Summary
Lipocalin (LCN) family members are small secreted proteins that bind to small hydrophobic molecules via their characteristic central β-barrels. A couple of LCN family members, including major urinary protein 1 (MUP1), retinol-binding protein 4 (RBP4), LCN2, and LCN13, have been reported to regulate insulin sensitivity and nutrient metabolism. LCN13 is expressed by multiple tissues, including the liver, pancreas, epididymis, and skeletal muscle, and is secreted into the bloodstream in mice. Obesity is associated with a downregulation of LCN13 expression and lower levels of circulating LCN13. LCN13 therapies overcome LCN13 deficiency in mice with either genetic or dietary obesity, leading to an improvement in hyperglycemia, hyperinsulinemia, insulin resistance, glucose intolerance, and hepatic steatosis. In hepatocytes, LCN13 directly suppresses hepatic gluconeogenesis and lipogenesis but increases fatty acid β oxidation. LCN13 also enhances insulin sensitivity in adipocytes. The potential mechanisms of the anti-diabetes and anti-steatosis actions of LCN13 are discussed.
Keywords: Obesity, Diabetes, Insulin, Steatosis, Lipocalin-13, lipocalin-2, retinol-binding protein-4, Gluconeogenesis, Lipogenesis, β-oxidation
I. INTRODUCTION
The alarmingly escalating epidemic of overweight and obesity is becoming a challenging public health problem globally. In the United States, the prevalence of obesity (body mass index (BMI) ≥ 30) and overweight (BMI 25.0 – 29.9) in adults was 35.7% and 33.1%, respectively, in 2009 – 2010 (Flegal et al., 2012). America’s obesity epidemic may not plateau until the prevalence of obesity hits approximately 42% in adults (Hill et al., 2010). It has been well defined that obesity is closely associated with various metabolic diseases, including insulin resistance, type 2 diabetes, hypertension, dyslipidemia, nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease (Despres and Lemieux, 2006; Kahn et al., 2006; Van Gaal et al., 2006).
Obesity develops as a result of an imbalance between food intake and energy expenditure (Morris and Rui, 2009; Spiegelman and Flier, 2001). The excess energy is stored as triacylglycerol in adipose tissues; moreover, in obesity, lipids spillover to other tissues which normally do not store a large amount of lipids, including the liver and skeletal muscle (Flier, 2004; Morris and Rui, 2009). Ectopic lipid accumulation in these tissues impairs their normal functions (called lipotoxicity), leading to insulin resistance and type 2 diabetes (Despres and Lemieux, 2006; Greenberg et al., 2011; Taube et al., 2009). Obesity is also associated with chronic, low-grade inflammation, particularly in adipose tissues and the liver, and proinflammatory cytokines further exacerbate insulin resistance (Hotamisligil, 2006; Shoelson and Goldfine, 2009; Tarantino et al., 2007; Taube et al., 2009). Inflammation is able to increase hepatic glucose production by augmenting the hyperglycemic response to glucagon (Chen et al., 2012; Sheng et al., 2012). Adipose tissues and the liver also secret a variety of molecules that modulate systemic insulin sensitivity and glucose and lipid metabolism in an endocrine fashion (Ahima and Osei, 2008; Esteve et al., 2009; Halberg et al., 2008; Rajala and Scherer, 2003). For example, adiponectin, secreted by adipocytes, functions as an endogenous insulin sensitizer to improve glucose metabolism (Berg et al., 2001; Yamauchi et al., 2002; Yamauchi et al., 2001). FGF21, mainly secreted by the liver, promotes fatty acid oxidation as well as enhances insulin sensitivity (Badman et al., 2007; Inagaki et al., 2007). Recently, several lipocalin (LCN) family members have been reported to be involved in the regulation of insulin sensitivity and nutrient metabolism (Cho et al., 2011; Guo et al., 2010; Hui et al., 2009; Jun et al., 2011; Law et al., 2010; Sheng et al., 2011; Yan et al., 2007; Yang et al., 2005; Zhou et al., 2009). In this review, we discuss these LCNs in the setting of obesity, focusing on LCN13.
