Abstract
Objective
To assess the current identification and management of patients with dementia in a primary care setting; to determine the accuracy of identification of dementia by primary care physicians; to examine reasons (triggers) for referral of patients with suspected dementia to the geriatric assessment team (GAT) from the primary care setting; and to compare indices of identification and management of dementia between the GAT and primary care network (PCN) physicians and between the GAT and community care (CC).
Design
Retrospective chart review and comparisons, based on quality indicators of dementia care as specified in the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia, were conducted from matching charts obtained from 3 groups of health care providers.
Setting
Semirural region in the province of Alberta involving a PCN, CC, and a GAT.
Participants
One hundred patients who had been assessed by the GAT randomly selected from among those diagnosed with dementia or mild cognitive impairment by the GAT.
Main outcome measures
Diagnosis of dementia and indications of high-quality dementia care listed in PCN, CC, and GAT charts.
Results
Only 59% of the patients diagnosed with dementia by the GAT had a documented diagnosis of dementia in their PCN charts. None of the 12 patients diagnosed with mild cognitive impairment by the GAT had been diagnosed by the PCN. Memory decline was the most common reason for referral to the GAT. There were statistically significant differences between the PCN and the GAT on all quality indicators of dementia, with underuse of diagnostic and functional assessment tools and lack of attention to wandering, driving, medicolegal, and caregiver issues, and underuse of community supports in the PCN. There was higher congruence between CC and the GAT on assessment and care indices.
Conclusion
Dementia care remains a challenge in primary care. Within our primary care setting, there are opportunities for synergistic collaboration among the health care professionals from the PCN, CC, and the GAT. Currently they exist as individual entities in the system. An integrated model of care is required in order to build capacity to meet the needs of an aging population.
Résumé
Objectif
Évaluer la façon actuelle d’identifier et de traiter les patients souffrant de démence dans un milieu de soins primaires; déterminer la précision avec laquelle la démence est identifiée par les médecins première ligne; examiner les raisons (déclencheurs) qui amènent à diriger les patients soupçonnés de démence vers l’équipe d’évaluation gériatrique (ÉÉG) du milieu de soins primaires; et comparer les éléments servant au diagnostic et au traitement de la démence utilisés par l’ÉÉG à ceux qu’utilisent les médecins du réseau de soins primaires (RSP), et à ceux qu’utilisent les soins communautaires (SC).
Type d’étude
Revue rétrospective de dossiers; on a aussi fait des comparaisons basées sur les indicateurs de qualité du traitement de la démence tals qu’énoncés par la Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia en se servant de dossiers appariés provenant de 3 groupes de soignants.
Contexte
Une région semi-rurale de l’Alberta comprenant un RSP, un établissement de SC et une ÉÉG.
Participants
On a choisi au hasard 100 patients déjà évalués par l’ÉÉG pour lesquels l’ÉÉG avait porté un diagnostic de démence ou de problème cognitif léger.
Principaux paramètres à l’étude
Un diagnostic de démence et des indices d’une grande qualité de soins pour la démence, tels qu’indiqués dans les dossiers de l’ÉÉG, des établissements de SC et des RSP.
Résultats
Seulement 59 % des patients chez qui l’ÉÉG avait porté un diagnostic de démence avaient un diagnostic de démence documenté dans leur dossier du RSP. Aucun des 12 patients pour lesquels l’ÉÉG avait porté un diagnostic de problème cognitif léger n’avait reçu ce diagnostic du RSP. Une diminution de la mémoire était la raison la plus fréquente pour diriger les patients à l’ÉÉG. Il y avait des différences significatives entre le RSP et l’ÉÉG pour tous les indices de qualité relatifs à la démence, notamment pour l’utilisation insuffisante des outils de diagnostic et d’évaluation fonctionnelle, et pour le manque d’attention portée aux questions relatives à l’errance, à la conduite automobile, aux problèmes d’ordre médicolégal et aux rapports avec les soignants, et pour le manque de recours au support communautaire offert par les RSP. Il y avait plus de similitude entre les SC et l’ÉÉG pour ce qui est de l’évaluation et des indices de traitement.
Conclusion
Le traitement de la démence demeure problématique en contexte de soins primaires. Notre milieu de soins primaires offre des occasions de collaboration entre les différents professionnels de la santé des RSP, des SC et de l’ÉÉG. À l’heure actuelle, ces organismes fonctionnent comme des entités individuelles dans le système. Un modèle de soins intégrés sera nécessaire pour mieux répondre aux besoins d’une population vieillissante.
