Abstract
Objective To systematically review and describe currently available approaches to supporting maintenance of weight loss in obese adults and to assess the evidence for the effectiveness of these interventions.
Design Systematic review with meta-analysis.
Data sources Medline, PsycINFO, Embase, and the Cochrane Central Register of Controlled Trials.
Study selection Studies were identified through to January 2014. Randomised trials of interventions to maintain weight loss provided to initially obese adults (aged ≥18) after weight loss of ≥5% body weight with long term (≥12 months) follow-up of weight change (main outcome) were included.
Study appraisal and synthesis Potential studies were screened independently and in duplicate; study characteristics and outcomes were extracted. Meta-analyses were conducted to estimate the effects of interventions on weight loss maintenance with the inverse variance method and a random effects model. Results are presented as mean differences in weight change, with 95% confidence intervals.
Results 45 trials involving 7788 individuals were included. Behavioural interventions focusing on both food intake and physical activity resulted in an average difference of −1.56 kg (95% confidence interval −2.27 to −0.86 kg; 25 comparisons, 2949 participants) in weight regain compared with controls at 12 months. Orlistat combined with behavioural interventions resulted in a −1.80 kg (−2.54 to −1.06; eight comparisons, 1738 participants) difference compared with placebo at 12 months. All orlistat studies reported higher frequencies of adverse gastrointestinal events in the experimental compared with placebo control groups. A dose-response relation for orlistat treatment was found, with 120 mg doses three times a day leading to greater weight loss maintenance (−2.34 kg, −3.03 to −1.65) compared with 60 mg and 30 mg three times a day (−0.70 kg, 95% confidence interval −1.92 to 0.52), P=0.02.
Conclusions Behavioural interventions that deal with both diet and physical activity show small but significant benefits on weight loss maintenance.
Introduction
Obesity is one of the greatest causes of preventable morbidity and mortality worldwide,1 with weight loss associated with reductions in risk of morbidity and mortality.2 Evidence from systematic reviews suggests that long term weight loss through changes in eating and physical activity is possible,3 even in adults who have already acquired obesity related illness,4 and effective weight loss programmes are now available.5 Wardle and colleagues reported that 28% of adults in the United Kingdom claimed to be actively trying to lose weight.6 In a population survey based in the United States, Nicklas and colleagues found that that 63% of obese participants had attempted to lose weight over the past 12 months, of whom 40% had succeeded in losing ≥5% of their initial weight and 20% had succeeded in losing ≥10%.7 Though formal behaviour change interventions and self guided efforts at individual behaviour change are successful in inducing weight loss, however, few people manage to maintain these changes in weight over the long term.8 Weight loss from behavioural interventions typically peaks at around six months into the weight loss attempt, followed by gradual regain of weight in most individuals.3 4 As maintenance of the weight loss is crucial to uphold health benefits,9 understanding how best to support people in sustaining weight loss is paramount to controlling the obesity epidemic and its consequences.
Compared with initiation of weight loss, the evidence base for maintenance of weight loss is in its infancy. A recent systematic review of 13 randomised controlled trials examining effects of “extended care” for weight loss maintenance reported an average 3.2 kg difference in weight regain between extended care and no or minimal additional contact.10 Other reviews that have examined weight loss maintenance studies confirm the potential of successful maintenance treatment, although there is considerable heterogeneity between studies. Currently available reviews are limited by not using meta-analyses,11 12 13 14 no separation of studies focused on weight loss or maintenance,11 12 the use of restrictive inclusion criteria focusing on specific subsets of non-surgical studies,10 11 12 inclusion of non-randomised trials,12 13 or a lack of systematic identification of studies.13 To date, no comprehensive systematic review of long term effects of non-surgical treatments for maintenance of weight loss tested in randomised controlled trials is available to examine the effects of different treatments in the prevention of weight regain. We describe currently available non-surgical interventions for weight loss maintenance and have synthesised the randomised controlled evidence for the effectiveness of interventions and intervention delivery features.
Methods
The systematic review was conducted in line with Cochrane recommendations15 following a pre-specified protocol.
Eligibility criteria
Types of studies—We included randomised controlled trials or cluster randomised controlled trials with participants randomised to a weight maintenance intervention compared with a control condition or another intervention, or both, and ≥12 months’ follow-up of weight outcomes from inception of the maintenance intervention.
Types of participants—Participants were adults (aged ≥18, no upper age limit) who had, or had had, an average BMI of ≥30 and lost ≥5% of their body weight/mass within 24 months before weight loss maintenance treatment. We excluded studies that recruited participants with established mental health conditions, including eating disorders, and conditions requiring treatment with antipsychotic drugs.
Type of interventions—Any behavioural/lifestyle, pharmacological (with European Medicines Agency approval for weight loss), food replacement/supplement, or alternative interventions, singly or in combination were included. We excluded surgical interventions.
Types of outcomes—Primary outcome was weight at 12 months from randomisation to the weight loss maintenance intervention. Weight could be reported as absolute weight change during the trial including the weight loss phase, weight change during the maintenance treatment period, or final weight values.
Types of reports—Full text reports in any language from 1946 to January 2014.
Electronic searches
We searched the electronic databases Medline, PsycINFO, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) using a comprehensive search strategy. We also conducted hand searches of all references of included studies and those of previous relevant reviews.10 13 14
Study selection
Three authors (SUD, FFS, and VA-S) double scanned all references identified through the search strategy for initial selection. Full texts of potentially eligible studies were obtained, and two authors (SUD, FFS) verified inclusion using a prespecified eligibility form.
Data extraction
We extracted risk of bias items, modes of intervention delivery, study design, study flow, participant details, outcome measures, and funding source. Risk of bias was assessed based on the Cochrane Collaboration’s tool.15 Intervention delivery was assessed based on the criteria of Davidson and colleagues,16 including intervention provider, format and delivery, setting, duration and intensity, and materials. Two researchers per study (from SUD, KK, VAS) coded intervention delivery based on published articles as well as publicly and freely available protocols and full manuals. Disagreements were resolved by a third researcher (FFS). One researcher (SUD) extracted study information and modes of intervention delivery, which were checked by another (KK). Two researchers (SUD, KK) independently assessed risk of bias. Disagreements were resolved by a third researcher (FFS).
