Abstract
OBJECTIVE
To provide a comprehensive evaluation of chromium (Cr) supplementation on metabolic parameters in a cohort of Type 2 DM subjects representing a wide phenotype range and to evaluate changes in “responders” and “non-responders”.
DESIGN
After pre-intervention testing to assess glycemia, insulin sensitivity (assessed by euglycemic clamps), Cr status, body composition, subjects were randomized in a double-blind fashion to placebo or 1,000 μg Cr. A sub-study was performed to evaluate 24 hour energy balance/substrate oxidation and myocellular/intra-hepatic lipid content.
RESULTS
There was not a consistent effect of chromium supplementation to improve insulin action across all phenotypes. Insulin sensitivity was negatively correlated to soleus and tibialis muscle intramyocellular lipids and intra-hepatic lipid content. Myocellular lipids were significantly lower in subjects randomized to Cr. At pre-intervention, “responders”, defined as insulin sensitivity change from baseline > 10%, had significantly lower insulin sensitivity and higher fasting glucose and A1c when compared to placebo and “non-responders”, i.e. insulin sensitivity change from baseline < 10%. Clinical response was significantly correlated (p < 0.001) to the baseline insulin sensitivity, fasting glucose and A1c. There was no difference in Cr status between “responders”, and “non-responders”.
CONCLUSIONS
Clinical response to chromium is more likely in insulin resistant subjects who have more elevated fasting glucose and A1c levels. Cr may reduce myocellular lipids and enhance insulin sensitivity in subjects with type 2 DM independent of effects on weight or hepatic glucose production. Thus, modulation of lipid metabolism by Cr in peripheral tissues may represent a novel mechanism of action.
Keywords: Chromium, nutrition, glucose, trace minerals
INTRODUCTION
Although lifestyle modification combined with pharmacologic intervention is the primary strategy to meet glycemic targets in patients with type 2 DM, alternative strategies, e.g., nutritional supplementation with over-the-counter agents, continue to be extensively practiced by a large number of patients (1, 2). There are more than 29,000 nutritional supplements available and patients pay over 12 billion dollars per year on these supplements (1, 2). One supplement that remains controversial, particularly as it relates to improving glycemia, is chromium (Cr), and to date, routine clinical use has not been suggested (3). However, several studies suggest that Cr may have more consistent effects in certain clinical states and conditions (4-6). We reported that Cr supplementation, provided as 1000 μg/d as Cr picolinate, improved glycemia, attenuated body weight gain, and enhanced insulin sensitivity in subjects with type 2 DM (6). When compared to studies evaluating a similar dose and formulation of Cr, the data agreed with some, but not all studies, and suggested that subject characteristics may be important when assessing clinical response (6-8). For example, Kleefstra et al (7) reported that Cr had no benefit in subjects with Type 2 DM, but when compared to the studies suggesting a benefit, e.g. Martin et al (6) and Wang et al (8), the subjects evaluated appeared to be more obese, were more advanced in their disease process and were taking other medications, e.g. metformin, in addition to high dose insulin (7).
Given the recent observations, several questions regarding Cr remain. In particular, if Cr does improve glycemia, what are the mechanisms and which patient population is more likely to respond? In pilot studies, a major parameter predicting clinical response to Cr was the pre-treatment insulin sensitivity and additional studies suggest more consistent effects in the presence of insulin resistance and poorer glycemic control (5,8). Does Cr status of the patient determine who does or does not respond? What is the mechanism for the reported weight effects if indeed Cr modulates weight? In this regard, definitive energy balance studies, i.e. dietary intake and energy expenditure, using well validated techniques have not been reported. As such, we sought to provide a comprehensive assessment of Cr supplementation by conducting randomized, double-blind, placebo-controlled evaluations in individuals with Type 2 DM and representing a wide phenotype range and levels of insulin sensitivity. The overall effects of Cr were evaluated in addition to characterizing the specific differences in metabolic and physiologic parameters, i.e. hepatic glucose production, Cr status, body weight/distribution, energy balance and myocellular/intrahepatic lipid content, in those who did or did not respond clinically to Cr.
RESEARCH DESIGN AND METHODS
Study Design
The design was double-blinded, randomized, and placebo-controlled. Type 2 DM subjects (age 30-70 yrs) who were in a BMI range of 25-40 and with a fasting plasma glucose ≥ 6.94 mmol/L (125 mg/dL) at time of screening were evaluated. Exclusions were: 1) medications known to affect glucose metabolism; 2) untreated thyroid or chronic liver, renal or cardiovascular disease; and 3) a history of drug and/or alcohol abuse, or psychiatric disease prohibiting adherence to protocol. All procedures were approved and conducted in compliance with institutional human research guidelines.
The main cohort of subjects were evaluated at the Pennington Biomedical Research Center (Parent Trial). After meeting entry criteria, subjects were instructed on a weight maintenance diet by the study dietitian and the following assessments obtained: 1) glycemic control, i.e. A1c and oral glucose tolerance testing; 2) body weight/fat distribution as assessed by abdominal computed tomography and DEXA scans; 3) Cr status, i.e. urinary and plasma Cr levels; and 4) insulin sensitivity as assessed with hyperinsulinemic-euglycemic clamps. After completing pre-intervention testing, each subject was randomized to receive either 1000 ug of Cr as chromium picolinate (CrPic) (two 500 ug tablets daily) or matching placebo (dicalcium diphosphate). In a substudy conducted only in subjects enrolled in the Parent Trial and at the Pennington site, dietary intake studies, myocellular and intrahepatic lipids, as assessed with magnetic resonance spectroscopy scans, and 24-hour energy expenditure and respiratory quotient, as assessed with use of whole body metabolic chambers, were completed before randomization and at end of study. Subjects were evaluated monthly for routine clinic visits, adverse event monitoring, and study medication compliance. Twenty-four weeks after randomization, subjects underwent repeat physiological testing identical to that described for the pre-intervention testing. As the major objective of the study was to evaluate the role of chromium supplementation on metabolic parameters in a cohort representing a wide phenotype range and range of glycemic control, subjects were evaluated at the additional site for which glycemic control, insulin sensitivity and body weight/adiposity were the primary measures obtained.
