Abstract
Objective
To develop and assess a semi-automated method for segmenting and counting individual renal cysts from mid-slice MR images in patients with autosomal dominant polycystic kidney disease (ADPKD)
Materials and Methods
A semi-automated method was developed to segment and count individual renal cysts from mid-slice MR images in 241 participants with ADPKD from the Consortium for Radiologic Imaging Studies of ADPKD (CRISP). For each subject, a mid-slice MR image was selected from each set of coronal T2-weighted MR images covering the entire kidney. The selected mid-slice image was processed with the semi-automated method to segment and count individual renal cysts. The number of cysts from the mid-slice image of each kidney was also measured by manual counting. The level of agreement between the semi-automated and manual cyst counts was compared using intra-class correlation (ICC) and a Bland-Altman plot.
Results
Individual renal cysts were successfully segmented using the semi-automated method in all 241 cases. The number of cysts in each kidney measured with the semi-automated and manual counting methods correlated well (ICC=0.96 for the right or left kidney), with a small average difference (-0.52, with higher semi-automated counts, for the right and 0.13, with higher manual counts, for the left) in the semi-automated method. There was, however, substantial variation in a small number of subjects: 6 of 241 (2.5%) participants had a difference in the total cyst count of more than 15.
Conclusion
We have developed a semi-automated method to segment individual renal cysts from mid-slice of MR images in ADPKD kidneys for a quantitative indicator of characterization and disease progression of ADPKD.
Keywords: kidney, polycystic kidney disease, renal cysts, magnetic resonance imaging, segmentation
Introduction
Autosomal dominant polycystic kidney Disease (ADPKD), the most common renal genetic disorder, is characterized by the progressive development and expansion of renal cysts. The decline in renal function in ADPKD strongly correlates with the severity and growth of these renal cysts [1]. In severe cases, cysts replace most of the functional parenchyma, leading to end-stage renal disease [2].
To study the relationship between kidney morphology and function in a prospective, longitudinal ADPKD cohort, we established the Consortium of Radiologic Imaging Study of PKD (CRISP) [3]. The data collected in this study, including magnetic resonance imaging (MRI), renal function and biomarkers relevant to the early course of ADPKD, showed that the MRI measurement of kidney volume is more sensitive than the glomerular filtration rate (GFR) measurement in evaluating the yearly progression of ADPKD, and that the growth seen in the kidney volume directly stems from increases in renal cyst volume [4].
In addition to total kidney and cyst volumes, the number of individual cysts in each kidney also provides important information about the characteristics and progression of ADPKD. For example, differences in kidney morphology between PKD1 and PKD2 genotypes are likely due to the earlier development rather than faster volumetric growth of cysts in PKD1 kidneys [5]. Generally, the number of individual cysts in each kidney is determined by manually counting them on the mid-section of an MR image set. Although this approach is straightforward, manual counting is time-consuming and labor-intensive, particularly in large kidneys with numerous cysts. Furthermore, it is extremely laborious to segment (i.e., to identify and partition an image into semantically interpretable regions) each cyst from the background renal parenchyma by manual delineation of individual cysts. We recently published a 3D semi-automated method to segment renal cysts from the entire volumetric MR slices [6]. However, this approach was exceedingly complex and successful only for ADPKD kidneys with mild and moderate cystic burdens. Thus, to overcome the limitations of the manual and the 3D segmentation of renal cysts, in the current study we developed and evaluated a semi-automated method for segmenting and counting individual renal cysts from mid-slice (2D) MR images in patients with ADPKD.
Materials and Methods
The study protocol for the CRISP (clinical trials registration: NCT01039987, registration date: Dec 23, 2009) has been previously described [3,4,7] and was approved by the institutional review board at each participating clinical center. Informed consent was obtained from all subjects who participated in the CRISP study.
Participants and MR Imaging
In the CRISP study launched in 1999, 241 ADPKD participants between 15 and 46 years old with relatively intact renal function were recruited. The clinical characteristics of the cohort and a detailed study protocol have been published previously [3,4,7]. MR images of kidneys were obtained at 3 mm fixed slice thickness in the coronal plane. Both three-dimensional spoiled gradient interpolated T1-weighted images without fat saturation and single-shot fast spin-echo T2-weighted images with fat saturation were acquired [3].
