Abstract
Drug hypersensitivity syndromes such as abacavir hypersensitivity and the severe cutaneous adverse drug reactions (SCAR) have been associated with significant short and long-term morbidity and mortality. More recently these immunologically mediated and previously unpredictable diseases have been shown to be associated with primarily Class I and also Class II HLA alleles. The case of the association of HLA-B*57:01 and abacavir hypersensitivity has created a translational roadmap for how this knowledge can be utilized in the clinic to prevent severe reactions. Although many hurdles exist to the widespread translation of such HLA screening approaches, our understanding of how drugs interact with the MHC has contributed to the discovery of new models that have provided considerable insights into the immunopathogenesis of SCAR and other T-cell mediated drug hypersensitivity syndromes. Future translation of this knowledge will facilitate the development of pre-clinical toxicity screening to significantly improve efficacy and safety of drug development and design.
Keywords: DRESS/DIHS/HSS, SJS/TEN, abacavir, carbamazepine, HLA, viral reactivation
CASE STUDY
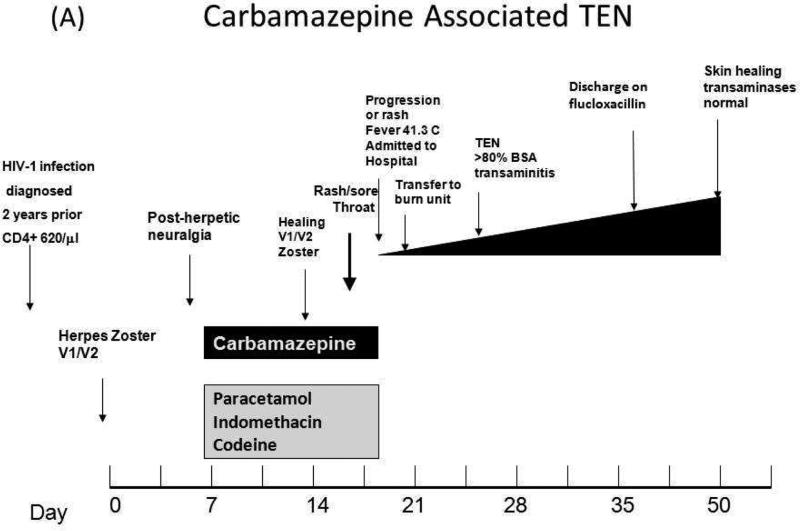
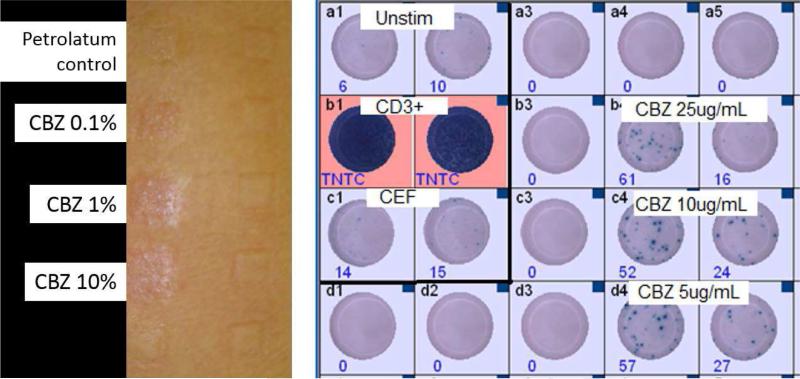
A 37 year old Thai woman with stable previously asymptomatic untreated HIV-1 disease (CD4+ count 640/μl) was diagnosed with multi-dermatomal Herpes Zoster infection of the trigeminal nerve (V1/V2) by her general practitioner. There was no clinical eye involvement and she was commenced on acyclovir 800 mg 5 ×/day for 7 days. The next day on follow-up with ophthamology she was not found to have eye involvement and was started on indomethacin, codeine and carbamazepine 200 mg bid p.o. for pain control. On day 7 following commencement of carbamazepine she was found to be well with evidence of healing Herpes Zoster infection. Prescription for carbamazepine 200 mg bid p.o., indomethacin suppositories 100 mg bid and paracetamol-codeine was repeated at this time. On day 10 following commencement of carbamazepine she was noted to have a generalized rash and complained of a sore throat. She was admitted to hospital on day 11 when the rash progressed and was associated with nausea, increasing throat soreness and a fever of 41.3 C and all drugs were ceased. Physical examination at this time showed lesions of the mouth and genitals and an extensive generalized rash with atypical target lesions and skin separation. On day 12 she was transferred to the burn unit with a diagnosis of probable carbamazepine-induced toxic epidermal necrolysis with body surface area involvement greater than 80%. There was no noted eye involvement. Laboratory tests also indicated impaired liver function: alkaline phosphatase was 298 U/L (40-135 U/L normal range) and ALT was 429 U/L (normal range 11-36 U/L). The rest of her hospitalization was uncomplicated and she was discharged on day 30 on flucloxacillin 500 mg qid p.o. She was given the advice to permanently avoid not only carbamazepine but all other aromatic amine anticonvulsants such as oxcarbazepine, phenytoin, phenobarbital and lamotrigine. Follow-up two weeks after discharge revealed her to have healing skin with normalization of liver function and cessation of trigeminal pain (Figure 1A). She remained clinically well from the standpoint of HIV and was started initially on zidovudine 250 mg bid, lamivudine 150 mg bid and indinavir 800 mg tid po 1.5 years later and then switched to Trizivir (zidovudine/lamivudine/abacavir) 3 years later because of concerns of fat redistribution. She remained clinically well and virologically suppressed (current HIV viral load < 40 copies/ml). Patch tests to 0.1, 1 and 10% carbamazepine in petrolatum and petrolatum negative control conducted 9.5 years following carbamazepine TEN when she was still virologically suppressed on Trizivir were strongly positive for all concentrations of carbamazepine and negative for petrolatum control (Figure 1B). The patient also had multiple positive INFγ ELISpot with PBMCs stimulated with carbamazepine with the last being over 17 years following the initial TEN diagnosis (Figure 1B). HLA typing was conducted revealing that the patient carried HLA-A*11:01, -B*15:02/58:01, -C*03:02/08;01 and HLA-DRB1*03:01/12:02.
Figure 1.
(A) Clincial timeline for the development of Carbamazepine associated TEN in the case study patient. (B) Negative petrolatum control and positive patch test for 0.1%-10% carbamazepine in the patient 9.5 years after the original TEN reaction (left) and positive PBMC INF-g Elispot for 5-10ug/mL carbamazepine for 100 000 cells/well for a sample taken 17 years after the clinical TEN reaction (right). Positive controls (CD3+ and CEF peptide pool) and unstimulated PBMCs are also shown.
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS (SCARs)
The immunologically mediated, “type B” ADRs are amongst the most dangerous off-target ADRs. Among the type B ADRs are a subset of reactions which can be characterized by severe cutaneous manifestations and are collectively referred to as severe cutaneous adverse reactions (SCAR). There are three phenotypically distinct SCARs (i) Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), (ii) acute generalized exanthematous pustulosis (AGEP) and (iii) Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome (DIHS) or hypersensitivity syndrome (HSS)1. Abacavir hypersensitivity syndrome (ABC HSR) is a distinct entity which does not clinically fit into any of these categories.
SJS and TEN are considered to be clinically and immunopathogenetically the same syndrome representing different severities across the spectrum. The level of skin detachment is used to demarcate the two syndromes. SJS is characterised by 1–10% detachment, there is 10-30% overlap and then TEN represents the most severe cases with >30% detachment. SJS/TEN can also be characterised by mucous membrane involvement and systemic symptoms including fever, liver chemistry elevations, intestinal and pulmonary manifestations, or the presence of lymphopenia. With prevalence of 2-6 cases / million per year, both syndromes are associated with high morbidity and mortality; 1-5% mortality for SJS and 30-50% for TEN. The most common drugs causing SJS/TEN are allopurinol, aromatic amine anticonvulsants (eg. carbamazepine, eslicarbazepine acetate, oxcarbazepine, fosphenytoin, phenytoin, phenobarbital, lamotrigine), antiretrovirals (particularly nevirapine), NSAIDS and sulfa antimicrobials).
AGEP is an acute febrile drug eruption characterized by numerous small, primarily nonfollicular, sterile pustules, arising within large areas of edematous erythema. In AGEP drug-specific T cells produce interleukin-8/CXCL8, leading to neutrophil recruitment resulting in acute widespread edematous erythema followed by a sterile pustular eruption. The onset of disease is typically rapid and often within 1-3 days of drug initiation. The condition is also characterised by fever and possible eosinophilia2, 3. Beta-lactam antibiotics, quinolones, hydroxycholoroquine, pristinamycin, sulfonamides, diltiazem, and terbinafine are all known to cause AGEP4. AGEP has been rarely associated with infections, non-drug antigens and viral reactivation3, 5-10. The prognosis is usually good, with resolution occurring within 15 days. Although an early report suggested a possible association between AGEP and HLA-B*51, it is currently uncertain as to whether AGEP is strictly HLA-restricted11.
