Abstract
Background
Signaling pathways that target I-κB kinase β (IKKβ) activation stimulate the expression of NF-κB-dependent genes and are thus thought to primarily promote inflammation and injury in solid organ grafts.
Methods
We examined the role of IKKβ in a mouse model of lung transplantation-mediated ischemia-reperfusion injury using NF-κB essential modulator (NEMO)-binding domain (NBD) peptide to pharmacologically inhibit IKK activation. As myeloid cells are primarily responsible for the production of acute inflammatory mediators following lung transplantation, we also investigated the effects of myeloid cell-specific IKKβ gene deletion on acute lung graft injury by transplanting mutant mice.
Results
When NBD was administered at a dose that partially inhibits IKKβ activation, we observed attenuated lung graft injury and blunted expression of intragraft pro-inflammatory mediators. Surprisingly, when the dose of NBD was increased to a level that completely ablates intragraft IKKβ activation, graft inflammation and injury were significantly worse compared to recipients treated with control peptide. Similar to lung recipients with pharmacologically ablated IKKβ activity, donor-recipient transplant combinations with a myeloid cell-specific IKKβ gene deletion had marked intragraft inflammation and poor lung function.
Conclusions
Our data show maintenance of IKKβ activity is critical for the return of lung graft homeostasis with important implications for targeting NF-κB-dependent signaling pathways for treating acute lung injury.
Keywords: Primary graft dysfunction, Ischemia-reperfusion, Acute lung injury, Lung transplantation, IKKβ
Introduction
Ischemia-reperfusion–induced acute lung injury, also known as primary graft dysfunction (PGD), is a major cause of early morbidity and mortality following lung transplantation (1–4). Ischemia-reperfusion injury (IRI) is exacerbated by the induction of pro-inflammatory signaling pathways that target the activation of the Nuclear Factor-κB (NF-κB) class of transcription factors (5). NF-κB activation is controlled by the I-κB Kinase (IKK) complex, which consists of 3 subunits: a regulatory subunit (IKKγ or NEMO/NF-κB Essential Modulator), and two catalytic subunits, IKKα and IKKβ (6–8). Two pathways for NF-κB activation have been identified. The alternative or non-canonical pathway, controlled by IKKα, is necessary for adaptive immune functions (9). The classical or canonical pathway, which requires both IKKβ and NEMO, regulates the expression of key pro-inflammatory mediators that promote innate immunity (10, 11). IKKβ stimulates activation of the canonical pathway through catalyzing the phosphorylation of I-κB, which in turn allows for the translocation of NF-κB complexes to the nucleus to drive the transcription of pro-inflammatory mediator genes as well genes involved in cell survival (12). In particular, IKKβ’s important role in inducing pro-inflammatory gene transcription has made it an attractive therapeutic target for treating inflammation (13). IKKβ activation requires association with NEMO via a C-terminal NEMO-binding Domain (NBD) on IKKβ (14). NBD peptide, a cell-permeable inhibitor spanning the NBD, disrupts NEMO-IKKβ complexes leading to inhibition of IKKβ activation and NF-κB dependent gene expression.
Previous reports in experimental models of chronic organ injury have shown that NBD peptide is effective at ameliorating tissue inflammation (15–17). However, despite the extensive characterization of IKKβ in such models it is unclear if it plays a role in regulating acute inflammatory responses in solid organs. In this study we asked if NBD peptide was effective at ameliorating acute graft injury following lung transplantation. NBD treatment, at a dose that partially inhibited IKKβ activity, decreased production of pro-inflammatory mediators and improved lung graft function. However, when NBD peptide dose was increased to completely suppress IKKβ activity, we unexpectedly observed augmented lung graft injury, which was associated with increased pro-inflammatory mediator production and inflammatory cell infiltration. Concordant with observations in high dose NBD peptide-treated lung recipients, graft injury and inflammation was also exacerbated in mice with genetic deletion of IKKβ within the myeloid cell compartment.
