. doi: 10.1242/dev.125.16.3189. [DOI] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axo-glial junctions. Curr. Opin. Neurobiol. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, et al. Axon–glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Trapp BD. Axonal degeneration and progressive neurologic disability in multiple sclerosis. Neurotox. Res. 2003;5:157–164. doi: 10.1007/BF03033380. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Carretto R, Jan YN. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127:237–244. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev. Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Campbell G, Goring H, Lin T, Spana E, Andersson S, Doe CQ, et al. RK2, a glial-specific homeodomain protein required for embryonic nerve cord condensation and viability in Drosophila. Development. 1994;120:2957–2966. doi: 10.1242/dev.120.10.2957. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Hilgers SL, Juang JL. Ultrastructure and blood–nerve barrier of chordotonal organs in the Drosophila embryo. J. Neurocytol. 1997;26:377–388. doi: 10.1023/a:1018564904170. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the insect. Annu. Rev. Entomol. 2000;45:151–174. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Chi C, Carlson SD. The perineurium of the adult housefly: ultrastructure and permeability to lanthanum. Cell Tissue Res. 1981;217:373–386. doi: 10.1007/BF00233587. [DOI] [PubMed] [Google Scholar]
- Condron B. Spatially discrete FGF-mediated signalling directs glial morphogenesis. Development. 1999;126:4635–4641. doi: 10.1242/dev.126.20.4635. [DOI] [PubMed] [Google Scholar]
- Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J. Neurosci. 2002;22:4906–4917. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, et al. Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur. J. Neurosci. 2003;17:411–416. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Fuerstenberg S, Peng CY. Neural stem cells: from fly to vertebrates. J. Neurobiol. 1998;36:111–127. [PubMed] [Google Scholar]
- Dumstrei K, Wang F, Nassif C, Hartenstein V. Early development of the Drosophila brain: V. Pattern of postembryonic neuronal lineages expressing DE-cadherin. J. Comp. Neurol. 2003;455:451–462. doi: 10.1002/cne.10484. [DOI] [PubMed] [Google Scholar]
- Ebersole TA, Chen Q, Justice MJ, Artzt K. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat. Genet. 1996;12:260–265. doi: 10.1038/ng0396-260. [DOI] [PubMed] [Google Scholar]
- Edenfeld G, Volohonsky G, Krukkert K, Naffin E, Lammel U, Grimm A, et al. The splicing factor crooked neck associates with the RNA-binding protein HOW to control glial cell maturation in Drosophila. Neuron. 2006;52:969–980. doi: 10.1016/j.neuron.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Fernandez JJ, VijayRaghavan K. The development of indirect flight muscle innervation in Drosophila melanogaster. Development. 1993;118:215–227. [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Sachse S, Rappert A, Menzel R. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat. Neurosci. 1999;2:473–478. doi: 10.1038/8144. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, et al. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc. Natl. Acad. Sci. USA. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J. Cell Biol. 2002;157:1247–1256. doi: 10.1083/jcb.200203050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granderath S, Klambt C. Glia development in the embryonic CNS of Drosophila. Curr. Opin. Neurobiol. 1999;9:531–536. doi: 10.1016/S0959-4388(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Hall SG, Bieber AJ. Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J. Neurobiol. 1997;32:325–340. [PubMed] [Google Scholar]
- Halter DA, Urban J, Rickert C, Ner SS, Ito K, Travers AA, et al. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development. 1995;121:317–332. doi: 10.1242/dev.121.2.317. [DOI] [PubMed] [Google Scholar]