DRESS/DIHS/HSS by definition is associated with fever, rash, eosinophilia and/or atypical lymphocytosis, cutaneous involvement and hepatitis typically occurring 2 or more weeks after first drug initiation. Although there have been many drugs described as causing DRESS/DIHS/HSS, drugs typically involved overlap considerably with those causing SJS/TEN and include antimicrobial sulphonamides, aromatic amine anticonvulsants, beta-lactam antibiotics, allopurinol, NSAIDs and antiretrovirals (nevirapine)1. The Japanese definition of DIHS also includes viral reactivation as a criteria for diagnosis and this tends to occur in the most severe cases with the most prevalent being reactivation of HHV-6 and other viruses in the Human Herpesvirus (HHV) family12, 13.
MANAGEMENT OF SCAR
The mainstay of treatment of SJS/TEN, ABC HSR and other SCAR is causality assessment and immediate withdrawal of the most likely implicated drug(s). Early and rigorous supportive care is crucial 14. For SJS/TEN this includes early ophthamology consultation and severe cases should be managed in an intensive care setting with dermatology consultation and care. Multidisciplinary care with ear nose and throat, gynecology and psychiatry may also be necessary for short and long-term complications of SJS/TEN. Current treatment remains controversial and there is really no good evidence that a specific treatment or combination of treatments has a benefit over rigorous supportive care at a specialized center. In small studies calcineurin inhibitors such as cyclosporine appear to have some benefit in halting disease progression and early reports have also suggested a possible benefit for tumor necrosis factor receptor antagonists and plasmaphoresis. Large controlled studies are lacking and there are no multicenter studies of a factorial design comparing different treatments. Although early studies suggested a potential benefit for intravenous immunoglobulin (IVIg) for SJS/TEN more recent studies and pooled analyses have not shown an effect on mortality. Long-term follow-up for SJS/TEN is necessary in view of the eye and mucous membrane complications. For DRESS/DIHS similar controversies apply and a study looking at IVIg was stopped prematurely because of adverse events including pulmonary embolism. Steroids are recommended for use in DRESS when there is severe internal organ involvement (ie renal, lung or ALT > 10 × ULN). AGEP is a self-limited disease with general recovery within 2 weeks of disease onset. Topical steroids have been successfully applied in both DRESS/DIHS and AGEP but without controlled data.
HLA IN SCAR
There are many known genetic associations between specific HLA alleles and drug hypersensitivity and SCARs, supporting an immunologically driven mechanism for the development of SJS/TEN and DRESS/DIHS/HSS (Table I). The best characterized examples are abacavir HSS and its association with HLA-B*57:01, carbamazepine SJS/TEN associated with HLA-B*15:02, as in the current patient, and allopurinol DRESS/DIHS/HSS and SJS/TEN associated with HLA-B*58:01. The prevalence of these reactions in a specific population correlates well with the prevalence of allele carriage (Figure 2). The antiretroviral drug nevirapine appears to be associated with different HLA allele and haplotype associations which are ethnicity and phenotype dependent15-22. ABC HSR now provides a roadmap for successful translation of laboratory based research into pharmacogenetics in the clinic (Figure 3).
Table I.
Pharmacogenomics of HLA associated drug hypersensitivity and related drug-induced syndromes
| Syndrome and Drug | Alleles | Populations | Year First Described | References |
|---|---|---|---|---|
| SJS/TEN (SCAR) | ||||
| Allopurinol | B*5801 | Han Chinese, Thai, European, Italian, Korean, Portuguese |
2005 | 92-100 |
| Carbamazepine | B*1502 | Han Chinese; Thai; Malaysian, Indian | 2004 | 38-40, 43, 44, 101-108 |
| B*1511 | Korean; Japanese | 2010 | 45, 109 | |
| HLA-B*1518, HLA-B*5901 and HLA-C*0704 | Japanese | 2010 | 110 | |
| A*3101 | Japanese; Northern European; Korean | 2011 | 45, 48, 49, 111 | |
| Oxcarbazepine | B*1502 | Han Chinese | 2010 | 112 |
| Lamotrigine | B*1502 – Positive | Han Chinese | 2010 | 108, 112 |
| B*1502 NO ASSOCIATION FOUND | Han Chinese | 2010 | 113, 114 | |
| Nevirapine | C*0401 | Malawian | 2013 | 22 |
| Phenytoin | B*1502; HLA-B*1301, Cw*0801 and DRB1*1602 | Han Chinese | 2007 | 101, 102, 108, 112 |
| Phenobarbital | B*51:01 | Japanese | 2013 | 115 |
| sulfamethoxazole | B*38 | European | 2008 | 94 |
| Methazolamide | B*59 B*5901, Cw*0102 alleles and B*5901-Cw*0102- A*2402 haplotype |
Japanese Korean and Japanese |
1997 2011 |
45 |
| Sulfonamides | A*29, B*12 and DR*7 | European | 1987 | 116 |
| Oxicam | B*73 | European | 2008 | 94 |
| A*2,B*12 | European | 1987 | 116 | |
| Strontium ranelate | Under investigation in post-marketing period | 2009 | 117, 118 | |
| Zonisamide | A*02:07 | Japanese | 2013 | 115 |
| DRESS-DIHS-HSS | ||||
| Abacavir | B*5701 | European, African | 2002 | 25, 26, 30 |
| Allopurinol | B*5801 (or B*58 haplotype) | Han Chinese, Korean, Japanese, Thai, European |
2005 | 92, 93, 95, 96, 98, 119-121 |
|
Nevirapine (Hepatitis) |
DRB1*01:01 (CD4+ >/=25%), DRB1*01:02, B*58:01 | Australian, European, South African | 2005 | 15-18 |
| Nevirapine (DRESS-DIHS) | Cw*8 or CW*8-B*14 Haplotype | Italian; Japanese | 2006 | 19, 20 |
| C*4 | Han Chinese | 2011 | 21 | |
| B*3505 | Asian, Black, white | 2011 | 17 | |
| 122 | ||||
| Carbamazepine | 8.1 AH (HLA A*0101 : Cw*0701 : B*0801 : DRB1*0301 : DQA1*0501 : DQB1*0201) |
Caucasians | 2006 | 123, 124 |
| A*3101 | Northern European; Japanese; Korean | 2011 | 45, 48, 49, 111 | |
| HLA-A11 and HLA-B51 (weak) | Japanese | 2011 | 111 | |
| Strontium ranelate | Under investigation in post-marketing period | 2009 | 118, 125 | |
| Dapsone | HLA-B*13:01 | Chinese patients treated for leprosy | 2013 | 126 |
| Delayed rash (non systemic) | ||||
| Efavirenz | DRB1*01 | French | 2008 | 127 |
| Nevirapine | DRB1*01 | French | 2008 | 127 |
| Cw*04 | African, Asian, European, Thai | 2009 | 17, 41 | |
| B*35:05; rs1576*G CCHCR1 status (GWAS) | Thai | 2009 | 128, 129 | |
| 122 | ||||
| Aminopenicllins | A*2, DR*52 | Italian | 1998 | 130 |
| Carbamazipine (or MPE) | A*3101 | Han Chinese, Northern European | 2006 | 39, 48 |
| Oxcarbazepine induced MPE | B*1502, B*3802 | Han Chinese | 2011, 2013 | 131, 132 |
| Drug Induced liver disease | ||||
| Amoxicillin-clavulanate; co-amoxiclav DILI | DRB1*1501; DRB1*07 protective; HLA-A*0201 and HLA-DQB1*0602 and rs3135388, a tag SNP of HLA-DRB1*1501 -DQB1*0602 |
European | 2009-2011 | 133-135 |
| B*1801 DRB1*0301-DQB1*0201 |
Spanish | 2013 | 116 | |
| Lumiracoxib | HLA-DRB1*1501-HLA-DQB1*0602-HLA- DRB5*0101-HLA-DQA1*0102 haplotype |
International, multicentre | 2010 | 136 |
| Ximelagatran | DRB1(*)07 and DQA1(*)02 | Swedish | 2008 | 137 |
| Diclofenac | ABCB11; C-24T; UGT2B7*2; IL-4 C-590-A | European | 2007 | 138-140 |
| Isoniazid | NAT2 slow acetylator; CYP2E1*5,*1B | European | 2009 | 139, 140 |
| Flucloxacillin | B*5701, HLA-DRB1*0107- DQB1*0103 | European | 2009 | 140, 141 |
| Lapatinib | DRB1*0701 -DQA2*0201-DQB1*0202/0202 | International, multicentre | 2011 | 142 |
| Ximelagatran | DRB1*07, DQA1*02 | European | 2008 | 137 |
| Fixed Drug Eruption | ||||
| Febrazone | B*22 | Italian | 1994 | 143, 144 |
| sulfamethoxazole | A*30-B*13-Cw*6 haploytpe | Turkish | 2001 | 145 |
| Agranuloytosis | ||||
| Clozapine | B*38, DR*4, DQw*3 | Jewish | 1990 | 146, 147 |
| (6672G>C) in HLA-DQB1 | North American | 2011 | 148 | |
| Cw7/B*18 or B*39 or B*44/DRB*5 | Caucasian | 2007 | 149 | |
| Levamisole | B*27 | South American | 1990 | 150 |
| Drug induced lupus erythematosis | ||||
| Hydralazine | DR*4 | European | 1980 | 151 |
|
Procainamide, Isonazid, Methyldopa, Quinidine |
DR*4 | Italian | 2009 | 152 |
| Other | ||||
| Aspirin (Uriticaria/angioedema) | DRB1*1302-DQB1*0609-DPB1*0201 haplotype | Korean | 2005 | 150 |
| Aspirin (Asthma) | DPB1*0301 | Korean | 2008 | 153 |
|
Gold sodium thiomalate (Mucocutaneaous reaction) |
DR*5 | Spanish | 1994 | 154 |
|
Gold sodium thiomalate (Proteinuria, thrombocytopenia or leakopenia) |
B*8, DR*3 | European | 1985 | 155 |
|
NSAIDS (Anaphylactoid and cutaneous reactions) |
DR*11 | Spanish | 1999 | 156 |
| D-penicillamine (myasthenia gravis) | DR*1 | Mixed Caucasian | 1983 | 157 |
| D-penicillamine (Proteinuria) | B*8, DR*3 | European | 1986 | 158 |
| Berylium (granulomatous lung disease) | HLA-DPB1 gene and DPR1 gene polymorphisms, DRB1*13 and DQB1*06 |
North American | 2003 | 159, 160 |
Figure 2.