Results
The effect of IKKβ activation blockade on acute lung graft injury
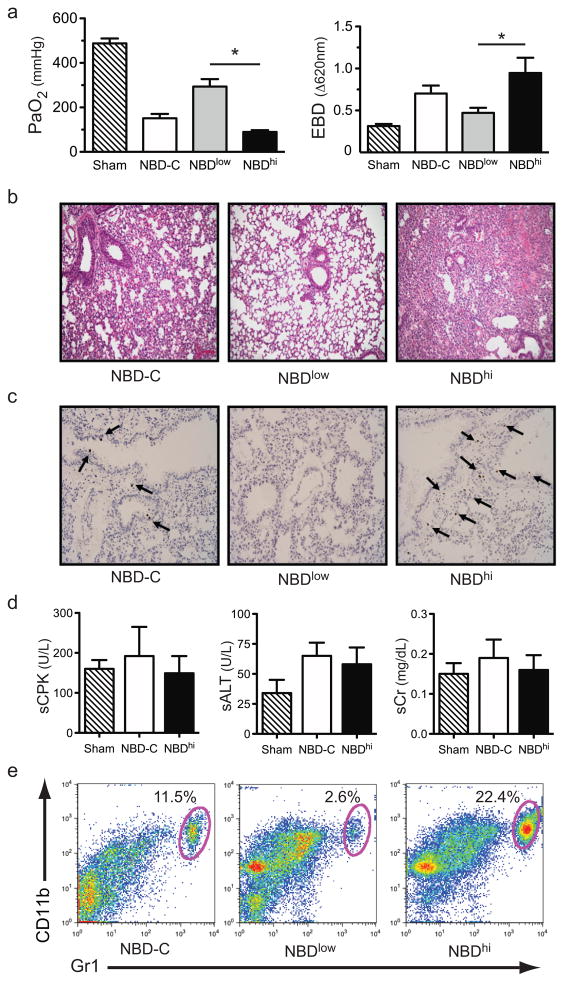
IKKβ activation stimulates the expression of pro-inflammatory cytokines associated with acute lung injury. However, the role of IKKβ in lung graft ischemia-reperfusion injury remains largely unclear. Therefore, we asked if pharmacological blockade of IKKβ activation with NBD peptide would prevent lung graft injury in a mouse model of orthotopic lung transplant-mediated ischemia-reperfusion injury recently developed in our laboratory (18, 19). Lung grafts were harvested from C57BL/6 (B6) donors and underwent cold ischemic storage for 18 hours. Five minutes prior to transplantation, B6 recipients were treated with a 25 μg/kg dose of control mutant peptide (NBD-C), 2.5μg/kg dose of NBD (NBDlow) or a 25 μg/kg dose of NBD (NBDhi). Measures of lung graft function and injury were assessed 24 hours after engraftment (Figs. 1a–c). NBDlow-treated lung recipients had significantly better graft function relative to recipient mice treated with NBD-C. Improvement of graft function as measured by arterial PaO2 was associated with attenuated pulmonary edema, decreased alveolar congestion and less prevalent apoptosis of stromal cells. Interestingly, NBDhi-treated recipients had significantly worse graft function, injury and more apoptotic stromal cells when compared to NBD-C or NBDlow-treated hosts. However, increased graft injury in NBDhi-treated lung recipients was not secondary to systemic solid organ dysfunction as there was little evidence of increased serum elevation of skeletal muscle, liver or kidney markers of injury relative to NBD-C-treated lung recipients or sham-operated mice (Fig. 1d). As granulocytes are key regulators of acute lung injury we also measured the percent abundance of these cells in the graft tissue (Fig. 1e). As compared to NBD-C or NBDlow-treated recipients there was a higher percent abundance of intragraft granulocytes in NBDhi-treated mice.
Figure 1.
The effects of pharmacological blockade of IKKβ on lung graft injury. (a) Sham-operated mice or B6 → B6 lung recipients treated with NBD-C, NBDlow or NBDhi and assessed for (left panel) PaO2 (N=5) or (right panel) EBD dye exclusion (N=5) 24 hours after engraftment. Data are shown as the mean ± standard error of the mean (SEM) *, p<0.05. (b) Representative (N=5) graft histology (200x magnification) and a (c) representative (N=5) graft tissue TUNEL assay (200x magnification) from indicated lung recipients 24 hrs following transplantation. (d) Measurement of serum creatine phosphokinase (sCPK), alanine aminotransferase (sALT) and creatinine (sCr) in NBDhi or NBD-C-treated B6 → B6 lung recipients (N=4) 24 hours after engraftment. Data are shown as the mean ± SEM. (e) Representative FACS analysis (N=5) of percent abundance of intragraft granulocytes in B6 → B6 lung recipients treated with NBD-C, NBDlow or NBDhi 24 hours after engraftment.
