Abstract
Background
Corpora amylacea (CA) are glycoproteinaceous (predominantly glial and extracellular) inclusions that accumulate in normal aging brain and, to a greater extent, in Alzheimer disease (AD). Previous pharmacological evidence suggested that up-regulation of endogenous heme oxygenase-1 (HO-1) in astrocytes promotes transformation of normal mitochondria to CA-like inclusions. Here, we determined whether 1) HMOX1 transfection fosters the accumulation of CA-like inclusions in cultured rat astroglia; 2) the HMOX1 transgene promotes CA formation in the brains of aging GFAP.HMOX1 mice; and 3) brain mitochondrial damage and CA biogenesis are augmented in persons with mild cognitive impairment (MCI), a harbinger of AD.
Methods
CA were ascertained in (i) neonatal rat astroglia transfected with flag-tagged human HO-1 cDNA, (ii) brain sections derived from 19 month-old GFAP.HMOX1 and wild-type (WT) mice, and (iii) post-mortem hippocampal sections from individuals with mild (MCI) and no cognitive impairment (NCI) after staining with PAS or antisera against HO-1, ubiquitin (Ub), manganese suoperoxide dismutase (MnSOD), α-synuclein or tyrosine hydroxylase (TH).
Results
HMOX1 transfection induced cytoplasmic vacuolation and the accumulation of PAS+ inclusions in cultured astroglia. Numerous CA-like inclusions stained with PAS− and immunoreactive for HO-1, Ub and MnSOD were observed in the brains of GFAP.HMOX1 mice, but were rarely encountered in age-matched, wild-type controls. Numbers of HO-1-positive CA were significantly increased in certain hippocampal strata of MCI subjects relative to NCI preparations. MnSOD and Ub proteins co-localized to CA in both the control and MCI specimens.
Conclusions
HO-1 promotes mitochondrial damage and CA biogenesis in astrocyte cultures and in the intact aging brain. CA formation is enhanced in the MCI hippocampus and thus occurs relatively early in the pathogenesis of AD. Glial HO-1 suppression may attenuate bioenergetic failure and slow disease progression in AD and other neurodegenerative conditions featuring accelerated accumulation of CA.
Keywords: Alzheimer disease, astrocyte, corpora amylacea, heme oxygenase-1, mild cognitive impairment, mitochondria, transgenic mice
Introduction
Alzheimer disease (AD) is a dementing illness characterized by gliosis, progressive neuronal degeneration, and the accumulation of neurofibrillary tangles and senile plaques in discrete regions of the basal forebrain, hippocampus, and association cortices (Selkoe, 1991). Mild cognitive impairment (MCI) refers to persons exhibiting cognitive dysfunction (often memory loss) that does not meet clinical criteria for AD or another dementing illness. Subjects with MCI often manifest hallmark neuropathological features of AD at autopsy and about half of persons with MCI will develop AD within an ensuing 5-year period (Chertkow, et al., 2001).
Corpora amylacea (CA) are glycoproteinaceous, ubiquitinated, cytoplasmic inclusions that accumulate in subpial and periventricular regions of human brain in the course of normal aging, and to a greater extent in AD and other neurodegenerative conditions (Cavanagh, 1999, Schipper and Cisse, 1995). In the central nervous system (CNS), CA are most frequently encountered in astrocytes and as extracellular deposits. CA may occasionally arise within neuritic processes and have been reported in various non-neural tissues. Although many of the histochemical, tinctorial and structural properties of CA have been delineated during the 150 years or so since their discovery, their subcellular origins and mechanisms responsible for their biogenesis have remained elusive. We demonstrated consistent co-localization of two mitochondrial proteins, sulfite oxidase and HSP60, to CA in subependymal regions of senescent and AD human brain, and in smears of isolated human CA (Schipper and Cisse, 1995). These findings suggested that effete mitochondria may be subcellular precursors of CA. We subsequently observed that prolonged (90 days) exposure of cultured neonatal rat astroglia to cysteamine (CSH) and subcutaneous administration of CSH to young adult rats elicited the formation of large, CA-like inclusions that stained for PAS, iron-mediated peroxidase activity, mitochondrial epitopes and ubiquitin (Ub) (Cisse and Schipper, 1995, Fenton, et al., 1998).
Heme oxygenase-1 (HO-1) is a 32 kDa stress protein that catalyzes the degradation of heme to biliverdin and bilirubin (in concert with biliverdin reductase) with liberation of equimolar concentrations of ferrous iron and carbon monoxide (CO) (Maines, 1997, Mancuso and Barone, 2009). Our laboratory demonstrated that CA in human brain (Schipper and Cisse, 1995, Schipper, et al., 1995) and in CSH-exposed astroglia (Sahlas, et al., 2002) are immunoreactive for HO-1, and that dexamethasone suppression of HO-1 interfered with the formation of CA in CSH-treated glial cultures (Sahlas, et al., 2002). These findings suggested that (i) degenerate, iron-laden mitochondria are subcellular progenitors of CA in senescent astroglia (and possibly other tissues) and (ii) the biogenesis of these aging-associated glial inclusions is contingent upon the antecedent up-regulation of HO-1. In the current study, we set out to ascertain whether 1) transfection of the HMOX1 gene engenders the accumulation of CA-like inclusions in cultured rat astroglia; 2) astroglial expression of the HMOX1 transgene accelerates formation of CA-like inclusions in aging mice and 3) mitochondrial damage and the biogenesis of CA are augmented in the brains of persons with MCI.
Materials and Methods
Primary astrocyte cultures and HO-1 transfection
Cell isolation and culture
Two-day-old postnatal Sprague-Dawley rat pups (Charles River Breeding Farms) were used to generate primary astroglial cultures. Rats were housed on a 14 h light–10 h dark lighting schedule with access to standard rat lab chow and water ad libitum. Twenty min prior to dissection, rat pups were separated from the mother and anesthetized in a CO2 chamber. Rats were decapitated and astroglia were isolated by mechanoenzymatic dissociation of cerebral tissue as previously described (Chopra, et al., 1995, Vaya, et al., 2007). Cells were grown in Ham’s F-12 and high glucose DMEM (50:50 v/v) supplemented with 10 mM HEPES, 5% heat-inactivated horse serum, 5% heat inactivated fetal bovine serum, and penicillin-streptomycin (50 U/ml and 50 mg/ml, respectively). Cells were seeded in 8-well chamber slides at a density of 1.25 × 105 cells/ml and incubated at 37°C in humidified 95% air-5% CO2 for 6 h. After washing twice with culture medium to remove adherent oligodendroglia and microglia, the cultures were maintained under the above-mentioned conditions for 6 days at which time more than 98% of the cells comprising the monolayer were astroglia as determined by immunohistochemical labeling for the astrocyte-specific marker, glial fibrillary acidic protein (GFAP) (Chopra, et al., 1995). To minimize cell stress, the cultures were maintained for 6 days prior to any manipulations. Gene transfections were initiated on in vitro day 7.