Manual Middle Section Cyst Counting
The T2-weighted MR images of the abdomen from each of the 241 patients were reviewed and cropped to generate separate images of the right and left hemi-abdomen. From the cropped image set of the hemi-abdomen, the mid-slice of the kidney was selected by reviewing and scrolling the image set. The kidney boundary was detected and segmented using image editing software (Analyze version 10.0, Mayo Clinic, Rochester, MN). Calyx and the renal pelvis were carefully identified and excluded (Figures 1 and 2).
Fig. 1. Mid-slice MR images from an ADPKD patient with mild cyst-burden illustrating (A) the initial segmented kidney, (B) manual cyst counting, and (C) individual cysts segmented using the semi-automated method.
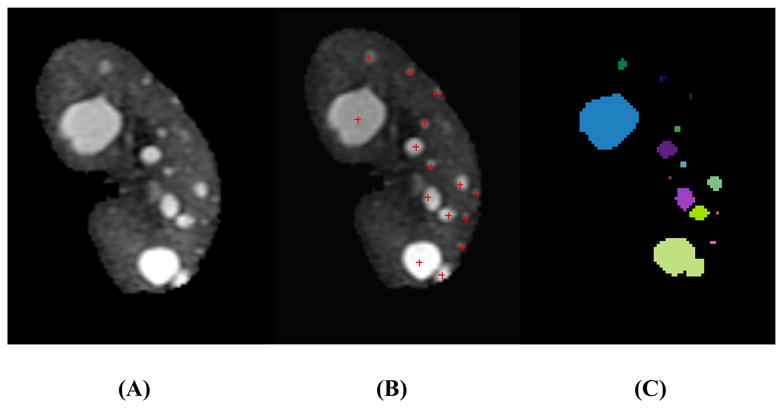
(A) Cysts were scattered and discretely defined by surrounding renal parenchyma. Most cysts were well separated without touching each other. (B) The manual counting method was performed using an in-house cyst-labeling computer program. Small poorly-defined foci of faint brightness were not counted as cysts because they were difficult to differentiate from background MR image noise in the parenchyma. (C) Individual renal cysts were segmented and color-coded using the semi-automated method.
Fig. 2. Mid-slice MR images from an ADPKD patient with severe cyst-burden illustrating (A) the initial segmented kidney, (B) manual cyst counting, and (C) individual cysts segmented using the semi-automated method.
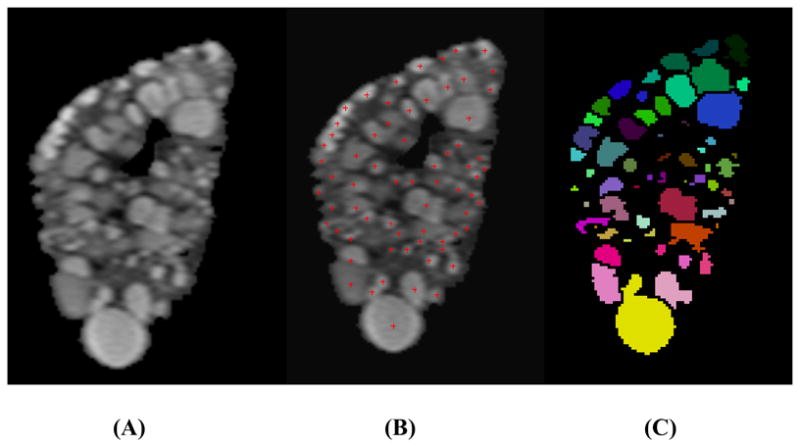
(A) The kidney was diffusely occupied by numerous cysts with barely discernible renal parenchyma. Some neighboring cysts shared borders. (B) Individual cysts were manually flagged and counted using an in-house cyst-labeling computer program. (C) Individual renal cysts were segmented and color-coded using the semi-automated method.
The number of cysts in each kidney was counted manually by a radiologist (HLS with 12-years of experience in body MR imaging). Only circular and spheroid structures ≥ 2 mm in diameter whose signal intensities were close to that of spinal fluid were considered cysts. Each identified and counted cyst was flagged by an electronic marker using an in-house cyst-labeling program (Figures 1B and 2B). The total number of cysts flagged with red marks was automatically computed.