Study Variables
Insulin Sensitivity
Hyperinsulinemic-euglyemic clamps were used to assess insulin sensitivity after an overnight fast (6, 9). After placement of catheters, a primed infusion of [6,6-2H2]glucose (75 μg/kg lean body mass/min) was started at 0700hr and continued for 5 hr. An insulin infusion (120 mU/m2/min) was started at 1000hr and continued for 2 hr. Blood glucose was monitored every 5 min and euglycemia maintained throughout the clamp by infusing 20% dextrose at a variable rate. The mean rate of exogenous glucose infusion during steady state (last 30 min) was corrected for changes in glycemia and divided by FFM to assess insulin sensitivity (9). Basal hepatic glucose production was calculated as previously described (6). Prior to, and during the last hour of the clamp, resting energy expenditure and respiratory quotient were assessed for each subject by indirect calorimetry for 45 min using the ventilated hood technique and substrate oxidation calculated (6).
Glycemic Parameters
Glucose tolerance was assessed by performing a 75-gram challenge with determination of glucose at 0, 0.5, 1, 1.5, 2, and 3 h after challenge. Glucose was measured using a glucose oxidase electrode on the Beckman Coulter DXC600. (Brea, CA). Insulin was measured by enzyme immunoassay on the Siemens 2000 (Los Angeles, CA). Hemoglobin A1c was determined spectrophotometrically on the Beckman Coulter DXC600. (Brea, CA). Day to day precision was assessed as <1.9% for glucose, <2.4% for HbA1C, and < 4.0% for insulin.
Body Weight/Fat Distribution
Fat-free mass, fat mass and body fat % were measured by dual-energy X-ray absorptiometry (DEXA) with CV for measurements assessed at 0.6%, 1.1%, and 1.1%, respectively. Total abdominal, visceral, and subcutaneous abdominal fat was measured by computed tomography as previously described (6). Overall, biologic, instrument and reader variability was assessed at approximately 10%.
Energy Balance
Dietary Intake
On test days, participants in the sub-study returned to the center and under supervision consumed a standard breakfast [460 kcal, fat 14.5g (28.4%), protein 13.6 g (11.8%), and carbohydrate 74.3 g (59.8 %), Cr content, estimated < 10 ug]. Food intake at both lunch (4 hours after breakfast) and dinner (4.5 hours after lunch) was measured. At dinner, food intake was measured using the Macronutrient Self-Selection Paradigm (10). Satiety ratings were collected using Visual Analogue Scales (11).
24 hour energy expenditure (EE) and Respiratory Quotient (RQ)
24h-EE and substrate oxidation were measured in a whole-room respiratory calorimeter (12). Participants entered the chamber at 0800h after an overnight fast. Meals were served at 0900h, 1330 and 1900h. Microwave motion detectors provided continuous monitoring of the participants’ spontaneous physical activity. 24hr EE and substrate oxidation (24 hr RQ, fat, carbohydrate and fat oxidation) were calculated from O2 consumption, CO2 production, and 24-h urinary nitrogen excretion. CV was determined to be 5% for 24hr EE and 7.8% for 24hr RQ.
Chromium Status
Serum was collected in lithium heparin chromium free monovetttes from Sarstedt (Numbrecht-Rommelsdorf, Germany) and was obtained at all time points during the OGTT and at 10 time points during the clamp. The samples were diluted with 1% nitric acid with 0.02% CTAC (cetlytrimethlyammonium chloride) and analyzed on a Varian graphite furnace atomic absorption spectrophotometer (Walnut Creek, CA) (13). Matrix matched calibration method was used which resulted in an analytical sensitivity of 0.01 ug/L. Urine samples, after first morning void, were collected in pre-screened urine collection cups over the entire duration of the OGTT and clamp studies and analyzed using graphite furnace atomic absorption (13). Quality control material were run each day and between run precision assessed as <10%.
Liver and Myocellular Lipid Content
Intramuscular (IMCL), extramuscular (EMCL), and intrahepatic (IHL) lipid stores were measured using 1H magnetic resonance spectroscopy (1H-MRS) using either a 1.5T(Picker Edge Eclipse) or GE 3-Tesla whole-body whole body imaging and spectroscopy system (General Electric, Milwaukee, WI). Once corrected for acquisition and relaxation time differences, data was shown to be highly correlated between the 1.5T and 3T systems in a subset of subjects run on both machines and was pooled into a common data set. Acquisition and processing techniques used in this project have been previously published by the current investigators (14, 15). To account for day-to-day variation in system performance, peak areas are expressed either relative to the peak area of an external phantom of known constant concentration or relative to an internal water peak assumed to be of constant concentration using the methodology described by Perseghin et al. (16) and Krssak et al. (17)
STATISTICAL ANALYSIS
SAS mixed procedure was used for analysis of variance to detect the effects of Cr. The least square means were used to determine significance of all pair wise differences. Unpaired two-tailed t-test was used to determine statistical differences between treatments. Changes between pre- and post-treatment were also tested by t-test with the hypothesis that change is null. Pearson correlation analysis was applied to analyze the relationships between assessments. Subjects randomized to Cr were classified as “responders” if the difference in insulin sensitivity at study end compared to pre-intervention was >10%, or as “non-responders” if the increase was < 10%. This stratification was based on data suggesting that a 10% increase in insulin sensitivity resulted in a significant clinical glycemic response to Cr assessed as an approximate 1% decrease in A1c (6). All the data analysis was carried out on SAS (SAS 9.1, 2003).