II. LIPOCALIN STRUCTURE AND FUNCTION
The LCN family contains a large number of diverse small secreted proteins, typically composed of 160-180 amino acids with an N-terminal signal peptide (Ganfornina et al., 2000). LCN family members are found in vertebrate and invertebrate animals, plants, and even bacteria. In mammals, LCN genes are clustered on the same chromosomal loci and are likely to evolve from an ancestral LCN gene through an in situ tandem duplication (Grzyb et al., 2006; Suzuki et al., 2004). The name of “lipocalin” is derived from the Greek words ‘lipos’ and ‘kalyx’ (means drinking vessel), indicating the common feature of the family members: they bind small hydrophobic molecules (Grzyb et al., 2006). LCN family members have low levels of primary amino acid sequence homology (usually < 30%) (Flower et al., 2000; Grzyb et al., 2006); however, all LCN family members contain one or more short structurally conserved regions (SCRs) (Flower et al., 1993). LCNs are generally divided into two subfamilies based on their SCR number. Kernel LCNs, including retinol-binding protein (RBP), β-lactoglobulin, and LCN2, contain three SCRs, while outlier LCNs, including crustacyanin, nitrophorins, and α-1-acid glycoprotein, contain one or two SCRs (Grzyb et al., 2006). Many LCNs have conserved cysteines which form disulfide bridges to stabilize their three-dimensional structures (Glasgow et al., 1998). Some LCNs are able to form dimmers or oligomers under specific conditions (e.g. a low pH and high calcium concentrations) (Grzyb et al., 2006).
Despite of very limited similarity in their amino acid sequences, all LCN family members share a highly-conserved tertiary structure with a characteristic central β-barrel. This β-barrel is formed by eight anti-parallel β-strands in a cylindrical manner with a ‘closed end’ on one side and an ‘open end’ on the opposite side (Flower, 1996; Flower et al., 1993). The ‘open end’ provides an access into the central cavity for small hydrophobic molecules (Grzyb et al., 2006; Schlehuber and Skerra, 2005). The interior of the β-barrel consists of hydrophobic amino acid residues which define the binding capacity of individual LCNs to various hydrophobic molecules (Grzyb et al., 2006). The eight β-strands, connected by seven loops between two consecutive β-strands, coil in a right-handed manner around a central axis and interact through transversal hydrogen bonds (Grzyb et al., 2006; Schlehuber and Skerra, 2005). The first loop is a large and flexible Ω-type loop which functions as a dynamic lid for the ‘open end’ of the β-barrel, and the other six are short hairpin-type loops (Flower et al., 1993).
The biological activity of LCN family members may largely depend on their ability to specifically bind to small hydrophobic molecules, including fatty acids, phospholipids, steroids, retinol, and pheromones (Flower et al., 2000; Grzyb et al., 2006; Schlehuber and Skerra, 2005; Zhou and Rui, 2010). LCNs control the transportation, stability, release, activation, and clearance of these bioactive hydrophobic molecules which in turn regulate diverse biological processes, including cellular metabolism, proliferation, differentiation and death, (Flower, 1996; Sharrow et al., 2002; Zhou and Rui, 2010). In rodents, the LCN family members have been documented to regulate chemical communication, reproduction, immune responses, and cancer development (Bratt, 2000; Chamero et al., 2007; Logdberg and Wester, 2000; More, 2006; Oehninger et al., 1995). Recently, LCNs have been reported to regulate insulin sensitivity and nutrient metabolism in obesity (Cho et al., 2011; Guo et al., 2010; Hui et al., 2009; Jun et al., 2011; Law et al., 2010; Sheng et al., 2011; Yan et al., 2007; Yang et al., 2005; Zhou et al., 2009).