Ablation of IKKβ activity exacerbates lung graft injury and inflammation
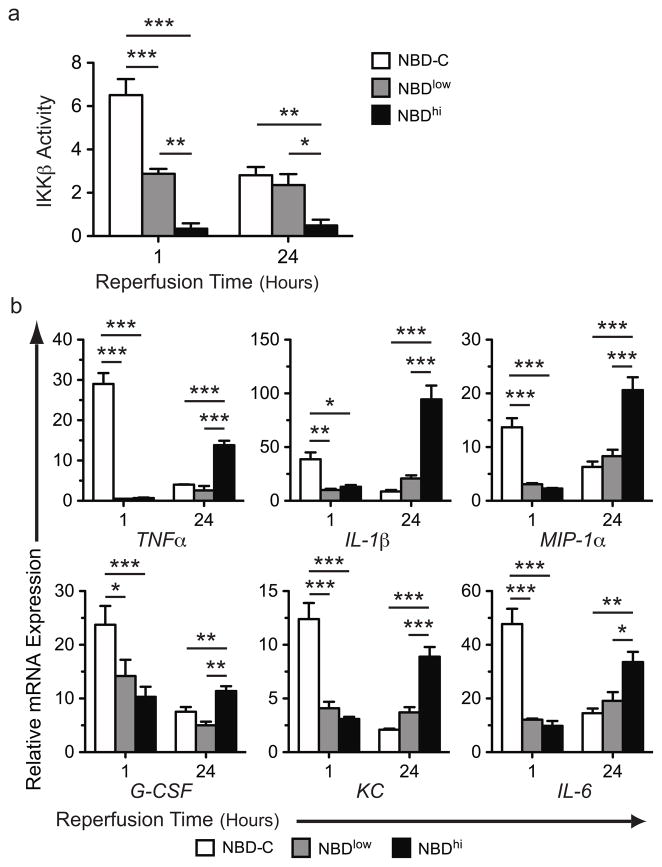
The relationship between NBDhi treatment and lung injury led us to investigate the temporal dynamics of IKKβ activation in graft recipients. We assessed intragraft IKKβ activity in lung recipients at 1 and 24 hours following NBD-C-, NBDlow- or NBDhi -treatment (Fig. 2a). In NBD-C-treated recipients we observed higher IKKβ activity at 1 hour following reperfusion relative to 24 hours post-engraftment. In comparison to NBD-C-treated animals NBDlow-treated recipients had significantly less intragraft IKKβ activity at 1 hour post-reperfusion but comparable IKKβ activity at 24 hours. In contrast, NBDhi-treated lung recipients had little intragraft IKKβ activity at both time points.
Figure 2.
The dynamics of intragraft IKKβ activity and inflammatory gene expression following pharmacological blockade. (a) B6 → B6 lung recipients were treated with NBD-C, NBDlow or NBDhi and evaluated for IKKβ activity at indicated times. Results are representative of 2 independent experiments. Results shown are from one representative experiment where N ≥ 4 per time point and is normalized to IKKβ activity of B6 lung tissue following a sham operation. (b) B6 → B6 lung recipients were treated as in (a) and analyzed for levels of intragraft inflammatory mediator transcripts by real-time quantitative RT-PCR at indicated times. Results shown as a mean ± SEM *, p < 0.05, **, p < 0.01, ***, p < 0.001.
These data suggested that the temporal dynamics of intragraft IKKβ activity were associated with changes in the expression levels of NF-κB-dependent gene transcripts. As we previously observed increased intragraft accumulation of granulocytes in NBDhi-treated lung recipients, we next set out to analyze transcript levels of a panel of pro-inflammatory NF-κB-dependent genes that promote granulocyte production and inflammation (Fig. 2b). Relative to NBD-C-treated lung graft recipients both NBDlow- and NBDhi-treatment were equivalently effective at blunting transcript levels of Granulocyte colony-stimulating factor (G-CSF), Interleukin-6 (IL-6), Macrophage inflammatory protein-1α (MIP-1α) and Chemokine (C-X-C motif) Ligand 1 (KC), at 1 hour following transplantation. However, at 24 hours post-engraftment these gene transcript levels were markedly higher in NBDhi -treated recipients than in NBDlow-treated or NBD-C-treated mice. Moreover, we observed a similar pattern of gene expression in Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α), which can promote endothelial cell injury and pulmonary edema. Thus, while a transient early reduction in IKKβ activity is beneficial, prolonged blockade of IKKβ activity is detrimental as it enhances the expression of pro-inflammatory gene transcripts and accumulation of neutrophils in lung grafts.
Myeloid-specific IKKβ deletion exacerbates acute lung graft injury
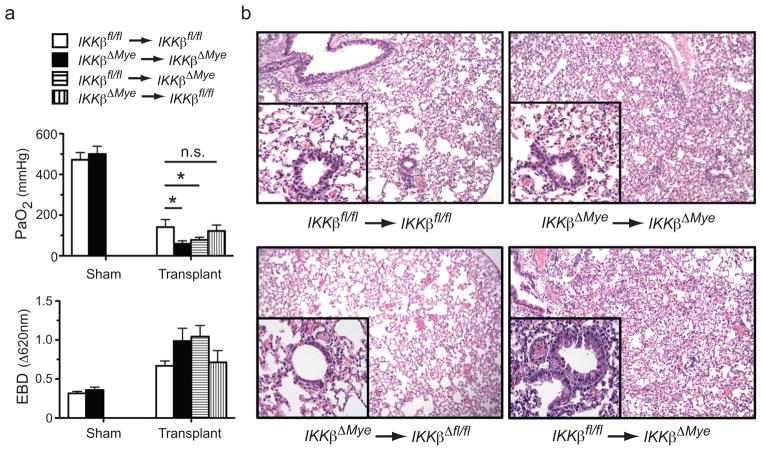
The myeloid compartment is a primary source of pro-inflammatory cytokines and chemokines associated with lung graft injury (20, 21). To specifically examine the role of IKKβ expression in the myeloid compartment on acute lung graft injury we utilized B6 mice carrying disrupted IKKβ alleles specifically in myeloid cells (IKKβΔmye), which were generated by crossing B6 mice expressing Cre recombinase driven by a lysozyme M promoter (C57BL/6LysMCre) to B6 IKKβ flox allele mice (IKKβfl/fl) (22–24). Following 18 hours of cold preservation IKKβΔmye and IKKβfl/fl lungs were transplanted into IKKβΔmye or IKKβfl/fl recipients and assessed for graft function and pulmonary edema (Fig. 3a). Twenty-four hours following engraftment IKKβΔmye → IKKβΔmye lung and IKKβ fl/fl → IKKβΔmye recipients had significantly worse graft function and greater pulmonary edema compared to control IKKβfl/fl → IKKβfl/fl mice. Additionally, analysis of lung graft histology showed that lung injury in IKKβΔmye → IKKβΔmye and IKKβfl/fl → IKKβΔmye lung recipients was associated with more prevalent inflammatory cell infiltrate sequestered to graft alveolar spaces (Fig. 3b). Interestingly, IKKβfl/fl → IKKβΔmye when compared to IKKβΔmye → IKKβΔmye lung recipients had a similar pattern of lung injury (Fig. 3a and b). By contrast, IKKβΔmye → IKKβ fl/fl like IKKβfl/fl → IKKβfl/fl lung recipients had notably milder lung injury indicating that IKKβ plays a more prominent role in graft-infiltrating myeloid cells than in myeloid cells of donor origin in promoting graft injury.