Data analysis
All inter-rater agreements for risk of bias items were assessed with Krippendorf’s α (which ranges from 1 (perfect agreement) to 0 (no agreement) and has been found to be superior to other reliability coefficients17). Studies reporting sufficient data to calculate mean differences in weight change in kg with 95% confidence intervals were considered for meta-analysis (RevMan version 5.1). Meta-analyses combined weight changes overall (that is, including an initial weight loss phase) or weight change of the maintenance phase only. When a choice of weight related outcome measures was available (such as completer only versus baseline observation carried forward) we chose the most conservative effect estimate provided. When studies reported average weight at baseline and follow-up we calculated weight change by subtracting the final from the initial weight at the start of maintenance. Standard deviations for weight change were imputed according to the formula provided Avenell and colleagues.3 Data that were reported only in graphical form18 19 20 were digitised with Engauge Digitizer, version 4.1 (http://sourceforge.net), to extract data in the most precise and replicable manner possible. All pooled effects were calculated as mean difference in weight change by using random effects model (inverse-variance approach). Mean differences were calculated for 12, 18, 24, 30, and 36 after the start of the maintenance intervention (outcomes at ≥3 months were integrated into the closest respective time points).15 Consistency across study effects was assessed with I2.21 I2 >25% and >50% were interpreted as an indicator for moderate and substantial heterogeneity, respectively. Publication bias was assessed by plotting the inverse of the standard errors of effect estimates with funnel plots to explore symmetry, which were assessed visually, as well as with use of Egger’s regression test using the “metabias” macros in STATA 13. The test for subgroup differences available in RevMan 5.1 was used to determine whether there was evidence for a difference in treatment effect between groups.
Types of comparisons
Comparisons were made for the following intervention types: behavioural/lifestyle based on both dietary and physical activity approaches; dietary, physical activity; pharmacological; food supplement; and meal replacement. Control conditions included no intervention, standard or minimal care or placebo controlled conditions, or conditions that controlled for a relevant intervention component to allow comparison. In addition, we examined three delivery modes (intensive v less intensive; internet v control; person v remote delivery—that is, internet or phone).
When studies tested multiple interventions against a comparison condition, we split the comparison group sample size by the number of intervention groups to capture heterogeneity across maintenance intervention arms. Studies that used a factorial design were treated as separate studies for the relevant factors. Findings for comparisons that could not be included in any meta-analyses are summarised narratively.
Sensitivity analyses
To examine the robustness of findings we conducted several sensitivity analyses:
Strength related sensitivity analyses—when studies contributed multiple study arms for a meta-analysis we examined the most intensive intervention arm. When judgment of intervention intensity was not possible, we combined both intervention arms with the methods outlined above
Dose-response related sensitivity analyses—when studies provided the intervention components in different doses these were analysed in separate subgroups
Focus related sensitivity analyses—when studies were combined for a particular general feature (such as physical activity), we examined the impact of specific focus areas (such as walking) separately when possible
Risk of bias analyses—we examined whether allocation concealment (adequate v unclear/no) and outcome assessment (adequate v unclear/no) influenced outcomes for the main findings.
Results
Study selection
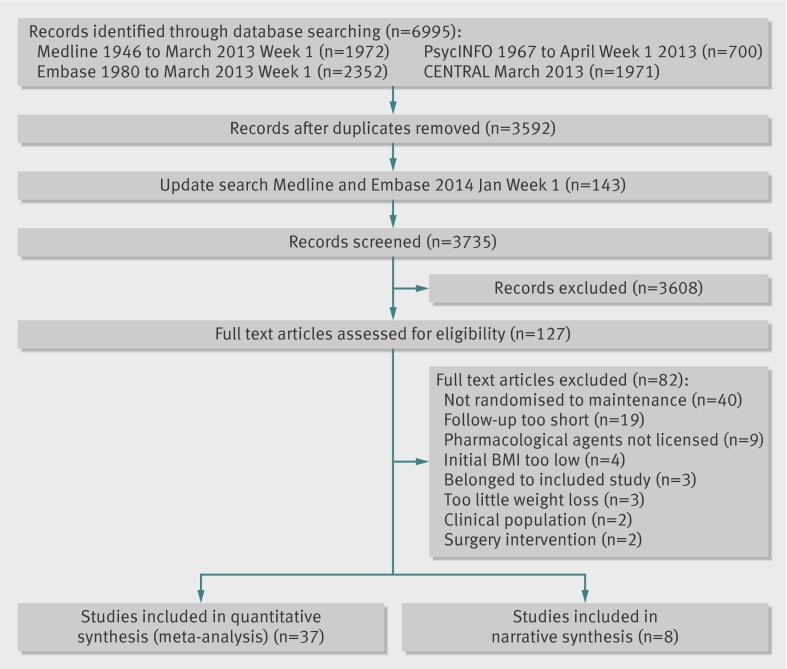
The search identified 3735 potentially relevant records, of which 127 were selected as potentially eligible; 42 papers describing 45 studies met inclusion criteria (fig 1). The table summarises overall details of the studies, with full details provided in appendix 1.
Fig 1 Flowchart of systematic process for report identification
Table.