The prevalence of dementia worldwide in 2010 was estimated to be 35.6 million; this is projected to double in 20 years and triple in 40 years.1 The number of individuals with dementia in Canada is projected to increase 2.5-fold by 2038,2 and the economic burden of dementia will increase from $15 billion in 2008 to $153 billion by 2038.2 Barriers to identifying dementia at the primary care level include time constraints,3–8 inadequate knowledge,4,5,7,9,10 an inadequate skill set,4,5,7,8,10 fear of making an incorrect diagnosis,7,9–11 lack of remuneration,3,5,6,11 and lack of coordination between physicians and community services.6,9 Thus, it is not surprising that two-thirds of all dementia and 91% of early dementia is missed in the primary care setting.12
Even when dementia is recognized in the primary care setting, research indicates that the quality of dementia care is suboptimal after diagnosis.7 Challenges in the management of the illness include initiation of dementia-specific medication,7,11–13 addressing behavioural problems,6,11 inappropriate psychoactive medication use,6 safety issues,12,14 management of caregiver stress and burden,4,12,14 coordination of care,6,11,15 and providing support for patients and their families.10,16 The inadequacies in the diagnosis and management of dementia are associated with higher rates of avoidable hospitalizations and earlier use of long-term care facilities.11,14,17
Different models of care have been proposed that are designed to offer skills and supports to primary care physicians in order to provide better dementia care, with clinical studies conducted to evaluate their effectiveness.18–22 Results are mixed, with studies reporting improvement in care,18–20 minimal change,21 and no change.22 Although limited in scope, research indicates that care management by interdisciplinary teams in the primary care setting results in better adherence to dementia care guidelines,19 less behavioural and psychological disturbance,23,24 and reductions in caregiver stress23 and depression.24 Findings also suggest that including geriatric specialists in the identification of dementia patients improves screening rates for dementia.12
In one of the health regions in Alberta, dementia care is delivered through a system involving family physicians in a primary care network (PCN), a geriatric assessment team (GAT), and community care (CC). In 2009, the PCN comprised a group of 50 family physicians serving a population of about 70 000 patients in cooperation with the local health region and other health professionals. The GAT assists with the assessment and management of the frail elderly in the health region and consists of a care of the elderly physician (a family physician who has extra training in geriatrics) and geriatric assessment nurses. Community care provides an integrated system of health and personal support services to clients living in the community using nurses and other allied health care professionals (eg, occupational therapists, social workers). Community care takes referrals from all health care professionals, patients, and families. As of 2009, the GAT had assessed and managed more than 400 patients, with approximately two-thirds of the patients referred for assessment of dementia and related issues. The turnaround time after referral for a GAT assessment averaged between 2 and 3 weeks. All referrals received comprehensive geriatric assessment, followed by detailed listing of problems, recommendations, interventions, and follow-up. Although it is assumed that this current approach to caring for patients with dementia has resulted in “better” care, the approach has not been systematically evaluated. This research addresses that deficiency. Thus, the objectives of this research were to determine the accuracy of the identification of dementia by primary care physicians; to examine the reasons (triggers) for referral of patients with suspected dementia to the GAT from the primary care setting; and to compare indices of the identification and management of dementia between the GAT and the PCN and between the GAT and CC. The overall goal is for the results to inform us on the consistency of assessments, knowledge of evolving partnerships in reducing gaps in dementia care, and opportunities for collaborations.
METHODS
Study sample
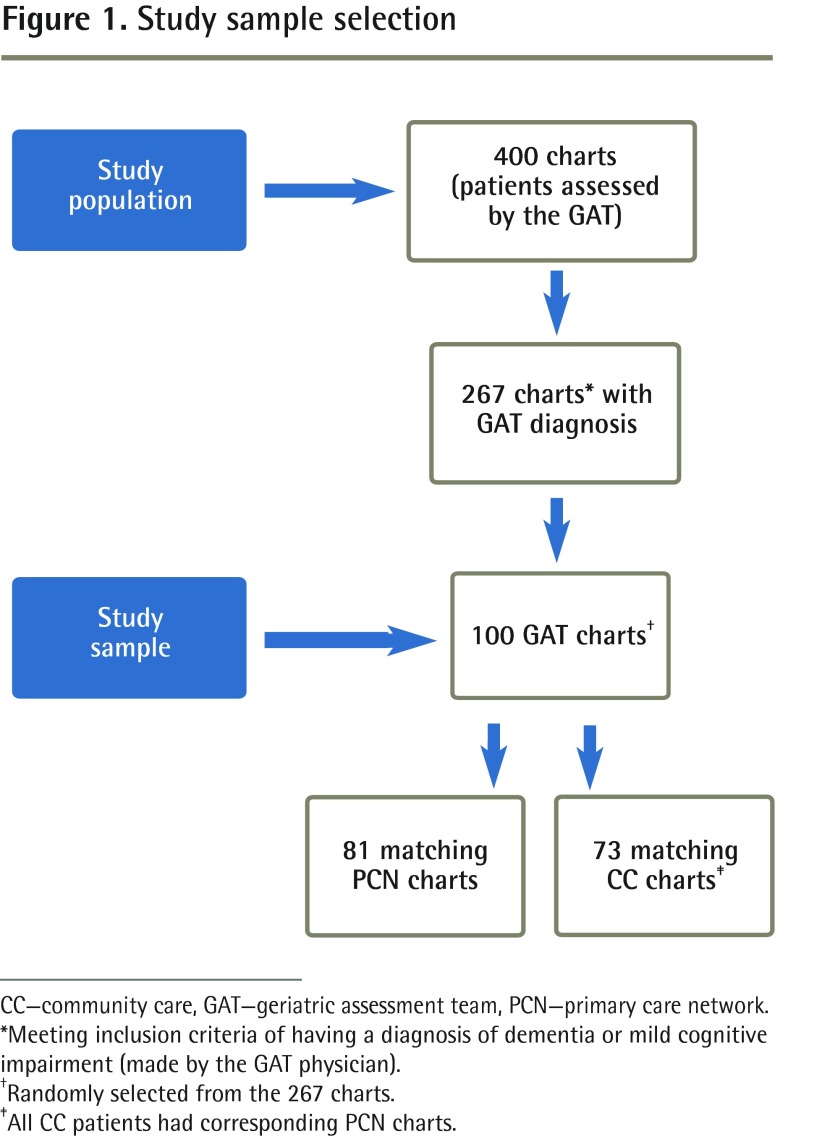
Using retrospective chart review methodology, the charts of 400 patients (aged 56 to 96 years) who had been assessed by the GAT between April 2005 and March 2009 were retrieved. From this sample, 267 charts of individuals who had been diagnosed with dementia (based on Diagnostic and Statistical Manual, fourth edition, criteria25) or mild cognitive impairment (MCI) by the GAT physician were identified. One hundred GAT charts were randomly selected from among those patients diagnosed with dementia or MCI to form the study sample (Figure 1).