Figure 3.
Graft injury in IKKβΔmye lung recipients. (a) IKKβfl/fl or IKKβΔmye mice underwent sham operations or IKKβfl/fl → IKKβfl/fl, IKKβΔmye → IKKβfl/fl, IKKβfl/fl → IKKβΔmye, IKKβΔmye → IKKβΔmye lung transplants were performed and evaluated for graft (left panel) PaO2 (N=4) or (right panel) Evans Blue dye (EBD) exclusion (N=4) 24 hours after engraftment. Results are shown as a mean ± SEM *, p < 0.05, n.s, p = 0.14 (b) Representative (N=5) histopathological analysis of indicated lung grafts 24 hours after transplantation (100x magnification, inset 400x magnification).
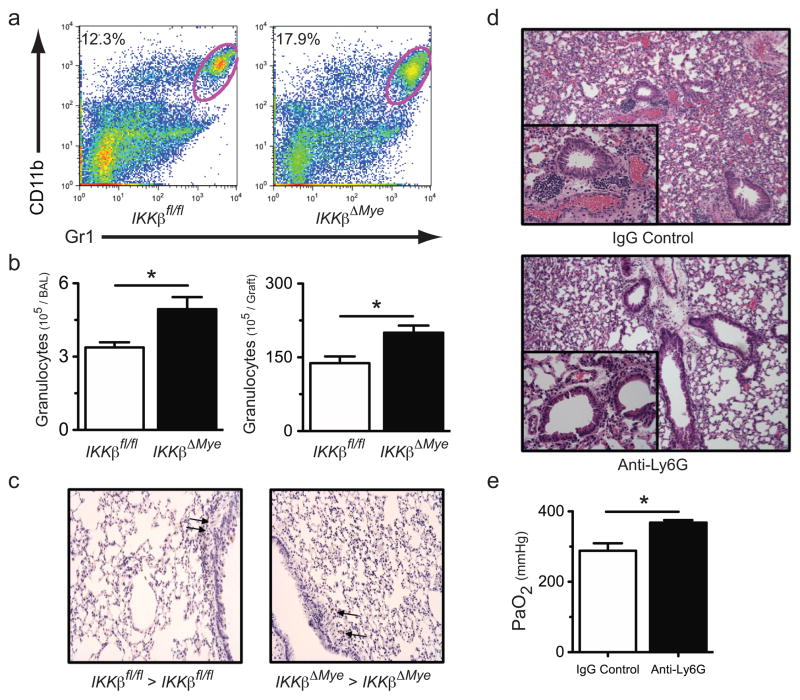
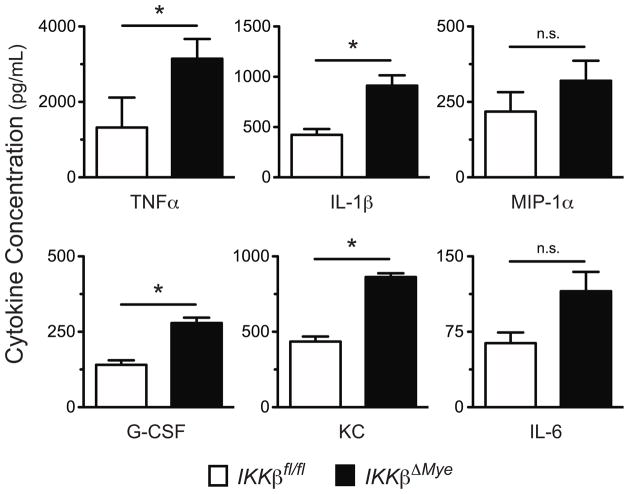
To better characterize IKKβ’s role in lung injury we analyzed the cellular infiltrate in IKKβΔmye → IKKβΔmye and IKKβfl/fl → IKKβfl/fl lung recipients by flow cytometric analysis (Fig. 4a). At 24 hours following transplantation IKKβΔmye → IKKβΔmye mice had a higher percent abundance of intragraft granulocytes as compared to IKKβfl/fl → IKKβfl/fl lung recipients. We also counted granulocytes in the bronchoalveolar lavage (BAL) and in the graft tissue of IKKβΔmye and IKKβfl/fl lung recipients at 24 hours following transplantation (Fig. 4b). Compared to IKKβfl/fl → IKKβfl/fl lung recipients IKKβΔmye → IKKβΔmye mice had significantly higher numbers of airway and graft tissue granulocytes. However, unlike in NBDhi-treated lung recipients we did not observe a higher abundance of apoptotic cells within the grafts of IKKβΔmye → IKKβΔmye lung recipients (Fig. 4c). To assess if granulocytes were responsible for the severe lung injury in IKKβΔmye → IKKβΔmye lung recipients we depleted granulocytes perioperatively with Ly6G specific antibodies and assessed pulmonary histology and lung function (Figs. 4d and e). As compared to control Ig-treated IKKβΔmye → IKKβΔmye lung recipients Ly6G treated IKKβΔmye → IKKβΔmye mice had less evidence of cellular infiltrate and significantly better PaO2 indicating that granulocytes promote lung injury in IKKβΔmye grafts. As in NBDhi treated lung recipients, the high numbers of granulocytes in IKKβΔmye → IKKβΔmye mice indicated the potential for the accumulation of inflammatory mediators associated with granulocyte-mediated tissue injury. Therefore, we measured levels of intragraft NF-κB-dependent granulocyte activity-associated inflammatory mediators in IKKβfl/fl →IKKβfl/fl and IKKβΔmye → IKKβΔmye lung recipients 24 hours following transplantation (Fig. 5). Relative to IKKβfl/fl → IKKβfl/fl lung recipients IKKβΔmye → IKKβΔmye mice had higher intragraft levels of G-CSF, KC, MIP-1α, TNF-α and IL-1β. Thus, IKKβ expression in the myeloid compartment is necessary to prevent ischemia-reperfusion mediated-acute lung injury and inflammation.
Figure 4.
Accumulation of granulocytes in IKKβΔmye lung recipients. IKKβfl/fl → IKKβfl/fl and IKKβΔmye → IKKβΔmye lung transplants were performed and assessed for intragraft granulocyte accumulation. (a) Representative FACS analysis (N=5) of the percent abundance of granulocytes in lung graft tissue, (b) total granulocyte counts in the (upper panel) airway and (lower panel) lung graft tissue 24 hours after engraftment. (c) Representative TUNEL assay (N=3) on graft tissue from IKKβfl/fl → IKKβfl/fl and IKKβΔmye → IKKβΔmye lung recipients 24 hrs following transplantation (200x magnification). (d) Representative histological analysis (N=5) of control IgG or Ly6G antibody-treated IKKβΔmye → IKKβΔmye lung recipients (200x magnification, inset 400x magnification). (e) IKKβΔmye → IKKβΔmye lung recipients (N=5) treated as in (d) and assessed for PaO2. For (b) and (d) results are shown as a mean ± SEM *, p < 0.05.