1 Overall details of studies included in review of interventions for weight loss maintenance (see appendix 1 for more detailed version of this table)
| Weight loss | Weight loss maintenance | ||||
|---|---|---|---|---|---|
| Intervention | Length (months) | Interventions | Length (months) | ||
| Agras 1996, USA | VLCD (800 kcal/day) + BT | 3 | a) Standard food—time dependent; b) standard food—weight dependent; c) prepackaged food—time dependent; d) prepackaged food—weight dependent | 9/15 | |
| Annunziato 2009, USA | Meal replacement (1100 kcal/day) + CBT | 2 | a) Lifestyle treatment (3 months) and meal replacement; b) lifestyle treatment (3 months) | 12 and 3/12 | |
| Borg 2002, Finland | LED (1200 kcal/day) for 2 weeks and VLED (500 kcal/day) for 6 weeks | 2 | a) Resistance training + group meetings; b) walking + group meetings; c) group meetings | 6/29 | |
| Christensen 2013, Denmark | a) LED (810 kcal/day) for 2 months, hypo-energetic diet + 2 formula products daily (~1200 kcal/day) for 2 months; b) VLED (415 kcal/day) for 2 months, hypo-energetic diet + 2 formula products daily (~1200 kcal/day) for 2 months | 4 | a) Weekly meetings with dietician and formula products (1/day); b) supervised exercise sessions; c) usual care | 12/12 | |
| Cussler 2008, USA | Moderate daily energy deficit (300-500 kcal/d) through diet and PA | 4 | a) Internet delivered lifestyle intervention; b) self directed weight management | 12/12 | |
| Dale 2009, New Zealand | Various (community recruitment) | — | a) Intensive support + high-carbohydrate diet; b) nurse support + high-carbohydrate diet; c) intensive support + high-monounsaturated-fat diet; d) nurse support + high-monounsaturated-fat diet | 24/24 | |
| Davidson 1999, USA | Orlistat 120 mg + controlled-energy diet | 12 | a) Orlistat 60g + 4 behaviour modification sessions; b) orlistat 120g + 4 behaviour modification sessions; c) placebo + 4 behaviour modification sessions | 12/12 | |
| Delbridge 2009, Australia | VLED (500-550 kcal/d) | 3 | a) High protein diet + monthly counselling; b) high carbohydrate diet + monthly counselling | 12/12 | |
| Elder 2012, USA | Reduce calorie diet + BT | 5 | a) Tapas acupressure technique–groups sessions ; b) social support—group social support sessions | 6/12 | |
| Fogelholm 2000, Finland | LED (weeks 1, 10-12) + VLED (weeks 2-9) + weekly group sessions | 4 | a) 2-3h walking (1000 Kcal) + weekly meetings; b) 4-6h walking (2000 Kcal) + weekly meetings; c) weekly meetings | 10/33 | |
| Harvey-Berino 2002, USA | Reduced energy intake of 1000 – 2500 kcal/day + increase in lifestyle activity + BT | 6 | a) Frequent in-person support; b) minimal in-person support; c) Internet support | 12/12 | |
| Harvey-Berino 2004, USA | Reduced energy intake of 1000 – 2500 kcal/day + increase in lifestyle activity + BT | 6 | a) Frequent in-person support; b) minimal in-person support; c) internet support | 12/12 | |
| Hill 1999, USA | Hypoenergetic diet (deficit of 4180 kJ/d) + brisk walking for 20-30 min 5 times/week + BT | 6 | a) Orlistat 30mg + dietary and behavioural counselling; b) orlistat 60mg + dietary and behavioural counselling; c) orlistat 120mg + dietary and behavioural counselling; d) placebo intervention + dietary/ behavioural counselling | 12/12 | |
| King 1989, USA | a) moderate energy restriction diet; b) increased physical activity | 12 | a) Mail/telephone contact for diet; b) mail/telephone contact for exercise | 12/12 | |
| Kramer 1986, USA | Weight loss programme | 4 | a) Skills focus programme; b) weight focus programme | 12/12 | |
| Lantz 2003, Sweden | VLCD (450 kcal/day) | 4 | a) Intermittent group: VLCD every 3 months for 2 weeks; b) on demand group: VLCD when weight regain occurred | 20/20 | |
| Larsen 2006, Denmark | Energy restriction (3300-4200 kJ/d) | 2 | a) CLA capsules + diet + educational diet programme; b) placebo + diet + educational diet programme | 12/12 | |
| Leermakers 1999, USA | Intake of 1200 kcal/day for women and 1500 kcal/ day for men + walking 30 minutes/day, 5 days/week + BT | 6 | a) Exercise focused maintenance; b) weight focused maintenance | 12/12 | |
| Lowe 2008, USA | Meal replacement 1100 kcal/day + increasing exercise (30 min most days) | 2 | a) CBT; b) CBT + EFMA (enhanced food monitoring accuracy); c) CBT + EFMA + reduced energy density eating | 12/15 | |
| Pasman 1997, Netherlands | VLCD (2 MJ/d) | 2 | a) Fibre supplement; b) no intervention control | 14/14 | |
| Pasman2 1997, Netherlands | VLCD (2 MJ/d) | 2 | a) 50 g carbohydrate +200 2g chromium-picolinate + 20 g fibre + 100 mg caffeine (CHO+); b) 50 g carbohydrate (CHO); c) no intervention control | 14/14 | |
| Perri 1984a, USA | BT | 3 | a) Maintenance booster session ; b) multicomponent maintenance programme | 12/21 | |
| Perri 1984b, USA | a) Non-BT; b) BT including exchange list diet plans; c) BT including exchange list diet plans + relapse prevention | 4 | a) Client-therapist contact by mail and telephone; b) no treatment control | 6/12 | |
| Perri 1986, USA | BT | 5 | a) Multicomponent maintenance programme; b) no treatment control | 12/18 | |
| Perri 1987, USA | BT | 5 | a) Peer self help group maintenance programme; b) therapist-contact maintenance programme | 7/18 | |
| Perri 1988, USA | BT | 5 | a) Post-treatment contact; b) post-treatment contact + social influence maintenance; c) post-treatment contact + aerobic exercise maintenance ; d) post-treatment contact + aerobic exercise + social influence maintenance ; e) no intervention control | 12/18 | |
| Perri 2001, USA | BT | 5 | a) Relapse prevention therapy (RPT); b) problem solving therapy (PST); c) no intervention control | 12/17 | |
| Perri 2008, USA | BT | 5 | a) Telephone counselling; b) face-to-face counselling; c) no intervention control | 12/12 | |
| Richelsen 2007, Scandinavia | VLED (600-800 kcal/day) | 2 | a) Lifestyle counselling for 3 years + orlistat 120 mg; b) lifestyle counselling for 3 years + placebo | 36/36 | |
| Riebe 2004, USA | Clinic based weight management programme | 6 | a) Trans theoretical model tailored mail; b) generic info about diet/exercise | 12/18 | |
| Ryttig 1995, Sweden | VLCD (330cal/day) | 3 | a) Hypocaloric diet + two sachets of meal replacement; b) hypocaloric diet | 12/12 | |
| Ryttig 1997, Sweden | VLCD (330cal/day) | 3 | a) Hypocaloric diet; b) hypocaloric diet + three sachets of meal replacement | 12/12 | |
| Sherwood 2013, USA | Various (community recruitment) | — | a) Guided intervention; b) self directed intervention | 24/24 | |
| Sjostorm 1997a, Europe | Hypocaloric diet + placebo 3 times/day | 12 | a) Diet + orlistat; b) diet + placebo | 12/12 | |
| Sjostorm 1997b, Europe | Hypocaloric diet + orlistat 120 mg 3 times/day | 12 | a) Diet + orlistat; b) diet + Placebo | 12/12 | |
| Sorenson 2011, Denmark | 600 kcal-deficit diet + orlistat | 3 | a) Gourmet cooking course; b) neurolinguistic programming (NLP) | 5/21 | |
| Svetkey 2008, USA | Weight loss programme (diet and exercise) | 6 | a) Monthly personal contact; b) unlimited access to an interactive technology intervention; c) self directed control | 30/30 | |
| Toubro 1997, Denmark | a) LED (2 MJ/day) + anorectic compound + weekly BT (8 weeks); b) conventional diet (5 MJ/day), + anorectic compound + weekly BT (17 weeks) | 2 or 4 | a) Ad lib, low fat high carbohydrate; b) fixed energy intake diet | 12/24 | |
| West 2011, USA | Weight loss programme (diet and exercise) | 6 | a) Skill based intervention; b) motivation focused maintenance programme | 12/12 | |
| Wikstrand 2010, Sweden | VLCD (800 kcal/day) + BT | 3 | a) Diet + corset + 2 meeting with GP; b) diet | 9/21 | |
| Wing 1996a, USA | a) Standard behaviour treatment (SBT); b) SBT + meal plans; c) SBT + food provision; d) SBT + food provision for free | 6 | a) Telephone assisted weight management group; b) no contact group | 12/12 | |
| Wing 1996b, USA | a) standard behaviour treatment (SBT); b) SBT + meal plans; c) SBT + food provision; d) SBT + food provision for free | 6 | a) Food provision + BT; b) BT | 12/12 | |
| Wing 2006, USA | Various (community recruitment) | — | a) Face-to-face group; b) internet; c) information only control group | 18/18 | |
LED=low energy diet; VLCD=very low calorie diet; VLED=very low energy diet; WLM=weight loss maintenance; WL=weight loss; BT=behavioural therapy.