RESULTS
A total of 137 subjects (70 Cr, 67 Placebo) were evaluated. As the overall goal was to evaluate a cohort of individuals with Type 2 diabetes over a wide range of phenotypes, insulin sensitivity values were observed to range from extreme insulin resistance to clinically insulin sensitive. Specifically, insulin sensitivity in the cohort ranged from 0.96 mg/FFMkg/min to 10.7 mg/FFMkg/min. There were no differences at baseline for clinical or biochemical characteristics of subjects randomized to either Cr or placebo (Table 1).
Table 1.
Subject Characteristics at Baseline
| Metabolic parameter | Chromium | Placebo |
|---|---|---|
| Gender (Male/Female) | 37/33 | 39/28 |
| Age (yr) | 58.7 ± 1.0 | 56.1 ± 1.1 |
| Body Weight (kg) | 89.0 ± 2.1 | 91.3 ± 1.8 |
| BMI (kg/m2) | 30.8 ± 0.5 | 31.5 ± 0.6 |
| A1c (%) | 6.9 ± 0.2 | 6.8 ± 0.2 |
| Insulin sensitivity (mg/FFMkg/min) | 5.0 ± 0.3 | 5.2 ± 0.3 |
| Fasting Glucose (mmol/l) | 7.4 ± 0.3 | 7.5 ± 0.3 |
| Fasting Insulin (IU/ml) | 17.4 ± 1.3 | 18.1 ± 1.7 |
| Triglyceride (mg/dl) | 177.4 ± 17.7 | 175.4 ± 19.0 |
Data are mean ± SE.
Insulin Sensitivity/Hepatic Glucose Production
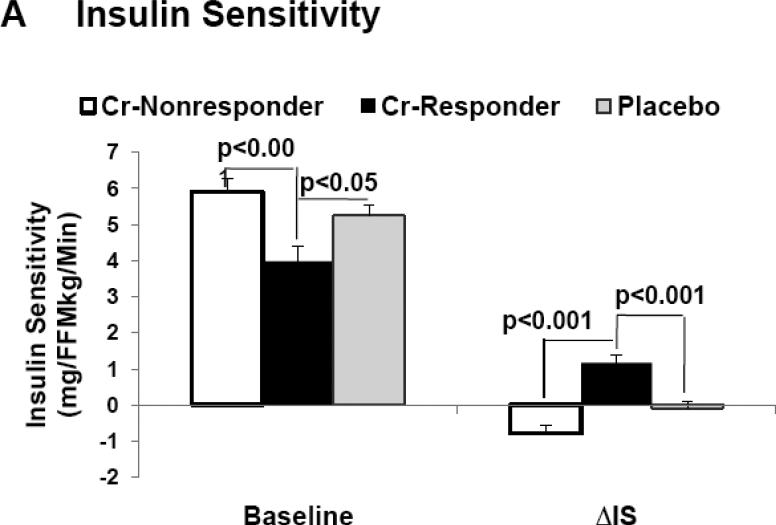
When evaluating the entire cohort and across all BMI ranges, insulin sensitivity increased in the Cr group (Δ = 0.35 mg/FFMkg/min), and slightly decreased in the placebo group (Δ = - 0.17 mg/FFMkg/min), but this difference was not considered statistically significant. However, approximately 51% of individuals randomized to Cr were classified as “responders”. In subjects classified as “responders”, there was a marked increase in insulin sensitivity from baseline as opposed to placebo (Δ =1.2 mg/kg/min, P < 0. 0001, vs Δ = −0.1 mg/kg/min, P = ns, respectively), and this difference was considered statistically significant between groups (Table 2). In addition, pre-intervention insulin sensitivity was observed to be significantly less in “responders” as opposed to both “non-responders” and placebo groups (Figure 1). Pre-intervention insulin sensitivity was also negatively correlated to the clinical response, i.e. Δ insulin sensitivity at end of study compared to baseline (r = −0.37, p < 0.001). Basal hepatic glucose production did not differ between treatment groups at end of study, nor were there were significant differences between “responders”, “non-responders” or placebo (Table 2).
Table 2.
Metabolic and phenotypic parameters at baseline and end of study.
| Placebo | Cr Non-responders | Cr-Responders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Baseline | End | P 1 | Baseline | End | P 1 | Baseline | End | P 1 | P 2 | P 3 |
| Glucose/Insulin | |||||||||||
| Fasting Glucose (mmol/L) | 7.5±0.3 | 7.6±0.2 | ns | 6.7±0.5 | 7.1±0.3 | ns | 8.5±0.5 | 7.4±0.3 | 0.010 | 0.002 | 0.003 |
| GHb (%) | 6.85±0.25 | 6.98±0.21 | ns | 6.29±0.18 | 6.48±0.17 | ns | 7.57±0.43 | 6.85±0.25 | 0.035 | 0.024 | 0.025 |
| Glucose AUC | 1172±35 | 1162±31 | ns | 1096±36 | 1126±42 | ns | 1310±66 | 1196±46 | 0.015 | 0.033 | 0.010 |