Cyst Area Measurements using Region-based Method
The total area of cysts in each kidney was measured by a different radiologist (JHW) using a region-based thresholding method [3,7]. Cysts that were brighter than the renal parenchyma could be measured by summing pixels with intensity values greater than those of the background renal parenchyma. On each renal MR image slice, a binary signal-intensity map was generated by determining a threshold signal intensity that visually distinguished the cyst and renal parenchymal regions. By summing the pixels of white regions in the binary map, the cystic area was measured in each slice.
Semi-automated Cyst Segmentation and Counting
The semi-automated segmentation program was implemented as an imageJ plugin [8]. T2-weighted MR images of ADPKD kidney contained regions of renal cysts (bright in pixel signal intensity) and regions of renal parenchyma (dark in pixel signal intensity). From the signal intensity distribution of the renal cyst and parenchyma pixels, a threshold was automatically determined by an iterative computation using the isodata algorithm [9]. A binary image was generated from the threshold that separated renal cyst regions (white) from parenchyma regions (black). If deemed appropriate, the threshold was interactively adjusted by the radiologist (HLS) to enhance the delineation between renal cyst and parenchyma regions. The renal cyst regions from the binary image were used as the initial candidates and superimposed over the original grey-scale MR image. The cyst candidate regions were frequently presented with variable signal intensities due to MR field inhomogeneity and further revised and filled to retain a relatively uniform signal intensity. A flood-filled algorithm was used to replace pixels appearing as holes within the cyst candidates. A connected components analysis (CCA) was applied to the cyst candidate region to extract and label candidates for individual cyst regions. To further break apart individual cysts from CCA regions, a Euclidean distance map (EDM) was generated from this region. In EDM, the value of each pixel was calculated to represent the distance to the nearest background pixel. By incorporating the watershed segmentation to EDM, loosely connected cysts were split at their necks [10]. A uniquely identifiable label and color was assigned to each connected component (Figures 1C and 2C). The area and count of individual cysts were automatically computed from the segmented cysts.
Data Analysis
The performance of the semi-automated segmentation method was evaluated and compared with the manual method in terms of counting the individual cysts in each kidney. The segmentation of each individual cyst could not be assessed because it was not available by the manual method. The level of agreement in cyst counts in each kidney between the semi-automated and manual method was assessed by means of intra-class correlation (ICC) [11] and corresponding confidence intervals. We also analyzed the inter-reader reliability in cyst counting between two readers using ICC. The cyst area measurements in each kidney between the semi-automated and region-growing methods were compared by means of a Spearman correlation. In addition, Bland-Altman analysis was used to visually assess systemic differences in cyst counting [12], and estimate the bias and limits of agreement. The statistical analysis was performed in Stata (version 12, Stata software); Bland-Altman plots were created using the batplot command.
Results
For all CRISP participants, we successfully segmented individual renal cysts with the semi-automated method. The numbers of cysts measured with the semi-automated segmentation method ranged from 2 to 69 (mean 26.0), with a median count of 25 (interquartile range of 16-33, with the middle 90% falling between 8 and 52) for the right, and 1 to 63 (mean 27.5), with a median count of 26 (inter-quartile range of 18-35, with the middle 90% falling between 8 and 49), for the left kidney. The number of cysts determined by the manual counting method ranged from 1 to 69 (mean 25.4), with a median count of 23 (interquartile range of 15-33, with the middle 90% falling between 6 and 53) for the right, and 1 to 68 (mean 27.6), with a median count of 27 (inter-quartile range of 18-36, with the middle 90% falling between 7 and 52), for the left kidney.