Risk of bias
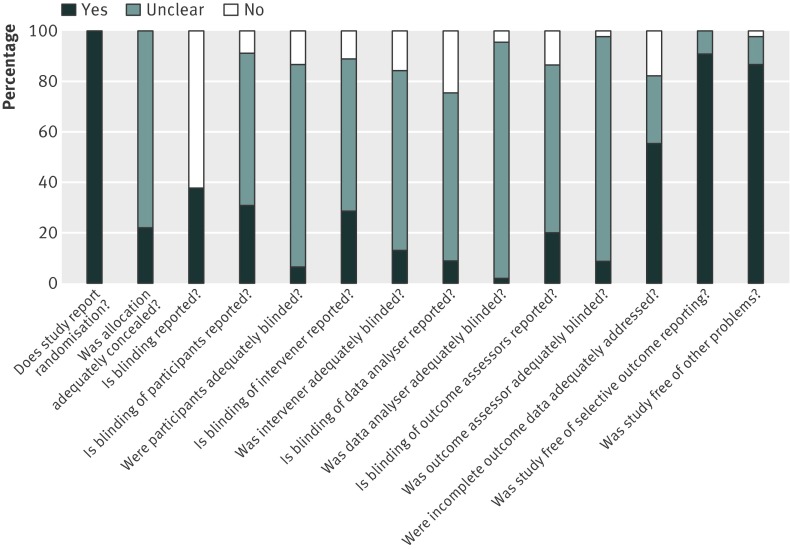
The average inter-rater agreement for risk of bias indicators was good (Krippendorf’s α =0.82). Allocation concealment was judged to be adequate in 10 studies and unclear in 35 (fig 2). Seventeen studies reported blinding, including descriptions of blinding of participants (n=14), interveners (n=13), data analysts (n=4), and outcome assessors (n=9). Blinding of participants, interveners, data analysts, and outcome assessors was judged to be adequate for three, none, one, and four studies, respectively. Data analysis of all participants (for example, last entry carried forward or baseline observation carried forward) was reported by 23 studies, and incomplete outcome data was judged to be adequately dealt with in 25 studies.
Fig 2 Risk of bias graph showing review authors’ judgments about each risk of bias item presented as percentages across all included studies
Study characteristics—intervention descriptions
Studies were published in 1984-2013. Most studies (n=28) were conducted in the US; other locations were Europe (15), New Zealand (n=1), and Australia (n=1). The weighted average age of participants was 47.3 and the weighted average BMI before weight loss was 35.2. Trials included a mix of overweight and obese women and men (n=29) or only women (n=13) or men (n=3). Studies sampling both women and men included a weighted average of 73.3% women.
Three studies recruited individuals who had lost weight in the community,22 23 24 with the 42 remaining providing weight loss treatment before weight loss maintenance. The 42 studies including a weight loss induction phase initially recruited 9451 individuals, of whom 7788 were included in the maintenance phase (average dropout of 28.4% before maintenance). Initial attrition was mainly because of study dropout, although failure to meet weight loss and/or adherence criteria prevented entry into the maintenance phase for individuals in 17 studies. A total of 6278 individuals completed weight maintenance treatment (additional average dropout of 20%). Fourteen studies disclosed funding from industry.18 19 25 26 27 28 29 30 31 32 33 34 35
Initial weight loss phases
Of the 42 studies that included a formal weight loss phase, the average weight loss across studies ranged from −4.03 kg36 to −21.3 kg,29 with a weighted average of averages of −10.8 kg. The length of weight loss treatment before the maintenance phase ranged from two to 12 months (median 4.0). Most advocated change in behaviour/lifestyle, diet, and physical activity (n=26), commonly referred to as “standard behavioural therapy.” Other studies used a diet only (n=11) or physical activity only (n=1) approach. Some studies used a combination of pharmacotherapy in addition to behavioural/lifestyle change (n=3) or placebo treatment for pharmacotherapy and behavioural/lifestyle change (n=1). The dietary approaches advocated were described as general energy deficit diets (n=14), very low energy diets (defined as ≤800 kcal (3344 kJ)/day, n=11), low energy diets (defined as ≤1500 kcal (6270 kJ)/day, n=7), and a combination of the two (n=4). Some studies provided no detail (n=6) or explicitly stated not using a dietary approach for weight loss (n=1).
The physical activity recommendations for weight loss varied considerably, with walking as the most commonly recommended activity (n=4). Intensity of recommended physical activity varied from 20-30 minutes three to five times a week27 to 60 minutes every day.23 Two studies provided exercise classes.26 37 Most studies provided no details of recommendations for physical activity.
Several studies (n=17) reported a formal weight loss criterion for entry into the weight loss maintenance treatment. Entry criteria ranged from 5% to 10% of original body weight or 4-8 kg of initial weight loss. Three studies recruiting from the community without a formal weight loss phase required objective evidence of either 5% weight loss in the previous six months22 or 10% weight loss in the previous one24 or two years.23
Weight loss maintenance phase
For maintenance of weight loss, most studies examined behavioural/lifestyle interventions for diet and physical activity (n=22). Some studies focused on dietary (n=3) or exercise approaches (n=2) only. Other interventions included pharmacological (n=5), meal replacement (n=5), food supplement (n=3), or other (n=2) interventions.
Most study arms for which dietary approaches were reported continuing to prescribe energy deficit diets (n=23), while others prescribed diets to maintain body weight (n=14). Some arms were not prescribed any diet (n=7), and others were prescribed a mixture of weight loss and weight loss maintenance diets, depending on weight maintenance goals (n=3). Most descriptions of interventions, however, were unclear or provided no detail as to whether dietary approaches targeted further weight loss or maintenance of existing weight loss.