cDNA plasmid construct
An HMOX1 construct was prepared consisting of pcDNA3.1/Zeo.CMV.Flag.HMOX1 containing the entire protein-coding region (866 bp) of the human HO-1 gene (Yoshida, et al., 1988) as previously described (Song, et al., 2006). Plasmid assembly was enabled using a forward primer (5′-TTC ATA CAA GCT TAT GGA GCG TCC GCA ACC-3′) containing a HindIII site and a reverse primer (5′-TCA ATG GAT CCT CAC ATG GCA TAA AGC CCT-3′) containing a BamHI site designed to match the multiple cloning sites in pcDNA3.1/Zeo CMV-Flag. The HMOX1 fragment was amplified by pfu DNA polymerase-catalyzed PCR with HMOX1 cDNA in pBluescript SKII(+) as a template, and adenine overhangs were added to the PCR product with Taq DNA polymerase. After purification of the PCR products, the HindIII/BamHI fragment of HMOX1 was subcloned into pGEM-T easy vector for color screening of recombinant clones. HMOX1 fragment (HindIII/BamHI) was excised from recombinant pGEM-T by digestion with HindIII and BamHI and inserted into the Hind III and BamHI sites of pcDNA3.1/Zeo.CMV.Flag. Identical plasmids minus the HMOX1 cDNA were used for sham (control) transfections. Correct orientation and sequence of the Flag-HMOX1 and Flag-only constructs were confirmed on sequencing gels.
Transfection
Upon reaching >90% confluence, cells (1 × 106) were transiently transfected with 4.6 – 5.6 μg HMOX1 cDNA-Lipofectamine 2000 complex according to manufacturer instructions (Invitrogen). This transfection dose range results in 3 – 4 fold increases in astroglial HO activity relative to sham-transfected cells (Song, et al., 2006), approximating the augmented HO-1 expression observed in Alzheimer-affected neural tissues (Schipper, et al., 1995). This transfection protocol was previously shown to promote whole-cell and mitochondrial oxidative stress and sequestration of non-transferrin-derived 55Fe or 59Fe iron by the mitochondrial compartment (Schipper, et al., 1999, Song, et al., 2006), autophagy, and cytoplasmic vacuolation (Zukor, et al., 2009). 0.3 μg plasmid DNA and 0.69 μl of Lipofectamine 2000 reagent were diluted individually in 17.25 μl opti-MEM I reduced serum medium for 5 min at room temperature with gentle mixing. The two solutions were combined, incubated at room temperature for 20 min to promote formation of DNA:lipid complexes and administered to the cells in 8-well chamber slides. Equal concentrations of empty vector were used as controls for the HMOX1 cDNA transfections. Following incubation for 6 h at 37°C, the transfection mixture was replaced with complete media without antibiotics.
GFAP.HMOX1 mice
Transgene construction
Conditional GFAP.HMOX1 transgenic (TG) mice were engineered to selectively over-expresses human HO-1 in the astrocytic compartment under temporal control by the Tet-Off system as recently described (Song, et al., 2012a, Song, et al., 2012b). Tet-controllable pGFAP.tTA and pTRE2.Flag.HMOX1 constructs were used to create the GFAP.HMOX1 transgenic mice. To inhibit transgene expression, doxycycline (Dox) was provided in the diet (200 mg/kg, sterile BIO-SERV, Frenchtown, NJ, USA) to breeding pairs and derived litters. To initiate transgene expression, the Dox diet was replaced with regular rodent chow. TG mice employed in this study were heterozygous. TG and WT mice used for all experiments were of either sex.
Surgical procedure
Brains of 19.5 month-old mice were fixed by transcardial perfusion as previously described (Schipper, et al., 1998), with minor modifications. Briefly, the animals were deeply anesthetized with rodent cocktail containing ketamine, xylazine, acepromazine, and saline and perfused transcardially with 200 ml ice-cold saline followed by 250 ml of cold 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS, pH 7.4) for light microscopic analysis. The brains were immediately removed and immersed in the same fixatives for 24 h at 4°C. The animals were bred and maintained in the Animal Care Facilities of Lady Davis Institute for Medical Research. All experimental protocols were approved by the Animal Care Committee of McGill University in accord with the guidelines of the Canadian Council on Animal Care.
MCI Evaluation
Subjects
Post-mortem brain tissue was procured from older Catholic clergy enrolled in the Religious Orders Study, a longitudinal clinical-pathologic study of aging and AD. Each subject signed an informed consent and an anatomical gift act donating his/her brain to Rush investigators at the time of death. Subjects underwent a uniform structured clinical evaluation that included a medical history, neuropsychological performance testing, neurological examination, and review of neuroimaging (when available) as previously described (Bennett, et al., 2002). This study was approved by the Human Investigations Committee of Rush University Medical Center and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The diagnosis of AD was made in accordance with established criteria by the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) (McKhann, et al., 1984). The diagnosis of MCI referred to individuals not found to have dementia by the examining physician but rated as impaired on neuropsychological tests by a neuropsychologist (Bennett, et al., 2003). The median interval from last clinical evaluation to autopsy was 7.7 months, and the median interval from last valid global cognitive score to autopsy was 7.9 months.
Histochemistry/immunohistochemistry/Immunofluoresence (HC/IHC/IF)
Periodic acid-Schiff (PAS) stain
For astroglial cultures, cells were harvested at 2–6 days post-transfection and washed twice with ice cold PBS (pH 7.4), and chamber dividers were removed by dipping slides in 100% methanol for 5 sec. Cells were fixed in 4% cold paraformaldehyde for 20 min at room temperature and washed with PBS before HC/IHC/IF. For mouse brain, coronal brain sections (40 μm) were cut on a vibratome (Lancer series 1000). Human brain sections were deparaffinized using toluene and rehydrated in an ethanol series, immersed in 1% periodic acid for 10 min at room temperature, incubated in Schiff reagent for 10 min followed by 0.52% NaHSO3 for 2 min (×3). The slides were washed with tap water between steps. The sections were dehydrated in an ethanol series, cleared with CitriSolv, mounted with Permount, and coverslipped.
IHC
For tissue sections, antigens were retrieved by boiling in 0.01M sodium citrate buffer (pH 6.0) for 5 min, followed by a 10 min cool-down period and washes in distilled water for 5 min and Tris-buffered saline (TBS) (5 min, ×2). Endogenous peroxidase activity was quenched by incubation in 1.5% H2O2/methanol for 10 min, followed by washing twice in TBS for 5 min. Immunolabelling was performed with the Vectastain elite ABC kit as per manufacturer protocol (Vector Laboratories). Briefly, sections were incubated for 20 min in blocking buffer (goat serum), followed by incubation in rabbit anti-HO-1 antibody (Enzo Life Sciences) or sheep anti-MnSOD antibody (Biodesign International) at 1:75, mouse monoclonal anti-Ub antibody (Chemicon) at 1:75 (in blocking buffer) or mouse anti-Flag antibody (Sigma-Aldrich) at 1:100 at 4°C overnight. Cells were washed in TBS for 5 min twice, followed by incubation with biotinylated anti-rabbit, anti-sheep, or anti-mouse secondary antibody in blocking buffer for 30 min at room temperature. Cells were incubated with avidin/biotin complex for 30 min with TBS washes before and after incubation. Sections were visualized using vector SG chromagen, followed by thorough rinsing in tap water. Sections were dehydrated in an ethanol series, cleared with CitriSolv, mounted with Permount, and coverslipped.