III. LIPOCALIN 13 REGULATION OF GLUCOSE METABOLISM
There have only been three reports in literatures that describe LCN13 action thus far (Cho et al., 2011; Sheng et al., 2011; Suzuki et al., 2004). The LCN13 gene was originally identified in 2004 by analyzing the epididymal cluster of the LCN genes on chromosome 2 in mice (Suzuki et al., 2004). This chromosomal locus also contains 12 additional LCN genes: LCN2-5, LCN8-13, C8γ (complement 8 γ), and PGDS (prostaglandin D2 synthase) (Suzuki et al., 2004). The LCN13 gene is predicted to have seven exons and to encode a polypeptide containing 176 amino acids. The first 19 amino acids of its N-terminus are hydrophobic and are predicted to function as a signal peptide responsible for LCN13 secretion (Suzuki et al., 2004). LCN13 protein was verified later using anti-LCN13 antibodies and also detected in the mouse bloodstream (Cho et al., 2011). LCN13 proteins are expressed and secreted by multiple tissues in mice, including the liver, pancreas, and skeletal muscles (Cho et al., 2011). Two distinct forms of LCN13 proteins are generated from a single full-length LCN13 cDNA, suggesting that LCN13 precursors are subjected to differential proteolytic cleavages and/or posttranslational modifications (e.g acylation and glycosylation) (Cho et al., 2011). However, the underlying mechanisms by which the LCN13 precursors are differentially processed and modified to generate the mature forms of LCN13 remain completely unknown.
The expression and secretion of LCN13 are not constant in mice and change in response to alterations of metabolic states. The levels of circulating LCN13 are lower in the fasting state than in the fed state (Cho et al., 2011), suggesting that LCN13 may be involved in nutrient sensing and metabolic regulation. Importantly, obesity is associated with downregulation of LCN13, and the levels of LCN13 in the bloodstream are extremely low in mice with either genetic (db/db) or high fat diet (HFD)-induced obesity (Cho et al., 2011). The expression of LCN13 in the liver is also lower in obese mice than in lean mice (Cho et al., 2011); however, factors that regulate the expression, secretion, and clearance of LCN13 are unknown.
To study the physiological function of LCN13, we have generated three useful regents: recombinant LCN13 proteins, LCN13 adenoviruses expressing LCN13 proteins, and LCN13 transgenic (Tg) mice containing the LCN13 transgene under the control of the constitutively-active chicken β-actin/rabbit β-globin hybrid promoter. Chronic treatments with recombinant LCN13 protein, via osmotic minipumps, do not prevent HFD-induced obesity; however, the treatments improve insulin resistance and glucose intolerance in HFD-fed mice (Cho et al., 2011). To overcome LCN13 deficiency in obese mice, we infected db/db (lack of functional leptin receptors) or HFD-fed mice with LCN13 adenoviruses via tail vein injection, with β-gal adenoviruses as controls. LCN13 adenoviral infection results in an increase in the levels of circulating LCN13 due to overexpression of recombinant LCN13 in the liver (Cho et al., 2011). Adenovirus-mediated overexpression of LCN13 reduces hyperglycemia, insulin resistance, and glucose intolerance in db/db mice as well as in HFD-fed mice (Cho et al., 2011). LCN13 Tg mice secret higher levels of LCN13 into the circulation than WT control mice, as expected (Cho et al., 2011). Although LCN13 Tg mice are not protected from HFD-induced obesity, they resist HFD-induced insulin resistance and glucose intolerance (Cho et al., 2011). Additionally, transgenic overexpression of LCN13 markedly reduces hyperglycemia, insulin resistance, and glucose intolerance in ob/ob mice that are deficient of leptin (Sheng et al., 2011). Moreover, neutralization of endogenous LCN13 through tail vein injection of anti-LCN13 antibodies induces glucose intolerance in normal mice (Sheng et al., 2011). Together, these results demonstrate that obesity is associated with LCN13 deficiency, which contributes to insulin resistance, hyperglycemia, and glucose intolerance in mice. These observations raise the possibility that LCN13 and its related molecules may have therapeutic potential for treating obesity-associated type 2 diabetes.