Figure 1.
Study sample selection
CC—community care, GAT—geriatric assessment team, PCN—primary care network.
*Meeting inclusion criteria of having a diagnosis of dementia or mild cognitive impairment (made by the GAT physician).
†Randomly selected from the 267 charts.
‡All CC patients had corresponding PCN charts.
Matching charts were requested from the PCN and CC. For the 100 selected GAT charts, 81 matching charts were available from the PCN and 73 matching charts were available from CC. Each CC chart had a corresponding PCN chart, leaving 19 of the 100 GAT charts unmatched. The reason for an unmatched chart might have been that the patient’s physician practised outside the community.
Chart extraction methodology
A participant list was developed from the 100 randomly selected GAT charts, with the corresponding participant records from the PCN and CC noted. Data collection from all 3 sets of charts included demographic characteristics, referral patterns (source of and reasons for referrals), and elements specific to the quality of dementia care, based on the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD3) indicators (Box 1).26–30 Recognition of dementia was based on documentation of dementia or MCI using liberal coding criteria (eg, “suspected dementia”). Any chart documentation related to behavioural disturbances, caregiver stress, safety, etc, with the requirements relatively generous (eg, any chart notation), was interpreted as being an “identified” issue. Ethics approval was obtained from the Health Ethics Research Board at the University of Alberta.
Box 1. The Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia indicators of quality of care.
The following are considered indicative of high-quality dementia care:
|
Data from the Third Canadian Consensus Conference on the Diagnosis and Treatment of Dementia.30
Statistical analysis
The data were analyzed to assess the recognition of dementia and MCI by primary care physicians, to examine triggers for specialist referral, and to assess comprehensiveness of care in the primary and community care settings through a comparison of care from GAT and PCN, and GAT and CC health care professionals, respectively. Descriptive nonparametric statistics were used to describe the sample; 2 × 2 contingency tables were created, with diagnostic accuracy examined through sensitivity and specificity; descriptive statistics (frequency counts expressed in percentages of total number of triggers with some of the referrals having multiple triggers) were used to examine triggers for referrals; and the McNemar test was used to compare data on selected indices of dementia care between the GAT and PCN and between GAT and CC. Specifically, the McNemar test was used to test the difference between correlated proportions for each variable of interest, with the McNemar test applied to a 2 × 2 contingency table with 2 dichotomous outcomes from the same group of participants to determine whether the row marginal frequency and the column marginal frequency were equal.
RESULTS
The demographic characteristics for the sample as a whole (N = 100) are provided in Table 1, compared with the matched sample from the PCN (n = 81) and the matched sample from CC (n = 73). The mean age and age range were relatively consistent across the 3 samples. The rest of the demographic characteristics were very similar across the GAT and PCN samples. However, compared with the GAT and PCN samples, the CC sample had higher percentages of women and those who were widowed, living alone, and living in assisted living. All patients had family physicians, and two-thirds of the sample had home care involvement.
Table 1.
Demographic characteristics of sample
| VARIABLE | GAT (N = 100) | PCN (N = 81) | CC (N = 73) |
|---|---|---|---|
| Age, y | |||
| • Mean (SD) | 80.9 (7.43) | 81.2 (7.35) | 82.2 (6.55) |
| • Range | 56–96 | 56–96 | 65–96 |
| Sex, % | |||
| • Female | 55 | 57 | 62 |
| Marital status, % | |||
| • Married, common-law | 52 | 51 | 43 |
| • Widowed | 40 | 41 | 51 |
| • Separated, divorced, never married | 8 | 9 | 7 |
| Living arrangements, % | |||
| • With spouse or family member | 57 | 57 | 49 |
| • Alone | 39 | 42 | 45 |
| • Other | 4 | 3 | 6 |
| Residence, % | |||
| • House or apartment | 72 | 72 | 62 |
| • Assisted living | 27 | 27 | 37 |
| • Long-term care | 1 | 1 | 1 |
| Family physician, % | |||
| • Yes | 100 | 100 | 100 |
| Home care involvement, % | |||
| • Yes | 67 | 65 | NA |
CC—community care, GAT—geriatric assessment team, NA—not applicable, PCN—primary care network.
Accuracy of identification of dementia
The GAT assessments were reviewed according to the CCCDTD3 guidelines30 and the adherence was high (Tables 2 and 3)28,29 on most of the dementia quality indicators. To determine the accuracy of the identification of dementia and MCI by the PCN physicians, we compared diagnostic data from the PCN and GAT. Among the 81 patients with both GAT and PCN charts (Table 4), 41 of the 69 patients diagnosed with dementia by the GAT had a diagnosis of dementia documented by the PCN (sensitivity of 59%). For MCI (Table 5), none of the patients diagnosed with MCI by the GAT had been identified as having MCI by the PCN physicians (sensitivity of 0%).
Table 2.
