Abstract
Aims/Introduction: Patients with type 1 diabetes mellitus often show a precipitous postprandial rise in blood glucose that cannot be controlled, even by intensive insulin therapy. The combined use of an α‐glucosidase inhibitor with insulin seems to be highly beneficial in such cases.
Materials and Methods: We investigated the efficacy and safety of miglitol, an α‐glucosidase inhibitor, for 12 weeks in 43 type 1 diabetes patients on intensive insulin therapy.
Results: Co‐administration of miglitol resulted in only a modest and temporal decrease in HbA1c level. However, it resulted in a significant reduction of plasma glucose level after breakfast (250.7 ± 102.0 mg/dL at 2 h after breakfast before treatment; 212.0 ± 95.8 mg/dL at 2 h after breakfast after treatment for 12 weeks, P = 0.01) and a significant reduction of insulin dosage (41.6 ± 17.1 U/day before treatment; 39.8 ± 17.4 U/day 12 weeks after treatment, P < 0.001). During the study period, 88.4% (38/43) of subjects experienced hypoglycemia, but all events were mild except for one case, which was considered to be moderate. No unexpected adverse events were observed during the study period.
Conclusions: Co‐administration of miglitol in type 1 diabetes patients on intensive insulin therapy resulted in an improvement of postprandial hyperglycemia with the reduction of insulin dosage. Considering the importance of postprandial hyperglycemia in the onset of cardiovascular disease, the combination therapy of miglitol and insulin could be advantageous in type 1 diabetes mellitus patients.
Keywords: α‐Glucosidase inhibitor, Insulin, Type 1 diabetes mellitus
Introduction
Multiple injections of insulin is the main form of treatment for type 1 diabetes mellitus (T1DM). However, despite the combination treatment of ultra‐rapid and long‐acting insulin, it is not always easy to achieve an ideal blood glucose diurnal profile. Improvement of postprandial blood glucose remains difficult in some patients.
The Diabetes Control and Complications Trial (DCCT) has confirmed that reductions in blood glucose level in T1DM can reduce the risk of microvascular complications and cardiovascular events1,2. However, at least in type 2 diabetes, not only total glucose exposure, but also glucose fluctuations seem to be associated with the onset of cardiovascular disease3–5. Accordingly, strict blood glucose control with consideration of glucose swings is recommended for the therapeutic aim of suppressing the onset or progression of macrovascular complications in both type 1 and 2 diabetes6.
α‐Glucosidase inhibitor (α‐GI), a drug that inhibits disaccharide‐hydrolyzing enzymes in the small intestine, suppresses postprandial elevation of blood glucose by delaying the digestion and absorption of sugars, and thus flattens out the postprandial fluctuations in plasma glucose. For type 2 diabetes, α‐GI is used in combination therapy with other oral hypoglycemic agents of different mechanisms of action or insulin. In patients with T1DM, the use of α‐GI, which inhibits the postprandial rise in blood glucose without involving endogenous insulin secretion, together with insulin therapy, is considered highly beneficial.
Unlike other types of α‐GI (acarbose, voglibose), miglitol is absorbed in the small intestine. It has been reported that miglitol, compared with similar drugs, strongly suppresses spikes in plasma glucose, particularly soon after meals (0.5–1 h postprandially)7. At the same time, because the time to peak postprandial glucose level is delayed by the administration of miglitol, the incidence of hypoglycemia in between meals decreases7. These findings show that miglitol reduces the range of fluctuations in plasma glucose throughout the day, and combined treatment with miglitol is thought to be effective in patients with T1DM with intensive insulin treatment and that it also prevents the occurrence of hypoglycemia.
In the present study, we investigated the efficacy and safety of 12‐week miglitol therapy in combination with insulin therapy in patients with T1DM.
Materials and methods
Subjects
The study subjects were patients with T1DM who visited Abe Clinic, Asahi Internal Medicine Department Clinic, Fukuoka City Medical Association Hospital, Matsuba Clinic, Matsunami General Hospital, Misaki Internal Medicine Clinic, Naka Memorial Clinic or Jinnouchi Hospital from August 2004 to September 2005, and had been diagnosed with T1DM by diabetes specialists in past medical treatment. The inclusion criteria were: (i) plasma glucose at 1 or 2 h after meal ≥180 mg/dL; (ii) HbA1c≥6.5%; (iii) patients treated with multiple self‐injections of insulin; and (iv) age ≥20 years.