Geographical distribution and frequency of the key drug HSR alleles associated with abacavir HSR, allopurinol DRESS/DIHS/HSS and SJS/TEN and carbamazepine SJS/TEN HLA-B*57:01, HLA-B*58:01 and HLA-B*1502, respectively. Red = HLA-B*57:01 frequency, Blue = HLA-B*58:01 frequency and Green = HLA-B*15:02 frequency.
Figure 3. The Abacavir-HLA-B*5701 Clinical Roadmap.
The abacavir-HLA-B*5701 example illustrates the necessary steps required to move from identification of the HSR and risk alleles to implementation of clinical screening prior to administration of the drug.
Abacavir
Abacavir (ABC) is a guanosine analogue associated with a hypersensitivity syndrome in the pre-marketing phase of its development characterized predominantly by fever, malaise, gastrointestinal symptoms in up to 8% of those starting treatment and mild-moderate rash was a late feature present in only 70%23. A strong association between the HLA class I allele, HLA-B*57:01, and ABC HSR was first reported in 200224-26. Later work, improved the clinical diagnosis of true immunologically mediated ABC HSR through the use of patch testing 27-30. Following this, a case-control study of black and white patients in the US, demonstrated that 100% of both white and black patch test-positive patients with a clinical history consistent with ABC HSR carried HLA-B*57:0130. It is now recommend by international guidelines that HLA-B*57:01 screening is carried out before the initiation of abacavir therapy31. Crucially the negative predictive value for this test is 100%. This means that individuals without the HLA-B*57:01 will not develop ABC HSR. Testing results in a reduction in the incidence of ABC HSR28, 30 and is cost-effective32, 33.
Due to its narrow HLA restriction and high positive predictive value of 55%, ABC HSR provided a unique model for the study of HLA-related drug hypersensitivity. The PPV refers to the number of HLA-B*57:01+ individuals who would develop HSR if given ABC therapy. Laboratory evidence has shown that ABC HSR is HLA-B*57:01 restricted and mediated by CD8+ T lymphocytes. Infiltrating CD8+ T cells are present within the skin of ABC HSR patients with rash34 and TNFs and INFγ are produced by ABC HSR patient PBMCs in vitro35, 36. In addition, CD8+ T cells from patients from ABC-naive patients carrying the HLA-B*57:01 allele proliferate in response to ABC in long term culture and are specifically activated by the drug. These T cells display a polyclonal response with the broad use of V beta receptors. This activation appears to be dependent upon peptide processing via the conventional MHC-I presentation pathway37.
Carbamazepine
Carbamazepine (CBZ) is a widely used anticonvulsant associated with SJS/TEN in individuals carrying HLA-B*15:0238, 39. Initial studies identified the association in the Han Chinese population and this has since been reproduced in individuals of Thai, Malaysian and Indian ethnicities40-44 and for other alleles on the B75 serotype 42, 43, 45. Like ABC, genetic testing for HLA-B*15:02 is readily available for routine clinical practice and has been associated with a positive predictive value of up to 3-7.7% for carbamazepine-induced SJS/TEN in the Han Chinese population 39 and is recommended by the FDA for individuals of East and Southeast Asian ethnicity. In a study of 4120 patients of Han Chinese background who tested negative for HLA-B*15:02 and subsequently received carbamazepine, none developed SJS/TEN 46. However, HLA-B*15:02 has not been found to be a risk factor for CBZ SJS/TEN in Caucasian populations where the carriage rate of this allele is <0.1% (Figure 2). The Taiwanese data as well as more recent European data suggests an association between HLA-A*31:01 and CBZ DRESS/DIHS. Some but not all studies in predominantly Caucasian populations have suggested an association between HLA-A*31:01 and CBZ associated SJS/TEN47-49.
Similar to abacavir HSR, CBZ-induced SJS/TEN has been shown to be mediated by CD8+ T cells. CBZ specific T cells have been isolated from SJS patients and exposure to drug activates granulysin release50. A dominant TCR V beta 11 clonotype VB-11-ISGSY has been identified in blister fluid and PBMCs in 84% of SJS/TEN patients, 14% of healthy controls and absent in CBZ tolerant controls. Furthermore, CBZ-dependent cytotoxicity can be blocked by anti-TCR-Vb-11 antibodies in these cells. Finally, both a VB-11-ISGSY clone and specific VB-11-ISGSY transfectants display cytotoxicity against HLA-B*1502 positive APCs in the presence of CBZ50. This study highlights the importance of both HLA type and TCR repertoire in CBZ induced SJS/TEN. However, it is important to note that the identified drug specific clonotypes were not present in all of the CBZ-SJS/TEN patients.
MODELS FOR THE IMMUNOPATHOGENESIS OF ABACAVIR HSR and CARBAMAZEPINE SJS/TEN
Several models have previously been suggested to explain the nature of immune activation during drug induced SCAR, including the hapten hypothesis where small molecule drugs are hypothesized to covalently bind to and modify self-proteins leading to immune recognition of a neoantigen51 and the pharmacological-interaction (p-i) concept which states that drugs can interact directly and non-covalently with the MHC and/or T-cell receptor inducing the formation of HLA:drug complexes which activate T-cell immune responses directly without requiring a specific peptide ligand52. Evidence now supports the altered peptide repertoire hypothesis, which in the case of ABC has now been verified by modelling and crystallography data showing that ABC binds non-covalently to the F anchor pocket of HLA-B*57:01, to alter the chemistry and shape of the antigen binding cleft53, 54. It has also been demonstrated that this binding alters the presented peptide repertoire with particular self-peptides presented only in the presence of ABC which are able to be recognized by T cells of hypersensitive patients53-55. The presentation of novel peptides therefore explains drug-induced hypersensitivity as the product of drug, HLA-type and the available T-cell repertoire that will respond to newly presented self-peptides (Figure E1 a video that shows this can be found in the online repository). Further insight into the interaction between CBZ and HLA-B*15:02 was provided by demonstrating that PBMCs from patients with CBZ SJS/TEN stimulated a specific population of CTL exhibiting cytotoxicity against B lymphoblastoid cell lines (B-LCLs) or keratinocyte transfectants expressing the HLA-B*15:02 allele. The effect could be blocked by anti-HLA-B antibodies. The study showed that endogenous peptide–loaded HLA-B*15:02 molecules presented CBZ to cytotoxic lymphocytes (CTLs) without the involvement of intracellular drug metabolism or antigen processing yet endogenous peptide binding was required to stabilize the HLA class I complex on the cell surface. Furthermore, the CBZ binding has been shown to be specific to members of the HLA-B75 serotype and modifications of the ring structure of CBZ altered HLA-B*15:02 binding and abrogated the CTL response. Finally, site directed mutagenesis has shown that the residues (Asn63, Ile95, and Leu156) in the peptide binding groove of HLA-B*15:02 are involved in CBZ presentation and CTL activation. In particular, Asn63 shared by members of the B75 family is the key residue. Supporting this, computational modelling shows that CBZ compounds are preferentially bound in the B pocket and consistently observed in the binding groove near Arg6256. An independent study also supports binding in this region and predicted that CBZ binds beneath the P4/P6 residues of the peptide, adjacent to position 15653. Similar to ABC it was demonstrated that the binding of CBZ to HLA-B*15:02 alters the repertoire of presented self-peptides53.
VIRAL REACTVATION IN DRESS/DIHS/HSS AND ADDITONAL IMMUNOPATHOGENETIC MODELS FOR SCAR
The reactivation of chronic persistent viruses in the HHV family including HHV-6/7, CMV and EBV has been described with most but not all drugs implicated in DRESS/DIHS/HSS (Table II). Viral reactivation has been an uncommon occurrence in other SCAR syndromes. The most prevalent HHV virus reported to reactivate has been HHV-657-63 and more recently HHV-7, EBV and/or CMV reactivation have also been observed in up to 76% of DRESS/DIHS/HSS patients62, 64, 65. Sequential reactivation of EBV, HHV-6 and CMV has been described to occur in some patients with DRESS/DIHS/HSS59, 60. When viral reactivation occurs it can be asymptomatic, cause recrudescence of DRESS/DIHS/HSS or cause organ specific viral disease, and this is highlighted by recurrent drug specific DRESS/DIHS/HSS and organ specific viral disease in cases where there has been inadvertent rechallenge of the implicated drug66. In addition there appears to be an EBV-driven expansion of CD8+ lymphocytes in many DRESS patients in both patients with or without EBV reactivation within the blood, skin, liver and lung. The DRESS/DIHS/HSS associated drugs CBZ, sulfamethoxazole and allopurinol have been shown to contribute to increased EBV production by EBV-transformed B lymphocytes from patients. It has been proposed that the general nature of this effect in DRESS patients may be due to inhibition of enzymes which promote EBV reactivation or via specific interactions of the drugs with enzymes regulating gene transcription but this is controversial62. Another complication of DRESS/DIHS/HSS is the development of autoimmune disease after resolution of the initial drug reaction and this has been reported in patients with prior HHV reactivation (Table III)67-70. Viruses such as EBV that can persist for the lifetime of the host and are re-activated during DRESS/DIHS/HSS and defective regulatory T cell function have also been proposed to be relevant to the pathogenesis of autoimmune diseases71. Alternatively, it has been suggested that another pre-existing predisposition to auto-immune disease may contribute to the development of a drug hypersensitivity syndrome63, 72.