Recommendations for physical activity mostly promoted a general increase in physical activity (n=22). Some interventions provided specific recommendations, including walking (n=8) or resistance training (n=1) or provided exercise classes (n=2). Nine studies advocated maintenance of the physical activity levels recommended in the previous weight loss phase. Many studies did not provide details of physical activity to maintain weight loss, were unclear, or did not provide physical activity recommendations.
The five studies prescribing pharmacotherapy used orlistat at different doses (30, 60, or 120 mg three times daily). The three studies examining dietary supplements included conjugated linoleic acid (n=1), fibre (n=1), carbohydrate, or a combination of carbohydrate, fibre, chromium picolinate, and caffeine (n=1). The five studies examining meal replacements included Optifast (n=2), Nutrilet (n=1), the Cambridge diet (n=1), or the option of food boxes containing food in line with the recommended diet (n=1). The two studies examining alternative treatments included the use of a corset (n=1) and acupressure (n=1).
Modes of delivery
Intervention provider—Intervention arms delivered in person were facilitated by one (n=59) or multiple (n=23) types of providers, including “therapists” (n=21), dietitians (n=21), general practitioners/physicians (n=14), nurses (n=11), nutritionists (n=11), physiotherapist/exercise instructors (n=6), peer support (n=3), students (n=3), peers (n=3), or acupressure practitioner (n=1).
Format and delivery—Most studies were delivered either in a group (n=42) or combined group and individual (n=17) format. Other delivery formats included individual (n=5), internet (n=5), mail (n=5), telephone (n=3), mail and telephone (n=2), or group, mail, and telephone (n=1).
Setting—Research settings were generally poorly described. Those that reported study settings included home, often through the internet or phone (n=19), clinics (n=15), community (n=3), interactive television studio (n=2), or gym based settings (n=1).
Duration and intensity—Most weight loss maintenance interventions lasted 12 months (n=42) and ranged from 3-36 months (median 12.0). Intensity of intervention arms ranged from a minimum of once every three months27 to a maximum of 17 intervention contacts a month,26 with a mean of 3.2 (SD 3.19) contacts a month. Longer interventions tended to offer more intervention contacts, r=0.45, P<0.001.
Materials—Most provided materials were paper based such as session handouts or self monitoring cards/booklets (n=35). Other materials included the provision of pharmacological agents and corresponding placebos, food supplements, or meal replacements (n=20). Some studies offered incentives and refunded money, provided lottery tickets, or coupons (n=6); one study provided participants with corsets (n=1).34 One trial provided two study arms with scales for regular self weighing and a toolbox of materials that were accessed depending on progress (n=2).23
Meta-analysis—intervention effectiveness
Table A in appendix 2 provides a summary of all meta-analytic findings.
Behavioural/lifestyle v control
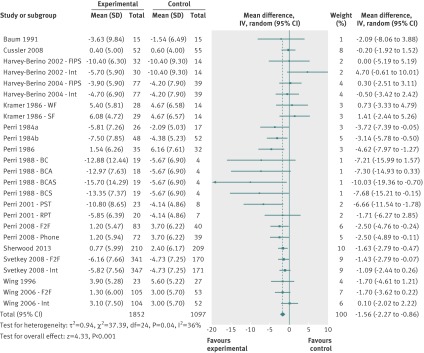
At 12 months, 15 behavioural/lifestyle studies including 25 comparisons20 23 24 36 38 39 40 41 42 43 44 45 46 47 48 showed a mean difference in weight change of −1.56 kg (95% confidence interval −2.27 to −0.86 kg; I2=36%; fig 3).
Fig 3 Mean difference in weight change at 12 months after initial weight loss in behavioural/lifestyle studies. BC=behaviour therapy + post-treatment therapy contact condition; BCA=behaviour therapy + post-treatment therapy contact + aerobic exercise maintenance condition; BCAS=behaviour therapy + post treatment therapy contact + aerobic exercise maintenance + social influence maintenance programme condition; F2F=face to face condition, FIPS=frequent in-person support condition; MIPS=minimal in-person support condition; Int=internet condition; PST=problem solving therapy condition; RPT=relapse prevention training condition; SF=skill focus condition, phone=telephone condition; WF=weight focus condition
Though Egger’s test was non-significant (P=0.14), inspection of the funnel plots does suggest small study bias (see fig A in appendix 2). Removal of four outlier comparisons with small numbers of participants and large effects that all came from a single study45 decreased the mean difference to −1.37 kg (95% confidence interval −2.02 to −0.73 kg; I2=27%). Sensitivity analysis including only the most intensive intervention arms from multi-arm trials found a mean difference of −1.69 kg (−2.47 to −0.92 kg; I2=40%) compared with controls. Sensitivity analysis by risk of bias items showed no differences between subgroups in relation to adequate allocation concealment or outcome assessment.
At 18 months, seven studies including 13 comparisons could be meta-analysed.20 23 24 42 44 45 49 The overall mean difference in weight change was −1.96 kg (95% confidence interval −2.73 to −1.20 kg; I2=15%). Sensitivity analysis including only the most intensive intervention arms from multi-arm studies found a mean difference in weight change of −2.22 kg (−3.18 to −1.26 kg; I2=26%). Sensitivity analysis for risk of bias items showed no significant differences in mean differences between subgroups in relation to adequate allocation concealment (adequate −1.49 kg (−2.24 to −0.73 kg) v unclear −2.92 kg (−4.31 to −1.54 kg), test for subgroup differences P=0.07; I2=69%) and outcome assessment (adequate −1.64 kg (−2.31 to −0.96 kg) v unclear −3.74 kg (−5.80 to −1.67 kg), test for subgroup differences P=0.06; I2=72%). At 24 and 30 months two studies reported outcomes.18 23 Overall mean differences in weight change remained significant at 24 months (−1.48 kg, −2.27 to −0.69 kg; I2=0%) but not at 30 months (−0.85 kg, −1.81 to 0.11 kg; I2=0%). No adverse events were reported for any behavioural/lifestyle weight loss maintenance treatments.20 24
Pharmacological interventions
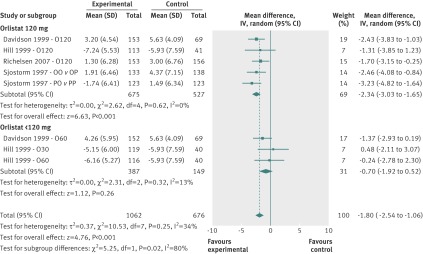
At 12 months, four pharmacological intervention studies including eight comparisons examined the effects of orlistat combined with lifestyle change compared with placebo and lifestyle change.18 19 27 50 The overall mean difference was −1.80 kg (95% confidence interval −2.54 to −1.06 kg; I2=34%) (fig 4). Subgroup analyses by dose of orlistat (120 mg v 60/30 mg three times daily) showed a dose-response relation, with studies that used 120 mg of orlistat displaying a mean difference of −2.34 kg (−3.03 to −1.65 kg, I2=0%) compared with −0.70 kg (−1.92 to 0.52 kg; I2=13%) in studies that used a 60/30 mg dose. The treatment effect was significantly greater in the subgroup of studies that used a 120 mg dose of orlistat (test for subgroup differences P=0.02; I2=81%).