| Insulin Sensitivitya | 5.22±0.29 | 5.11±0.26 | ns | 5.91±0.36 | 5.39±0.42 | ns | 3.98±0.43 | 5.17±0.51 | 0.001 | 0.001 | 0.001 |
| Body Fat | |||||||||||
| Weight (kg) | 91.3±1.8 | 92.4±1.8 | ns | 88.0±2.9 | 89.0±3.0 | ns | 89.6±3.1 | 90.0±3.0 | ns | ns | ns |
| Body Fat (%) | 32.4±1.1 | 32.8±1.1 | ns | 33.0±1.4 | 33.3±1.3 | ns | 33.3±1.4 | 32.7±1.3 | ns | ns | ns |
| FFM (kg) | 61.2±1.5 | 61.8 ±1.5 | ns | 58.0±1.7 | 57.6±1.8 | ns | 61.0±2.5 | 62.5±2.7 | 0.02 | ns | ns |
| Visceral Fat | 5.70±0.33 | 5.60±0.33 | ns | 5.66±0.42 | 5.80±0.48 | ns | 5.80±0.55 | 5.82±0.53 | ns | ns | ns |
| SubQ Fat | 9.97±0.63 | 10.24±0.64 | ns | 9.35±0.73 | 9.15±0.71 | ns | 10.36±0.87 | 11.17±0.72 | ns | ns | ns |
| Myocellular Lipids | |||||||||||
| Soleus EMCL (ASU) | 0.014±0.002 | 0.019±0.003 | 0.055 | 0.013±0.003 | 0.016±0.004 | ns | 0.023±0.008 | 0.013±0.001 | ns | 0.011 | ns |
| Soleus IMCL(ASU) | 0.008±0.001 | 0.009±0.001 | ns | 0.007±0.002 | 0.007±0.001 | ns | 0.010±0.003 | 0.006±0.001 | ns | 0.033 | ns |
| Tibialis EMCL(ASU) | 0.016±0.002 | 0.029±0.005 | 0.008 | 0.027±0.009 | 0.040±0.010 | ns | 0.037±0.018 | 0.018±0.004 | ns | 0.017 | 0.026 |
| Tibialis IMCL(ASU) | 0.007±0.001 | 0.007±0.001 | ns | 0.005±0.002 | 0.007±0.002 | ns | 0.010±0.004 | 0.006±0.001 | ns | ns | 0.053 |
| Liver Parameters | |||||||||||
| IHL (ASU) | 0.11±0.02 | 0.16±0.03 | ns | 0.10±0.04 | 0.13±0.05 | ns | 0.20±0.08 | 0.12±0.05 | 0.039 | 0.055 | 0.035 |
| HGP (mmol/dl/min) | 2.37±0.12 | 2.17 ± 0.09 | ns | 2.32±0.11 | 2.21 ±0.11 | ns | 2.48±0.10 | 2.31 ±0.08 | ns | ns | ns |
| Metabolic Chamber | |||||||||||
| Energy Bal (Kcal/d) | −132±46 | −116±44 | ns | 8±42 | −76 ±63 | ns | −163±75 | −191±70 | ns | ns | ns |
| 24 hour EE (Kcal/d) | 2324±72 | 2311 ±63 | ns | 2043±85 | 2122±104 | ns | 2203±152 | 2184±153 | ns | ns | ns |
| Sleep EE (Kcal/d) | 1903±54 | 1915±55 | ns | 1699±72 | 1757±78 | ns | 1821±129 | 1800±121 | ns | ns | ns |
| NENS EE (Kcal/d) | 1850±60 | 1837±52 | ns | 1617±72 | 1703±91 | ns | 1747±123 | 1747±127 | ns | ns | ns |
| Activity % | 15.5±1.6 | 16.3±1.7 | ns | 13.9±1.9 | 14.2±1.7 | ns | 17.5±2.6 | 15.6±2.3 | ns | ns | ns |
| Fuel Utilization | |||||||||||
| Fasting RQ | 0.83±0.01 | 0.82±0.01 | ns | 0.83±0.01 | 0.82±0.01 | ns | 0.83±0.01 | 0.80±0.01 | ns | ns | ns |
| Clamp RQ | 0.86±0.01 | 0.86±0.01 | ns | 0.87±0.01 | 0.87±0.01 | ns | 0.85±0.01 | 0.84±0.02 | ns | ns | ns |
| 24 hr RQ | 0.87±0.01 | 0.88±0.01 | ns | 0.86+0.01 | 0.88±0.01 | ns | 0.88±0.01 | 0.89±0.01 | ns | ns | ns |
| Cho Ox | 288.4±15.4 | 308.2±18.6 | ns | 255.6±21.5 | 290.6±23.8 | ns | 290.0±21.3 | 299.7±25.7 | ns | ns | ns |
P 1 is t-test p-value of change in parameters versus null. P 2 is t-test p-value of CrPic “Responders” vs placebo. P 3 is t-test p-value of CrPic “Responders” vs CrPic “Nonresponders”. ASU, arbitrary scanning units; Bal, balance; IHL, intrahepatic lipid content; HGP, Hepatic Glucose Production; NENS, Non-exercise, non-sleep energy expenditure
Insulin Sensitivity Units = mg/FFMkg/Min
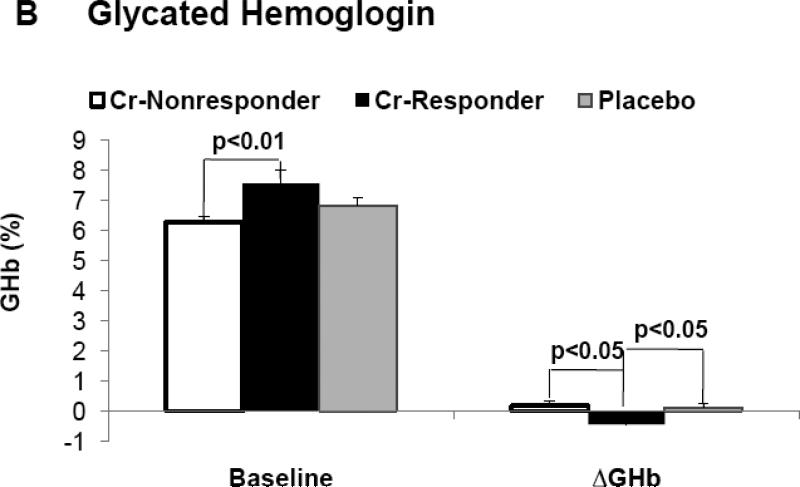
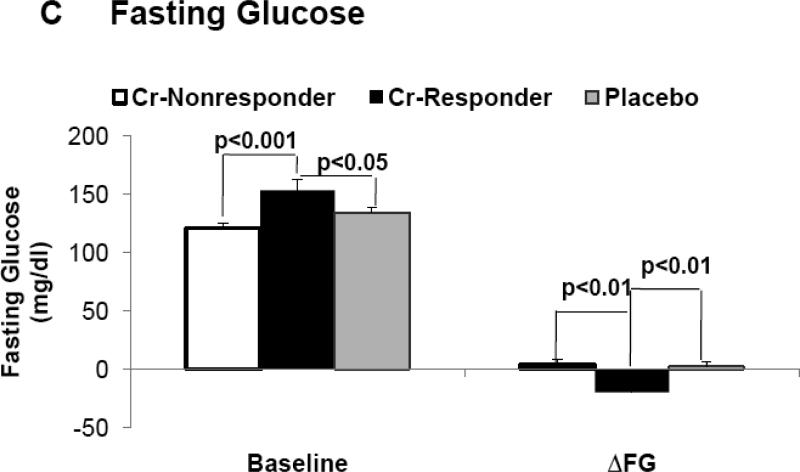
Figure 1.