Comparison of family physician and GAT data on selected indices of dementia assessment and care
| VARIABLE | PCN, % (N = 81) | GAT, % (N = 81) | P VALUE |
|---|---|---|---|
| Diagnosis of dementia documented | |||
| • On referral to GAT | 52 | 37 | .02 |
| Cognitive testing | |||
| • Any cognitive testing performed | 44 | 100 | < .001 |
| Cognitive tests used* | |||
| • MMSE | 44 | 100 | < .001 |
| • MoCA | 7 | 32 | < .001 |
| • CDT | 12 | 88 | < .001 |
| • Other | 3 | 15 | .01 |
| ADLs | |||
| • Assessment of BADLs28 | 17 | 100 | < .001 |
| • Assessment of IADLs29 | 17 | 100 | < .001 |
| Safety | |||
| • Driving status explored | 30 | 99 | < .001 |
| • Wandering explored | 17 | 88 | < .001 |
| Medicolegal | |||
| • Personal directive explored | 6 | 99 | < .001 |
| • EPOA explored | 10 | 99 | < .001 |
| • DMC assessment explored | 5 | 39 | < .001 |
| • DMC assessment provided | 4 | 36 | < .001 |
| • Elder abuse explored | 1 | 26 | < .001 |
| BPSD | |||
| • Identification of BPSD | 46 | 100 | < .001 |
| Caregiver stress | |||
| • Caregiver coping or stress explored | 20 | 53 | < .001 |
| CC services | |||
| • Referral to CC services | 16 | 57 | < .001 |
ADLs—activities of daily living, BADLs—basic activities of daily living, BPSD—behavioural and psychological symptoms of dementia, CC—community care, CDT—clock-drawing test, DMC—decision-making capacity, EPOA—enduring power of attorney, GAT—geriatric assessment team, IADLs—instrumental activities of daily living, MMSE—Mini-Mental State Examination, MoCA—Montreal Cognitive Assessment, PCN—primary care network.
Out of those for whom cognitive testing was performed.
Table 4.
Contingency table of dementia diagnosis: Sensitivity = 0.59; specificity = 0.92; PPV = 0.98; NPV = 0.28.
|
GAT (CRITERION STANDARD)
|
||||
|---|---|---|---|---|
| YES | NO | TOTALS | ||
| PCN | YES | 41 | 1 | 42 |
| NO | 28 | 11 | 39 | |
| TOTALS | 69 | 12 | 81 | |
GAT—geriatric assessment team, NPV—negative predictive value, PCN—primary care network, PPV—positive predictive value.
Table 5.
Contingency table of MCi diagnosis: Sensitivity = 0; specificity = 0.99; PPV = 0; NPV = 0.85.
|
GAT (CRITERION STANDARD)
|
||||
|---|---|---|---|---|
| YES | NO | TOTALS | ||
| PCN | YES | 0 | 1 | 1 |
| NO | 12 | 68 | 80 | |
| TOTALS | 12 | 69 | 81 | |
GAT—geriatric assessment team, MCI—mild cognitive impairment, NPV—negative predictive value, PCN—primary care network, PPV—positive predictive value.
Triggers for referral from primary care
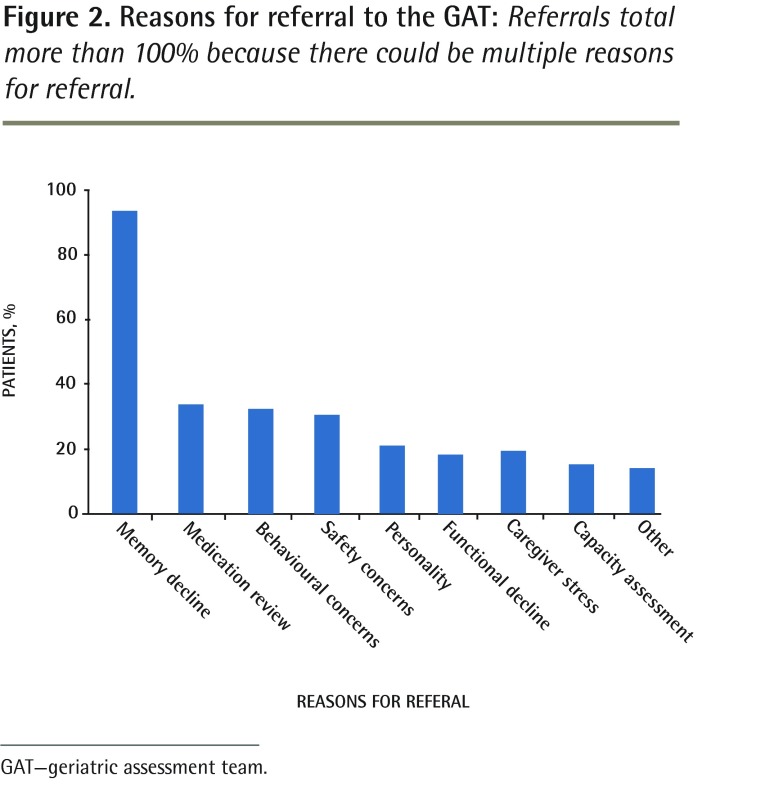
The reasons for referral from the PCN to the GAT are provided in Figure 2. Memory decline was the most frequent reason for referral, followed by requests for medication review, assessment of behavioural disturbances, and concerns about safety. Less common reasons for referral included concerns about personality change, functional decline, caregiver stress, and decision-making capacity (DMC) assessment. Other reasons for referral included fatigue, frailty, and placement for supportive living or long-term care.
Figure 2.
Reasons for referral to the GAT: Referrals total more than 100% because there could be multiple reasons for referral.
GAT—geriatric assessment team.