LCN13 is able to directly regulate glucose metabolism in both adipocytes and hepatocytes. Recombinant LCN13 dose-dependently enhances insulin signaling and insulin-stimulated glucose uptake in cultured adipocytes (Cho et al., 2011). LCN13 also increases the ability of insulin to suppress glucose production in primary hepatocyte cultures (Cho et al., 2011). These observations suggest that LCN13 acts as an insulin sensitizer to improve glucose metabolism in these two cell types. The livers secrets LCN13, and neutralization of endogenous LCN13 increases glucose production in primary hepatocytes and impairs glucose tolerance in mice (Cho et al., 2011; Sheng et al., 2011). These observations suggest that hepatic LCN13 is able to suppress hepatic glucose production in an autocrine/paracrine fashion. In the absence of insulin, LCN13 still stimulates glucose uptake in adipocytes and inhibits glucose production and the expression of key gluconeogenic genes (e.g. phosphoenolpyruvate carboxykinase and glucose-6-phosphatase) in primary hepatocytes (Cho et al., 2011). These results indicate that LCN13 is able to regulate glucose metabolism by an additional insulin-independent mechanism. Since skeletal muscles, immune cells, and the brain (particularly the hypothalamus) play an important role in the regulation of systemic insulin sensitivity and glucose metabolism, it will be important to determine whether LCN13 regulates the activity of these tissues in the future.
The molecular mechanism of LCN13 action is currently unknown. Like other LCN family members, LCN13 is predicted to bind to small hydrophobic molecules via its central β-barrel. These small hydrophobic molecules may be bioactive and regulates insulin sensitivity and glucose metabolism. LCN13 may exert its biological action by controlling the transportation, stability, release, activation, and/or clearance of these molecules. Additionally, several LCN family members have been reported to bind to and activate their cognate receptors on the plasma membrane (Berry et al., 2011; Zhou and Rui, 2010). Similarly, LCN13 may modulate insulin sensitivity and nutrient metabolism by binding to and activating its cognate receptors. In the future, it will be important to identify and characterize the putative LCN13-bound small molecules (ligands), the putative LCN13 receptors, and the signaling pathways that link LCN13 ligands and LCN13 receptors to LCN13 metabolic responses. It will also be interesting to determine whether the two forms of LCN13 have different specificity and/or affinity for LCN13 ligands, and whether these two forms differentially regulate the activation of LCN13 receptors.
IV. LIPOCALIN 13 REGULATION OF LIPID METABOLISM
LCN13 appears to not have anti-obesity capability, but it markedly decreases hepatic steatosis in obese mice (Sheng et al., 2011). LCN13 Tg mice resist HFD-induced hyperlipidemia and hepatic steatosis (Sheng et al., 2011). LCN13 Tg mice also resist hyperlipidemia and hepatic steatosis induced by leptin deficiency (Sheng et al., 2011). Similarly, adenovirus-mediated overexpression of LCN13 ameliorates hepatic steatosis in db/db mice (Sheng et al., 2011). LCN13 is able to suppress the hepatic lipogenic program in mice (Sheng et al., 2011). The expression of key liver lipogenic genes, including stearoyl-coenzyme A desaturase 1 (SCD1), fatty acid synthase (FAS), carbohydrate-responsive element-binding protein (ChREBP), peroxisome proliferator-activated receptor-γ (PPARγ), and sterol regulatory element-binding protein (SREBP-1c), is lower in LCN13 Tg mice as well as in mice infected with LCN13 adenoviruses (Sheng et al., 2011). In contrast, LCN13 increases the expression of carnitine palmitoyltransferase-1α (CPT-1α) in the liver (Sheng et al., 2011). CPT-1α promotes fatty acid β oxidation (Schreurs et al., 2010). Therefore, LCN13 protects obesity-associated hepatic steatosis presumably by both decreasing lipogenesis and increasing fatty acid β oxidation in the liver. In addition to anti-diabetes, LCN13 also has an anti-steatosis effect; thus, LCN13 and its related molecules may have therapeutic potential for treating both type 2 diabetes and NAFLD.
In agreement with its anti-steatosis effects, LCN13 directly suppresses lipogenesis in primary hepatocytes in a dose-dependent manner (Sheng et al., 2011). It inhibits the expression of SCD1, FAS, SREBP-1c, ChREBP, and PPARγ in hepatocytes (Sheng et al., 2011). Moreover, LCN13 directly stimulates the expression of CPT-1α, fatty acid β oxidation, and ketogenesis in primary hepatocytes (Sheng et al., 2011). Conversely, in primary hepatocytes, neutralization of endogenous LCN13 with anti-LCN13 antibody increases the expression of lipogenic genes and lipogenesis but decreases CPT-1α expression and fatty acid β oxidation (Sheng et al., 2011). These observations suggest that liver-derived LCN13 regulates lipogenesis and fatty acid β oxidation in an autocrine/paracrine fashion. Because the liver cross-talks with other tissues, including adipose tissues, skeletal muscles and the brain, it will be interesting to study whether LCN13 regulates lipid metabolism and lipid levels in the liver indirectly by regulating the crosstalk among these tissues.