Demonstrates baseline (pre-intervention) values and change in each parameter from baseline when assessed at end of study, i.e. delta (Δ) for insulin sensitivity (1A), fasting glucose (1B), and glycated hemoglobin (1C) in “responders”, “non-responders” and placebo groups.
Glycemic Control
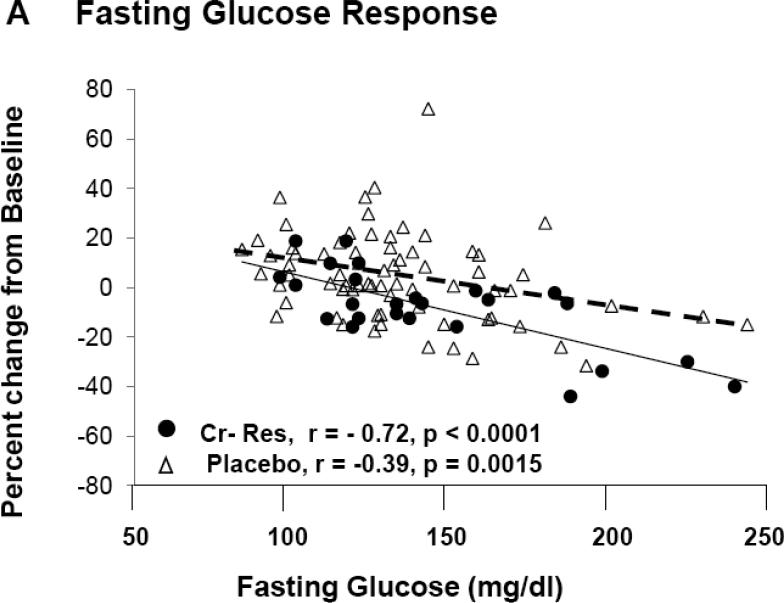
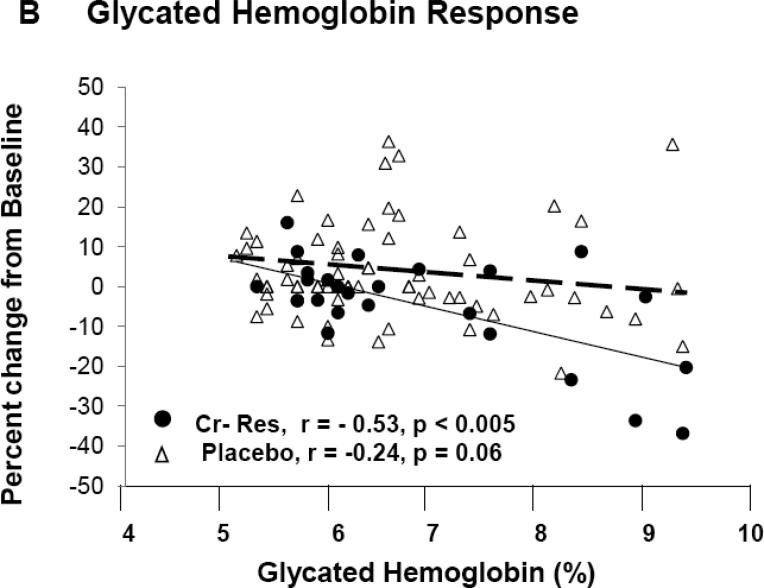
In the entire cohort, differences between placebo and Cr groups for A1c (Δ = 0.12 %) and fasting glucose (Δ = 5.4 mg/dl) were not felt to be statistically significant. However, subjects classified as “responders” had significantly lower A1c levels, fasting glucose, and glucose AUC at end of study when compared to pre-intervention, and when compared to placebo and “non-responders” at end of study (Table 2). Fasting glucose and A1c levels, at pre-intervention, were significantly greater in “responders” as opposed to both “non-responders” and placebo groups (Figure 1B and 1C). For both A1c and fasting glucose, the preintervention value significantly correlated to the clinical response as assessed by decrease in A1c, i.e., Δ A1c, (r = −0.57, p < 0.001) and fasting glucose, i.e., Δ fasting glucose (r = −0.63, p < 0.001) for all chromium subjects. In order to evaluate the role of each subject's response as a function of the baseline value, the % change in each glycemic parameter was plotted versus the baseline level for each individual subject. As demonstrated in Figure 3A, there was a statistically significant and negative correlation for the % change in fasting glucose for both Cr “responders” and placebo groups, but, as expected, there was no correlation of % change vs baseline fasting glucose in subjects identified by insulin sensitivity testing as “non-responders” (r = 0.07, p = ns) (data not shown). In addition, there was a statistically signifcant and negative correlation for the % change of glycated hemoglobin as a function of the individual baseline value for Cr “responders”, and this value approached significance in the placebo group (Figure 3B). There was no correlation of % change in glycated hemoglobin when plotted versus the baseline level in subjects identified by insulin sensitivity testing as “non-responders” (r = 0.05, p = ns) (data not shown).
Figure 3.