IF
1). Human brain samples
Following deparaffinization and rehydration, antigens were retrieved by incubation in saponin solution (0.1% saponin, 0.1% BSA, 1mM CaCl, 1 mM MgCl, 5% non-fat milk, 0.1% NaN3) for 30 min at room temperature and blocked with 3% BSA for 30 min followed by incubation in mouse monoclonal anti-Ub antibody (1:75 in saponin) at 4°C overnight. Sections were incubated with rhodamine conjugated anti-mouse secondary antibodies (ICN Pharmaceutical Inc. - CAPPEL; 1:75 in PBS) for 1 h at room temperature, followed by washes in PBS and saponin. Sections were incubated in sheep anti-MnSOD antibody (1:50 in saponin) at 4°C overnight followed by incubation in FITC conjugated anti-sheep secondary antibodies (Jackson ImmunoResearch Laboratory; 1:75 in PBS) for 1 h at room temperature and exposure to saturated Sudan black solution for 10 min to quench autofluorescence. Sections were washed with running water for 10 minutes, mounted with GelTol and coverslipped.
2). Mouse brain samples
After antigen retrieval as described for IHC, vibratome sections were permeabilized with 0.01% Triton X100 for 10 min. The slides were blocked with the solutions provided with Vector mouse or rabbit antibody kit or 3% BSA for sheep polyclonal antibody, respectively, for 20 min, followed by incubation with mouse monoclonal anti-Flag (1:100) or anti-α-synuclein (Pierce; 1:250) or anti-myelin basic protein (COVANCE; 1:50), rabbit polyclonal anti-ubiquitin (Pierce; 1:500) or sheep polyclonal anti-MnSOD (Pierce; 1:200) at 4°C overnight. Sections were then incubated with Alexa Fluor 594 or 488 conjugated anti-mouse (Life Technologies Inc.), Cy3 labeled anti-rabbit (Jackson ImmunoResearch), or FITC-linked anti-sheep, all at 1:100 concentrations at 4°C overnight. For co-staining, sections were re-blocked and re-blotted with primary polyclonal rabbit anti-HO-1 (1:100) or anti-Flag (Bioss; 1:200) or anti-GFAP (Chemicon; 1:100) or anti-neurofilament 200 (Sigma; 1:50) or rabbit monoclonal anti-CD11b; (abcam; 1:50), or sheep anti-tyrosine hydroxylase (TH) antibody (Pierce; 1:500), or goat anti-PECAM-1 (CD31) antibody (Santa Cruz; 1:50) at 4°C overnight followed by incubation with Cy3- anti-rabbit or FITC- anti-sheep (1:100) or Alexa Fluor 488 anti-goat (Life technologies; 1:100). The slides were washed with TBS, quenched with Sudan black, counter-stained for nuclei with DAPI (4′,6-diamidino-2-phenylindole; 1 μg/ml; Sigma), mounted with ProLong Gold (Life technologies) and coverslipped.
The HC and IHC preparations were examined using a Leica DM LB2 microscope. The IF sections were observed under a Carl Zeiss LSM 5 Pascal laser-scanning confocal imaging microscope.
Quantification
Following HC/IHC staining, numbers of Ub- and HO-1-positive CA per unit area were determined in six sub-regions of the hippocampus (stratum oriens, pyramidal layer, stratum radiatum, molecular layer, granule cell layer and hilus of the dentate gyrus) at 1000× magnification with the aid of an ocular grid. For each sub-region surveyed, fields were selected at random and areas of at least 1 mm2 were evaluated by a single investigator unaware of the tissue source. For HO-1 transfected and control monolayers, single astrocytes were randomly selected and photographed at 1000× magnification. Successful transfection was confirmed by Flag-HO-1 immunostaining. Densities of PAS-positive cytoplasmic inclusions and PAS-negative vacuoles were analyzed using Adobe Photoshop 7.0. For aging GFAP.HMOX1 and WT mice (n=4 per group), numbers of PAS-positive inclusions were counted in various brain regions at 1000× magnification within coronal sections cut at bregma – 4.52 mm.
Statistical analyses
Multiple regression analysis was used to examine the relation of numbers of HO-1-positive CA to clinical diagnosis (NCI and MCI). Tukey’s studentized range was used for post hoc comparisons involving multiple tests of all possible pairs among the two diagnostic groups. All analyses were computed using with SAS/STAT® software Version 8 on a SunUltraSparc workstation. Data were expressed as means ± SEM. Main effects between groups were determined using Student’s t-test. Statistical significance was set at P < 0.05.
Results
Transfected astrocytes
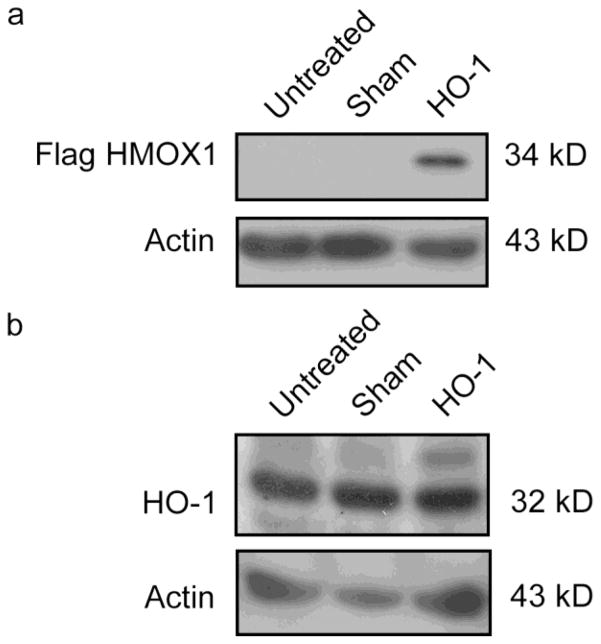
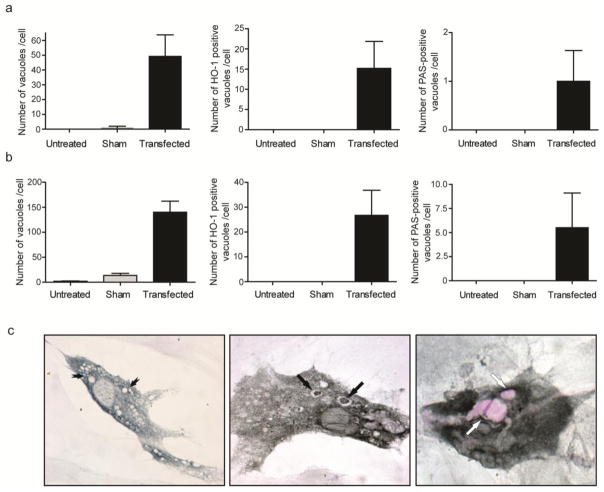
Flag-tagged HO-1 protein was expressed at 3 days following transient transfection of primary rat astrocytes with pcDNA3.1/Zeo.CMV.Flag.HMOX1 relative to untreated and sham-transfected controls (Fig. 1a and b). HO-1 transgene expression was identified with anti-Flag antibody (Fig. 1a), or as a band approximately 2 kDa heavier than the endogenous form in blots stained with anti-HO-1 antisera (Fig. 1b). Flag IHC revealed a mosaic of transfected and non-transfected cells within each field (Fig. 2c) as previously described (Zukor, et al., 2009). Under oil immersion, Flag-HO-1 immunoreactivity appeared as a punctate cytoplasmic precipitate predominantly localized to the perinuclear region. HMOX1-transfected cells exhibited numerous cytoplasmic vacuoles ranging from 5 – 50 μm in diameter (Figs 2c, f, i and insets, 3a–f). At 2–6 days post-transfection, a sub-population of inclusions exhibited PAS-positive (pink-purple) material characteristic of CA (Figs 2f, i and insets, 3a–f). Nineteen-29% of the CA-like inclusions contained HO-1-immunopositive material either surrounding or embedded within the vacuoles (Fig. 4). Seventy-nine-93% of the PAS-negative vacuoles also exhibited Flag-HO-1 immunoreactivity (Fig. 4). Little or no cytoplasmic vacuolation or PAS-positive inclusions was observed in non-transfected astrocytes in close proximity to cells expressing the HMOX1 transgene or in the untransfected or sham-transfected monolayers (Fig. 4).