The molecular mechanism by which LCN13 regulates lipid metabolism is currently unknown. As discussed above, LCN13 is predicted to bind to bioactive hydrophobic molecules. These small hydrophobic molecules may regulate hepatocyte lipogenesis and fatty acid oxidation. LCN13 may regulate the transportation, stability, release, activation, and/or clearance of these bioactive molecules, thereby indirectly regulating lipid metabolism in the liver. Alternatively, LCN13 may bind to and activate its cognate receptors on hepatocytes, thereby directly regulating lipid metabolism in the liver. In the future, it is essential to identify and characterize these putative hydrophobic molecules and LCN13 receptors in order to advance our understanding of LCN13 action. Interestingly, LCN13 decreases hepatic steatosis but not adiposity in obese mice, suggesting that LCN13 decreases lipogenesis and increases fatty acid oxidation specifically in hepatocytes but not in adipocytes. Adipocytes do respond to LCN13 by increasing glucose uptake (Cho et al., 2011). It will be interesting to further study the differential effects of LCN13 on adipocytes and hepatocytes in order to gain insight into the molecular mechanism of LCN13 action.
V. REGULATION OF NUTRIENT METABOLISM BY OTHER LCN FAMILY MEMBERS
Three other LCN family members, major urinary protein 1 (MUP1), RBP4 and LCN2, have also been reported to be involved in the regulation of insulin sensitivity and glucose metabolism in obesity. MUP1 is abundantly expressed in mouse livers and secreted into the circulation (Zhou and Rui, 2010). MUP genes are highly polymorphic, and the MUP gene subfamily contains 21 paralogous genes clustered on chromosome 4 in mice (Hastie et al., 1979). The polymorphic MUP genes serve as a specific genetic marker of individual identity (Zhou and Rui, 2010). MUP expression is sexually dimorphic and is higher in males (Geertzen et al., 1973; Lane and Neuhaus, 1972). Each individual adult male mouse normally secretes 8-14 different MUP isoforms, and this specific pattern of MUP isoforms serves as an individual identity signature (Zhou and Rui, 2010). MUPs bind to various volatile pheromones via their central β-barrels and function as pheromone carriers (Zhou and Rui, 2010). The MUP-pheromone complexes are excreted into the urine and mediate chemical signaling in rodents (Zhou and Rui, 2010). Interestingly, both the expression of MUP1 in the liver and the levels of circulating MUP1 are significantly lower in mice with either genetic (db/db) or HFD-induced obesity (Hui et al., 2009; Zhou et al., 2009). Like LCN13, recombinant MUP1 ameliorates hyperglycemia and glucose intolerance in both db/db and HFD-fed mice (Hui et al., 2009; Zhou et al., 2009). At cellular levels, MUP1 inhibits the expression of gluconeogenic genes and glucose production in hepatocytes in an autocrine/paracrine fashion (Zhou et al., 2009). MUP1 also improves skeletal muscle insulin sensitivity in obese mice presumably by increasing mitochondrial oxidative capacity (Hui et al., 2009).
RBP4 is secreted into the circulation from both adipose tissues and the liver (Kotnik et al., 2011). In contrast to LCN13 and MUP1, RBP4 levels are higher in both rodents and humans with obesity (Broch et al., 2010; Graham et al., 2006; Stefan et al., 2007; Yang et al., 2005). However, clinical study results are not consistent, and several studies report no correlation between circulating RBP4 levels and the severity of obesity and insulin resistance as reviewed by Kotnik et al (Kotnik et al., 2011). Transgenic overexpression of RBP4 or administration of recombinant RBP4 proteins promotes insulin resistance and glucose intolerance in normal mice; conversely, genetic deletion of RBP4 improves insulin sensitivity (Yang et al., 2005). RBP4 promotes insulin resistance in skeletal muscles, adipocytes, and hepatocytes (Ost et al., 2007; Yang et al., 2005). RBP4 also stimulates cytokine secretion by macrophages, thus indirectly promoting insulin resistance (Deng et al., 2009; Norseen et al., 2012). In adipocytes, retinol-bound RBP4 activates STRA6 (stimulated by retinoic acid 6), the RBP4 receptor on the plasma membrane (Berry et al., 2011). STRA6 in turn activates the JAK2/STAT5 pathway, leading to SOCS3 expression in adipocytes (Berry et al., 2011). SOCS3 has been reported to induce insulin resistance (Rui et al., 2002; Ueki et al., 2004a; Ueki et al., 2004b).