Table II.
DRESS associated viral re-activation
| Drug | Viral reactivation | References |
|---|---|---|
| Carbamazepine | HHV-6, HHV-7, CMV, EBV | 13, 57-60, 62, 161, 162 |
| Phenobarbital, Phenobarbital /Zonisamide | HHV-6, HHV-7, CMV, EBV | 13, 59, 60 |
| Zonisamide | HHV-6, HHV-7, CMV | 13, 60 |
| Sulfasalazine/ salazosulfapyridine | HHV-6 | 13, 58 |
| Ibuprofen | HHV-6 | 58 |
| Aspirin | HHV-6 | 163 |
| Mexiletine | HHV-6, EBV, CMV | 13, 59 |
| Allopurinol | HSV-2, HHV-6, HHV-7, CMV, EBV | 13, 60, 62, 65, 164 |
| Amoxicillin | HHV-6 | 165 |
| Vancomycin/Teicoplanin | HHV-6 | 166 |
| Isoniazid, Rifampin, Ethambutol and Pyrazinamide | HHV-7 | 167 |
| Sulfamethoxazole | HHV-6, HHV-7, EBV | 62 |
Table III.
Reported DRESS associated autoimmune diseases
| Disease | References |
|---|---|
| Lupus erythematosus | 63, 67 |
| Autoimmune thyroiditis | 63, 168 |
| Graves’ disease | 72, 168 |
| Thrombotic thrombocytopenic purpure | 169 |
| Type 1 diabetes mellitus | 68, 70, 72 |
| Autoimmune hemolytic anemia | 72 |
| Autoimmune hepatitis | 170 |
| Juvenile idiopathic arthritis, Rheumatoid Arthritis (preceding DRESS) | 171-173 |
| Graft-versus-host disease | 69 |
| Kawasaki disease | 163 |
Several factors are necessary but not sufficient for the pathogenesis of ADRs as exemplified by ABC and CBZ. The HLA-allele-drug interaction as in the altered peptide model is a key factor but does not explain why some individuals with susceptibility alleles are free from adverse effects, in other words why only 55% of HLA-B*57:01 positive patients treated with ABC, and 3% of HLA-B*15:02 positive patients treated with CBZ develop HSR and SJS/TEN respectively. The CBZ example suggests the role of available TCR clonal types. The final requirement for a drug induced adverse reaction is the presence of a self-peptide that will bind to the drug-HLA complex and activate the appropriate T cell. Although viral reactivation appears to be a complication of DRESS/DIHS/HSS associated with many drugs, given the time course of this reactivation and the fact that multiple HHV have been shown to reactivate, it does not explain the immunopathogenesis or onset of DRESS/DIHS and its specific and varied clinical syndromes. Furthermore DRESS/DIHS/HSS appears relatively unique in its association with HHV reactivation. Substantial evidence supports a model of heterologous immunity mediated organ transplant rejection that is likely to apply to at least some drug hypersensitivity syndromes. In organ transplantation, pre-existing class I restricted effector memory T-cell responses to prevalent viral infections can mediate organ rejection73-78. It has been shown that allo-HLA reactivity of virus-specific memory T cells is common77, 79. Both naïve and memory CD4+ and CD8+ T cells frequently cross-react against allogeneic HLA molecules and this allo-recognition exhibits exquisite peptide and HLA specificity and is dependent on both public and private specificities of the T cell receptor80. Finally, allo-HLA cross-reactive responses show tissue specificity depending on presentation of tissue-specific self peptides27, 77, 78, 81-84. Similarly, the persistence of patch test reactivity in patients with previous ABC skin symptoms more than 9 years after the ADR and negative skin patch testing in ABC-naive individuals, despite ABC responsive cells in circulation, supports the presence of tissue specific resident memory cells homing to the skin as a result of a prior systemic reaction27, 85.
In keeping with this, HSV specific CD8+ effector memory T-cells may reside in the epidermis poised to kill keratinocytes presenting an HSV epitope in the context of the appropriate HLA molecule. In the setting of CBZ associated SJS/TEN for instance it could be proposed that altered peptide presentation by HLA-B*15:02 in the presence of CBZ may be cross recognised by tissue resident-memory CD8+ T-cells specific for viral peptides and mediate SJS/TEN86-88.
CONCLUSIONS
We report a case study of a HLA-B*15:02, CBZ induced SJS/TEN patient who has shown a durable drug-specific immune response 17 years after her initial reaction, as also observed in HLA-B*57:01 positive ABC HSR patients27, 85. Our case was also unusual in having a positive patch test 9.5 years after the original reaction as patch testing has been reported to be less than 30% sensitive in SJS/TEN. The less than 100% negative predictive value and <50% sensitivity of patch testing for most SCAR (abacavir hypersensitivity>DRESS/DIHS/AGEP>>SJS/TEN) mean that clinical diagnosis is still the gold standard that drives management. Similarly in terms of ex vivo assays ELISpot appears to be more sensitive than lymphocyte transformation tests but these are also adjunctive research tests not available to most centers and they lack 100% sensitivity/negative predictive value. Current evidence for the pathogenesis of HLA-mediated drug hypersensitivity, including ABC and CBZ supports a complex model of HLA-drug non-covalent interactions which result in an altered repertoire of self-peptides presented to the available T-cell population. Both the speed and tissue specificity of the immunological response also support the stimulation of pre-existing memory cell response. Furthermore, although Ag-specific T-cell responses are actively maintained, they are reversible and short lived in the absence of drug exposure to provide the stimulating antigen89-91. It is known that the patient is HSV-2, VZV, CMV and HIV positive and it is possible that a viral epitope(s) (or overlapping epitope(s)) may be responsible for the previous systemic immune response and subsequent maintenance of the CBZ-HLA-B*15:02-self peptide responsive memory T cells.
Although very different clinical phenotypes, there are significant immunopathogenetic parallels between ABC HSR and CBZ SJS/TEN suggesting that many SCARs may share common immunopathogenetic mechanisms. An increased understanding of structural and biochemical basis of how drugs interact with HLA molecules, the functional consequences and the pathogenesis of the incomplete positive predictive value and varying clinical phenotypes will provide a strategy for pre-clinical screening and approaches to improve the safety and cost-effectiveness of drug development. HLA screening to prevent life-threatening immunologically mediated drug reactions such as CBZ SJS/TEN and others can be useful and cost-effective measures to improve drug safety. This has been evidenced by marked decreases in the incidence of CBZ associated SJS/TEN in Taiwan, related to decreased off-label use of CBZ, but also due to the recommendation and government funding of HLA-B*15:02 screening prior to CBZ prescription. However, there are population specific considerations for many of these drugs and testing may not be available in all jurisdictions. There are also numerous hurdles that exist to clinical translation. The ABC “roadmap” for genetic screening to prevent ABC HSR, from discovery through to translation of a genetic test in routine clinical practice acts as a successful example that can be applied to the development of screening tests for other drugs to improve patient care (Figure 3).