Figure 5.
Inflammatory mediator expression in IKKβΔmye lung recipients. IKKβfl/fl → IKKβfl/fl (N=4) and IKKβΔmye → IKKβΔmye (N=4) lung transplants were performed and compared for intragraft inflammatory mediator expression by multiplex ELISA at 24 hours following engraftment. Results are shown as a mean ± SEM *, p < 0.05.
Discussion
The recognition that NF-κB canonical pathway activation stimulates the expression of genes that promote tissue inflammation has made it a target for the development of anti-inflammatory drugs (13). Experimental approaches to prevent ischemia-reperfusion injury have primarily involved the selective inhibition of the expression or activity of target genes of the canonical pathway such as TNF-α, IL-1β, IL-6 and IL-8 (15, 25). Although these approaches have shown some promise the redundant pro-inflammatory activities of many genes regulated by NF-κB have suggested that more comprehensive approaches are needed to attenuate graft tissue inflammation. IKKβ activation is critical to promote the transcription of target genes of the NF-κB canonical pathway. Indeed, IKKβ-specific inhibitors have been recently shown to be effective at ameliorating tissue injury and organ dysfunction in animal models of inflammatory arthritis, colitis and allergic airway disease (15–17). Consistent with these reports we found that a transient reduction of IKKβ activity is effective at suppressing lung graft injury and inflammatory gene expression following prolonged cold preservation. Surprisingly, complete pharmacological inhibition of IKKβ activity had the unintended effect of exacerbating lung graft inflammation and injury. Similarly, intestinal epithelial cell-specific IKKβ ablation promotes chronic inflammation and cause severe apoptotic damage in the intestinal mucosa (26). In this context IKKβ activation appears to be necessary for promoting epithelial cell survival. In NBDhi-treated animals we observed increased numbers of apoptotic cells lining the airway along increased intragraft TNF-α transcripts levels. By contrast, IKKβΔmye → IKKβΔmye lung recipients had a very few apoptotic graft cells. As IKKβ has been shown to be critical in promoting the expression of pro-survival genes including Bcl2, which is highly expressed within lung graft airway epithelial cells, the resistance to apoptotic cell death in IKKβΔmye → IKKβΔmye recipients may be the result of maintaining IKKβ activity within graft parenchymal cells (27, 28). Moreover, as apoptosis has been shown to be enhanced by TNF-α when NF-κB activation is inhibited these observations indicate IKKβ activity plays a critical role in promoting lung graft parenchymal cell survival by stimulating NF-κB translocation (29). Consistent with these data have been several observations where toll-like receptor-mediated NF-κB activation in lung parenchymal cells was necessary to limit acute inflammation (30, 31).