- Hartenstein V. The Atlas of Drosophila Development. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hebbar S, Fernandes JJ. Glial remodeling during metamorphosis influences the stabilization of motor neuron branches in Drosophila. Dev. Biol. 2010;340:344–354. doi: 10.1016/j.ydbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Kinrade EF, Georgiou M. The Drosophila neuregulin vein maintains glial survival during axon guidance in the CNS. Dev. Cell. 2001;1:679–690. doi: 10.1016/s1534-5807(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J. Neurosci. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M, Margolis B. Septate and paranodal junctions: kissing cousins. Trends Cell Biol. 2003;13:557–561. doi: 10.1016/j.tcb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y. glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82:1025–1036. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Hoyle G. High blood potassium in insects in relation to nerve conduction. Nature. 1952;169:281–282. doi: 10.1038/169281a0. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Glial cells of an insect ganglion. J. Comp. Neurol. 1986;246:85–103. doi: 10.1002/cne.902460106. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH. Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J. Comp. Neurol. 1988;273:436–444. doi: 10.1002/cne.902730312. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Rouxs Arch. Dev. Biol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Jacobs JR. The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Prog. Neurobiol. 2000;62:475–508. doi: 10.1016/s0301-0082(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Jacobs JR, Goodman CS. Embryonic development of axon pathways in the Drosophila CNS. II. Behavior of pioneer growth cones. J. Neurosci. 1989;9:2412–2422. doi: 10.1523/JNEUROSCI.09-07-02412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jhaveri D, Rodrigues V. Sensory neurons of the Atonal lineage pioneer the formation of glomeruli within the adult Drosophila olfactory lobe. Development. 2002;129:1251–1260. doi: 10.1242/dev.129.5.1251. [DOI] [PubMed] [Google Scholar]
- Jhaveri D, Sen A, Rodrigues V. Mechanisms underlying olfactory neuronal connectivity in Drosophila—the atonal lineage organizes the periphery while sensory neurons and glia pattern the olfactory lobe. Dev. Biol. 2000;226:73–87. doi: 10.1006/dbio.2000.9855. [DOI] [PubMed] [Google Scholar]
- Jones BW. Glial cell development in the Drosophila embryo. Bioessays. 2001;23:877–887. doi: 10.1002/bies.1129. [DOI] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kim J, Jones BW, Zock C, Chen Z, Wang H, Goodman CS, et al. Isolation and characterization of mammalian homologs of the Drosophila gene glial cells missing. Proc. Natl. Acad. Sci. USA. 1998;95:12364–12369. doi: 10.1073/pnas.95.21.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinrade EF, Brates T, Tear G, Hidalgo A. Roundabout signalling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development. 2001;128:207–216. doi: 10.1242/dev.128.2.207. [DOI] [PubMed] [Google Scholar]
- Klambt C, Goodman CS. The diversity and pattern of glia during axon pathway formation in the Drosophila embryo. Glia. 1991;4:205–213. doi: 10.1002/glia.440040212. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 1997;17:7425–7432. doi: 10.1523/JNEUROSCI.17-19-07425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, Poeck B, Roth H, Ernst R, Keller A, Porsch M, et al. Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3beta adaptin gene ruby. Genetics. 2000;155:213–223. doi: 10.1093/genetics/155.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NJ. Vertebrate-like tight junctions in the insect eye. Exp. Cell Res. 1981;132:482–488. doi: 10.1016/0014-4827(81)90126-9. [DOI] [PubMed] [Google Scholar]
- Lane NJ. Novel arthropod cell junctions with restrictive intercellular ‘linkers’. J. Neurocytol. 1989;18:661–669. doi: 10.1007/BF01187085. [DOI] [PubMed] [Google Scholar]
- Lane NJ, Swales LS. Intercellular junctions and the development of the blood–brain barrier in Manduca sexta. Brain Res. 1979;168:227–245. doi: 10.1016/0006-8993(79)90166-5. [DOI] [PubMed] [Google Scholar]
- Lee BP, Jones BW. Transcriptional regulation of the Drosophila glial gene repo. Mech. Dev. 2005;122:849–862. doi: 10.1016/j.mod.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28:793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131:181–190. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood–brain barrier chemoprotective mechanisms in Drosophila. J. Neurosci. 2009;29:3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne TV, Luer K, Technau GM, Klambt C. CNS midline cells in Drosophila induce the differentiation of lateral neural cells. Development. 1997;124:4949–4958. doi: 10.1242/dev.124.24.4949. [DOI] [PubMed] [Google Scholar]