Supplementary Material
Acknowledgments
Declaration of Funding
The author's work was supported by funding from 1R01AI103348 NIH/NIAID and the NHMRC
Abbreviations
- ADR
Adverse drug reactiony
- SCAR
Severe cutaneous adverse drug reaction
- SJS
Steven-Johnson syndrome
- TEN
Toxic epidermal necrolysis
- AGEP
Acute generalized exanthematous pustulosis
- HSS
Hypersensitivity syndrome
- HSR
Hypersensitivity reaction
- DRESS
Drug reaction with eosinophilia and systemic symptoms
- DIHS
Drug-induced hypersensitivity syndrome
- NSAIDS
Non-steroidal anti-inflammatory drugs
- ABC
Abacavir
- CBZ
Carbamazepine
- HLA
Human leukocyte antigen
- TCR
T cell receptor
- PBMC
Peripheral blood mononuclear cell
- INF-g
Interferon gamma
- HHV
Human Herpesvirus
- CMV
Cytomegalovirus (HHV-5)
- EBV
Epstein–Barr virus (HHV-4)
- HSV2
Herpes simplex virus (HHV-2)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Drs. Phillips and Mallal have equity in IIID Pty Ltd which has a patent for HLA-B*57:01 testing for abacavir hypersensitivity
References
- 1.Pirmohamed M, Aithal GP, Behr E, Daly A, Roden D. The phenotype standardization project: improving pharmacogenetic studies of serious adverse drug reactions. Clin Pharmacol Ther. 2011;89:784–5. doi: 10.1038/clpt.2011.30. [DOI] [PubMed] [Google Scholar]
- 2.Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013 doi: 10.1111/bjd.12502. [DOI] [PubMed] [Google Scholar]
- 3.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001;28:113–9. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 4.Speeckaert MM, Speeckaert R, Lambert J, Brochez L. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol. 2010;20:425–33. doi: 10.1684/ejd.2010.0932. [DOI] [PubMed] [Google Scholar]
- 5.Raison-Peyron N. “Cutaneous adverse drug reactions” are not always drug-induced. Eur J Dermatol. 2013;23:439–42. doi: 10.1684/ejd.2013.2055. [DOI] [PubMed] [Google Scholar]
- 6.Feio AB, Apetato M, Costa MM, Sa J, Alcantara J. [Acute generalized exanthematous pustulosis due to Coxsackie B4 virus]. Acta Med Port. 1997;10:487–91. [PubMed] [Google Scholar]
- 7.Haro-Gabaldon V, Sanchez-Sanchez-Vizcaino J, Ruiz-Avila P, Gutierrez-Fernandez J, Linares J, Naranjo-Sintes R. Acute generalized exanthematous pustulosis with cytomegalovirus infection. Int J Dermatol. 1996;35:735–7. doi: 10.1111/j.1365-4362.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 8.Klein N, Hartmann M, Helmbold P, Enk A. [Acute generalized exanthematous pustulosis associated with recurrent urinary tract infections]. Hautarzt. 2009;60:226–8. doi: 10.1007/s00105-008-1604-1. [DOI] [PubMed] [Google Scholar]
- 9.Manzano S, Guggisberg D, Hammann C, Laubscher B. [Acute generalized exanthematous pustulosis: first case associated with a Chlamydia pneumoniae infection]. Arch Pediatr. 2006;13:1230–2. doi: 10.1016/j.arcped.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Naides SJ. Rheumatic manifestations of parvovirus B19 infection. Rheum Dis Clin North Am. 1998;24:375–401. doi: 10.1016/s0889-857x(05)70014-4. [DOI] [PubMed] [Google Scholar]
- 11.Bernard P, Lizeveaux-Parneix V, Miossec V, Bonnetblanc J, Drouet M. HLA et prédisposition génétique dans les pustuloses exanthématiques aiguës généralisées (PEAG) et dans les exanthèmes maculo-papuleux (EMP). Ann Dermatol Venereol. 1995;122:S38–S9. [Google Scholar]
- 12.Tohyama M, Hashimoto K. New aspects of drug-induced hypersensitivity syndrome. J Dermatol. 2011;38:222–8. doi: 10.1111/j.1346-8138.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 13.Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 14.Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–306. doi: 10.2217/pgs.12.108. [DOI] [PubMed] [Google Scholar]
- 15.Martin AM, Nolan D, James I, Cameron P, Keller J, Moore C, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–9. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 16.Phillips E BJ, Sanne I, Lederman M, Hinkle J, Rousseau F, James I, Mallal S. Associations between HLA-DRBA*0102, HLA-B*5801 and hepatotoxicity in patients who initiated nevirapine containing regimens in South Africa.. 18th Conference on retroviruses and opportunistic infections. [Conference proceeding].; Boston, MA, USA. Feb 27-March 1; 2011. Paper#949. [Google Scholar]
- 17.Yuan J, Guo S, Hall D, Cammett AM, Jayadev S, Distel M, et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011 doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips E, Bartlett JA, Sanne I, Lederman MM, Hinkle J, Rousseau F, et al. Associations between HLA-DRB1*0102, HLA-B*5801, and hepatotoxicity during initiation of nevirapine-containing regimens in South Africa. J Acquir Immune Defic Syndr. 2013;62:e55–7. doi: 10.1097/QAI.0b013e31827ca50f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littera R, Carcassi C, Masala A, Piano P, Serra P, Ortu F, et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621–6. doi: 10.1097/01.aids.0000238408.82947.09. [DOI] [PubMed] [Google Scholar]
- 20.Gatanaga H, Yazaki H, Tanuma J, Honda M, Genka I, Teruya K, et al. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. 2007;21:264–5. doi: 10.1097/QAD.0b013e32801199d9. [DOI] [PubMed] [Google Scholar]
- 21.Gao S, Gui XE, Liang K, Liu Z, Hu J, Dong B. HLA-Dependent Hypersensitivity Reaction to Nevirapine in Chinese Han HIV-Infected Patients. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/AID.2011.0107. [DOI] [PubMed] [Google Scholar]
- 22.Carr DF, Chaponda M, Jorgensen AL, Castro EC, van Oosterhout JJ, Khoo SH, et al. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin Infect Dis. 2013;56:1330–9. doi: 10.1093/cid/cit021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutrell AG, Hernandez JE, Fleming JW, Edwards MT, Moore MA, Brothers CH, et al. Updated clinical risk factor analysis of suspected hypersensitivity reactions to abacavir. Ann Pharmacother. 2004;38:2171–2. doi: 10.1345/aph.1E202. [DOI] [PubMed] [Google Scholar]
- 24.Symonds W, Cutrell A, Edwards M, Steel H, Spreen B, Powell G, et al. Risk factor analysis of hypersensitivity reactions to abacavir. Clin Ther. 2002;24:565–73. doi: 10.1016/s0149-2918(02)85132-3. [DOI] [PubMed] [Google Scholar]
- 25.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–2. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 26.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–32. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 27.Phillips EJ, Wong GA, Kaul R, Shahabi K, Nolan DA, Knowles SR, et al. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–81. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- 28.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 29.Phillips E SS, Arribas J, Fitch N, Givens N. Characteristics of abacavir hypersensitivity diagnoses according to HLA-B*5701 status and subsequent abacavir patch test result.. 11th European AIDS Conference Madrid, [Conference proceedings].; Spain. 2007.p. P9.p. 7/04. [Google Scholar]
- 30.Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, et al. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111–8. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- 31.Martin MA, Klein TE, Dong BJ, Pirmohamed M, Haas DW, Kroetz DL. Clinical pharmacogenetics implementation consortium guidelines for hla-B genotype and abacavir dosing. Clin Pharmacol Ther. 2012;91:734–8. doi: 10.1038/clpt.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes DA, Vilar FJ, Ward CC, Alfirevic A, Park BK, Pirmohamed M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics. 2004;14:335–42. doi: 10.1097/00008571-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Schackman BR, Scott CA, Walensky RP, Losina E, Freedberg KA, Sax PE. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy for HIV. AIDS. 2008;22:2025–33. doi: 10.1097/QAD.0b013e3283103ce6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–5. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 35.Almeida CA, Martin AM, Nolan D, Lucas A, Cameron PU, James I, et al. Cytokine profiling in abacavir hypersensitivity patients. Antivir Ther. 2008;13:281–8. [PubMed] [Google Scholar]
- 36.Martin AM, Almeida CA, Cameron P, Purcell AW, Nolan D, James I, et al. Immune responses to abacavir in antigen-presenting cells from hypersensitive patients. AIDS. 2007;21:1233–44. doi: 10.1097/QAD.0b013e3280119579. [DOI] [PubMed] [Google Scholar]
- 37.Chessman D, Kostenko L, Lethborg T, Purcell AW, Williamson NA, Chen Z, et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity. 2008;28:822–32. doi: 10.1016/j.immuni.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 39.Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- 40.Kulkantrakorn K, Tassaneeyakul W, Tiamkao S, Jantararoungtong T, Prabmechai N, Vannaprasaht S, et al. HLA-B*1502 Strongly Predicts Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Thai Patients with Neuropathic Pain. Pain Pract. 2011 doi: 10.1111/j.1533-2500.2011.00479.x. [DOI] [PubMed] [Google Scholar]
- 41.Likanonsakul S, Rattanatham T, Feangvad S, Uttayamakul S, Prasithsirikul W, Tunthanathip P, et al. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res Ther. 2009;6:22. doi: 10.1186/1742-6405-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB, et al. Association of HLAB*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol. 2009;75:579–82. doi: 10.4103/0378-6323.57718. [DOI] [PubMed] [Google Scholar]