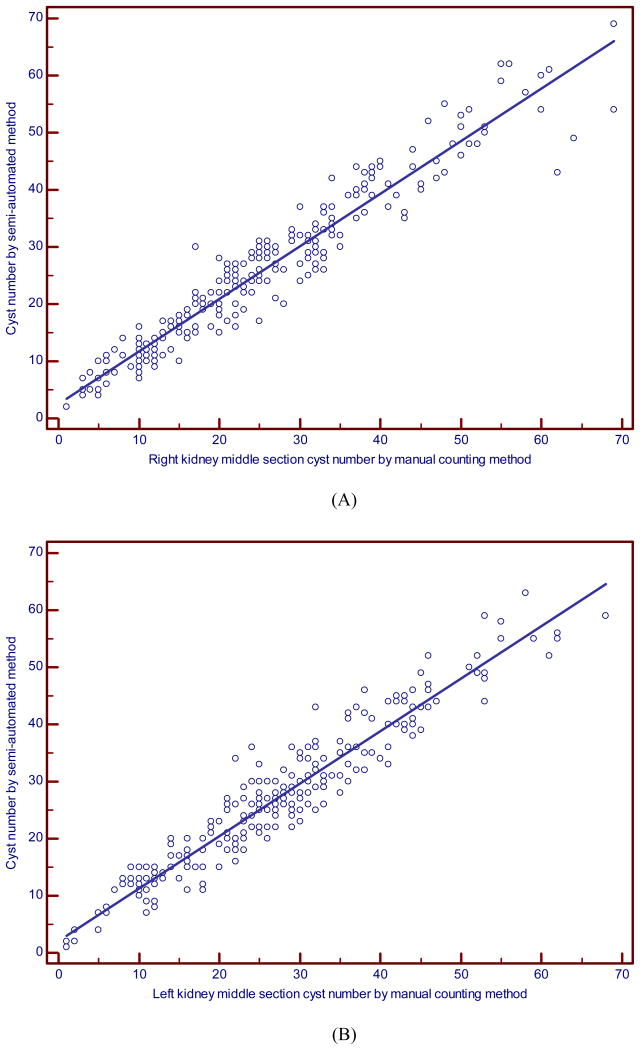
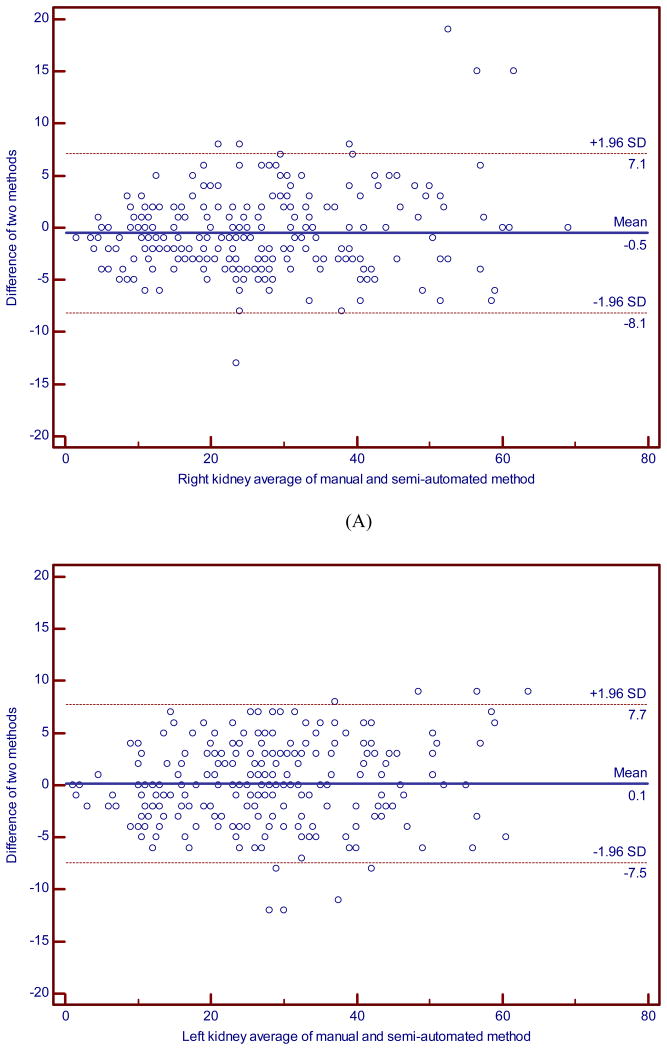
The average difference in the total numbers of cysts, i.e., the manual minus semi-automated segmentation count, was very small: -0.52 (SD = 3.9; inter-quartile range from -3 to 2) for the right, and 0.13 (SD = 3.9; inter-quartile range from -3 to 3) for the left kidney. However, the variability for some individual subjects was not trivial. Six of 241 (2.5%) participants had a difference in the total cyst count larger than 15 in magnitude, and the middle 90% of the distribution had differences of 10 or less in magnitude; as noted by the inter-quartile range, most differences were 3 or less in magnitude. The two methods correlated well (ICC = 0.96; 95% CI, 0.95 to 0.97 for the right, ICC = 0.96; 95% CI, 0.95 to 0.97 for the left, ICC=0.97; 0.96 to 0.98 for the right and left combined) (Fig. 3). Although we found a high level of average agreement and overall variability as demonstrated by the high ICCs, Bland-Altman analyses revealed large differences for some individual measurements. More specifically, the limits of agreement (LOA) were (-8.1, 7.1) for the right side and (-7.5, 7.7) for the left side. Figures 4A and 4B showed a relatively spread of differences between the LOA over most of the range (e.g., for average counts above 10), with some outliers in the differences (with 1.7% and 3.7% outside the LOA for the right and left sides, respectively).