Fig. 1. Expression of Flag-tagged HO-1 at 3 days post-transfection.
(a) Western blot of Flag-HO-1 for untreated, sham transfected, and HMOX1-transfected cells at 3 days post transfection. Flag-tagged HO-1 is detectable in HMOX1-transfected cells but not in control preparations. (b) Western blot of endogenous HO-1 for untreated, sham-transfected and HMOX1-transfected cells at 3 days post transfection. Flag-tagged HO-1 is only detectable in HMOX1-transfected cells as a 34 kDa band approximately 2 kDa heavier than endogenous HO-1. The transgene yields product that is immunopositive for both the Flag peptide and HO-1 protein
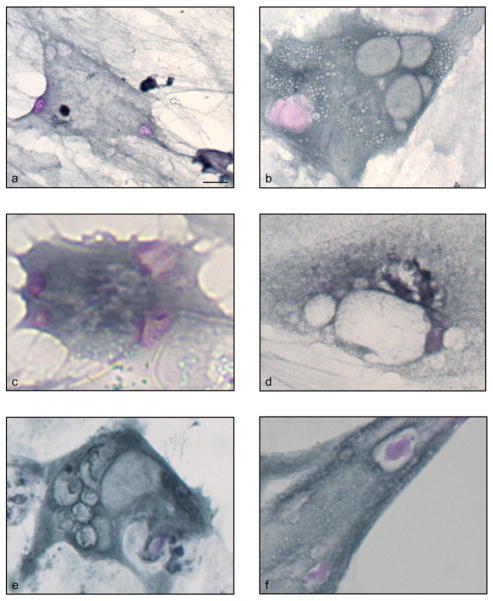
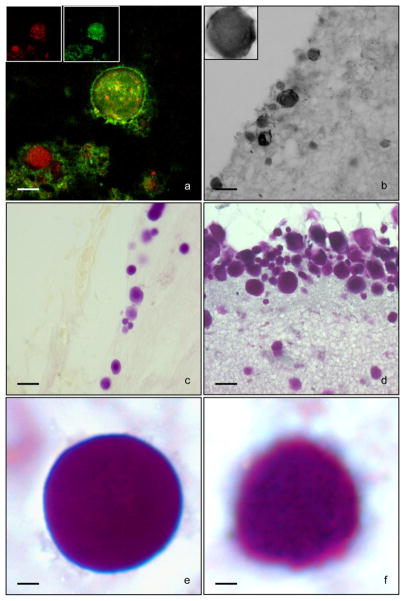
Fig. 2. PAS HC and anti-Flag IHC of HMOX1-transfected and control astroglial cultures.
Depicted are non-transfected (a, d, and g), sham-transfected (b, e, and h) and HMOX1-transfected (c, f, and i) cells at 2, 3, and 6 days post-transfection. Numerous PAS+ structures (arrowheads) and vacuoles (arrows) are present in Flag-HO-1 positive cells (c, f, i; insets). Some PAS-positive structures are immunoreactive for Flag-HO-1 (i, inset). Non-transfected (a, d, and g; insets) and sham-transfected (b, e, and h; insets) cells are devoid of PAS-positive structures and rarely contain vacuoles. Non-transfected cells within HMOX1-transfected chambers serve as a negative internal control for PAS staining and vacuolation (c, f, and i). Magnification bars = 25 μm in panels a–i and 10 μm in corresponding insets
Fig. 3. Spectrum of PAS-positive pathology in HMOX1-transfected astroglia.
Depicted are PAS+ structures (a–f), vacuolation (a–f) and macroautophagy (e) in HMOX1-transfected glia. Magnification bars = 10 μm in panels a–f
Fig. 4. Quantification of PAS-positive cytoplasmic inclusions and PAS-negative vacuoles in HMOX1-transfected and control rat astrocytes.
CA-like structures were identified on morphological grounds (panel c) and quantified as total vacuoles (tailed arrows), vacuoles containing or surrounded by Flag-HO-1-immunoreactive material (black arrows), and PAS-positive inclusions (white arrows). Calculations were expressed as number of vacuoles per cell at day 2 (panel a) and day 3 (panel b) post-transfection. N = 4–5 glial monolayers per group from several independent cultures.