- Nelson KS, Furuse M, Beitel GJ. The Drosophila claudin kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010 doi: 10.1534/genetics.110.114959. (doi: 10.1534/ genetics.110.114959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer JN, Kopczynski CC, Fetter RD, Bland KS, Chen WY, Goodman CS. Wrapper, a novel member of the Ig superfamily, is expressed by midline glia and is required for them to ensheath commissural axons in Drosophila. Neuron. 1998;21:991–1001. doi: 10.1016/s0896-6273(00)80618-2. [DOI] [PubMed] [Google Scholar]
- Ohara R, Yamakawa H, Nakayama M, Ohara O. Type II brain 4.1 (4.1B/KIAA0987), a member of the protein 4.1 family, is localized to neuronal paranodes. Brain Res. Mol. Brain Res. 2000;85:41–52. doi: 10.1016/s0169-328x(00)00233-3. [DOI] [PubMed] [Google Scholar]
- Parker RJ, Auld VJ. Roles for glia in the Drosophila nervous system. Semin. Cell Dev. Biol. 2006;17:66–77. doi: 10.1016/j.semcdb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- Peles E, Joho K, Plowman GD, Schlessinger J. Close similarity between Drosophila neurexin IV and mammalian Caspr protein suggests a conserved mechanism for cellular interactions. Cell. 1997;88:745–746. doi: 10.1016/s0092-8674(00)81920-0. [DOI] [PubMed] [Google Scholar]
- Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003;44:808–817. doi: 10.1167/iovs.02-0473. [DOI] [PubMed] [Google Scholar]
- Pereanu W, Shy D, Hartenstein V. Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev. Biol. 2005;283:191–203. doi: 10.1016/j.ydbio.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J. Neurosci. Res. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. The fine structure of neuroglia in the lamina ganglionaris of the housefly, Musca domestica L. J. Neurocytol. 1983a;12:213–241. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. Glial membrane specializations and the compartmentalization of the lamina ganglionaris of the housefly compound eye. J. Neurocytol. 1983b;12:243–275. doi: 10.1007/BF01148464. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Schofield PK, Treherne JE. Octopamine reduces potassium permeability of the glia that form the insect blood–brain barrier. Brain Res. 1985;360:344–348. doi: 10.1016/0006-8993(85)91252-1. [DOI] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood–brain barrier formation in drosophila. Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Sen A, Shetty C, Jhaveri D, Rodrigues V. Distinct types of glial cells populate the Drosophila antenna. BMC Dev. Biol. 2005;5:25. doi: 10.1186/1471-213X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J. Neurosci. 2003a;23:8221–8230. doi: 10.1523/JNEUROSCI.23-23-08221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development. 2003b;130:1825–1835. doi: 10.1242/dev.00413. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia. 2000;30:122–133. doi: 10.1002/(sici)1098-1136(200004)30:2<122::aid-glia2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev. Biol. 2001;238:47–63. doi: 10.1006/dbio.2001.0411. [DOI] [PubMed] [Google Scholar]
- Shaw SR. Early visual processing in insects. J. Exp. Biol. 1984;112:225–251. doi: 10.1242/jeb.112.1.225. [DOI] [PubMed] [Google Scholar]
- Shishodo E, Ono N, Kojima T, Saigo K. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development. 1997;124:2119–2128. doi: 10.1242/dev.124.11.2119. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Dickie MM, Appel SH. Mutant mice (quaking and jimpy) with deficient myelination in the central nervous system. Science. 1964;144:309–311. doi: 10.1126/science.144.3616.309. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld MJ, Jacobs JR. Apoptosis of the midline glia during Drosophila embryogenesis: a correlation with axon contact. Development. 1995;121:569–578. doi: 10.1242/dev.121.2.569. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the Achilles’ heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Stollewerk A, Klämbt C. The midline glial cells are required for compartmentalisation of axon commissures in the embryonic CNS of Drosophila. Dev. Genes Evol. 1997;207:401–409. doi: 10.1007/s004270050129. [DOI] [PubMed] [Google Scholar]