Demonstrates the percentage change for fasting glucose (3A) and glycated hemoglobin (3B) when plotted versus the individual baseline value for “responders” and placebo groups. There was no correlation for % change in fasting glucose (r = 0.07, p = ns) nor glycated hemoglobin (r = 0.05, p = ns) for “non-responders” (Data not shown).
Chromium Status
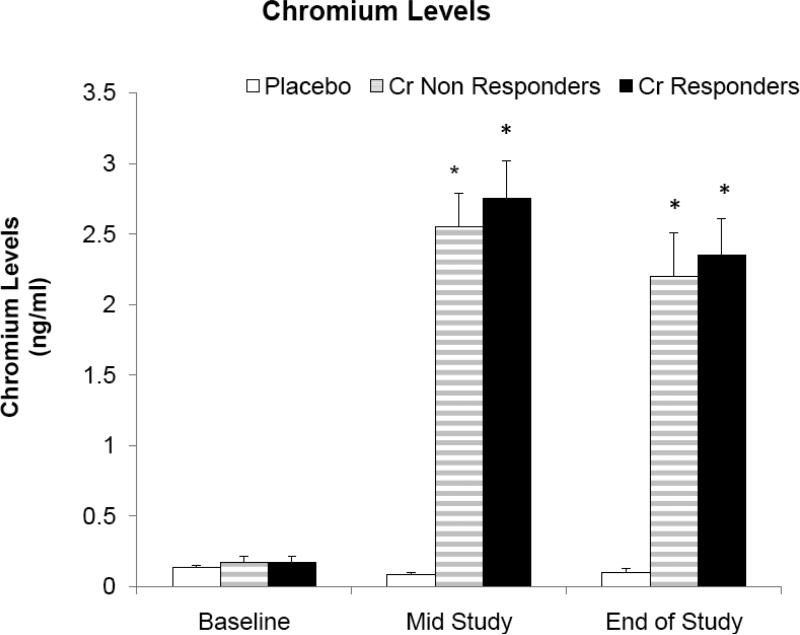
Cr levels in serum or urine did not differ at baseline for subjects randomized to either Cr or placebo (Table 1). There was a significant increase in serum Cr in subjects randomized to Cr as opposed to placebo at mid-point (2.40 + 0.19 vs 0.16 + 0.05 ng/dl, p < .0001) and end of study (2.62 + 0.09 + 0.17 + 0.04 ng/dl, p < .0001). In addition, there was a significant increase in urine Cr in subjects randomized to Cr as opposed to placebo at mid-point (11.68 + 3.39 vs 0.10 + 0.03 ng/dl, p < .0001) and end of study (6.5 + 1.13 + 0.13 + 0.06 ng/dl, p < .0001). There was no difference in either serum or urine Cr between “responders” and “non-responders” when assessed at baseline, mid-point or end of study, although both groups differed significantly from placebo (Figure 2, Table 2).
Figure 2.
Demonstrates serum Cr levels in “responders”, “non-responders” and placebo when assessed at baseline, mid-point or end of study. *p < .001 vs placebo
Body Weight/Fat Distribution
Body weight did not significantly increase from baseline for subjects randomized to either Cr or placebo (0.8 + 0.5 kg vs 0.7 + 0.6 kg, resp). There was no difference between treatment groups for body weight, % body fat, fat-free mass, or abdominal fat depots, i.e. TAF, VAF, or SubQ AF. In addition, “responders” did not differ from “non-responders” for body fat/composition measures (Table 2).
Energy Balance
Mixed linear models indicated that change in food intake, percent kcal from fat, carbohydrate, and protein, did not differ by group. AUC analysis indicated that change in hunger, desire to eat, fullness, and prospective food consumption did not differ by group (data not shown).
24-hour EE, sleep EE or % activity did not differ in subjects randomized to Cr (n = 30) vs placebo (n = 27). Non-exercise/non-sleep EE was increased from pre-intervention levels in subjects randomized to Cr (63 + 33, p < 0.05) as opposed to placebo ( –33 + 30, p = ns) and these differences were statistically different between Cr and placebo groups at study end (p < 0.03). Subjects randomized to Cr appeared to be in a more negative energy balance compared to pre-intervention testing as compared to placebo (−63 + 45, p = 0.06 vs 12 + 36, p = ns), but these differences were not statistically significant between groups at end of study. Both “responders” and “non-responders” trended to a more negative energy balance compared to placebo, but there were no significant changes in 24 hour EE, Sleep EE, % activity, fasting RQ, 24 hrRQ, carbohydrate or protein oxidation (Table 2).
Myocellular and Intra-hepatic Lipids
MRS scanning was completed on 56 subjects in the sub-study (27 Cr, 26 placebo). There was a statistically significant negative correlation between insulin sensitivity and soleus muscle IMCL (r = −0.33, p <.02), tibialis muscle IMCL (r = -0.33, p < .03) and IHL (r = −0.42, p < .01) for all subjects. Compared to pre-intervention, subjects randomized to placebo had an increase in soleus muscle EMCL (0.006 + 0.003 ASU, p = 0.05) compared to decrease with Cr (−0.003 + 0.003 ASU) and the value approached significance between groups (p = 0.07). There was an increase in tibialis muscle EMCL (0.009 + 0.003 ASU, p < .01) and IHL (0.17 + 0.08 ASU, p < .04) in the placebo group whereas there were no significant changes from pre-intervention for Cr. When compared with “non-responders”, “responders” had a significant decrease in tibialis muscle IMCL (p = 0.05), tibialis muscle EMCL (p < 0.03), and IHL (p < 0.04). When compared to placebo, “responders” had a significant decrease in soleus muscle EMCL (p < 0.02), soleus muscle ICML (p < 0.04), tibialis muscle EMCL (p < 0.02), and it approached significance for IHL (p = 0.055) (Table 2).