Aging GFAP.HMOX1 transgenic mice
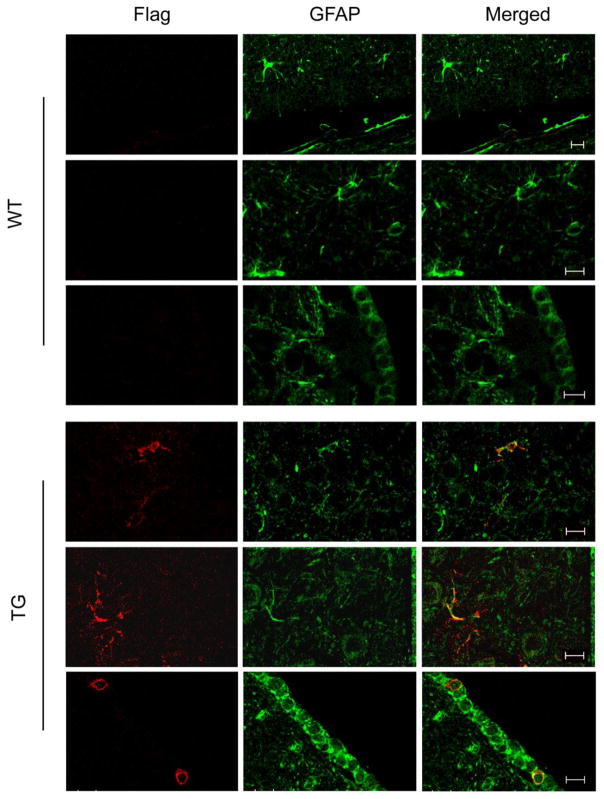
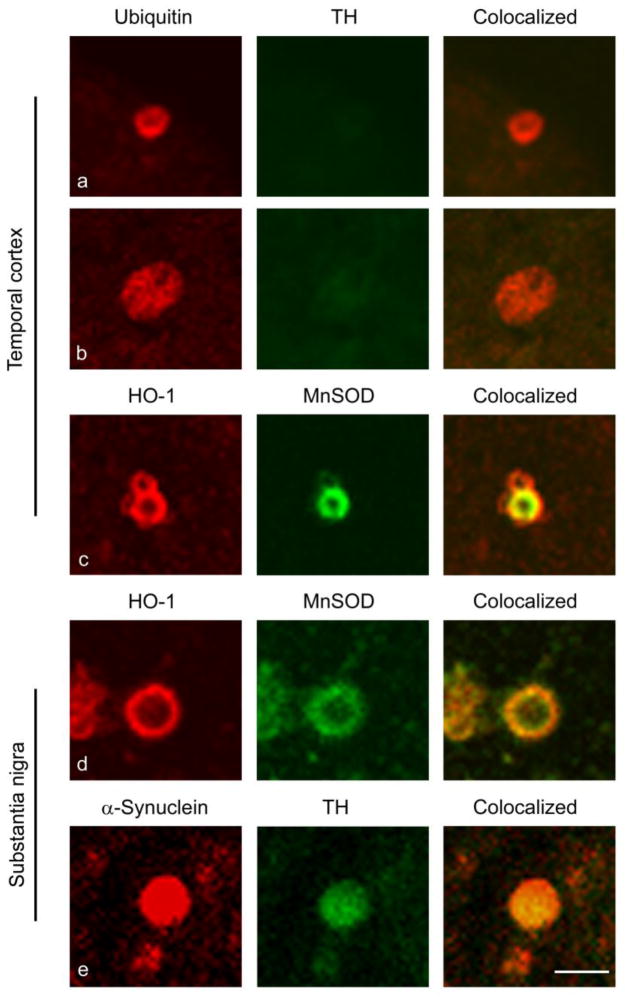
Flag-HO-1 immunoreaction product was not observed in WT littermates (Fig. 6) or TG mice on Dox. In the 19.5 month-old GFAP.HMOX1 mice (off Dox), Flag-tagged human HO-1 protein was detected by IF as a homogeneous cytoplasmic precipitate in astrocytes (Fig. 5e, 6), ependymocytes (Fig. 6) and ependymal tanycytes. The specific expression of astroglial HO-1 transgene was further confirmed by co-localization of Flag-HO-1 and GFAP (Fig. 6). Transgene expression was not detected in other CNS cell types, as demonstrated by co-immunostaining of Flag-HO-1 and specific markers for oligodendroglia (myelin basic protein; Zeller, et al., 1985), microglia (CD11b; Perego, et al., 2011), neurons (200 kDa neurofilament; Leapman, et al., 1997), cerebrovascular endothelial cells (PECAM-1; Muller, et al., 2002) (Fig. 7), or in non-neural tissues (heart, liver, spleen, lung, kidney, stomach, intestines, and gonads). Substantial numbers of PAS-positive inclusions were observed in grey matter (e.g. substantia nigra compacta, hippocampus) and white matter (e.g. brachium inferior colliculus, optic tract) regions (0.029–0.043/mm2 per brain) of the 19.5 month-old GFAP.HMOX1 transgenic mice; similar PAS-positive concretions were rarely encountered in the 19 month-old WT brain (0–0.005/mm2 per brain; Fig. 5a, b) or in 12 month-old transgenic animals (Song, et al., 2012a, Song, et al., 2012b). Virtually all CA-like structures exhibited Flag-HO-1 (Fig. 5c, d), Ub (Fig. 8a, b) and MnSOD (Fig. 8c, d) immunoreactivity. Although CA were largely non-reactive for α-synuclein and TH, small numbers of extracellular CA-like inclusions in the substantia nigra exhibited co-localization of these epitopes (Fig. 8e). Despite the disparities in the densities of PAS-positive inclusions between the TG and WT animals, the inclusions in both strains did not differ with respect to the pattern or intensity of Ub, MnSOD, and α-synuclein immunoreactivity. Overall, glial and neuronal expression of MnSOD and Ub, unrelated to the presence of CA, was more prominent in the substantia nigra and striatum of the old GFAP.HMOX1 transgenic mice than in the WT controls (data not shown).
Fig. 6. Colocalization of Flag-HO-1 and GFAP in aging GFAP.HMOX1 mice.
Confocal IF imaging depicts astroglial expression of Flag-HO-1 (red) and GFAP (green) and their co-localization (yellow) from two randomly-selected fields of striatum of GFAP.HMOX1 mice at 19.5 month age (top and middle panels of TG images). Similar colocalization was observed in ependymocytes lining the lateral ventricle of aging TG mice (bottom panels of TG images). Expression of the HMOX1 transgene was not detected in WT preparations. Magnification bars = 10 μm for all panels
Fig. 5. PAS-positive inclusions in aging GFAP.HMOX1 mice.
PAS-positive inclusions (circles) are depicted in the brachium inferior colliculus of a female GFAP.HMOX1 mouse at 19.5 month age (b). PAS-staining inclusions were rarely encountered in the corresponding region of a female age-matched WT littermate (a). Astrocytes in the alveus hippocampus and optic tract of a 19.5 month old male GFAP.HMOX1 exhibit anti-Flag IF (red) and DAPI-positive (blue) nuclei (e); CA-like inclusions co-localize with anti-Flag IF (red) in the substantia nigra of a male GFAP.HMOX1 mouse at 19.5 month age (c, d). Magnification bar = 100 μm for a and b; length bar in the inset of b = 100 μm; and magnification bar = 18 μm for c–e
Fig. 7. Expression of transgenic HO-1 by non-astrocytic cell types in the aging GFAP.HMOX1 mouse brain.