Fig 4 Mean difference in weight change at 12 months after initial weight loss in pharmacological intervention studies. O120=orlistat 120 mg 3 times daily; O60=orlistat 60 mg 3 times daily; O30=orlistat 30 mg 3 times daily; OO v OP=comparison between groups who lost weight using orlistat 120 mg and low energy diet for 1 year followed by randomisation to continued orlistat 120 mg treatment or placebo condition; PO v PP=comparison between groups who lost weight using placebo and low energy diet for 1 year followed by randomisation to continued orlistat 120 mg treatment or placebo condition
One study testing the 120 mg dose provided long term follow-up at 18 and 36 months, with mean differences of −2.10 kg (95% confidence interval −4.09 to −0.11 kg) and −2.20 kg (−4.01 to −0.39 kg), respectively.18
All pharmacological studies reported increased adverse events in those taking orlistat compared with placebo groups (see table B in appendix 2). There was an increased frequency of gastrointestinal events in participants taking orlistat, with some studies reporting increased numbers withdrawing for this reason16 50. Gastrointestinal events were described as mild to moderate in intensity16 50 and occurring in the early phase of the intervention.16 19 50 No differences in other types of adverse events were reported. Two studies report minimal systemic absorption of orlistat.16 43 Two studies reported small significant decreases in vitamin status in participants taking orlistat, which remained within the normal clinical ranges and were treatable through vitamin supplementation.16 19
Other interventions
There was no evidence of effectiveness for the following interventions: dietary interventions versus control condition,35 37 high carbohydrate and low fat diets versus other types of diets,22 33 51 physical activity interventions versus control conditions,35 37 42 adding aerobic exercise to a dietary intervention versus diet alone,42 45 adding physical activity (such as walking and resistance training) to a dietary intervention versus diet alone,26 28 or adding meal replacements to dietary recommendations versus dietary recommendations alone.25 48 52 53 54 Details are in table A, appendix 2.
Other studies found no evidence for the effectiveness of nutritional supplements in addition to a dietary regimen versus the diet itself and placebo supplements55 or no supplements,30 31 Tapas acupressure technique versus social support,56 mailing computer generated individualised reports based on the “transtheoretical” model versus generic action oriented information,57 gourmet cooking versus neurolinguistic programming based therapy,32 a motivation focused versus a skill focused intervention,58 or providing participants with a corset over nine months versus no corset.34 One study reported that wearing a corset was perceived as uncomfortable34—no other comments about weight loss maintenance treatments were reported.
Mode of delivery of intervention
There was no evidence of effectiveness for more intensive interventions in terms of frequency of contact22 or number of intervention components59 versus less intensive versions of the intervention,22 59 internet delivered lifestyle/behavioural interventions versus control groups,20 23 38 39 40 43 or the delivery of a lifestyle/behavioural intervention through face to face contact versus remote delivery (such as phone/internet) of the same intervention.20 23 39 40 43
Discussion
Principal findings
This comprehensive systematic review of interventions for maintenance of non-surgical weight loss suggests that it is possible to reduce weight regain through behavioural and pharmacological means. Lifestyle interventions targeting both dietary intake and physical activity are effective in reducing weight regain after initial weight loss in obese adults within 12 months of weight loss. There is some evidence that these effects can be further sustained at 24 months and limited evidence beyond 24 months. The strength of the evidence, however, is limited; there was moderate heterogeneity and some evidence for potential risk of bias in terms of allocation concealment and outcome assessment as well as publication bias. All but 23 trials reported results only for those participants who completed the interventions, so results should be interpreted with caution.
Orlistat added to a lifestyle intervention seemed to be more effective than placebo and lifestyle intervention. Heterogeneity of effects was explained by a dose-response effect, with a dose of 120 mg three times a day reaching an effect estimate of −2.34 kg and treatment with lower doses a non-significant effect estimate of −0.70 kg. There was also evidence for sustainable effectiveness with orlistat 120 mg three times daily over 36 months. All orlistat studies report significant increases in adverse effects in the form of an increased number of gastrointestinal events in participants taking orlistat compared with placebo, with some studies also reporting slight decreases in vitamin concentrations, which were small and treatable with supplementation. Undesirable gastrointestinal side effects could limit acceptability at the individual level and therefore the impact of the treatment at population level.60, Orlistat, however, is normally prescribed alongside behaviour change. The behavioural interventions in orlistat trials included in our review provided extremely limited detail about the behaviour change components, and it is unclear if effects could be further optimised by pairing orlistat with the best evidence based behavioural interventions.
For interventions focused on diet or physical activity alone, using nutritional supplements or food replacements, we found no evidence for effectiveness of these interventions. Only a few studies tested these specific intervention components, and further research is required to confirm this finding. We found no significant evidence that specific modes of intervention delivery were more effective, although face to face interventions displayed a tendency to be more effective than remotely delivered ones (such as internet or telephone). Face to face interventions have limited scalability for population use, and it is highly desirable to use new technologies and methods for intervention design to develop methods that are both effective and scalable.
Strengths and limitations
The main strength of this review is the comprehensive and rigorous search and the meta-analytic synthesis of available evidence from randomised trials of non-surgical interventions for weight loss maintenance. Our findings, however, are applicable only to the contexts in which the studies have been conducted. Most included studies were conducted in the US and Scandinavia. Research in different countries and cultural settings would add to the generalisability.
Although energy prescriptions were poorly described in some weight loss maintenance intervention arms, participants seemed to receive advice to follow a regimen that continued to create an energy deficit, which was perhaps an unrealistic expectation for the long term. This obscures the important distinction between weight loss and weight loss maintenance.
All but three trials in this review provided a standardised weight loss treatment before allocating participants to different weight loss maintenance arms. While this practice might be sensible in terms of trial management, it could limit the generalisability of the findings to those who respond well to the initial weight loss treatment and does not provide an evidence base for weight loss maintenance that takes into account the many ways in which individuals initially lose weight.