DISCUSSION
This study demonstrated that in a well-characterized cohort of Type 2 diabetic subjects representing a wide phenotype, e.g. lean to obese, and wide range of glycemic control, and including both insulin sensitive and insulin resistant subjects, there was not a consistent effect of chromium supplementation to improve insulin action or glycemic control. However, in subjects responding to Cr, the effect of Cr to improve glycemia was secondary to enhanced insulin sensitivity in muscle. There was no effect of Cr on hepatic glucose production or on body weight/fat distribution. In addition, in those subjects responding to Cr, fasting glucose and A1c were significantly higher and insulin sensitivity significantly lower when assessed at pre-intervention. It was also observed that the clinical response to Cr was significantly related to the baseline A1c, fasting glucose, and insulin resistance and that Cr status did not differentiate between “responders” and “non-responders”. An interesting observation was that myocellular and intra-hepatic lipid content was significantly related to insulin sensitivity and both were reduced in subjects randomized to Cr.
An intriguing finding from this study was the effect of Cr to decrease tissue lipids. Accumulation of lipids in muscle and liver is postulated to impair insulin receptor signaling and contribute to insulin resistance (18). Several pre-clinical studies demonstrate an effect for Cr on lipid metabolism. Specifically, Sreejayan et al (19) reported that Cr treatment decreased liver triglyceride levels and lipid accumulation in animal models. Horvath et al (20) reported that Cr activates Glut-4 trafficking via a cholesterol-dependent mechanism and concluded that Cr3+ supplementation may lower blood glucose by altering the plasma membrane composition of cholesterol in fat and muscle cells. However, there have been no reports assessing myocellular lipids in human studies evaluating Cr supplementation. Myocellular and intrahepatic lipid content were negatively correlated to insulin sensitivity and both were decreased in subjects randomized to Cr who had improved insulin sensitivity. Tissue lipid content may decrease with lifestyle or drug-induced improvement in insulin sensitivity, and in the case of pharmacologic therapy, can improve tissue lipids without reduction in body weight (21). The observations support the findings of enhanced insulin sensitivity and reduction in myocellular lipids without an effect on weight in “responders”. In addition, fat-free mass was increased from baseline in “responders” and differed significantly from the placebo group. This observation in Cr treated subjects has been reported previously, but the mechanism is not precisely known (6).
The measured response to an identical pharmacologic or lifestyle intervention will vary greatly among individuals, and the variation may be secondary to differences in genetic or physiologic makeup in addition to differences in other subject characteristics. The data demonstrated that chromium supplementation did not have a consistent and significant effect on metabolic parameters when evaluated over the entire cohort. As stated, the overall goal was to evaluate clinical chromium supplementation across a wide phenotype range and range of glycemia to specifically address the controversy surrounding chromium and recommended clinical use. However, it was concluded that the likelihood for a response to Cr is greater when insulin resistance is present and glucose control poorer prior to supplementation which confirms observations reported in both human and animal studies (5, 8, 22). In addition to the data as outlined in Figure 1 for which statistical differences were noted for baseline insulin sensitivity and fasting glucose in “responders” and “non-responders”, the individual responses for such an effect were assessed. Figure 3 demonstrates the percentage change for glycemic parameters obtained at end of study when plotted versus baseline value for fasting glucose and glycated hemoglobin. As demonstrated, the data suggests that below a fasting glucose value of approximately 175 mg/dl and a glycated hemoglobin of 8%, a consistent effect for chromium supplementation to improve glycemia is not observed. Specifically, a considerable overlap is seen for individual responses for fasting glucose and glycated hemoglobin for those classified as “responders” versus those subjects randomized to placebo. Interestingly, as glycemic control worsens, e.g. fasting glucose > 175 mg/dL and glycated hemoglobin > 8%, it appears that the likelihood for an effect of chromium supplementation was greater. Clearly, there were too few subjects in these higher glycemic ranges to make a definitive statement in this regard. However, the trend appears to be consistent with findings from prior clinical studies suggesting that a formulation of chromium that also contained biotin, and when administered as an adjuvant to current prescription anti-diabetic medication, improved glycemic control especially those patients with poor glycemic control on oral therapy (26). This observation may partially explain the reported discrepancies in response to Cr in humans and why Cr supplementation appears to have a more predictable response in hyperinsulinemic or obese states (3,8,23). The mechanism by which hyperinsulinism, insulin resistance, and/or obesity plays a role in Cr response, or how Cr contributes to development of these phenotypes is currently unknown. But, this study clearly demonstrates that simply increasing Cr levels in serum is not sufficient for a response as the Cr status did not differ between “responders” and “nonresponders”. Therefore, a very relevant question is the specific mechanism that accounts for the whole-body response to chromium when circulating levels don't appear to differentiate between “responders” and “non-responders”. At this time, the reasons are not known for this observation, and mechanistic studies evaluating gene expression, cellular protein abundance, cytosolic enzyme activity, etc. are clearly required in order to address this observation.
The decision to use a specified change in insulin sensitivity instead of fasting glucose or A1c levels to stratify subjects as to clinical response was based on the following rationale. First, although A1c represents the “gold standard” for assessing glycemic control, it is well known that the decrease in A1c with treatment depends on the baseline level, with a greater percent decrease observed if the levels are higher prior to treatment (24). As such, using a pre-specified percentage decrease in A1c to define a “responder” would have to vary according to the baseline level. Secondly, it has been shown that Cr can improve insulin sensitivity with minimal effect on glycemia as observed in studies evaluating subjects who have glucose intolerance (25). Therefore, having to evaluate response on glycemia alone could potentially classify patients incorrectly as “non-responders” although they may have had a significant improvement for insulin sensitivity. Third, in preliminary studies, insulin resistance was demonstrated as the most predictive factor in assessing response to Cr (8). Thus, the rationale to stratify subjects based on changes in insulin sensitivity seemed not only sound, but highly clinically relevant.