Study Protocol
The study consisted of a 4‐ to 10‐week observation period and a 12‐week treatment period. During the treatment period, all subjects were instructed to take a 50 mg miglitol tablet immediately before meals three times a day. Throughout the observation and treatment periods, the dosage of insulin was adjusted to achieve blood glucose control as judged by the attending physician; however, changes in the type of insulin or drugs were prohibited. Also, subjects were instructed to follow the same diet and exercise therapy. A meal tolerance test was carried out at the start of treatment and after 12 weeks of treatment in order to measure postprandial plasma glucose. Plasma glucose was measured before breakfast and at 0.5, 1 and 2 h after the start of breakfast. Each participating medical facility tried to prepare similar content for breakfast. The content of breakfast served before and after the treatment in each patient was the same. All undesirable events (symptoms and signs; abnormal fluctuations in laboratory values) that appeared after the start of the administration of miglitol were collected as adverse events. Adverse events, other than those for which any relation to the study drug could be ruled out, were taken as adverse drug reactions. General blood tests, blood chemistry tests, and urinalysis including HbA1c and 1,5‐anhydroglucitol (1,5‐AG) were carried out at the start of treatment and at 4, 8 and 12 weeks of treatment. In order to check for symptoms of hypoglycemia, each subject was provided with a hypoglycemia notebook in which they recorded their condition at the time of the appearance of hypoglycemic symptoms during the treatment period. The present study was carried out in accordance with the Declaration of Helsinki and with approval from the Institutional Review Board of the medical institution. A signed written informed consent was obtained from each patient before starting the study.
Statistical Analysis
Statistical analysis of both efficacy and safety was carried out for data from all the subjects who proceeded to the treatment phase. The basic statistics for efficacy analysis were calculated at each time‐point and a paired one‐sample t‐test for the baseline was carried out. Data at 12 weeks of treatment or at the time of withdrawal were used as data for the end of treatment. For safety analysis, the numbers and incidence of adverse events and adverse drug reactions were calculated. Level of significance was two‐tailed 5%.
Results
The investigational drug was given to 43 patients (22 men, 21 women; mean age 43.7 ± 13.2 years, ±SD, Table 1) with body mass index of 22.9 ± 2.8 kg/m2 and disease duration of 13.0 ± 7.2 years. The details of insulin therapy and the number of patients who received each therapy are as follows: once‐daily injections of long‐acting insulin with bolus doses of rapid‐acting insulin given three times daily at mealtime (n = 18); once‐daily injections of NPH insulin with bolus doses of rapid‐acting insulin given three times daily at mealtimes (n = 7); twice‐daily injections of NPH insulin with bolus doses of rapid‐acting insulin given three times daily at mealtimes (n = 5); once‐daily injections of long‐acting insulin with bolus doses of regular insulin given three times daily at mealtime (n = 4); once‐daily injections of NPH insulin with bolus doses of regular insulin given three times daily at mealtimes (n = 2); three times daily injections of premixed insulin (n = 4); and twice‐daily injections of premixed insulin (n = 3). Treatment was discontinued in four patients. Two patients withdrew because of diarrhea and edema. The remaining two patients wished to not continue in the present study.
Table 1. Characteristics of study subjects before treatment.
| No. patients | 43 |
| Sex (male/female) | 22/21 |
| Age (years) | 43.7 ± 13.2 |
| Body mass index (kg/m2) | 22.9 ± 2.8 |
| Duration of diabetes (years) | 13.0 ± 7.2 |
| Instructed calorie intake (kcal/day) | 1633 ± 196 |
| Instructed calorie intake (kcal/kg/day) | 27.4 ± 4.2 |
| Daily insulin dosage (U/day) | 41.6 ± 17.1 |
| HbA1c (%) | 7.68 ± 0.89 |
| Fasting glucose (mg/dL) | 217.6 ± 82.1 |
| 1 h postprandial glucose (mg/dL) | 274.6 ± 91.8 |
| 2 h postprandial glucose (mg/dL) | 250.7 ± 102.0 |
Data are number of patients (categorized data) or mean ± SD (quantitative data).
Figure 1 shows the serial changes in plasma glucose levels after a meal, before and after treatment. Levels at the start of treatment were 217.6 ± 82.1 mg/dL (n = 43) before breakfast, 261.4 ± 83.8 mg/dL (n = 43) at 0.5 h, 274.6 ± 91.8 mg/dL (n = 43) at 1 h and 250.7 ± 102.0 mg/dL (n = 43) at 2 h after the meal. At the end of treatment they were 208.7 ± 88.0 mg/dL (n = 41), 199.5 ± 85.7 mg/dL (n = 39), 199.4 ± 91.3 mg/dL (n = 39) and 212.0 ± 95.8 mg/dL (n = 39), respectively, and the differences at 0.5, 1 and 2 h after the meal were significant (one‐sample t‐test; P < 0.001, P < 0.001 and P = 0.01, respectively).
Figure 1.
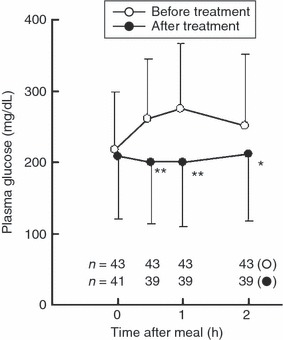
Miglitol reduces postprandial glucose levels. Serial changes in plasma glucose levels before and after breakfast. The treatment with miglitol was discontinued in four subjects and the meal tolerance test after treatment was not carried out with them. Thus, the postprandial blood glucose data from these four cases are not included. In two of these four discontinued cases, only fasting blood glucose level was measured and included as fasting blood glucose level. Data are mean ± SD. *P < 0.01, **P < 0.001 compared with before treatment.