- Stollewerk A, Klambt C, Cantera R. Electron microscopic analysis of Drosophila midline glia during embryogenesis and larval development using beta-galactosidase expression as endogenous cell marker. Microsc. Res. Tech. 1996;35:294–306. doi: 10.1002/(SICI)1097-0029(19961015)35:3<294::AID-JEMT8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood–brain barrier in Drosophila. J. Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Thomas S, Rodrigues F, Silies M, Naffin E, Wenderdel S, et al. Drosophila neurexin IV stabilizes neuron–glia interactions at the CNS midline by binding to Wrapper. Development. 2009;136:1251–1261. doi: 10.1242/dev.032847. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. The IgLON protein Lachesin is required for the blood–brain barrier in Drosophila. Mol. Cell. Neurosci. 2006;32:91–101. doi: 10.1016/j.mcn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Swales LS, Lane NJ. Insect intercellular junctions: rapid freezing by jet propane. J. Cell Sci. 1983;62:223–236. doi: 10.1242/jcs.62.1.223. [DOI] [PubMed] [Google Scholar]
- Swales LS, Lane NJ. Embryonic development of glial cells and their junctions in the locust central nervous system. J. Neurosci. 1985;5:117–127. doi: 10.1523/JNEUROSCI.05-01-00117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, et al. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axoglial junction. J. Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Bhat MA. Myelination and regional domain differentiation of the axon. Results Probl. Cell Differ. 2009;48:1–28. doi: 10.1007/400_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Pillai AM, Pribisko AL, Labasque M, Dupree JL, Faivre-Sarrailh C, Bhat MA. In vivo deletion of immunoglobulin domains 5 and 6 in neurofascin (Nfasc) reveals domain-specific requirements in myelinated axons. J. Neurosci. 2010;30:4868–4876. doi: 10.1523/JNEUROSCI.5951-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev. Biol. 1987;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- Truman JW. Metamorphosis of the central nervous system of Drosophila. J. Neurobiol. 1990;21:1072–1084. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- Vincent S, Vonesch JL, Giangrande A. Glide directs glial fate commitment and cell fate switch between neurones and glia. Development. 1996;122:131–139. doi: 10.1242/dev.122.1.131. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood–retinal barrier dysfunction in macular edema. Doc. Ophthalmol. 1999;97:217–228. doi: 10.1023/a:1002136712070. [DOI] [PubMed] [Google Scholar]
- Virchow R. Cellular Pathology as Based Upon Physiological and Pathological Histology. John Churchill; London: 1860. [DOI] [PubMed] [Google Scholar]
- Ward RE, IV, Lamb RS, Fehon RG. A conserved functional domain of Drosophila coracle is required for localization at the septate junction and has membrane-organizing activity. J. Cell Biol. 1998;140:1463–1473. doi: 10.1083/jcb.140.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SR, Kearney JB, Guardiola AR, Crews ST. Single-cell mapping of neural and glial gene expression in the developing Drosophila CNS midline cells. Dev. Biol. 2006;294:509–524. doi: 10.1016/j.ydbio.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SR, Banerjee S, Blauth K, Rogers SL, Bhat MA, Crews ST. Neurexin IV and Wrapper interactions mediate Drosophila midline glial migration and axonal ensheathment. Development. 2009;136:1147–1157. doi: 10.1242/dev.030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Rizzolo LJ. Remodeling of junctional complexes during the development of the outer blood–retinal barrier. Anat. Rec. 1997;249:380–388. doi: 10.1002/(SICI)1097-0185(199711)249:3<380::AID-AR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Wolff T. EGF receptor signaling: putting a new spin on eye development. Curr. Biol. 2003;13:R813–R814. doi: 10.1016/j.cub.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern Formation in the Drosophila Retina. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- Wood RL. Intercellular attachment in the epithelium of Hydra as revealed by electron microscopy. J. Biophys. Biochem. Cytol. 1959;6:343–352. doi: 10.1083/jcb.6.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi-Hartenstein A, Salvaterra PM, Hartenstein V. Early development of the Drosophila brain: IV. Larval neuropile compartments defined by glial septa. J. Comp. Neurol. 2003;455:435–450. doi: 10.1002/cne.10483. [DOI] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc. Natl. Acad. Sci. USA. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]