Interestingly, NBDhi-treated lung recipients also had elevated inflammatory gene expression despite the complete absence of intragraft IKKβ activity. Graft resident myeloid cells are potential major producers of pro-inflammatory mediator production. NBD has been previously shown to inhibit IKKβ activity and myeloid cell inflammatory responses in a dose dependent manner (32). However, enhanced inflammatory effects of pharmacologically inhibiting IKKβ in macrophages and neutrophils have also been reported (33). We observed augmented pro-inflammatory gene expression was coincident with the recruitment of large amounts of neutrophils, which are a major source of pre-transcribed pro-inflammatory mediators such as IL-1β that in turn can act to promote the expression of inflammatory mediators in an IKKβ independent manner (34). To further define the impact of IKKβ activity on graft injury we performed orthotopic lung transplants using mice carrying a myeloid lineage-specific deletion of IKKβ. Previous reports have shown that IKKβ expression in the airway epithelium can reduce bronchial injury, attenuate inflammatory cytokine production and drive the production of mucus indicating that it may be beneficial to inhibit IKKβ in lung parenchymal cells (31). However, in myeloid cells IKKβ may additionally act to limit inflammatory gene expression as it is a negative regulator of caspase-1-mediated IL-1β secretion and attenuator of STAT-1-dependent expression of inducible nitric oxide synthase (33, 35). Accordingly, IKKβΔmye → IKKβΔmye and IKKβfl/fl → IKKβΔmye but not IKKβΔmye → IKKβfl/fl lung recipients developed more severe ischemia-reperfusion injury as compared to IKKβfl/fl→ IKKβfl/fl mice. Consistent with this observation, IKKβΔmye→ IKKβΔmye lung recipients had evidence of higher NF-κB dependent inflammatory mediator expression, enhanced accumulated neutrophils in graft tissue and a comparable loss of lung function. As we have previously observed the rapid replacement of donor hematopoietic cells with recipient hematopoietic cells within lung grafts (19, 36) these data taken collectively suggest that regulation of IKKβ activity just within the graft-infiltrating myeloid cells is a major determinant of ischemia-reperfusion mediated-lung graft injury.
Pro-inflammatory and chemotactic mediators released by neutrophils facilitate rapid clearance of respiratory pathogens and potentiate lung alloimmune responses. However, signals promoting granulocyte influx and accumulation in the lung need to be tightly regulated to limit collateral tissue damage and minimize lung injury (20, 21). We show that neutrophilic alveolar infiltration, a pathologic hallmark of acute lung injury, increases in amplitude when IKKβ activity is pharmacologically disrupted or when IKKβ is deleted from myeloid cells in the setting of ischemia-reperfusion injury. This exaggerated inflammatory response was associated with elevated intragraft levels of canonical NF-κB dependent chemokines KC and MIP-1α, which promote myeloid cell chemotaxis and G-CSF and IL-6, which promote granulocyte production. The functional significance of this pattern of expression of pro-inflammatory mediators was demonstrated by the selective depletion of neutrophils, which led to significantly improved lung function in IKKβΔmye → IKKβΔmye mice. Taken together, our findings support an intrinsic anti-inflammatory role for IKKβ activity that is important for limiting acute lung graft inflammation. Recognition of the complications of targeting IKKβ over prolonged periods of time should be taken inconsideration when developing therapies to treat acute lung injury.
Methods
Mice
C57BL/6 (B6) mice were purchased from Jackson Laboratories. IKKβfl/fl mice and C57BL/6LysMCre mice, both on C57BL/6 background, were a generous gift from M. Karin (University of California San Diego). All mice were maintained in the facilities of Washington University School of Medicine in accordance with institutional guidelines.
Mouse lung transplantation
All mouse lung transplant protocols were approved by the Washington University School of Medicine Animal Studies Committee. Left orthotopic lung transplants were performed as previously described (18, 19).