Comparison with other studies
This systematic review adds to our knowledge of weight loss maintenance by providing a comprehensive evaluation of the evidence base to identify what works in helping patients to keep weight off after initial weight loss. Previous reviews have focused on a narrow set of methods for weight loss maintenance,10 criteria for inclusion of study by weight loss and follow-up periods of limited clinical relevance,14 and narrative rather than systematic reviews methods.13 While the overall findings of our review might be seen as encouraging, further research is needed to provide more rigorous evaluations of well described replicable interventions with an explicit theoretical underpinning over periods of more than 24 months.
Conclusions and policy implications
Comprehensive behavioural interventions targeting dietary and physical activity behaviours are moderately effective in slowing regain of weight in obese adults after initial weight loss for follow-up periods of up to 24 months. Orlistat treatment in addition to behaviour change is effective in reducing weight regain, with clear evidence of a dose-response relation and some evidence for effectiveness over 36 months. Side effects of this drug should be considered and discussed with patients before it is prescribed.
What is already known on this topic
Behaviour change leads to moderate clinically meaningful changes in weight
After initial weight loss, most people regain lost weight
Maintenance of weight loss is crucial to uphold the health benefits of initial weight loss
What this study adds
Behavioural interventions dealing with diet and physical activity show small but significant benefits on weight loss maintenance for up to 24 months
Pharmacological support from orlistat 120 mg three times daily also shows small but significant benefits on weight loss maintenance for up to 36 months
Overall effects of behavioural/lifestyle interventions, both with and without orlistat, on weight loss maintenance are small, and further research needs to focus on increasing effectiveness of interventions
Contributors: FFS and AA conceived the review. FFS, SUD, AA, and VAS developed the review protocol. All authors were involved in data extraction. SUD and FFS analysed the data. All authors reviewed and contributed to the manuscript drafted by SUD. All authors read and agreed the final version. FFS is guarantor.
Funding: FFS is funded by Fuse, the Centre for Translational Research in Public Health, a UKCRC Public Health Research Centre of Excellence. Fuse gratefully acknowledges funding from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration. The Health Services Research Unit, University of Aberdeen, is funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate. The views expressed are not necessarily those of the funding bodies. This research was conducted independently of the funders, and the funders had no influence on the research process.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Transparency: The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the review being reported; that no important aspects of the review have been omitted; and that any discrepancies from the study as planned have been explained.
Cite this as: BMJ 2014;348:g2646
Web Extra. Extra material supplied by the author
Appendix 1: Full details of included studies
Appendix 2: Summary of meta-analysis findings (table A), adverse events (table B), funnel plot (fig A)
References
- 1.Haslam DW, James WPT. Obesity. Lancet 2005;366:1197-209. [DOI] [PubMed] [Google Scholar]
- 2.Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev 2007;8:503-13. [DOI] [PubMed] [Google Scholar]
- 3.Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8:iii-iv,1-182. [DOI] [PubMed] [Google Scholar]
- 4.Dombrowski SU, Avenell A, Sniehotta FF. Behavioural interventions for obese adults with additional risk factors for morbidity: systematic review of effects on behaviour, weight and disease risk factors. Obes Facts 2010;3:377-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardle J, Griffith J, Johnson F, Rapoport L. Intentional weight control and food choice habits in a national representative sample of adults in the UK. Int J Obes 2000;24:534-40. [DOI] [PubMed] [Google Scholar]
- 7.Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese US adults. Am J Prev Med 2012;42:481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82:222-5S. [DOI] [PubMed] [Google Scholar]
- 9.Penn L, White M, Lindström J, den Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PLoS One 2013;8:e57143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev 2012;13:509-17. [DOI] [PubMed] [Google Scholar]
- 11.Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev 2010;11:306-21. [DOI] [PubMed] [Google Scholar]
- 12.Ramage S, Farmer A, Apps Eccles K, McCargar L. Healthy strategies for successful weight loss and weight maintenance: a systematic review. Appl Physiol Nutr Metab 2014;39:1-20. [DOI] [PubMed] [Google Scholar]
- 13.Simpson SA, Shaw C, McNamara R. What is the most effective way to maintain weight loss in adults? BMJ 2011;343:d8042. [DOI] [PubMed] [Google Scholar]
- 14.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs 2009;24:58-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S, eds. Cochrane handbook of systematic reviews of intervention. Cochrane Collaboration, 2011.
- 16.Davidson KW, Goldstein M, Kaplan RM, Kaufman PG, Knatterud GL, Orleans CT, et al. Evidence-based behavioral medicine: what is it and how do we achieve it? Ann Behav Med 2003;26:161-71. [DOI] [PubMed] [Google Scholar]
- 17.Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas 2007;1:77-89. [Google Scholar]
- 18.Richelsen B, Tonstad S, Rossner S, Toubro S, Niskanen L, Madsbad S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 2007;30:27-32. [DOI] [PubMed] [Google Scholar]
- 19.Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet 1998;352:167-72. [DOI] [PubMed] [Google Scholar]
- 20.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139-48. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale KS, McAuley KA, Taylor RW, Williams SM, Farmer VL, et al. Determining optimal approaches for weight maintenance: a randomized controlled trial. CMAJ 2009;180:E39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563-71. [DOI] [PubMed] [Google Scholar]
- 24.Sherwood NE, Crain AL, Martinson BC, Anderson CP, Hayes MG, Anderson JD, et al. Enhancing long-term weight loss maintenance: 2 year results from the Keep It Off randomized controlled trial. Prev Med 2013;56:171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annunziato RA, Timko CA, Crerand CE, Didie ER, Bellace DL, Phelas S, et al. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eat Behav 2009;10:176-83. [DOI] [PubMed] [Google Scholar]
- 26.Borg P, Kukkonen-Harjula K, Fogelholm M, Pasenen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes 2002;26:676-83. [DOI] [PubMed] [Google Scholar]
- 27.Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 1999;281:235-42. [DOI] [PubMed] [Google Scholar]