Identification and comprehensiveness of care
Comparison of PCN and GAT data
The data on quality indicators of dementia care from the PCN charts and corresponding GAT charts are provided in Table 2. There were statistically significant differences between PCN and GAT data on all measures, with the GAT more likely to administer a cognitive test (44% vs 100%; P < .001); to assess basic activities of daily living (BADLs) and instrumental activities of daily living (IADLs) (17% vs 100%; P < .001); and to attend to safety issues such as driving (30% vs 99%; P < .001) and wandering (17% vs 88%; P < .001). There was very little in the way of documentation in the PCN charts related to personal directives (PD) and enduring power of attorney (EPOA), whereas by far most GAT charts had documentation in these 2 areas (P < .001). Exploration of DMC was documented in only 5% of the primary care charts, with assessment of DMC provided in 4% of cases. On the other hand, DMC was documented as being explored in 39% of GAT charts, with assessment of DMC documented in 36% of these patients. Behavioural and psychological symptoms of dementia (BPSD) were documented in 46% of the primary care charts. Conversely, the GAT inquired about BPSD on 100% of the referrals (P < .001). Two domains not routinely addressed in the primary care setting or by the GAT were elder abuse and caregiver stress. Referrals to CC were less likely to occur from the PCN setting than from the GAT (16% vs 57%; P < .001).
Comparison of CC and GAT data
The data on quality indicators of dementia care from CC charts and the corresponding GAT charts are provided in Table 3. As can be seen, CC patients had a documented diagnosis of dementia about half the time (45%). With respect to cognitive testing, 79% of CC charts had some type of cognitive testing documented versus 100% of the corresponding GAT charts, a difference that was statistically significant (P < .001). The Mini-Mental State Examination26 was the most commonly used instrument (77% by CC and 100% by GAT; P < .001). The clock-drawing test was used less commonly by CC as compared with the GAT (47% vs 88%, respectively; P < .001). The Montreal Cognitive Assessment27 was used infrequently in both settings, but with higher rates of use by the GAT (6% vs 32%, respectively; P < .001). Overall, at least 2 types of tests were documented as used to assess cognitive functioning in half of the CC and GAT charts, with 3 tests documented as used to assess cognitive functioning on one-third of the CC and the GAT charts. In terms of assessing BADLs and IADLs, documentation on BADLs was high in both CC and GAT charts (93% vs 100%, respectively), a difference that was not statistically significant (P = .06). However, only 89% of the CC charts had documentation for IADLs, compared with 100% of the GAT charts (P = .01). Driving status was documented in 70% of the CC charts and in 99% of the GAT charts (P < .001). The same pattern of findings was evident for inquiry on PD and EPOA, with documentation in 73% and 64%, respectively, of the CC charts compared with 99% for both PD and EPOA in the GAT charts (P < .001). Exploration of elder abuse was documented in only 30% of GAT charts and fewer (14%) of the CC charts (P = .03). Documentation of BPSD was high for CC (88%) but BPSD was always documented in the GAT charts (100%), a difference that was statistically significant (P = .004). Documentation of inquiry into caregiver stress was found in approximately half the CC and GAT charts (P > .83). Finally, of the 73 patients who were common to CC and GAT, GAT referred 59% to CC for further care.
Table 3.
Comparison of CC and GAT data on selected indices of dementia assessment and care
| VARIABLE | CC, % (N = 73) | GAT, % (N = 73) | P VALUE |
|---|---|---|---|
| Diagnosis of dementia documented | |||
| • On referral to GAT | 45 | 44 | > .999 |
| Cognitive testing | |||
| • Any cognitive testing performed | 79 | 100 | < .001 |
| Cognitive tests used* | |||
| • MMSE | 77 | 100 | < .001 |
| • MoCA | 6 | 32 | < .001 |
| • CDT | 47 | 88 | < .001 |
| • Other | 7 | 15 | .39 |
| Number of cognitive tests used* | |||
| • 1 | 38 | 9 | |
| • 2 | 55 | 54 | |
| • 3 | 34 | 31 | |
| • 4 | 2 | 6 | |
| ADLs | |||
| • Assessment of BADLs28 | 93 | 100 | .06 |
| • Assessment of IADLs29 | 89 | 100 | .01 |
| Safety | |||
| • Driving status explored | 70 | 99 | <.001 |
| • Wandering explored | 73 | 88 | .04 |
| Medicolegal | |||
| • Personal directive explored | 73 | 99 | <.001 |
| • EPOA explored | 64 | 99 | <.001 |
| • DMC assessment explored | NA† | 36 | NA |
| • DMC assessment provided | NA† | 33 | NA |
| • Elder abuse explored | 14 | 30 | .03 |
| BPSD | |||
| • Identification of BPSD | 88 | 100 | .004 |
| Caregiver stress | |||
| • Caregiver coping or stress explored | 55 | 52 | 0.832 |
| CC services | |||
| • Referral to CC services | NA‡ | 59 | NA |
ADLs—activities of daily living, BADLs—basic activities of daily living, BPSD—behavioural and psychological symptoms of dementia, CC—community care, CDT—clock-drawing test, DMC—decision-making capacity, EPOA—enduring power of attorney, GAT—geriatric assessment team, IADLs—instrumental activities of daily living, MMSE—Mini-Mental State Examination, MoCA—Montreal Cognitive Assessment, NA—not applicable.
Out of those for whom cognitive testing was performed.
CC does not conduct capacity assessments.
CC cannot refer to itself.
DISCUSSION
Results from our study indicate that identification and management of dementia remains a challenge in primary care settings, with recognition of MCI being even more difficult. Our results are consistent with previous studies.8,12,31–34 With the aging of the population, the number of patients presenting with dementia will increase substantially over the next several decades.1,2 This will place a great strain on primary care physicians unless the barriers, such as lack of training,4,5,7 time constraints,3–8 and issues of reimbursement,3,5,6,11 are addressed. Specifically, changes are needed at the individual (eg, practitioner19,21,32), system (eg, support staff, funding, resources, partners in care6,10,15), and societal level (eg, public education,5,11 ongoing research and knowledge translation activities related to dementia care). Results of this research have identified current strengths and areas for improvement in dementia care at each of these levels, including an urgent need for system coordination.