- 43.Tassaneeyakul W, Tiamkao S, Jantararoungtong T, Chen P, Lin SY, Chen WH, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51:926–30. doi: 10.1111/j.1528-1167.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 44.Then SM, Rani ZZ, Raymond AA, Ratnaningrum S, Jamal R. Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pac J Allergy Immunol. 2011;29:290–3. [PubMed] [Google Scholar]
- 45.Kim SH, Lee KW, Song WJ, Jee YK, Lee SM, Kang HR, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97:190–7. doi: 10.1016/j.eplepsyres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364:1126–33. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 47.Amstutz U, Ross CJ, Castro-Pastrana LI, Rieder MJ, Shear NH, Hayden MR, et al. HLA-A 31:01 and HLA-B 15:02 as genetic markers for carbamazepine hypersensitivity in children. Clin Pharmacol Ther. 2013;94:142–9. doi: 10.1038/clpt.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D, Carrington M, et al. HLAA*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20:1034–41. doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- 50.Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128:1266–76. e11. doi: 10.1016/j.jaci.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Park BK, Naisbitt DJ, Gordon SF, Kitteringham NR, Pirmohamed M. Metabolic activation in drug allergies. Toxicology. 2001;158:11–23. doi: 10.1016/s0300-483x(00)00397-8. [DOI] [PubMed] [Google Scholar]
- 52.Pichler WJ, Beeler A, Keller M, Lerch M, Posadas S, Schmid D, et al. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55:17–25. doi: 10.2332/allergolint.55.17. [DOI] [PubMed] [Google Scholar]
- 53.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. doi: 10.1038/nature11147. [10.1038/nature11147]. 2012;advance online publication. [DOI] [PubMed] [Google Scholar]
- 54.Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norcross MA, Luo S, Lu L, Boyne MT, Gomartelli M, Rennels AD, et al. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. 2012 doi: 10.1097/QAD.0b013e328355fe8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2011.12.990. [DOI] [PubMed] [Google Scholar]
- 57.Kano Y, Inaoka M, Shiohara T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia. Arch Dermatol. 2004;140:183–8. doi: 10.1001/archderm.140.2.183. [DOI] [PubMed] [Google Scholar]
- 58.Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–4. [PubMed] [Google Scholar]
- 59.Kano Y, Hiraharas K, Sakuma K, Shiohara T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. 2006;155:301–6. doi: 10.1111/j.1365-2133.2006.07238.x. [DOI] [PubMed] [Google Scholar]
- 60.Seishima M, Yamanaka S, Fujisawa T, Tohyama M, Hashimoto K. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–9. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- 61.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 62.Picard D, Janela B, Descamps V, D'Incan M, Courville P, Jacquot S, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46ra62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 63.Ushigome Y, Kano Y, Ishida T, Hirahara K, Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721–8. doi: 10.1016/j.jaad.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 64.Aihara M, Sugita Y, Takahashi S, Nagatani T, Arata S, Takeuchi K, et al. Anticonvulsant hypersensitivity syndrome associated with reactivation of cytomegalovirus. Br J Dermatol. 2001;144:1231–4. doi: 10.1046/j.1365-2133.2001.04239.x. [DOI] [PubMed] [Google Scholar]
- 65.Descamps V, Mahe E, Houhou N, Abramowitz L, Rozenberg F, Ranger-Rogez S, et al. Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol. 2003;148:1032–4. doi: 10.1046/j.1365-2133.2003.05330.x. [DOI] [PubMed] [Google Scholar]
- 66.Harding DJ, Subramaniam K, MacQuillan G, Davis J, Nolan D. Severe drug-induced hypersensitivity syndrome with a shared HLA-B allele. Med J Aust. 2012;197:411–3. doi: 10.5694/mja12.10477. [DOI] [PubMed] [Google Scholar]
- 67.Aota N, Hirahara K, Kano Y, Fukuoka T, Yamada A, Shiohara T. Systemic lupus erythematosus presenting with Kikuchi-Fujimoto's disease as a long-term sequela of drug-induced hypersensitivity syndrome. A possible role of Epstein-Barr virus reactivation. Dermatology. 2009;218:275–7. doi: 10.1159/000187619. [DOI] [PubMed] [Google Scholar]
- 68.Chiou CC, Chung WH, Hung SI, Yang LC, Hong HS. Fulminant type 1 diabetes mellitus caused by drug hypersensitivity syndrome with human herpesvirus 6 infection. J Am Acad Dermatol. 2006;54:S14–7. doi: 10.1016/j.jaad.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 69.Kano Y, Sakuma K, Shiohara T. Sclerodermoid graft-versus-host disease-like lesions occurring after drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;156:1061–3. doi: 10.1111/j.1365-2133.2007.07784.x. [DOI] [PubMed] [Google Scholar]
- 70.Sekine N, Motokura T, Oki T, Umeda Y, Sasaki N, Hayashi M, et al. Rapid loss of insulin secretion in a patient with fulminant type 1 diabetes mellitus and carbamazepine hypersensitivity syndrome. JAMA. 2001;285:1153–4. doi: 10.1001/jama.285.9.1153. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182:8071–9. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 72.Chen YC, Chang CY, Cho YT, Chiu HC, Chu CY. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol. 2013;68:459–65. doi: 10.1016/j.jaad.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: Implications for graft-versus-host disease. Journal of Experimental Medicine. 1994;179:1155–61. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gamadia LE, Remmerswaal EB, Surachno S, Lardy NM, Wertheim-Van Dillen PM, Van Lier RAW, et al. Cross-reactivity of cytomegalovirus-specific CD8+ T cells to allo-major histocompatibility complex class I molecules. Transplantation. 2004;77:1879–85. doi: 10.1097/01.tp.0000131158.81346.64. [DOI] [PubMed] [Google Scholar]
- 75.Koelle DM, Chen HB, McClurkan CM, Petersdorf EW. Herpes simplex virus type 2-specific CD8 cytotoxic T lymphocyte cross-reactivity against prevalent HLA class I alleles. Blood. 2002;99:3844–7. doi: 10.1182/blood.v99.10.3844. [DOI] [PubMed] [Google Scholar]
- 76.Landais E, Morice A, Long HM, Haigh TA, Charreau B, Bonneville M, et al. EBV-specific CD4+ T cell clones exhibit vigorous allogeneic responses. Journal of Immunology. 2006;177:1427–33. doi: 10.4049/jimmunol.177.3.1427. [DOI] [PubMed] [Google Scholar]
- 77.Amir AL, D'Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–57. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 78.D'Orsogna LJ, Roelen DL. Doxiadis, II, Claas FH. Screening of viral specific T-cell lines for HLA alloreactivity prior to adoptive immunotherapy may prevent GvHD. Transpl Immunol. 2011;24:141. doi: 10.1016/j.trim.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Morice A, Charreau B, Neveu B, Brouard S, Soulillou JP, Bonneville M, et al. Cross-reactivity of herpesvirus-specific CD8 T cell lines toward allogeneic class I MHC molecules. PLoS One. 2010;5:e12120. doi: 10.1371/journal.pone.0012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amir AL, van der Steen DM, Hagedoorn RS, Kester MG, van Bergen CA, Drijfhout JW, et al. Allo-HLA-reactive T cells inducing graft-versus-host disease are single peptide specific. Blood. 2011;118:6733–42. doi: 10.1182/blood-2011-05-354787. [DOI] [PubMed] [Google Scholar]
- 81.Deckers JGM, Daha MR, Van Der Kooij SW, Van Der Woude FJ. Epithelial- and endothelial-cell specificity of renal graft infiltrating T cells. Clinical Transplantation. 1998;12:285–91. [PubMed] [Google Scholar]
- 82.Yard BA, Claas FHJ, Paape ME, Bruijn JA, Daha MR, Van Es LA, et al. Recognition of a tissue-specific polymorphism by graft infiltrating T-cell clones isolated from a renal allograft with acute rejection. Nephrology Dialysis Transplantation. 1994;9:805–10. [PubMed] [Google Scholar]
- 83.Deckers JGM, Boonstra JG, Van Der Kooij SW, Daha MR, Van Der Woude FJ. Tissue-specific characteristics of cytotoxic graft-infiltrating T cells during renal allograft rejection. Transplantation. 1997;64:178–81. doi: 10.1097/00007890-199707150-00034. [DOI] [PubMed] [Google Scholar]
- 84.D'Orsogna LJ, Roelen DL, van der Meer-Prins EM, van der Pol P, Franke-van Dijk ME, Eikmans M, et al. Tissue specificity of cross-reactive allogeneic responses by EBV EBNA3A-specific memory T cells. Transplantation. 2011;91:494–500. doi: 10.1097/TP.0b013e318207944c. [DOI] [PubMed] [Google Scholar]