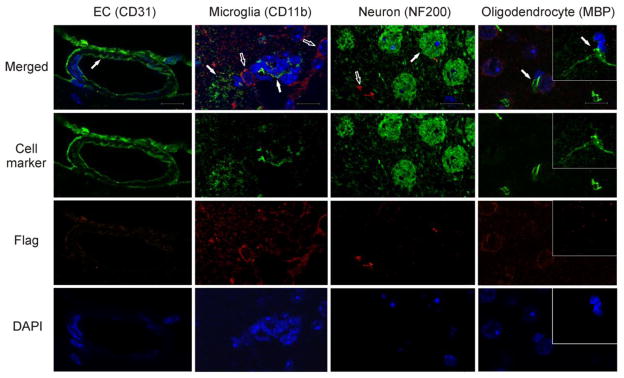
There was no co-localization of Flag-HO-1 (red, empty arrows) with markers for neurons, microglia, oligodendroglia, or vascular endothelial cells (green, filled arrows) in the same brain regions described in Figure 6. EC, endothelial cell; MBP, myelin basic protein; NF200, 200 kDa neurofilament. Magnification bars = 18 μm for all panels
Fig. 8. Ubiquitin, MnSOD and α-synuclein immunoreactivity in CA-like structures of aging GFAP.HMOX1 mice.
Confocal IF imaging depicts ubiquitin expression (red) in CA-like inclusions (a and b) and co-localization of MnSOD (green) and HO-1 (red) to CA-like structures (c and d) in temporal cortex and substantia nigra of the GFAP.HMOX1 mice at 19.5 month age. Occasional CA co-immunoreactive for α-synuclein (red) and TH (green) were noted in the substantia nigra of GFAP.HMOX1 mice at 19.5 month age (e). Magnification bar = 5 μm for all panels
Human neuropathology
Demographics
Post-mortem brain specimens used in these analyses were procured from 18 individuals: 9 MCI and 9 NCI. Persons with MCI and NCI were similar with respect to age and years of formal education (Table 1).
Table 1.
Demographic, clinical, and MMSE data for persons enrolled in this study
| Total (N=18) | NCI (N=9) | MCI (N=9) | |
|---|---|---|---|
| Age (years), M ± SD (range) | 82.3 ± 6.0 (72 – 97) | 80.1 ± 5.9 (72 – 90) | 84.5 ± 6.1 (76 – 97) |
| Sex (%), (Male/Female) | 58.9/41.4 | 77.8/22.2 | 40/60 |
| Education (years), M ± SD (range) | 19.45 ± 2.9 (15 – 25) | 19.9 ± 2.8 (16 – 25) | 19.0 ± 3.0 (15 – 24) |
| MMSE (scores), M ± SD (range) | 27.65 ± 1.55 (25 – 30) | 28.4 ± 1.3 (26 – 30) | 26.9 ± 1.8 (25 – 30) |
HO-1 positive CA
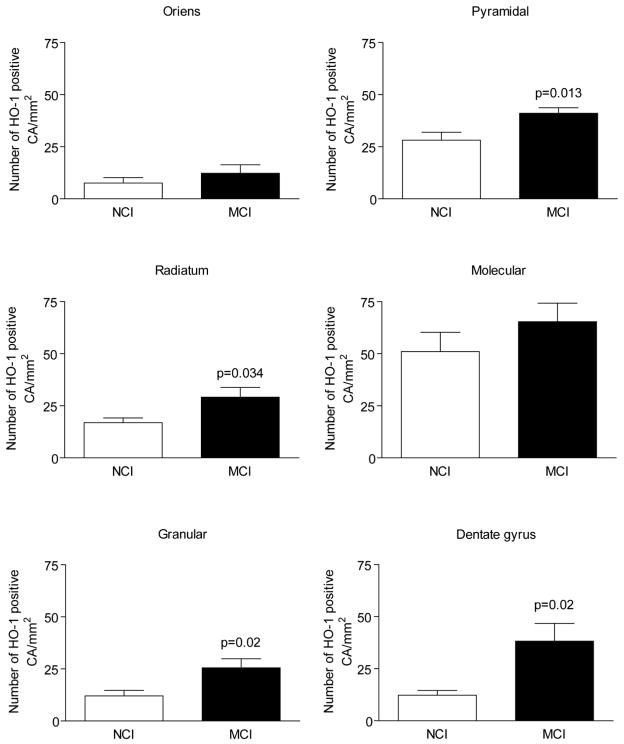
HO-1 positive CA were ~10–40 μm in diameter (Fig. 9b and inset). HO-1 consistently decorated PAS-positive CA and exhibited homogeneous, stippled, central target, and rim staining patterns (Fig. 9d–f). There were statistically significant increases in the numbers of HO-1 positive CA in the stratum radiatum, dentate gyrus, pyramidal cell layer, and granule cell layer of the MCI hippocampus relative to respective NCI values (P < 0.05; Fig. 10). No differences in CA numbers between the groups were observed in the other hippocampal regions surveyed (P > 0.05; Fig. 10). There were no significant differences in the numbers of HO-1- and PAS-positive CA in any of the hippocampal sub-regions (p>0.05 for each comparison; data not shown).
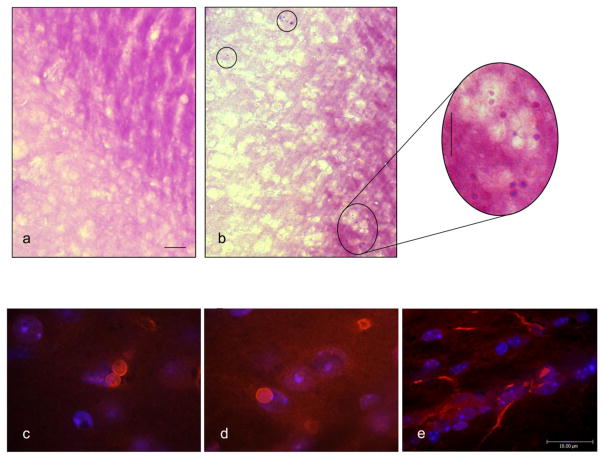
Fig. 9. PAS, Ub, MnSOD and HO-1 staining of human hippocampal CA.
Photomicrographs depicting CA in human brain stained with (a) ubiquitin (red), MnSOD (green), (b) HO-1 (black) (c) PAS (purple) and (d-f) dual PAS/HO-1. Magnification bars = 10 μm in panel a, 25 μm in panels b–d and 2.5 μm in panels e and f
Fig. 10. HO-1-positive CA in sub-regions of the human hippocampus.
Numbers of HO-1 positive CA are significantly increased in several hippocampal sub-regions of the MCI brain relative to NCI values
Ub and MnSOD IF
Dual immunolabeling for MnSOD and Ub showed consistent co-localization of the two proteins within CA by confocal microscopy regardless of diagnostic category. As in the case of HO-1 labeling, the Ub and MnSOD reaction products exhibited homogeneous, speckled, rim or central staining patterns in both NCI and MCI specimens (Fig. 9a).
Discussion
CA formation in HMOX1-transfected astrocytes
In the present study, we demonstrate that HO-1 overexpression, at levels commensurate with those prevailing in the AD brain, engenders profound morphological alterations in the cytoplasmic compartment of cultured astroglia. Autophagic vacuoles were consistently encountered within HO-1 transfected glia at post-transfection days 2 – 6. At two days post HMOX1 transfection, LM analysis revealed abundant vacuoles ranging in size from 5 – 50 μm which stained negatively for PAS. From 3 – 6 days post transfection, a sub-population of these autophagic vacuoles stained positively for PAS and the Flag-HO-1 fusion protein. These observations confirm our earlier ultrastructural findings of cytoplasmic inclusions bearing CA-like morphology in HMOX1-transfected astrocytes (Zukor, et al., 2009).