In summary, with use of “state of the art” metabolic techniques and in a well characterized cohort of individuals with Type 2 diabetes representing a wide range of phenotype, glycemic paramters, and parameters assessing whole body insulin action, a consistent effect of chromium was not observed. However, this study is the first to show that Cr levels after supplementation do not differ between “responders” and “non-responders”, and provides the first comprehensive assessment of physiological and biochemical characteristics of individuals who responded to Cr. Specifically, “response” to chromium is more likely in insulin resistant individuals who have more elevated fasting glucose and A1c levels. Another novel finding was that tissue lipids are decreased in subjects randomized to Cr. Thus, it may be postulated that Cr alters insulin sensitivity through modulation of lipid metabolism in peripheral tissues and may represent a unique mechanism of action for trace minerals. The mechanism for this effect is the focus of ongoing studies.
Acknowledgments
Supported by R55 DK060126 and R01 DK060126 awarded to William T. Cefalu, M.D., and K23 DK068052 awarded to Corby K. Martin, Ph.D. This work was partially supported by a NORC Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK.
Abbreviations
- AUC
area under the curve
- CrPic
chromium picolinate
- CT
Computed Tomography
- DEXA
dual-energy X-ray absorptiometry
- EE
energy expenditure
- EMCL
extramyocellular lipids
- FFA
free fatty acid
- FM
fat mass
- FFM
fat free mass
- IHL
intrahepatic lipids
- IMCL
intramyocellular lipids
- MRS
magnetic resonance spectroscopy
- OGTT
oral glucose tolerance test
- REE
resting energy expenditure
- RQ
respiratory quotient
- SubQ AF
subcutaneous abdominal fat
- EE
energy expenditure
- TA
anterior tibialis
- TAF
total abdominal fat
- VAF
visceral abdominal fat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have made substantial contributions to the conception and design of the study, acquisition of data and analysis and interpretation of data. In addition, all authors have reviewed the manuscript and provided important intellectual content and have approved the final submitted version. The manuscript is not under consideration elsewhere. There are no conflicts of interest with any of the authors and the submitted data. As the funding was from the National Institute of Health, there is no problem with employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
REFERENCES
- 1.Gibson JE, Taylor DA. Can claims, misleading information and manufacturing issues regulating dietary supplements be improved in the United States of America? J Pharmacol Exp Ther. 2005;314:939–944. doi: 10.1124/jpet.105.085712. [DOI] [PubMed] [Google Scholar]
- 2.Neuhouser ML. Dietary supplement use by American women: challenges in assessing patterns of use, motives and costs (Review). J Nutr. 2003;133:1992S–1996S. doi: 10.1093/jn/133.6.1992S. [DOI] [PubMed] [Google Scholar]
- 3.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2742–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 4.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30(8):2154–63. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 5.Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev. 2008 Jan-Feb;24(1):41–51. doi: 10.1002/dmrr.755. [DOI] [PubMed] [Google Scholar]
- 6.Martin J, Wang ZQ, Zhang XH, Wachtel D, Volaufova J, Matthews DE, Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006;29(8):1826–32. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 7.Kleefstra N, Houweling ST, Jansman FGA, Groenier KH, Gans ROB, Meyboom-de Jong B, Bakker SJL, Bilo HJG. Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetes in an obese Western population: a randomized, double-blind, placebo-controlled trial. Diabetes Care. 2006;29(3):521–5. doi: 10.2337/diacare.29.03.06.dc05-1453. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, Anderson RA, Pinsonat P, Cefalu WT. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;56(12):1652–5. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 10.Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiology & Behavior. 1998;3(5):919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 11.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003;41:572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- 13.Nunes M, Nunes S. Fast determination of chromium in human serum by electrothermal atomic absorption spectrometry. J.Anal. At. Spectrom. 2002;17:1335–1338. [Google Scholar]
- 14.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelly DE, Ravussin E, Newcomer BR. Muscle-Associated Triglyceride Measured by Computed Tomography and Magnetic Resonance Spectroscopy. Obesity Research. 2006;14(1):73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso AA, Ravussin E, the Pennington CALERIE group Effect of Calorie Restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size and ectopic lipid in overweight Subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 17.Krssak M, Falk Petersen F, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 18.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sreejayan N, Dong F, Kandadi MR, Yang X. Ren J Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity (Silver Spring) 2008;16(6):1331–7. doi: 10.1038/oby.2008.217. [DOI] [PubMed] [Google Scholar]
- 20.Horvath EM, Tackett L, McCarthy AM, Raman P, Brozinick JT, Elmendorf JS. Antidiabetogenic effects of chromium mitigate hyperinsulinemia-induced cellular insulin resistance via correction of plasma membrane cholesterol imbalance. Mol Endocrinol. 2008;22(4):937–50. doi: 10.1210/me.2007-0410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Teranishi T, Ohara T, Maeda K, Zenibayashi M, Kouyama K, Hirota Y, Kawamitsu H, Fujii M, Sugimura K, Kasuga M. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2007 Oct;56(10):1418–24. doi: 10.1016/j.metabol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;136(2):415–20. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 23.Morris BW, MacNeil S, Stanley K, Gray TA, Fraser R. The inter-relationship between insulin and chromium in hyperinsulinaemic euglycaemic clamps in healthy volunteers. J Endocrinol. 1993;139:339–45. doi: 10.1677/joe.0.1390339. [DOI] [PubMed] [Google Scholar]
- 24.Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care. 2006;29(9):2137–9. doi: 10.2337/dc06-1120. [DOI] [PubMed] [Google Scholar]
- 25.Cefalu WT, Bell-Farrow AD, Stegner J, Wang ZQ, King T, Morgan T, Terry JG. Effect of chromium picolinate on insulin sensitivity in vivo. J Trace Elem Exp Med. 1999;12:71–83. [Google Scholar]
- 26.Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;24(1):41–51. doi: 10.1002/dmrr.755. [DOI] [PubMed] [Google Scholar]