- 85.Schnyder B, Adam J, Rauch A, Thurnheer MC, Pichler WJ. HLA-B*57:01(+) abacavir-naive individuals have specific T cells but no patch test reactivity. J Allergy Clin Immunol. 2013;132:756–8. doi: 10.1016/j.jaci.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, et al. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol. 2012;188:2173–8. doi: 10.4049/jimmunol.1102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lang A, Brien JD, Nikolich-Zugich J. Inflation and long-term maintenance of CD8 T cells responding to a latent herpesvirus depend upon establishment of latency and presence of viral antigens. J Immunol. 2009;183:8077–87. doi: 10.4049/jimmunol.0801117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lang A, Nikolich-Zugich J. Functional CD8 T cell memory responding to persistent latent infection is maintained for life. J Immunol. 2011;187:3759–68. doi: 10.4049/jimmunol.1100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allam A, Conze DB, Giardino Torchia ML, Munitic I, Yagita H, Sowell RT, et al. The CD8+ memory T-cell state of readiness is actively maintained and reversible. Blood. 2009;114:2121–30. doi: 10.1182/blood-2009-05-220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–34. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 91.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–74. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Genin E, Schumacher M, Roujeau JC, Naldi L, Liss Y, Kazma R, et al. Genome-wide association study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Europe. Orphanet J Rare Dis. 2011;6:52. doi: 10.1186/1750-1172-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–9. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 95.Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLAB*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet. 2011;12:118. doi: 10.1186/1471-2350-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–9. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 97.Cristallo AF, Schroeder J, Citterio A, Santori G, Ferrioli GM, Rossi U, et al. A study of HLA class I and class II 4-digit allele level in Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Immunogenet. 2011;38:303–9. doi: 10.1111/j.1744-313X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 98.Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21:303–7. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 99.Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, et al. Allopurinol Hypersensitivity: A Systematic Review of All Published Cases, 1950-2012. Drug Saf. 2013 doi: 10.1007/s40264-013-0084-0. [DOI] [PubMed] [Google Scholar]
- 100.Goncalo M, Coutinho I, Teixeira V, Gameiro AR, Brites MM, Nunes R, et al. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013;169:660–5. doi: 10.1111/bjd.12389. [DOI] [PubMed] [Google Scholar]
- 101.Locharernkul C, Loplumlert J, Limotai C, Korkij W, Desudchit T, Tongkobpetch S, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49:2087–91. doi: 10.1111/j.1528-1167.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 102.Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007;48:1015–8. doi: 10.1111/j.1528-1167.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q, Zhou JQ, Zhou LM, Chen ZY, Fang ZY, Chen SD, et al. Association between HLAB*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure. 2011;20:446–8. doi: 10.1016/j.seizure.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 104.Wu XT, Hu FY, An DM, Yan B, Jiang X, Kwan P, et al. Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsy Behav. 2010;19:405–8. doi: 10.1016/j.yebeh.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Wang J, Zhao LM, Peng W, Shen GQ, Xue L, et al. Strong association between HLAB*1502 and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol. 2011;67:885–7. doi: 10.1007/s00228-011-1009-4. [DOI] [PubMed] [Google Scholar]
- 106.Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol. 2011;50:221–4. doi: 10.1111/j.1365-4632.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- 107.Tangamornsuksan W, Chaiyakunapruk N, Somkrua R, Lohitnavy M, Tassaneeyakul W. Relationship Between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2013;149:1025–32. doi: 10.1001/jamadermatol.2013.4114. [DOI] [PubMed] [Google Scholar]
- 108.Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MH, Kwan P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013;54:1307–14. doi: 10.1111/epi.12217. [DOI] [PubMed] [Google Scholar]
- 109.Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51:2461–5. doi: 10.1111/j.1528-1167.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 110.Ikeda H, Takahashi Y, Yamazaki E, Fujiwara T, Kaniwa N, Saito Y, et al. HLA class I markers in Japanese patients with carbamazepine-induced cutaneous adverse reactions. Epilepsia. 2010;51:297–300. doi: 10.1111/j.1528-1167.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 111.Niihara H, Kakamu T, Fujita Y, Kaneko S, Morita E. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. J Dermatol. 2011 doi: 10.1111/j.1346-8138.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- 112.Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11:349–56. doi: 10.2217/pgs.09.162. [DOI] [PubMed] [Google Scholar]
- 113.An DM, Wu XT, Hu FY, Yan B, Stefan H, Zhou D. Association study of lamotrigine-induced cutaneous adverse reactions and HLA-B*1502 in a Han Chinese population. Epilepsy Res. 2010;92:226–30. doi: 10.1016/j.eplepsyres.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Shi YW, Min FL, Liu XR, Zan LX, Gao MM, Yu MJ, et al. Hla-B alleles and lamotrigine-induced cutaneous adverse drug reactions in the Han Chinese population. Basic Clin Pharmacol Toxicol. 2011;109:42–6. doi: 10.1111/j.1742-7843.2011.00681.x. [DOI] [PubMed] [Google Scholar]
- 115.Kaniwa N, Sugiyama E, Saito Y, Kurose K, Maekawa K, Hasegawa R, et al. Specific HLA types are associated with antiepileptic drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese subjects. Pharmacogenomics. 2013;14:1821–31. doi: 10.2217/pgs.13.180. [DOI] [PubMed] [Google Scholar]
- 116.Stephens C, López-Nevot MÁ, Ruiz-Cabello F, Ulzurrun E, Soriano G, Romero-Gómez M, Moreno-Casares A, Lucena MI, Andrade RJ. HLA alleles influence the clinical signature of amoxicillin-clavulanate hepatotoxicity. PLoS One. 2013 Jul 9;8(7):e68111. doi: 10.1371/journal.pone.0068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HY, Lie D, Lim KS, Thirumoorthy T, Pang SM. Strontium ranelate-induced toxic epidermal necrolysis in a patient with post-menopausal osteoporosis. Osteoporos Int. 2009;20:161–2. doi: 10.1007/s00198-008-0677-0. [DOI] [PubMed] [Google Scholar]
- 118.Musette P, Brandi ML, Cacoub P, Kaufman JM, Rizzoli R, Reginster JY. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723–32. doi: 10.1007/s00198-009-1097-5. [DOI] [PubMed] [Google Scholar]
- 119.Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–22. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 120.Tohkin M, Kaniwa N, Saito Y, Sugiyama E, Kurose K, Nishikawa J, et al. A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.41. [DOI] [PubMed] [Google Scholar]
- 121.Chiu L, Hu M, Ng M, Yeung C, Chan J, Chang M, et al. Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol. 2012 doi: 10.1111/j.1365-2133.2012.10894.x. [DOI] [PubMed] [Google Scholar]
- 122.Phillips E, Lucas M, Keane N, Lucas A, McKinnon E, Mallal S. HLA-B*35 is associated with nevirapine hypersensitivity in the contemporary Western Australian HIV cohort study.. Eur Ann of Allerg and Clin Immunology; 4th International Drug Hypersensitivity Meeting. 22-25 April 2010 (oral presentation)..2010. p. 48. [Google Scholar]
- 123.Alfirevic A, Jorgensen AL, Williamson PR, Chadwick DW, Park BK, Pirmohamed M. HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics. 2006;7:813–8. doi: 10.2217/14622416.7.6.813. [DOI] [PubMed] [Google Scholar]
- 124.Pirmohamed M, Lin K, Chadwick D, Park BK. TNFalpha promoter region gene polymorphisms in carbamazepine-hypersensitive patients. Neurology. 2001;56:890–6. doi: 10.1212/wnl.56.7.890. [DOI] [PubMed] [Google Scholar]
- 125.Jonville-Bera AP, Crickx B, Aaron L, Hartingh I, Autret-Leca E. Strontium ranelate-induced DRESS syndrome: first two case reports. Allergy. 2009;64:658–9. doi: 10.1111/j.1398-9995.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- 126.Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med. 2013;369:1620–8. doi: 10.1056/NEJMoa1213096. [DOI] [PubMed] [Google Scholar]
- 127.Vitezica ZG, Milpied B, Lonjou C, Borot N, Ledger TN, Lefebvre A, et al. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–1. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- 128.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139–46. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 129.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, et al. Genome-wide association study identifies variations in 6p21.3 associated with nevirapine-induced rash. Clin Infect Dis. 2011;53:341–8. doi: 10.1093/cid/cir403. [DOI] [PubMed] [Google Scholar]
- 130.Romano A, De Santis A, Romito A, Di Fonso M, Venuti A, Gasbarrini GB, et al. Delayed hypersensitivity to aminopenicillins is related to major histocompatibility complex genes. Ann Allergy Asthma Immunol. 1998;80:433–7. doi: 10.1016/s1081-1206(10)62997-3. [DOI] [PubMed] [Google Scholar]
- 131.Hu FY, Wu XT, An DM, Yan B, Stefan H, Zhou D. Pilot association study of oxcarbazepine-induced mild cutaneous adverse reactions with HLA-B*1502 allele in Chinese Han population. Seizure. 2011;20:160–2. doi: 10.1016/j.seizure.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 132.Lv YD, Min FL, Liao WP, He N, Zeng T, Ma DH, et al. The association between oxcarbazepine-induced maculopapular eruption and HLA-B alleles in a northern Han Chinese population. BMC Neurol. 2013;13:75. doi: 10.1186/1471-2377-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hautekeete ML, Horsmans Y, Van Waeyenberge C, Demanet C, Henrion J, Verbist L, et al. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology. 1999;117:1181–6. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 134.Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–47. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O'Donohue J, Oien KA, Donaldson P, Underhill J, Clare M, MacSween RN, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–20. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singer JB, Lewitzky S, Leroy E, Yang F, Zhao X, Klickstein L, et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat Genet. 2010;42:711–4. doi: 10.1038/ng.632. [DOI] [PubMed] [Google Scholar]