Family physicians play a key role in dementia care.35,36 Our study results indicate that there is underuse of diagnostic and functional assessment tools, lack of attention to caregiver issues, and underuse of community supports.14,16,36 Our results also indicate that quality indicators such as assessment of wandering and driving14 were inadequately assessed in the primary care setting, which is of concern, as both are matters of individual and public safety.37,38 Attention to medicolegal issues also was suboptimal. This might reflect either deficiencies in important elements of dementia care or reliance on the GAT to complete necessary and underused assessments. Although access to the GAT was attained in a relatively short time, the quality indicators requiring attention that were not addressed by the primary care physicians might have existed for an extended period of time requiring attention, and in some situations might have delayed important interventions (eg, addressing wandering or other risks, or addressing reversible causes of cognitive decline). Understanding the reasons for referral serves a 2-fold purpose. They serve as red flags for screening patients for dementia that should be recognized by primary care physicians. They also can help primary care physicians identify patients that require assessment and management by a specialized team.
It is interesting to note that there was greater congruence between the GAT and CC in assessment and care of patients with dementia and MCI than between PCN and GAT. One of the reasons for this finding could be that the GAT and CC are located in the same building, which facilitates communication and collaboration.23,24 For example, despite two-thirds of the patients being “in” CC, more than half of this sample was referred to CC by GAT for additional services, highlighting the unmet needs of patients and caregivers. In our study, CC strengths included a comprehensive approach to assessment, with attention to assessment of overall functional status, use of cognitive tests to assess cognitive status, identification of dementia-related issues such as BPSD, and attention to safety issues. Thus, results from our study support the inclusion of CC as a valuable health care partner in dementia care.35 However, our results also indicate that there is considerable duplication in assessments (eg, multiple cognitive tests and functional inquiries for the same patient) across the settings. This duplication not only wastes valuable health care resources, but also has the potential to confuse patients and families as a result of their receiving information from different sources at the same time.39–41 This observation is helpful in streamlining communication and information sharing between the care partners involved. For example, the cognitive testing or a functional inquiry completed by CC could be used in GAT assessments. Inclusion of this information in the referral package or availability of an electronic database accessible to the PCN, CC, and the GAT will decrease the potential for duplication.
Overall, results from this study and from the existing literature suggest there is a need to transition from a fragmented model of dementia care6,15,16,34 to a more integrated model within our primary care setting.23,24,42,43 In our region, there are opportunities for synergistic collaboration of health care professionals from the PCN, CC, and the GAT.19,36,39 Collaborative models of care can result in increased adherence to dementia care guidelines, increased use of community agencies and implementation of safety measures, and greater confidence in caregiving.18–20,44 Further system changes, including administrative coordination and collaborative research efforts, are needed to facilitate PCN integration with CC. Results from our research add to the growing body of knowledge in this area, and underscore the importance of the need for more collaborative models of dementia care.
Limitations
This study was retrospective in nature. As such, the data might be limited by variability in documentation of information related to identification and management across the respective charts. This limitation is particularly relevant for primary care, where conversations about the indicators of high-quality care might have occurred but not been recorded in the charts. We have no information on the number of different physicians treating patients in this sample, which might have been useful in interpreting the data, as the practice style and knowledge of each physician might influence the accuracy of dementia diagnosis. Further, although the GAT followed CCCDTD3 guidelines,30 the accuracy of diagnosis and management might be limited by there only being a single care of the elderly physician on the team. A more rigorous methodology would involve chart audit by knowledgeable peers to confirm agreement. A further limitation is that 19% of the corresponding PCN charts were not available, which reduced the sample size. However, given the consistency in the pattern of findings from the remaining 81 charts, it is unlikely that data from an additional 19 charts would have substantially altered the findings. Finally, the PCN was less likely than the GAT to record a diagnosis of dementia or MCI early, but it is unknown whether this would lead to less optimal outcomes (eg, more mortality or disability). A prospective study or an examination of long-term data could help to inform us on these issues.
Conclusion
Increased longevity and the aging of the baby boomers have resulted in an increasing number of individuals with dementia. The effects of the disease are substantial from both a human and financial perspective. Despite the presence of various services to attend to the needs of seniors, early identification and management of dementia in the primary care setting continues to be challenging. Our findings on reasons for referral can be used to inform screening of patients for dementia assessments and to target the most appropriate patients for further evaluation by the GAT. The awareness of this information and distinction needs to become part of dementia care in a collaborative environment. Results from this study demonstrate there are inconsistencies in assessment of dementia in primary care settings, with these results underscoring the need for integration. The presence of 3 types of service providers gives us the opportunity to move toward a more collaborative model of care that would allow for coordination and consistency in the provision of services, and avoidance of duplication of services, which in turn will assist in building capacity to meet the needs of an aging population. Further research will show if collaborative models improve overall quality of dementia diagnosis and care at the primary care level.
Acknowledgments
We thank the members of the Westview primary care network, community care, and geriatric health team for their collaborative efforts in this project.
EDITOR’S KEY POINTS
Increased longevity and the aging of the baby boomers have resulted in an increasing number of individuals with dementia. The effects of the disease are substantial from both a human and a financial perspective. Despite the presence of various services to attend to the needs of seniors, early identification and management of dementia in the primary care setting continues to be challenging.