Evans Blue Dye (EBD) exclusion and lung function
EBD was administered intravenously 4 hours prior to sacrifice, at which point lung grafts were excised and flushed with 20 mL of PBS. To extract EBD, lung tissue was homogenized in 5 mL of formamide. The homogenate was incubated at 37°C for 24 hours and centrifuged at 3500 g for 30 min. The optical density of the supernatant was measured at 620 nm and expressed as milligrams of EBD per gram of wet lung weight. To assess graft function arterial blood gases were measured using an iSTAT Portable Clinical Analyzer (iSTAT) at a FiO2 of 1.0 after the right pulmonary hilum was clamped for 5 minutes.
IKKβ assay
All reagents used in the assay were from Cell Signaling unless otherwise specified. Lung tissue was homogenized in 1x lysis buffer supplemented with 1mM PMSF, 1mM NaF & 10 ng Aprotinin and then sonicated for 5 sec pulses four times. Lysates were clarified by microcentrifugation, incubated with IKKβ specific antibodies overnight and immunoprecipitated with Protein G agarose beads (Amersham). Beads were equilibrated in Kinase buffer, incubated with 1.5mM IκBα (Ser32) Biotinylated Peptide and 20mM ATP for 30 min at 25 °C and transferred to streptavidin-coated plates. Phosphorylated IκBα was detected with Phospho-specific IκBα (Ser32/36-clone 5A5) antibodies and quantified using a DELFIA Detection Kit (Perkin Elmer) and Europium labeled anti-mouse IgG antibodies (Perkin Elmer) in accordance with manufacturer recommendations.
TUNEL Assay
Lung grafts were perfused with 20 mL isotonic sodium chloride solution and 20 mL HistoChoice (Amresco Inc, Solon, OH). Specimens were fixed, cut, mounted, de-paraffinized, and then steam-treated with Dako target retrieval solution (Dako, Carpinteria, CA) and quenched with 3% hydrogen peroxide. Assessment of lung cell apoptosis was performed with a TUNEL kit (Promega, Madison, WI) in accordance with manufacturer’s instructions.
Multiplex ELISA
Approximately 10 mg graft tissue specimens were flash frozen in liquid nitrogen, homogenized in 1.0 ml of T-PER reagent (Pierce, Rockford, IL) and clarified by centrifugation at 10,000 rpm for 5 minutes. Lysates were then normalized for protein concentration using a colorimetric BCA Protein Assay kit (Pierce, Rockford, IL). 50 μl aliquots of lysate were analyzed for cytokine and chemokine levels with Bioplex mouse cytokine bead suspension array kits (Bio-Rad Laboratories, Hercules, CA.) and a Bioplex array reader (Luminex Corp., Austin, TX) in accordance with manufacturer’s recommendations. Data was acquired and processed with Bio-Plex Manager Software, version 5.0.
NBD peptides and treatment
NBD peptide is a fusion peptide (DRQIKIWFQNRRMKWKKTALDWSWLQTE) comprised of a N-terminal cell-permeable Antennapedia leader peptide (italicized sequence) and C-terminal peptide analog of the NEMO-Binding Domain (underlined sequence). The NBD-C control peptide, DRQIKIWFQNRRMKWKKTALDASALQTE, has the identical Antennapedia leader peptide but is fused to a C-terminal NEMO-Binding Domain that has been mutated with two W → A substitutions (in bold lettering). These W → A mutations in the NEMO-Binding Domain have been shown to prevent association with IKKβ but do not affect the TNF-α induced phosphorylation of c-Jun or DNA binding of Oct-1 (14). NBD and NBD-C peptides were custom synthesized by Anaspec Inc. to an equal or greater than 95% purity and were unreactive to the Limulus assay. NBD and NBD-C was reconstituted in DMSO and given intravenously to lung graft recipients 5 minutes prior to transplantation at a 25 μg/kg (NBD-C), 2.5 μg/kg (NBDlow) or 25 μg/kg (NBDhi) dose.
Real Time PCR
Lung tissue was disrupted using a rotor-stator homogenizer (Omni) and RNA was extracted using a RNeasy Kit (Qiagen) in accordance with manufacturers’ instructions. Quantitative real-time, reverse transcription polymerase (RT-PCR) was conducted on an ABI 7900 using TaqMan Gene Expression Assay system (Applied Biosystems) in accordance with manufacturer’s recommendations. Amplification of target sequences was conducted as follows: 50°C for 20 min and 95°C for 10 min, followed by 38–45 cycles of 95°C for 15 sec and 60°C for 1 min. All primers and MGB-probes were purchased as kits from Applied Biosystems and can be identified in the following manner: TNFα (Mm00443258_m1), IL-1β (Mm00434227_g1), IL-6 (Mm00446191_m1), G-CSF (Mm00438334_m1), KC (Mm00433859_m1), MIP-1α (Mm99999057_m1) and 18S rRNA (Hs03003631_g1).