- 137.Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenomics J. 2008;8:186–95. doi: 10.1038/sj.tpj.6500458. [DOI] [PubMed] [Google Scholar]
- 138.Daly AK, Aithal GP, Leathart JB, Swainsbury RA, Dang TS, Day CP. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology. 2007;132:272–81. doi: 10.1053/j.gastro.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 139.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Semin Liver Dis. 2009;29:400–11. doi: 10.1055/s-0029-1240009. [DOI] [PubMed] [Google Scholar]
- 140.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Drug Metab Rev. 2012;44:116–26. doi: 10.3109/03602532.2011.605790. [DOI] [PubMed] [Google Scholar]
- 141.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–9. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 142.Spraggs CF, Budde LR, Briley LP, Bing N, Cox CJ, King KS, et al. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J Clin Oncol. 2011;29:667–73. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- 143.Pellicano R, Ciavarella G, Lomuto M, Di Giorgio G. Genetic susceptibility to fixed drug eruption: evidence for a link with HLA-B22. J Am Acad Dermatol. 1994;30:52–4. doi: 10.1016/s0190-9622(94)70007-9. [DOI] [PubMed] [Google Scholar]
- 144.Pellicano R, Lomuto M, Ciavarella G, Di Giorgio G, Gasparini P. Fixed drug eruptions with feprazone are linked to HLA-B22. J Am Acad Dermatol. 1997;36:782–4. doi: 10.1016/s0190-9622(97)80347-7. [DOI] [PubMed] [Google Scholar]
- 145.Ozkaya-Bayazit E, Akar U. Fixed drug eruption induced by trimethoprim-sulfamethoxazole: evidence for a link to HLA-A30 B13 Cw6 haplotype. J Am Acad Dermatol. 2001;45:712–7. doi: 10.1067/mjd.2001.117854. [DOI] [PubMed] [Google Scholar]
- 146.Lieberman JA, Yunis J, Egea E, Canoso RT, Kane JM, Yunis EJ. HLA-B38, DR4, DQw3 and clozapine-induced agranulocytosis in Jewish patients with schizophrenia. Arch Gen Psychiatry. 1990;47:945–8. doi: 10.1001/archpsyc.1990.01810220061007. [DOI] [PubMed] [Google Scholar]
- 147.Valevski A, Klein T, Gazit E, Meged S, Stein D, Elizur A, et al. HLA-B38 and clozapine-induced agranulocytosis in Israeli Jewish schizophrenic patients. Eur J Immunogenet. 1998;25:11–3. doi: 10.1046/j.1365-2370.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 148.Athanasiou MC, Dettling M, Cascorbi I, Mosyagin I, Salisbury BA, Pierz KA, et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72:458–63. doi: 10.4088/JCP.09m05527yel. [DOI] [PubMed] [Google Scholar]
- 149.Dettling M, Cascorbi I, Opgen-Rhein C, Schaub R. Clozapine-induced agranulocytosis in schizophrenic Caucasians: confirming clues for associations with human leukocyte class I and II antigens. Pharmacogenomics J. 2007;7:325–32. doi: 10.1038/sj.tpj.6500423. [DOI] [PubMed] [Google Scholar]
- 150.Diez RA. HLA-B27 and agranulocytosis by levamisole. Immunol Today. 1990;11:270. doi: 10.1016/0167-5699(90)90109-m. [DOI] [PubMed] [Google Scholar]
- 151.Batchelor JR, Welsh KI, Tinoco RM, Dollery CT, Hughes GR, Bernstein R, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet. 1980;1:1107–9. doi: 10.1016/s0140-6736(80)91554-8. [DOI] [PubMed] [Google Scholar]
- 152.Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99–105. doi: 10.1007/s00403-008-0895-5. [DOI] [PubMed] [Google Scholar]
- 153.Kim SH, Hur GY, Choi JH, Park HS. Pharmacogenetics of aspirin-intolerant asthma. Pharmacogenomics. 2008;9:85–91. doi: 10.2217/14622416.9.1.85. [DOI] [PubMed] [Google Scholar]
- 154.Rodriguez-Perez M, Gonzalez-Dominguez J, Mataran L, Garcia-Perez S, Salvatierra D. Association of HLA-DR5 with mucocutaneous lesions in patients with rheumatoid arthritis receiving gold sodium thiomalate. J Rheumatol. 1994;21:41–3. [PubMed] [Google Scholar]
- 155.Speerstra F, Reekers P, van de Putte LB, Vandenbroucke JP. HLA associations in aurothioglucose- and D-penicillamine-induced haematotoxic reactions in rheumatoid arthritis. Tissue Antigens. 1985;26:35–40. doi: 10.1111/j.1399-0039.1985.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 156.Quiralte J, Sanchez-Garcia F, Torres MJ, Blanco C, Castillo R, Ortega N, et al. Association of HLA-DR11 with the anaphylactoid reaction caused by nonsteroidal anti-inflammatory drugs. J Allergy Clin Immunol. 1999;103:685–9. doi: 10.1016/s0091-6749(99)70243-5. [DOI] [PubMed] [Google Scholar]
- 157.Garlepp MI, Dawkins RL, Christiansen FT. HLA antigens and acetylcholine receptor antibodies in penicillamine induced myasthenia gravis. Br Med J (Clin Res Ed) 1983;286:1442–3. doi: 10.1136/bmj.286.6375.1442-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pachoula-Papasteriades C, Boki K, Varla-Leftherioti M, Kappos-Rigatou I, Fostiropoulos G, Economidou J. HLA-A,-B, and -DR antigens in relation to gold and D-penicillamine toxicity in Greek patients with RA. Dis Markers. 1986;4:35–41. [PubMed] [Google Scholar]
- 159.Maier LA, McGrath DS, Sato H, Lympany P, Welsh K, Du Bois R, et al. Influence of MHC class II in susceptibility to beryllium sensitization and chronic beryllium disease. J Immunol. 2003;171:6910–8. doi: 10.4049/jimmunol.171.12.6910. [DOI] [PubMed] [Google Scholar]
- 160.Rosenman KD, Rossman M, Hertzberg V, Reilly MJ, Rice C, Kanterakis E, et al. HLA class II DPB1 and DRB1 polymorphisms associated with genetic susceptibility to beryllium toxicity. Occup Environ Med. 2011;68:487–93. doi: 10.1136/oem.2010.055046. [DOI] [PubMed] [Google Scholar]
- 161.Morito H, Kitamura K, Fukumoto T, Kobayashi N, Kuwahara M, Asada H. Drug eruption with eosinophilia and systemic syndrome associated with reactivation of human herpesvirus 7, not human herpesvirus 6. J Dermatol. 2012;39:669–70. doi: 10.1111/j.1346-8138.2011.01365.x. [DOI] [PubMed] [Google Scholar]
- 162.Descamps V, Bouscarat F, Laglenne S, Aslangul E, Veber B, Descamps D, et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br J Dermatol. 1997;137:605–8. doi: 10.1111/j.1365-2133.1997.tb03795.x. [DOI] [PubMed] [Google Scholar]
- 163.Kawakami T, Fujita A, Takeuchi S, Muto S, Soma Y. Drug-induced hypersensitivity syndrome: drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome induced by aspirin treatment of Kawasaki disease. J Am Acad Dermatol. 2009;60:146–9. doi: 10.1016/j.jaad.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 164.Hamaguchi Y, Fujimoto M, Enokido Y, Wayaku T, Kaji K, Echigo T, et al. Intractable genital ulcers from herpes simplex virus reactivation in drug-induced hypersensitivity syndrome caused by allopurinol. Int J Dermatol. 2010;49:700–4. doi: 10.1111/j.1365-4632.2009.04441.x. [DOI] [PubMed] [Google Scholar]
- 165.Mardivirin L, Valeyrie-Allanore L, Branlant-Redon E, Beneton N, Jidar K, Barbaud A, et al. Amoxicillin-induced flare in patients with DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms): report of seven cases and demonstration of a direct effect of amoxicillin on Human Herpesvirus 6 replication in vitro. Eur J Dermatol. 2010;20:68–73. doi: 10.1684/ejd.2010.0821. [DOI] [PubMed] [Google Scholar]
- 166.Tamagawa-Mineoka R, Katoh N, Nara T, Nishimura Y, Yamamoto S, Kishimoto S. DRESS syndrome caused by teicoplanin and vancomycin, associated with reactivation of human herpesvirus-6. Int J Dermatol. 2007;46:654–5. doi: 10.1111/j.1365-4632.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 167.Draz N, Datta S, Webster DP, Cropley I. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome secondary to antituberculosis drugs and associated with human herpes virus-7 (HHV-7). BMJ Case Rep. 2013:2013. doi: 10.1136/bcr-2013-010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Funck-Brentano E, Duong T, Family D, Bouaziz JD, Ortonne N, Bagot M, et al. [Auto-immune thyroiditis and drug reaction with eosinophilia and systemic symptoms (DRESS) associated with HHV-6 viral reactivation]. Ann Dermatol Venereol. 2011;138:580–5. doi: 10.1016/j.annder.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 169.Sandouk Z, Alirhayim Z, Khoulani D, Hassan S. DRESS syndrome and thrombotic thrombocytopaenic purpura: are they related? BMJ Case Rep. 2012:2012. doi: 10.1136/bcr-2012-007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kinyo A, Belso N, Nagy N, Palvolgyi A, Nagy I, Korom I, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205–6. doi: 10.2340/00015555-1014. [DOI] [PubMed] [Google Scholar]
- 171.Bejia I, Ben Hammouda S, Riahi K, Zinelabidine F, Mediouni B, Touzi M, et al. DRESS syndrome induced by sulphasalazine in rheumatoid arthritis. Joint Bone Spine. 2006;73:764–5. doi: 10.1016/j.jbspin.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 172.Michel F, Navellou JC, Ferraud D, Toussirot E, Wendling D. DRESS syndrome in a patient on sulfasalazine for rheumatoid arthritis. Joint Bone Spine. 2005;72:82–5. doi: 10.1016/j.jbspin.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 173.Pinana E, Lei SH, Merino R, Melgosa M, De La Vega R, Gonzales-Obeso E, et al. DRESS-syndrome on sulfasalazine and naproxen treatment for juvenile idiopathic arthritis and reactivation of human herpevirus 6 in an 11-year-old Caucasian boy. J Clin Pharm Ther. 2010;35:365–70. doi: 10.1111/j.1365-2710.2009.01081.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.