Fig. 3. Scatter plot of the manual versus semi-automated renal cyst counts for (A) the right and (B) left kidney.
The two methods correlated well (ICC = 0.96; 95% CI, 0.95 to 0.97 for the right, ICC = 0.96; 95% CI, 0.95 to 0.97 for the left). Lines represent the line of identity.
Fig. 4. Bland-Altman plot for the manual versus semi-automated renal cyst counts for (A) the right and (B) left kidney.
The cyst area measurements between the semi-automated segmentation and region-growing method were highly correlated with a Spearman correlation of 0.958 and a mean difference of -1.025 in the semi-automated method minus region growing method (SD=11.35). For the inter-reader reliability, the analysis (using log-transformed data) showed that the intra-class correlation (ICC) between the two readers was very high, with an estimate of 0.989 (95% CI of 0.986, 0.992). The mean difference (in the original scale) between the readers was nearly zero for the left (mean=0.004; SD=2.50) and right (mean=0.241; SD=2.74) sides. The middle half of the differences was 2 or less in magnitude (with a total range of up to a difference of 9).
Discussion
CRISP demonstrated that both the total renal volume and total cyst volume could be reliably measured by MR imaging and used to monitor the disease progression of ADPKD [3,4]. In addition to volumetric measurements, other renal cyst measurements, including the number and size of cysts, might be closely related to ADPKD pathogenesis and the genetic variations found in the family members of patients with ADPKD [5,13,14]. In particular, Harris et al. [5] reported that PKD1 kidneys have a greater number of cysts than PKD2 kidneys, and that PKD1 has a more severe disease progression because more cysts develop earlier, not because they grow faster, implicating the disease gene in cyst initiation but not expansion. In an MR imaging study of a pediatric ADPKD cohort, Cadnapaphornchai et al. [15] found that cyst number increases more rapidly in hypertensive ADPKD children. The number of renal cysts was estimated in these two studies by manual counting on a mid-slice of MR images.
A reliable method for measuring cyst numbers in multiple MR image slices and changes over time is important to accurately evaluate the disease progression in ADPKD, particularly when longitudinal change in the total kidney volume is relatively small, e.g., in children. Although the manual cyst counting method may be straightforward and easy to perform for ADPKD kidneys, it is likely subject to high variability and errors, particularly in kidneys with a severe cyst burden.
The semi-automated and manual methods showed excellent intra-class correlations with relatively small paired differences, although there were some large differences for a small percentage of the measurements. We reviewed these cases to investigate the causes for these large differences. When a number of cysts are weakly discernible from the background renal parenchyma, the segmentation outcome of these cysts is highly sensitive to the threshold value that is adjusted and determined by the radiologist on each image to differentiate cysts from the background parenchyma. There are also regional variations in MR signal intensity within a kidney that may not be uniformly resolved by a single global threshold value. As a result, a considerable number of faintly-defined cysts may appear or disappear with a subtle adjustment of a threshold. On the other hand, manual counting relies on human perception, which is subjective, but at the same time more adaptable to regional signal intensity fluctuations within the kidney. The majority of large differences between the two methods are likely attributable to the intrinsic signal intensity heterogeneity of cysts. One potential approach to identify these cases with large signal intensity heterogeneity would be to compute and analyze the intensity histogram covering the entire kidney region for the spread of the intensity distribution and to use well-defined simple cysts as the reference to normalize MR signal intensities. Some other differences between the two methods may be related to image quality that could be improved with further technical advances in the future.