Serum injury markers, granulocyte analysis and depletion
Serum markers of skeletal muscle (sCPK), liver (sALT) and kidney (sCr) injury were measured with an autoanalyzer (Antech Diagnositics, Memphis TN). Bronchoalveolar lavage (BAL) fluid and lung tissue digest were prepared as previously described (18). Lung cell isolates were analyzed by FACS analysis through staining with Gr1 (RB6-8C5), CD11b (M1/70) and neutrophil counts were conducted by multiplying the percent abundance of Gr1hi CD11bhi cells by the number total number of live cells isolated immediately following lung tissue digestion. Neutrophils were counted in the BAL with a HEMAVET analyzer (Drew Scientific). Neutrophils were depleted as previously described (37) with a 250 μg dose of Ly6G-specific antibodies (1A8; Bio-X-Cell) administered intravenously 2 hours prior to surgery.
Statistical Analysis
Unpaired two-tailed Student’s t-test was used to evaluate pairs of means for significant differences (α = 0.05). Statistical testing of multiple means for significance were made using one-way ANOVA/post-hoc Tukey’s multiple comparison test (α = 0.05), and two-way ANOVA/post-hoc Bonferroni test for single and multiple independent variables, respectively. Data was analyzed using GraphPad Prism, version 5.0b (GraphPad Software, Inc.).
Acknowledgments
This work is supported by grants from the National Institutes of Health, National Heart, Lung and Blood Institute R01 HL041281 and K12 HL089968, and research fellowship grants from the International Society for Heart and Lung Transplantation.
Abbreviations
- IKKβ
I-κB kinase β
- NBD
NF-κB Essential Modulator (NEMO) Binding Domain peptide
- NBD-C
NF-κB Essential Modulator (NEMO) Binding Domain Control peptide
- IKKβΔmye
mice with a IKKβ genetic deletion in myeloid cells
- IKKβfl/fl
control mice for IKKβΔmye mice, which retain IKKβ flox alleles
- EBD
Evans Blue Dye
References
- 1.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167 (4):490. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 2.Huang HJ, Yusen RD, Meyers BF, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8 (11):2454. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86 (1):189. doi: 10.1016/j.athoracsur.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009;6 (1):39. doi: 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]
- 5.Ross SD, Kron IL, Gangemi JJ, et al. Attenuation of lung reperfusion injury after transplantation using an inhibitor of nuclear factor-kappaB. Am J Physiol Lung Cell Mol Physiol. 2000;279 (3):L528. doi: 10.1152/ajplung.2000.279.3.L528. [DOI] [PubMed] [Google Scholar]
- 6.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395 (6699):297. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 7.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91 (2):243. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 8.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18 (49):6867. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 9.Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293 (5534):1495. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 10.Li ZW, Chu W, Hu Y, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189 (11):1839. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12 (1):85. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 12.Luo JL, Kamata H, Karin M. The anti-death machinery in IKK/NF-kappaB signaling. J Clin Immunol. 2005;25 (6):541. doi: 10.1007/s10875-005-8217-6. [DOI] [PubMed] [Google Scholar]
- 13.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3 (1):17. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 14.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289 (5484):1550. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 15.Tas SW, Vervoordeldonk MJ, Hajji N, May MJ, Ghosh S, Tak PP. Local treatment with the selective IkappaB kinase beta inhibitor NEMO-binding domain peptide ameliorates synovial inflammation. Arthritis Res Ther. 2006;8 (4):R86. doi: 10.1186/ar1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10 (6):617. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 17.Shibata W, Maeda S, Hikiba Y, et al. Cutting edge: The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179 (5):2681. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki M, Krupnick AS, Kornfeld CG, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7 (6):1672. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 19.Krupnick AS, Lin X, Li W, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4 (1):86. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121 (6):1069. doi: 10.1067/mtc.2001.113603. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Fernandez LG, Doctor A, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291 (5):L1018. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 22.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11 (2):191. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 23.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8 (4):265. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 24.Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170 (9):4630. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- 25.Yamane M, Liu M, Kaneda H, Uhlig S, Waddell TK, Keshavjee S. Reperfusion-induced gene expression profiles in rat lung transplantation. Am J Transplant. 2005;5 (9):2160. doi: 10.1111/j.1600-6143.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- 26.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446 (7135):552. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 27.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9 (5):575. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki M, Gelman AE, Tietjens JR, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007;37 (6):625. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274 (5288):787. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 30.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11 (11):1173. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 31.Broide DH, Lawrence T, Doherty T, et al. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci U S A. 2005;102 (49):17723. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279 (36):37219. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 33.Greten FR, Arkan MC, Bollrath J, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130 (5):918. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solt LA, Madge LA, Orange JS, May MJ. Interleukin-1-induced NF-kappaB activation is NEMO-dependent but does not require IKKbeta. J Biol Chem. 2007;282 (12):8724. doi: 10.1074/jbc.M609613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fong CH, Bebien M, Didierlaurent A, et al. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med. 2008;205 (6):1269. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreisel D, Richardson SB, Li W, et al. Cutting Edge: MHC Class II Expression by Pulmonary Nonhematopoietic Cells Plays a Critical Role in Controlling Local Inflammatory Responses. J Immunol. 185(7):3809. doi: 10.4049/jimmunol.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83 (1):64. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]