Levels of circulating LCN2, also called neutrophil gelatinase-associated lipocalin, are higher in mice and humans with obesity (Esteve et al., 2009). The relative contribution of LCN2 to insulin resistance in obesity remains controversial, and three groups described different metabolic phenotypes of LCN2 knockout (KO) mice. Guo el al reported that LCN2 KO mice are susceptible to HFD-induced obesity and insulin resistance (Guo et al., 2010). Law et al showed that LCN2 KO mice resist HFD-induced insulin resistance and glucose intolerance (Law et al., 2010). Jun et al demonstrated that LCN2 deficiency has a very mild effect on HFD-induced insulin resistance and glucose intolerance (Jun et al., 2011). The reasons underlying the discrepancy between these three studies are currently unknown.
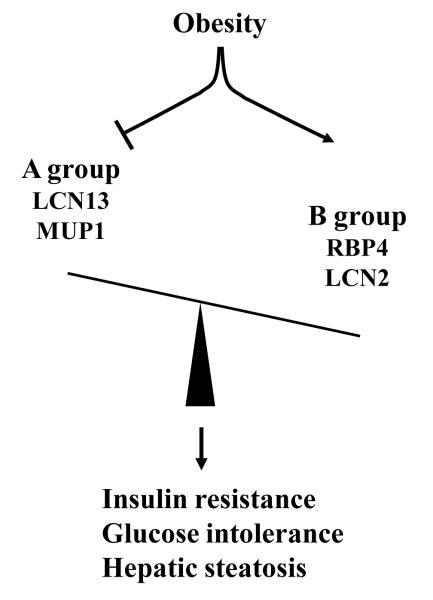
In summary, obesity is associated with an altered secretion of several LCN family members. These LCNs can be divided into two groups: A and B (Fig. 1). The A group contains LCN13 and MUP1 and enhances insulin sensitivity and improves glucose metabolism. Obesity is associated with deficiency of these group members. The B group contains RBP4 and LCN2 and promotes insulin resistance, and obesity is associated with an increase in the expression and secretion of these LCNs. These two groups have opposing effects on insulin sensitivity and nutrient metabolism; therefore, the ratios of A to B LCNs are likely to be an important factor to determine insulin sensitivity. Obesity is associated with a marked decrease in this A to B LCN ratios; therefore, one goal for treatment of insulin resistance and type 2 diabetes is to restore the normal balance between A and B LCNs.
Figure 1. A model of lipocalin action in obesity.
Lipocalins known to regulate nutrient metabolism can be divided into two groups: A and B. The A group (LCN13 and MUP1) ameliorates insulin resistance, glucose intolerance, and hepatic steatosis in obesity, whereas the B group (RBP4 and LCN2) has opposite effects. Obesity is associated with both deficiency of the A group of LCNs and increased expression/secretion of the B group of LCNs, thus disrupting balance between A and B LCNs. This A and B LCN imbalance contributes to metabolic disorders in obesity, including type 2 diabetes and NAFLD.
VI. CONCLUSIONS AND FUTURE DIRECTIONS
LCN13 is a LCN family member that is secreted into the bloodstream by multiple tissues. It has an anti-diabetes and anti-steatosis effect in mice with obesity. Obesity is associated with LCN13 deficiency, which is likely to contribute to insulin resistance, hepatic steatosis, and glucose intolerance in obese mice. Aside from LCN13, MUP1, RBP4, and LCN2 are also involved in the regulation of insulin sensitivity and nutrient metabolism, particularly in the setting of obesity. In general, these LCN family members fall into two distinct categories. LCNs in the first category contains LCN13 and MUP1 and have an anti-insulin resistance and anti-diabetes effect, whereas LCNs in the second category, including RBP4 and LCN2, have an opposite effect. Obesity is associated with a downregulation of the first category LCNs and an upregulation of the second category LCNs, resulting in a sharp decrease in the first to the second category LCN ratios. This imbalance between the first and the second category LCNs may contribute to metabolic disorders in obesity.