The authors found inconsistencies in the assessment of dementia in the primary care settings studied, underscoring the need for integration in dementia care. Reasons for referral—most commonly memory decline, but also medication review, assessment of behavioural disturbances, and concerns about safety—can be used to inform screening of patients for dementia assessments and to target the most appropriate patients for further evaluation.
POINTS DE REPÈRE DU RÉDACTEUR
L’augmentation de la longévité et le vieillissement des baby-boomers ont eu comme effet d’augmenter les cas de démence. Les effets de cette maladie sont importants tant sur le plan humain que financier. Malgré la présence de plusieurs services pour répondre aux besoins des aînés, l’identification et le traitement de la démence dans le contexte des soins primaires demeurent problématiques.
Les auteurs ont observé des inconsistances dans l’évaluation de la démence au niveau des établissements de soins primaires, soulignant le besoin d’une meilleure intégration du traitement de cette condition. On peut se servir des raisons de consulter – le plus souvent des problèmes de mémoire, mais aussi une revue de la médication, une évaluation pour des troubles du comportement et des inquiétudes pour la sécurité – pour déterminer quels patients nécessitent une évaluation de la démence et préciser ceux qui ont le plus besoin d’une évaluation plus poussée.
Footnotes
This article has been peer reviewed.
Contributors
Dr Parmar conceived and designed the research, interpreted the data, reviewed and revised the manuscript, and approved the version to be published.
Dr Dobbs contributed to the concept and design of the study, analysis and interpretation of the data, and drafting and revising the manuscript, and approved the version to be published. Ms McKay contributed to data entry and cleaning, the literature reviews, and analysis and interpretation of data; assisted with revisions to all drafts of the manuscript and creation of tables, figures, and references; and approved the version to be published. Ms Kirwan provided assistance with literature reviews and revisions to early drafts of the manuscript, and approved the version to be published. Dr Cooper provided substantial contributions to acquisition of data, analysis and interpretation of data, and drafting of an early version of the manuscript, and approved the version to be published. Dr Marin contributed to study design, made revisions to later drafts of the manuscript with substantial input on the discussion of findings in terms of clinical and practice implications, and gave approval of the version to be published. Dr Gupta provided assistance with the literature reviews and synopsis of results from the relevant literature, revised early drafts of the manuscript, and approved the version to be published.
Competing interests
The authors received unrestricted educational grants from Janssen-Ortho Inc, Lundbeck, and Pfizer Canada Inc.
References
- 1.World Health Organization . Dementia: a public health priority. Geneva, Switz: WHO Press; 2012. Alzheimer’s Disease International. [Google Scholar]
- 2.Smetanin P, Kobak P, Briante C, Stiff D, Sherman G, Ahmad S. Rising tide: the impact of dementia on Canadian society. Toronto, ON: Alzheimer Society of Canada; 2010. [Google Scholar]
- 3.Koch T, Iliffe S. Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: a systematic review. BMC Fam Pract. 2010;11:52. doi: 10.1186/1471-2296-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Hout H, Vernooij-Dassen M, Bakker K, Blom M, Grol R. General practitioners on dementia: tasks, practices and obstacles. Patient Educ Couns. 2000;39(2–3):219–25. doi: 10.1016/s0738-3991(99)00034-8. [DOI] [PubMed] [Google Scholar]
- 5.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306–14. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinton L, Franz CE, Reddy G, Flores Y, Kravitz RL, Barker JC. Practice constraints, behavioral problems, and dementia care: primary care physicians’ perspectives. J Gen Intern Med. 2007;22(11):1487–92. doi: 10.1007/s11606-007-0317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pimlott NJG, Persaud M, Drummond N, Cohen CA, Silvius JL, Seigel K, et al. Family physicians and dementia in Canada. Part 2. Understanding the challenges of dementia care. Can Fam Physician. 2009;55:508–9. e1–7. Available from: www.cfp.ca/content/55/5/508.full.pdf+html. Accessed 2014 Apr 3. [PMC free article] [PubMed] [Google Scholar]
- 8.Ganguli M, Rodriguez E, Mulsant B, Richards S, Pandav R, Vander Bilt J, et al. Detection and management of cognitive impairment in primary care: the Steel Valley seniors survey. J Am Geriatr Soc. 2004;52(10):1668–75. doi: 10.1111/j.1532-5415.2004.52459.x. [DOI] [PubMed] [Google Scholar]
- 9.Turner S, Iliffe S, Downs M, Wilcock J, Bryans M, Levin E, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461–7. doi: 10.1093/ageing/afh140. [DOI] [PubMed] [Google Scholar]
- 10.Iliffe S, Wilcock J. The identification of barriers to the recognition of, and response to, dementia in primary care using a modified focus group approach. Dementia. 2005;4(1):73–85. [Google Scholar]
- 11.Foster NL. Barriers to treatment: the unique challenges for physicians providing dementia care. J Geriatr Psychiatry Neurol. 2001;14(4):188–98. doi: 10.1177/089198870101400404. [DOI] [PubMed] [Google Scholar]
- 12.Valcour VG, Masaki KH, Curb D, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160(19):2964–8. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 13.Hutchings D, Vanoli A, Mckeith I, Brotherton S, Mcnamee P, Bond J. Cholinesterase inhibitors and Alzheimer’s disease: patient, care and professional factors influencing the use of drugs for Alzheimer’s disease in the United Kingdom. Dementia. 2010;9(3):427–43. [Google Scholar]