Using TEM, dynamic secondary ion mass spectrometry and an identical cell culture protocol as described herein, we previously demonstrated that HMOX1 transfection promotes mitochondrial membrane damage, cytoplasmic vacuolation, macroautophagy and the trapping of redox-active iron within autophagic vacuoles independently of classical iron mobilization pathways (Zukor, et al., 2009). Furthermore, CA-like cytoplasmic inclusions were consistently encountered in the HMOX1-transfected astroglia whereas they were not apparent in control cells. Iron was detected within the outer rims of the CA-like inclusions (Zukor, et al., 2009), akin to reports of histochemically detectable iron within human and experimental CA (Cisse and Schipper, 1995). Oxygen signatures co-localized to iron within CA, further implicating mitochondria in their biogenesis (Zukor, et al., 2009). Many of the CA-like inclusions exhibited strong sulfur signals reminiscent of Gomori-positive astrocytic granules which are mitochondria-derived (Brawer, et al., 1994) and may be homologous to nascent human CA (Cisse and Schipper, 1995, Schipper, 2004).
Here, we provide direct evidence using HMOX1-transfected astrocytes and immunohistochemical/PAS staining that HO-1 over-expression induces the biogenesis of cytoplasmic inclusions with morphological, tinctorial and immunochemical profiles reminiscent of human CA (Schipper, et al., 1995). The PAS-positive inclusions were consistently immunoreactive for HO-1, as previously documented in CA-like structures induced in cultured rat astroglia by cysteamine (CSH) exposure (Sahlas, et al., 2002). On the other hand, not all HO-1 positive vacuoles exhibited PAS staining commensurate with the notion that glycation is a relatively late stage in CA formation (Cisse and Schipper, 1995).
CA formation in the GFAP.HMOX1 mouse brain
Corroborating our findings in HMOX1-transfected astrocyte cultures, numerous CA-like inclusions were observed in the brains of 19.5 month-old mice engineered to overexpress human HO-1 in the astrocytic compartment. The inclusions were apparent in temporal and other cortical grey matter, hippocampus and other deep grey structures, periventricular regions, white matter tracts (e.g. optic tract, brachium inferior colliculus) and certain brainstem nuclei (e.g. substantia nigra). This topography shows considerable overlap with the distribution of CA in the aging human brain (Cavanagh, 1999). Similar concretions were rarely encountered in age-matched WT littermates or in GFAP.HMOX1 mice continuously expressing the transgene until sacrifice at 48 weeks of age (Song, et al., 2012a). As in senescent and degenerating human neural tissues (Schipper and Cisse, 1995, Schipper, et al., 1995), CA in the GFAP.HMOX1 mouse brain were glycoproteinacious (stained with PAS) and immunoreactive for HO-1, Ub and MnSOD. In conjunction with ultrastructural evidence of mitophagy in the transgenic astrocytes (Song, et al., 2012a, Song, et al., 2012b), the presence of MnSOD, an antioxidant enzyme localized to the mitochondrial matrix, implicates oxidatively-damaged mitochondria in the biogenesis of CA, as previously posited (Cisse and Schipper, 1995, Schipper and Cisse, 1995). As such, the accumulation of CA may be linked mechanistically with the progressive bioenergy deficits which have been amply documented in the aging and Alzheimer-diseased brain using various biochemical and neuroimaging techniques (Schipper, 2004). Indeed, by attenuating enzymatic activity, the augmented oxidation and other posttranslational modifications of HO-1 and biliverdin reductase recently reported in AD brain (Barone, et al., 2011a, Barone, et al., 2011b, Barone, et al., 2012) may confer some degree of neuroprotection by curtailing the mitochondriotoxic effects of excessive HO-1 expression in affected astroglia.
In the majority of brain regions surveyed, CA were entirely devoid of α-synuclein and TH immunoreactivities. In the GFAP.HMOX1 mouse substantia nigra, small numbers of extracellular CA-like inclusions stained positively for α-synuclein and TH. α-Synuclein-containing CA have been previously reported (Buervenich, et al., 2001, Wilhelmus, et al., 2011) and may be of neuronal (e.g. dopaminergic cell) or glial origin. In aging-related neurodegenerative conditions, the induction of HO-1 may assist in the detoxification of α-synuclein aggregates by (i) stimulating proteasomal degradation of the protein (Song, et al., 2009) and (ii) compartmentalization of the latter within nascent CA (current study), as may also occur with the crosslink-promoting enzyme, transglutaminase 1 (Wilhelmus, et al., 2011) and other potentially deleterious substrates.
CA formation in MCI
Previous studies showed that (i) numbers of CA are increased in the AD brain and (ii) HO-1 is significantly up-regulated in hippocampal astrocytes of subjects with MCI (Schipper, et al., 2006). In light of these observations, we queried whether enhanced CA formation would already be manifest in the MCI hippocampus and therefore represent a fairly early event in the pathogenesis of AD. Using well-characterized material from the Religious Orders Study, we demonstrated that numbers of HO-1-positive CA are significantly increased in certain hippocampal regions of the MCI brain relative to normal (NCI) values. Quantities of HO-1-positive CA per unit area did not differ from those labeled with the classical CA markers, Ub and PAS, supporting earlier observations that CA in human brain are consistently immunoreactive for HO-1 (Schipper, et al., 1995). We also showed that MnSOD protein co-localizes to CA in the aging and degenerating human hippocampus. This finding is consistent with our in vitro data (vide supra) and earlier observation that human CA contain mitochondrial remnants and epitopes (Schipper and Cisse, 1995).
Taken together, the human neuropathological, whole animal and in vitro data strongly suggest that glial CA are derived from dystrophic mitochondria engaged in a complex macroautophagic process which, in turn, is contingent on the antecedent overexpression of HO-1. The results of the current investigation, in conjunction with a previous observation that astroglial HO-1 is already maximally induced in the brains of individuals with MCI (Schipper, et al., 2006), indicate that HO-1-related mitochondrial damage, mitophagy and CA formation are relatively early events in the pathogenesis of AD rather than ‘graveyard’ pathological features which may be refractory to intervention. Further preclinical and clinical studies may determine whether timely suppression of glial HO-1 activity in affected neural tissues (Schipper, et al., 2009) will ameliorate bioenergetic failure and functional decline in AD and other aging-associated CNS disorders.
Highlights.
HMOX1 transfection promotes corpora amylacea (CA) biogenesis in rat astrocytes.
CA formation is accelerated in the brains of aging GFAP.HMOX1 mice.
CA biogenesis is augmented in mild cognitive impairment, a prodromal stage of AD.