The mechanisms of LCN13 action remain largely unknown. In the future, we will determine whether LCN13 exerts its biological action directly via binding to and activating its cognate receptors or indirectly by regulating the transportation, stability, release, and/or clearance of its bound bioactive molecules (ligands). It is important to identify and characterize the putative LCN13 receptors and LCN13 ligands in order to completely understand LCN13 actions. The intracellular signaling pathways that act downstream of LCN13 receptors or LCN13 ligands and mediate LCN13’s metabolic responses should be examined. Additionally, human LCN13 has not been identified yet. It is also unclear whether LCN13 has a similar anti-diabetes and anti-steatosis effect in humans. The relevance of LCN13’s metabolic functions described in mice to human obesity and obesity-associated metabolic disorders should be vigorously examined in order to evaluate the therapeutic potentials of LCN13 and LCN13-related molecules in the treatments of type 2 diabetes, NAFLD, and other obesity-associated metabolic diseases. Furthermore, more A and B LCNs, in addition to LCN13, MUP1, RBP4, and LCN2 as described in Fig. 1, are expected to be identified and characterized, and their contributions to obesity-associated metabolic disorders will be investigated.
ACKNOWLEDGEMENTS
We thank Crystal W. Rui for helpful discussion. This work was supported by RO1 DK 065122 from NIH.
REFERENCES
- Ahima RS, Osei SY. Adipokines in obesity. Front Horm Res. 2008;36:182–97. doi: 10.1159/000115365. [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci U S A. 2011;108:4340–5. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt T. Lipocalins and cancer. Biochim Biophys Acta. 2000;1482:318–26. doi: 10.1016/s0167-4838(00)00154-0. [DOI] [PubMed] [Google Scholar]
- Broch M, Gomez JM, Auguet MT, Vilarrasa N, Pastor R, Elio I, Olona M, Garcia-Espana A, Richart C. Association of retinol-binding protein-4 (RBP4) with lipid parameters in obese women. Obes Surg. 2010;20:1258–64. doi: 10.1007/s11695-010-0200-5. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Chen Z, Sheng L, Shen H, Zhao Y, Wang S, Brink R, Rui L. Hepatic TRAF2 regulates glucose metabolism through enhancing glucagon responses. Diabetes. 2012;61:566–73. doi: 10.2337/db11-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KW, Zhou Y, Sheng L, Rui L. Lipocalin-13 Regulates Glucose Metabolism by both Insulin-Dependent and Insulin-Independent Mechanisms. Mol Cell Biol. 2011;31:450–7. doi: 10.1128/MCB.00459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, Shah SV, Sun D, Michalek S, Grizzle WE, Garvey T, Mobley J, Zhang HG. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Esteve E, Ricart W, Fernandez-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32(Suppl 2):S362–7. doi: 10.2337/dc09-S340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower DR, North AC, Attwood TK. Structure and sequence relationships in the lipocalins and related proteins. Protein Sci. 1993;2:753–61. doi: 10.1002/pro.5560020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Gutierrez G, Bastiani M, Sanchez D. A phylogenetic analysis of the lipocalin protein family. Mol Biol Evol. 2000;17:114–26. doi: 10.1093/oxfordjournals.molbev.a026224. [DOI] [PubMed] [Google Scholar]
- Geertzen HG, Ouderaa F. J. v., Kassenaar AA. Isolation and metabolism of male sex-dependent urinary protein from rats. Acta Endocrinol (Copenh) 1973;72:197–208. doi: 10.1530/acta.0.0720197. [DOI] [PubMed] [Google Scholar]
- Glasgow BJ, Abduragimov AR, Yusifov TN, Gasymov OK, Horwitz J, Hubbell WL, Faull KF. A conserved disulfide motif in human tear lipocalins influences ligand binding. Biochemistry. 1998;37:2215–25. doi: 10.1021/bi9720888. [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–10. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzyb J, Latowski D, Strzalka K. Lipocalins - a family portrait. J Plant Physiol. 2006;163:895–915. doi: 10.1016/j.jplph.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–85. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–68. x–xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie ND, Held WA, Toole JJ. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979;17:449–57. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Hill AL, Rand DG, Nowak MA, Christakis NA. Infectious disease modeling of social contagion in networks. PLoS Comput Biol. 2010;6:e1000968. doi: 10.1371/journal.pcbi.1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hui X, Zhu W, Wang Y, Lam KS, Zhang J, Wu D, Kraegen EW, Li Y, Xu A. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J Biol Chem. 2009;284:14050–7. doi: 10.1074/jbc.M109.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine Regulation of the Fasting Response by PPARalpha-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jun LS, Siddall CP, Rosen ED. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am J Physiol Endocrinol Metab. 2011;301:E825–35. doi: 10.1152/ajpendo.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–11. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- Lane SE, Neuhaus OW. Multiple forms of 2 u, a sex-dependent urinary protein of the adult male rat. Biochim Biophys Acta. 1972;263:433–40. doi: 10.1016/0005-2795(72)90095-5. [DOI] [PubMed] [Google Scholar]
- Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–82. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logdberg L, Wester L. Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta. 2000;1482:284–97. doi: 10.1016/s0167-4838(00)00164-3. [DOI] [PubMed] [Google Scholar]
- More L. Mouse major urinary proteins trigger ovulation via the vomeronasal organ. Chem Senses. 2006;31:393–401. doi: 10.1093/chemse/bjj043. [DOI] [PubMed] [Google Scholar]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–59. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, Usheva A, Davis RJ, Smith U, Kahn BB. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010–9. doi: 10.1128/MCB.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehninger S, Coddington CC, Hodgen GD, Seppala M. Factors affecting fertilization: endometrial placental protein 14 reduces the capacity of human spermatozoa to bind to the human zona pellucida. Fertil Steril. 1995;63:377–83. doi: 10.1016/s0015-0282(16)57372-5. [DOI] [PubMed] [Google Scholar]
- Ost A, Danielsson A, Liden M, Eriksson U, Nystrom FH, Stralfors P. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 2007;21:3696–704. doi: 10.1096/fj.07-8173com. [DOI] [PubMed] [Google Scholar]
- Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 Block Insulin Signaling by Ubiquitin-mediated Degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–8. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- Schlehuber S, Skerra A. Lipocalins in drug discovery: from natural ligand-binding proteins to “anticalins”. Drug Discov Today. 2005;10:23–33. doi: 10.1016/S1359-6446(04)03294-5. [DOI] [PubMed] [Google Scholar]
- Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010;11:380–8. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- Sharrow SD, Vaughn JL, Zidek L, Novotny MV, Stone MJ. Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci. 2002;11:2247–56. doi: 10.1110/ps.0204202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Cho KW, Zhou Y, Shen H, Rui L. Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid beta-oxidation. J Biol Chem. 2011;286:38128–35. doi: 10.1074/jbc.M111.256677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Zhou Y, Chen Z, Ren D, Cho KW, Jiang L, Shen H, Sasaki Y, Rui L. NF-kappaB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat Med. 2012 doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Goldfine AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15:373–4. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Haring HU. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007;30:1173–8. doi: 10.2337/dc06-2342. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Lareyre JJ, Sanchez D, Gutierrez G, Araki Y, Matusik RJ, Orgebin-Crist MC. Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene. 2004;339:49–59. doi: 10.1016/j.gene.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22:293–303. doi: 10.1111/j.1440-1746.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- Taube A, Eckardt K, Eckel J. Role of lipid-derived mediators in skeletal muscle insulin resistance. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00241.2009. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004a;24:5434–46. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004b;101:10422–7. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–40. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang L, Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem. 2009;284:11152–9. doi: 10.1074/jbc.M900754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Rui L. Major urinary protein regulation of chemical communication and nutrient metabolism. Vitam Horm. 2010;83:151–63. doi: 10.1016/S0083-6729(10)83006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]