- 28.Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight maintenance after a very-low- energy diet in premenopausal obese women: A randomized controlled trial. Arch Intern Med 2000;160:2177-84. [DOI] [PubMed] [Google Scholar]
- 29.Lantz H, Peltonen M, Agren L, Torgerson JS. Intermittent versus on-demand use of a very low calorie diet: a randomized 2-year clinical trial. J Intern Med 2003;253:463-71. [DOI] [PubMed] [Google Scholar]
- 30.Pasman WJ, Westerterp-Plantenga MS, Muls E, Vansant G, van Ree J, Saris WH. The effectiveness of long-term fibre supplementation on weight maintenance in weight-reduced women. Int J Obes Relat Metab Disord 1997;21:548-55. [DOI] [PubMed] [Google Scholar]
- 31.Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effectiveness of long-term supplementation of carbohydrate, chromium, fibre and caffeine on weight maintenance. Int J Obes Relat Metab Disord 1997;21:1143-51. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen LB, Greve T, Kreutzer M, Pedersen U, Nielsen CM, Toubro S, et al. Weight maintenance through behaviour modification with a cooking course or neurolinguistic programming. Can J Diet Pract Res 2011;72:181-5. [DOI] [PubMed] [Google Scholar]
- 33.Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ 1997;314:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wikstrand I, Torgerson J, Bostrom KB. Very low calorie diet (VLCD) followed by a randomized trial of corset treatment for obesity in primary care. Scand J Prim Health Care 2010;28:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen P, Frederiksen R, Bliddal H, Riecke BF, Bartels M, Henriksen M, et al. Comparison of three different weight maintenance programs risk, bone, and vitamins in sedentary older adults. Obesity (Silver Springs) 2013;21:1982-90. [DOI] [PubMed] [Google Scholar]
- 36.Baum JG, Clark HB, Sandler J. Preventing relapse in obesity through posttreatment maintenance systems—comparing the relative efficacy of 2 levels of therapist support. J Behav Med 1991;14:287-302. [DOI] [PubMed] [Google Scholar]
- 37.King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs exercise in weight maintenance. The effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med 1989;149:2741-6. [DOI] [PubMed] [Google Scholar]
- 38.Cussler EC, Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, et al. Maintenance of weight loss in overweight middle-aged women through the internet. Obesity 2008;16:1052-60. [DOI] [PubMed] [Google Scholar]
- 39.Harvey-Berino J, Pintauro S, Buzzell P, DiGuilio M, Casey Gold B, Moldovan C, et al. Does using the Internet facilitate the maintenance of weight loss? Int J Obes 2002;26:1254-60. [DOI] [PubMed] [Google Scholar]
- 40.Harvey-Berino J, Pintauro S, Buzzell P, Gold EC. Effect of internet support on the long-term maintenance of weight loss. Obes Res 2004;12:320-9. [DOI] [PubMed] [Google Scholar]
- 41.Kramer FM, Jeffery RW, Snell MK, Forster JL. Maintenance of successful weight-loss over 1 year—effects of financial contracts for weight maintenance or participation in skills training. Behav Ther 1986;17:295-301. [Google Scholar]
- 42.Perri MG, Lauer JB, McAdoo WG, Lauer JB, Yancey DZ. Enhancing the efficacy of behaviour therapy for obesity—effects of aerobic exercise and a multicomponent maintenance program. J Consult Clin Psychol 1986;54:670-75. [DOI] [PubMed] [Google Scholar]
- 43.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med 2008;168:2347-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perri MG, McAdoo WG, Spevak PA, Newlin DB. Effect of a multicomponent maintenance program on long-term weight-loss. J Consult Clin Psychol 1984;52:480-1. [DOI] [PubMed] [Google Scholar]
- 45.Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol 1988;56:529-34. [DOI] [PubMed] [Google Scholar]
- 46.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol 2001;69:722-6. [PubMed] [Google Scholar]
- 47.Perri MG, Shapiro RM, Ludwig WW, Twentyman CT, McAdoo WG. Maintenance strategies for the treatment of obesity—an evaluation of relapse prevention training and posttreatment contact by mail and telephone. J Consult Clin Psychol 1984;52:404-13. [DOI] [PubMed] [Google Scholar]
- 48.Wing RR, Jeffery RW, Hellerstedt WL, Burton LR. Effect of frequent phone contacts and optional food provision on maintenance of weight loss. Ann Behav Med 1996;18:172-6. [DOI] [PubMed] [Google Scholar]
- 49.Perri MG, Lauer JB, Yancey DZ, Lauer JB, Jordan RC, Yancey DZ, et al. Effects of peer support and therapist contact on long-term weight-loss. J Consult Clin Psychol 1987;55:615-7. [DOI] [PubMed] [Google Scholar]
- 50.Hill JO, Hauptman J, Anderson JW, Fujioka K, O’Neil PM, Smith DK, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-y study. Am J Clin Nutr 1999;69:1108-16. [DOI] [PubMed] [Google Scholar]
- 51.Delbridge EA, Prendergast LA, Pritchard JE, Proietto J. One-year weight maintenance after significant weight loss in healthy overweight and obese subjects: does diet composition matter? Am J Clin Nutr 2009;90:1203-14. [DOI] [PubMed] [Google Scholar]
- 52.Agras WS, Berkowitz RI, Arnow BA, Telch CF, Marnell M, Henderson J, et al. Maintenance following a very-low-calorie diet. J Consult Clin Psychol 1996;64:610-3. [DOI] [PubMed] [Google Scholar]
- 53.Ryttig KR, Flaten H, Rossner S. Long-term effects of a very low calorie diet (Nutrilett) in obesity treatment. A prospective, randomized, comparison between VLCD and a hypocaloric diet + behavior modification and their combination. Int J Obes 1997;21:574-9. [DOI] [PubMed] [Google Scholar]
- 54.Ryttig KR, Rossner S. Weight maintenance after a very low calorie diet (VLCD) weight reduction period and the effects of VLCD supplementation. A prospective, randomized, comparative, controlled long-term trial. J Intern Med 1995;238:299-306. [DOI] [PubMed] [Google Scholar]
- 55.Larsen T, Toubro S, Gudmundsen O, Astrup A. Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. Am J Clin Nutr 2006;83:606-12. [DOI] [PubMed] [Google Scholar]
- 56.Elder CR, Gullion CM, DeBar LL, Funk KL, Lindberg NM, Ritenbaugh C, et al. Randomized trial of Tapas acupressure technique for weight loss maintenance. BMC Complement Altern Med 2012;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riebe D, Blissmer B, Greene G, Caldwell M, Ruggiero L, Stillwell KM, et al. Long-term maintenance of exercise and healthy eating behaviors in overweight adults. Prev Med 2005;40:769-78. [DOI] [PubMed] [Google Scholar]
- 58.West DS, Gorin AA, Subak LL, Foster G, Bragg C, Hecht J, et al. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes 2011;35:259-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe MR, Tappe KA, Annunziato RA, Riddell LJ, Coletta MC, Crerand CE, et al. The effect of training in reduced energy density eating and food self-monitoring accuracy on weight loss maintenance. Obesity 2008;16:2016-23. [DOI] [PubMed] [Google Scholar]
- 60.Ogden J, Sidhu S. Adherence, behavior change, and visualization: a qualitative study of the experiences of taking an obesity medication. J Psychosom Res 2006;61:545-52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Full details of included studies
Appendix 2: Summary of meta-analysis findings (table A), adverse events (table B), funnel plot (fig A)