The semi-automated method provides several key advantages. For example, the ability to segement individual cysts by the semi-automated method allows us to study the characteristics of renal cysts in ADPKD. We can investigate both the size and spatial (e.g., medullary vs cortical) distribution of cysts from the segmented individual cysts within the kidney. While unecessary for small kidneys with a few cysts that can be counted easily, for large kidneys with numerous cysts, the semi-automated method is faster and more efficient than the manual method. In the CRISP cohort, most of the kidneys contain numerous renal cysts and thus the semi-automated method was preferred by the radiologists. Since no specific reference measurement was available to compare each individual renal cyst, the size and locational accuracy of segmentation of individual cysts could not be assessed.
Although counting cysts from a three-dimensional (3D) MR image set representing the whole kidney volume is preferred to counting only from limited MR image slices, manually counting numerous cysts on a 3D MR image set is exceedingly laborious and may not be accurate [16]. A recent semi-automated method for cyst segmentation and counting from the 3D kidney volume MR image set was reported to have a relatively good performance for kidneys with mild and moderate cystic burdens [6]. However, the 3D semi-automated method failed to reliably segment individual cysts from advanced ADPKD kidneys in which the renal parenchyma is largely replaced by innumerable cysts. These cysts are touching and inseparable from each other without discernible background renal parenchyma. The segmentation of the cysts in advanced ADPKD, which requires a labor-intensive manual editing of each cyst even after an initial segmentation by computer, is impractical. On the other hand, our 2D segmentation method based on mid-slice MR images was successful for the entire spectrum of cyst severity in all CRISP participants. Although this method is limited to an area as opposed to volumetric data set, it could be applied to additional MR slices sampled over a volumetric data set (e.g., two additional slices corresponding to the mid-slice of the anterior half and the mid-slice of the posterior half of the series) to approximate and estimate the number of individual cysts in a 3D MR imaging representation of the kidney. We investigated this issue further for a small subset of the participants (16 cases) by comparing the 3D individual cyst count to the estimated cyst count from the mid-slice times the number of slices in each kidney; results yielded a correlation of 0.80 and a linear regression (of log-transformed counts, with the product of the mid-slice count times the number of slices as the predictor) gave similar predictions. The mean predicted count was 70.0 (SD=19.9) versus the actual mean count of 71.9 (21.7) to give a resulting mean difference of -1.83 (SD=16.2).
The semi-automated method we developed requires a prior segmentation of the kidney from abdominal MR images. There are a number of image segmentation methods for the kidney published in the literature, ranging from simple manual delineation to the application of advanced object-detection algorithms [17]. In our approach, we focused on the development of a simple and robust segmentation scheme to minimize the operator dependence. The kidney was segmented semi-automatically in each slice of the MR images by placing a seed point over the kidney area. Renal hila and the extra-parenchymal collecting system were edited and excluded by an operator. The segmented kidney was also used to estimate the kidney volume. Although a fully-automated segmentation method would be preferred for the segmentation of both kidney and individual renal cysts, this works well only for normal kidneys [18,19]. With the progression of ADPKD, the morphology and MR signal intensities of kidneys become too complex to reliable segment kidney and individual cysts without monitoring and editing by an expert radiologist [20].
The study has some limitations. First, the semi-automated method focused on 2D images, which do not include the total number of cysts in the whole kidney. Second, in this analysis, we identified and subdivided cyst brightness in T2-weighted MR images. Complex and hemorrhagic cysts may appear as a gray or dark signal on T2-weighted images and cannot be identified or segmented in any study using either manual or semi-automated methods. These cysts need to be carefully identified in the visualization and comparison of T1- and T2-weighted images. Third, microscopic cysts are “invisible” by MRI and cannot be segmented. A recent study showed that a large number of microscopic cysts in the ADPKD kidney cannot be detected in vivo with MR imaging because of imaging resolution limitations [16]. Nevertheless, the collective volume of these tiny cysts to the overall kidney size is relatively insignificant [16]. Finally, even if the segmentation process is semi-automated, it may still require a manual edit of the threshold after the initial segmentation by computer. When renal cysts are overestimated or not labeled automatically by the program, the radiologist edits the threshold on MR images manually in order to eliminate an incorrectly segmented structure or to add new cysts.
In conclusion, we have developed a semi-automated method to segment and count renal cysts from a mid-slice of MR image data set in ADPKD kidneys with a wide range of cyst severity. This method facilitates the assessment of the number and spatial distribution of renal cysts for the characterization and disease progression of ADPKD.