- 14.Pimlott NJG, Siegel K, Persaud M, Slaughter S, Cohen C, Hollingworth G, et al. Management of dementia by family physicians in academic settings. Can Fam Physician. 2006;52:1108–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Franz CE, Barker JC, Kim K, Flores Y, Jenkins C, Kravitz RL, et al. When help becomes a hindrance: mental health referral systems as barriers to care for primary care physicians treating patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2010;18(7):576–85. doi: 10.1097/jgp.0b013e3181a76df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantegreil-Kallen I, Turbelin C, Angel P, Flahault A, Rigaud A. Dementia management in France: health care and support services in the community. Dementia. 2006;5(3):317–26. [Google Scholar]
- 17.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–72. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns R, Nichols LO, Martindale-Adams J, Graney MJ, Lummus A. Primary care interventions for dementia caregivers: 2-year outcomes from the REACH study. Gerontologist. 2003;43(4):547–55. doi: 10.1093/geront/43.4.547. [DOI] [PubMed] [Google Scholar]
- 19.Vickrey BG, Mittman BS, Connor KI, Pearson ML, Della Penna RD, Ganiats TG, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713–26. doi: 10.7326/0003-4819-145-10-200611210-00004. [DOI] [PubMed] [Google Scholar]
- 20.Downs M, Turner S, Bryans M, Wilcock J, Keady J, Levin E, et al. Effectiveness of educational interventions in improving detection and management of dementia in primary care: cluster randomized controlled study. BMJ. 2006;332(7543):692–6. doi: 10.1136/bmj.332.7543.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chodosh J, Berry E, Lee M, Connor K, DeMonte R, Ganiats T, et al. Effect of a dementia care management intervention on primary care provider knowledge, attitudes, and perceptions of quality of care. J Am Geriatr Soc. 2006;54(2):311–7. doi: 10.1111/j.1532-5415.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 22.Waldorff FB, Almind G, Makela M, Moller S, Waldemar G. Implementation of a clinical dementia guideline: a controlled study on the effect of a multifaceted strategy. Scand J Prim Health Care. 2003;21(3):142–7. doi: 10.1080/02813430310005136. [DOI] [PubMed] [Google Scholar]
- 23.Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–57. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 24.Johansson G, Eklund K, Gosman-Hedstrom G. Multidisciplinary team, working with elderly persons living in the community: a systematic literature review. Scand J Occup Ther. 2010;17(2):101–16. doi: 10.1080/11038120902978096. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 29.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 30.Approved recommendations. Montreal, QC: Canadian Consensus Conference on Diagnosis and Treatment of Dementia; 2007. Third Canadian Consensus Conference on Diagnosis and Treatment of Dementia. [Google Scholar]
- 31.Adams WL, McIlvain HE, Lacy NL, Magsi H, Crabtree BF, Yenny SK, et al. Primary care for elderly people: why do doctors find it so hard? Gerontologist. 2002;42(6):835–42. doi: 10.1093/geront/42.6.835. [DOI] [PubMed] [Google Scholar]
- 32.Boise L, Neal MB, Kaye J. Dementia assessment in primary care: results from a study in three managed care systems. J Gerontol A Biol Sci Med Sci. 2004;59(6):M621–6. doi: 10.1093/gerona/59.6.m621. [DOI] [PubMed] [Google Scholar]
- 33.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138(11):927–37. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 34.Boustani M, Callahan CM, Unverzagt FW, Austrom MG, Perkins AJ, Fultz BA, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20(7):572–7. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings JL, Frank JC, Cherry D, Kohatsu ND, Kemp B, Hewett L, et al. Guidelines for managing Alzheimer’s disease: part II. Treatment. Am Fam Physician. 2002;65(12):2525–34. [PubMed] [Google Scholar]
- 36.Olafsdóttir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linköping study. Dement Geriatr Cogn Disord. 2000;11(4):223–9. doi: 10.1159/000017241. [DOI] [PubMed] [Google Scholar]
- 37.Chow TW, MacLean CH. Quality indicators for dementia in vulnerable community-dwelling and hospitalized elders. Ann Intern Med. 2001;135(8 Pt 2):668–76. doi: 10.7326/0003-4819-135-8_part_2-200110161-00005. [DOI] [PubMed] [Google Scholar]
- 38.Lyketsos CG, Colenda CC, Beck C, Blank K, Doraiswamy MP, Kalunian DA, et al. Position statement of the American Association For Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14(7):561–72. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 39.Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–12. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 40.Robinson A, Elder J, Emden C, Lea E, Turner P, Vickers J. Information pathways into dementia care services: family carers have their say. Dementia. 2009;8(1):17–37. [Google Scholar]
- 41.Robinson A, Emden C, Lea E, Elder J, Turner P, Vickers J. Information issues for providers of services to people with dementia living in the community in Australia: breaking the cycle of frustration. Health Soc Care Community. 2009;17(2):141–50. doi: 10.1111/j.1365-2524.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 42.Woodward CA, Abelson J, Tedford S, Hutchison B. What is important to continuity in home care? Perspectives of key stakeholders. Soc Sci Med. 2004;58(1):177–92. doi: 10.1016/s0277-9536(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 43.Donaldson MS. Continuity of care: a reconceptualization. Med Care Res Rev. 2001;58(3):255–90. doi: 10.1177/107755870105800301. [DOI] [PubMed] [Google Scholar]
- 44.Cockerill R, Jaglal S, Charles LL, Chambers L, Brazil K, Cohen C. Components of coordinated care: a new instrument to assess caregivers’ and care recipients’ experiences with networks of dementia care. Dementia. 2006;5(1):51–66. [Google Scholar]