Glial HO-1 suppression may confer neuroprotection in Alzheimer disease.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research and the Mary Katz Claman Foundation (to HMS) and the National Institute on Aging (to DAB). WS is a senior scientist of the Mary Katz Claman Foundation. We are indebted to the nuns, priests and brothers who participated in the Religious Orders Study.
Footnotes
Disclosures
Hyman Schipper has served as consultant to Osta Biotechnologies, Molecular Biometrics Inc., TEVA Neurosciences, and Caprion Pharmaceuticals. Wei Song, Hillel Zukor, Adrienne Liberman, Sagi Kaduri, Zoe Arvanitakis, and David A. Bennett have no disclosures to declare. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, Perluigi M, Mancuso C, Butterfield DA. Biliverdin reductase--a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta. 2011a;1812:480–487. doi: 10.1016/j.bbadis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C, Butterfield DA. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer’s disease and amnestic mild cognitive impairment. J Alzheimers Dis. 2011b;25:623–633. doi: 10.3233/JAD-2011-110092. [DOI] [PubMed] [Google Scholar]
- Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Reichard G, Small L, Schipper HM. The origin and composition of peroxidase-positive granules in cysteamine-treated astrocytes in culture. Brain Res. 1994;633:9–20. doi: 10.1016/0006-8993(94)91516-4. [DOI] [PubMed] [Google Scholar]
- Buervenich S, Olson L, Galter D. Nestin-like immunoreactivity of corpora amylacea in aged human brain. Brain Res Mol Brain Res. 2001;94:204–208. doi: 10.1016/s0169-328x(01)00166-8. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. Corpora-amylacea and the family of polyglucosan diseases. Brain Res Brain Res Rev. 1999;29:265–295. doi: 10.1016/s0165-0173(99)00003-x. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bergman H, Schipper HM, Gauthier S, Bouchard R, Fontaine S, Clarfield AM. Assessment of suspected dementia. Can J Neurol Sci. 2001;28(Suppl 1):S28–41. doi: 10.1017/s0317167100001189. [DOI] [PubMed] [Google Scholar]
- Chopra VS, Chalifour LE, Schipper HM. Differential effects of cysteamine on heat shock protein induction and cytoplasmic granulation in astrocytes and glioma cells. Brain Res Mol Brain Res. 1995;31:173–184. doi: 10.1016/0169-328x(95)00049-x. [DOI] [PubMed] [Google Scholar]
- Cisse S, Schipper HM. Experimental induction of corpora amylacea-like inclusions in rat astroglia. Neuropathol Appl Neurobiol. 1995;21:423–431. doi: 10.1111/j.1365-2990.1995.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Fenton H, Finch PW, Rubin JS, Rosenberg JM, Taylor WG, Kuo-Leblanc V, Rodriguez-Wolf M, Baird A, Schipper HM, Stopa EG. Hepatocyte growth factor (HGF/SF) in Alzheimer’s disease. Brain Res. 1998;779:262–270. doi: 10.1016/s0006-8993(97)00958-x. [DOI] [PubMed] [Google Scholar]
- Leapman RD, Gallant PE, Reese TS, Andrews SB. Phosphorylation and subunit organization of axonal neurofilaments determined by scanning transmission electron microscopy. Proc Natl Acad Sci U S A. 1997;94:7820–7824. doi: 10.1073/pnas.94.15.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10:579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlas DJ, Liberman A, Schipper HM. Role of heme oxygenase-1 in the biogenesis of corpora amylacea. Biogerontology. 2002;3:223–231. doi: 10.1023/a:1016223109601. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Bernier L, Mehindate K, Frankel D. Mitochondrial iron sequestration in dopamine-challenged astroglia: role of heme oxygenase-1 and the permeability transition pore. J Neurochem. 1999;72:1802–1811. doi: 10.1046/j.1471-4159.1999.0721802.x. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Cisse S. Mitochondrial constituents of corpora amylacea and autofluorescent astrocytic inclusions in senescent human brain. Glia. 1995;14:55–64. doi: 10.1002/glia.440140108. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Cisse S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol. 1995;37:758–768. doi: 10.1002/ana.410370609. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr Alzheimer Res. 2009;6:424–430. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Song W, Patel A, Qureshi HY, Han D, Schipper HM, Paudel HK. The Parkinson disease-associated A30P mutation stabilizes alpha-synuclein against proteasomal degradation triggered by heme oxygenase-1 over-expression in human neuroblastoma cells. J Neurochem. 2009;110:719–733. doi: 10.1111/j.1471-4159.2009.06165.x. [DOI] [PubMed] [Google Scholar]
- Song W, Su H, Song S, Paudel HK, Schipper HM. Over-expression of heme oxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. J Cell Physiol. 2006;206:655–663. doi: 10.1002/jcp.20509. [DOI] [PubMed] [Google Scholar]
- Song W, Zukor H, Lin SH, Hascalovici J, Liberman A, Tavitian A, Mui J, Vali H, Tong XK, Bhardwaj SK, Srivastava LK, Hamel E, Schipper HM. Schizophrenia-Like Features in Transgenic Mice Overexpressing Human HO-1 in the Astrocytic Compartment. Journal of Neuroscience. 2012a;32:10841–10853. doi: 10.1523/JNEUROSCI.6469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Zukor H, Lin SH, Liberman A, Tavitian A, Mui J, Vali H, Fillebeen C, Pantopoulos K, Wu TD, Guerquin-Kern JL, Schipper HM. Unregulated brain iron deposition in transgenic mice over-expressing HMOX1 in the astrocytic compartment. J Neurochem. 2012b;123:325–336. doi: 10.1111/j.1471-4159.2012.07914.x. [DOI] [PubMed] [Google Scholar]
- Vaya J, Song W, Khatib S, Geng G, Schipper HM. Effects of heme oxygenase-1 expression on sterol homeostasis in rat astroglia. Free Radic Biol Med. 2007;42:864–871. doi: 10.1016/j.freeradbiomed.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Verhaar R, Bol JG, van Dam AM, Hoozemans JJ, Rozemuller AJ, Drukarch B. Novel role of transglutaminase 1 in corpora amylacea formation? Neurobiol Aging. 2011;32:845–856. doi: 10.1016/j.neurobiolaging.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Biro P, Cohen T, Muller RM, Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. European journal of biochemistry/FEBS. 1988;171:457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Zeller NK, Behar TN, Dubois-Dalcq ME, Lazzarini RA. The timely expression of myelin basic protein gene in cultured rat brain oligodendrocytes is independent of continuous neuronal influences. J Neurosci. 1985;5:2955–2962. doi: 10.1523/JNEUROSCI.05-11-02955.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukor H, Song W, Liberman A, Mui J, Vali H, Fillebeen C, Pantopoulos K, Wu TD, Guerquin-Kern JL, Schipper HM. HO-1-mediated macroautophagy: a mechanism for unregulated iron deposition in aging and degenerating neural tissues. J Neurochem. 2009;109:776–791. doi: 10.1111/j.1471-4159.2009.06007.x. [DOI] [PubMed] [Google Scholar]