Acknowledgments
The CRISP study is supported by cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK056943, DK056956, DK056957, DK056961) and by the National Center for Research Resources General Clinical Research Centers at each institution (RR000039, Emory University; RR00585, Mayo College of Medicine; RR23940, Kansas University Medical Center; RR000032, University of Alabama at Birmingham) and the National Center for Research Resources Clinical and Translational Science Awards at each institution (RR025008, Emory; RR024150, Mayo College of Medicine; RR033179, Kansas University Medical Center; RR025777 and UL1TR000165, University of Alabama at Birmingham; RR024153 and UL1TR000005, University of Pittsburgh School of Medicine). The investigators are indebted to the radiologists, radiology technologists, imaging engineering, and study coordinators in CRISP.
Disclosures: K.T.B., A.B.C. and J.J.G. are consultants to Otsuka Corporation, and V.E.T. received research support from Otsuka Corporation. M.M. is a consultant to Otsuka Corporation and Alexion Pharmaceuticals.
References
- 1.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 3.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O'Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP. Renal structure in early autosomal-dominant polycystic kidney disease (adpkd): The consortium for radiologic imaging studies of polycystic kidney disease (crisp) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 5.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2006;17:3013–3019. doi: 10.1681/ASN.2006080835. [DOI] [PubMed] [Google Scholar]
- 6.Bae K, Park B, Sun H, Wang J, Tao C, Chapman AB, Torres VE, Grantham JJ, Mrug M, Bennett WM, Flessner MF, Landsittel DP, Bae KT. Segmentation of individual renal cysts from mr images in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2013;8:1089–1097. doi: 10.2215/CJN.10561012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF, Jr, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The consortium for radiologic imaging studies of polycystic kidney disease cohort. Clin J Am Soc Nephrol. 2006;1:64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 8.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with imagej. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 9.Ridler TW, Calvard S. Picture thresholding using an iterative selection method. IEEE Transactions on Systems, Man, and Cybernetics. 1978;8:630–632. [Google Scholar]
- 10.Leymarie F, Levine MD. Fast raster scan distance propagation on the discrete rectangular lattice. CVGIP: Image Understanding. 1992;55:84–94. [Google Scholar]
- 11.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Fick-Brosnahan GM, Belz MM, McFann KK, Johnson AM, Schrier RW. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: A longitudinal study. Am J Kidney Dis. 2002;39:1127–1134. doi: 10.1053/ajkd.2002.33379. [DOI] [PubMed] [Google Scholar]
- 14.Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, Guay-Woodford LM, Bae KT. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–116. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW. Magnetic resonance imaging of kidney and cyst volume in children with adpkd. Clin J Am Soc Nephrol. 2011;6:369–376. doi: 10.2215/CJN.03780410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grantham JJ, Mulamalla S, Grantham CJ, Wallace DP, Cook LT, Wetzel LH, Fields TA, Bae KT. Detected renal cysts are tips of the iceberg in adults with adpkd. Clin J Am Soc Nephrol. 2012;7:1087–1093. doi: 10.2215/CJN.00900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zollner FG, Svarstad E, Munthe-Kaas AZ, Schad LR, Lundervold A, Rorvik J. Assessment of kidney volumes from mri: Acquisition and segmentation techniques. AJR Am J Roentgenol. 2012;199:1060–1069. doi: 10.2214/AJR.12.8657. [DOI] [PubMed] [Google Scholar]
- 18.Shim H, Chang S, Tao C, Wang JH, Kaya D, Bae KT. Semiautomated segmentation of kidney from high-resolution multidetector computed tomography images using a graphcuts technique. J Comput Assist Tomogr. 2009;33:893–901. doi: 10.1097/RCT.0b013e3181a5cc16. [DOI] [PubMed] [Google Scholar]
- 19.Khalifa F, Elnakib A, Beache GM, Gimel'farb G, El-Ghar MA, Ouseph R, Sokhadze G, Manning S, McClure P, El-Baz A. 3d kidney segmentation from ct images using a level set approach guided by a novel stochastic speed function. Med Image Comput Comput Assist Interv. 2011;14:587–594. doi: 10.1007/978-3-642-23626-6_72. [DOI] [PubMed] [Google Scholar]
- 20.Bae KT, Commean PK, Lee J. Volumetric measurement of renal cysts and parenchyma using mri: Phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr. 2000;24:614–619. doi: 10.1097/00004728-200007000-00019. [DOI] [PubMed] [Google Scholar]