Figure 2 shows the serial changes in HbA1c levels during the 12‐week treatment. HbA1c level decreased slightly from 7.68 ± 0.89% before treatment to 7.56 ± 0.87% at 4 weeks, 7.57 ± 0.92% at 8 weeks and 7.59 ± 0.97% at 12 weeks of treatment, and the decrease was significant at 4 weeks of treatment (one‐sample t‐test, P = 0.023).
Figure 2.
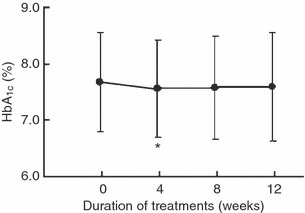
Serial changes in HbA1c levels during the treatment period. Data are mean ± SD. *P < 0.05 compared with before treatment.
Figure 3 shows the serial changes in 1,5‐AG levels during the 12‐week treatment. 1,5‐AG rose from 2.87 ± 2.06 μg/mL at the start of treatment to 4.94 ± 3.55 μg/mL at 4 weeks of treatment, 5.16 ± 4.16 μg/mL at 8 weeks and 4.87 ± 4.01 μg/mL at 12 weeks of treatment, and the increases at 4, 8 and 12 weeks of treatment were significant (one‐sample t‐test, all P < 0.001).
Figure 3.
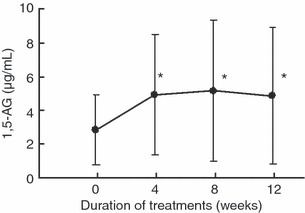
Serial changes in 1, 5‐AG levels during the treatment period. Data are mean ± SD. *P < 0.001 compared with before treatment.
The incidence of adverse events was 97.7%, and the major symptoms and signs were hypoglycemia and digestive symptoms. Hypoglycemia was observed in 38 of the 43 (88.4%) patients, and the frequency of hypoglycemia over the course of 30 days was 4.11 ± 3.99 times/person/30 days. One patient with loss of consciousness that required treatment with glucagon was judged to have developed moderate hypoglycemia, whereas the others were considered to have developed mild hypoglycemia and improved without treatment when patients took glucose, supplements or meals. The major digestive symptoms were flatulence (20.9% [9/43]), abdominal distention (14.0% [6/43]) and diarrhea (11.6% [5/43)]), most of which occurred in the early phase of treatment.
Bodyweight was 60.9 ± 10.0 kg at the start of treatment, 61.3 ± 10.1 kg at 4 weeks, 61.0 ± 10.5 kg at 8 weeks and 60.9 ± 10.8 kg at 12 weeks of treatment. Although there was a significant increase at 4 weeks compared with the start of treatment (P = 0.015), no such increase was noted at 12 weeks of treatment.
The dosage of insulin at the end of treatment, including subjects for whom administration of the test drug was discontinued, was decreased in 17 subjects, increased in 2 and was unchanged in the remaining 24 subjects. The total amount of insulin used per day was 41.6 ± 17.1 U/day before treatment and 39.8 ± 17.4 U/day at the end of the treatment, with a change of −1.9 ± 3.0 U/day (one‐sample t‐test, P < 0.001).
The subjects were categorized into two groups: the 17 in whom the dosage of insulin was decreased and the other 26. The background data are shown in Table 2, and the HbA1c and plasma glucose levels in Table 3. In the insulin reduction group, the change in insulin dosage was −4.9 ± 2.8 U/day. In the insulin dose increase or no change group, the change in insulin dosage was +0.1 ± 2.8 U/day. Although no marked differences in background data were observed between the two groups, the age was somewhat higher and the HbA1c values somewhat lower for the subjects for whom insulin was reduced. In both groups, a similar inhibition of postprandial elevation of plasma glucose level as a result of administration of miglitol was observed, and favorable control over plasma glucose levels was shown after the reduction in dosage of insulin. With regard to bodyweight change, a modest decrease in bodyweight was observed in the insulin reduction group, whereas an increase in bodyweight was observed in the insulin dose increase or no change group, at the end‐point. The change in bodyweight was significantly different between the two groups (insulin reduction group, −0.5 ± 1.2 kg vs insulin dose increase or no change group, +0.6 ± 1.2 kg; P = 0.006).
Table 2. Characteristics of patients categorized by the change in daily insulin dosage after administration of miglitol.
| Insulin dose reduction group | Insulin dose increase or no change group | P‐values | |
|---|---|---|---|
| No. patients | 17 | 26 | – |
| Sex (male/female) | 9/8 | 13/13 | – |
| Age (years) | 46.7 ± 12.5 | 41.8 ± 13.5 | 0.236 |
| Body mass index (kg/m2) | 22.6 ± 2.6 | 23.2 ± 3.0 | 0.534 |
| Duration of diabetes (years) | 12.0 ± 6.9 | 13.7 ± 7.5 | 0.433 |
| Instructed calorie intake (kcal/day) | 1599 ± 159 | 1655 ± 216 | 0.360 |
| Instructed calorie intake (kcal/kg/day) | 27.2 ± 3.8 | 27.5 ± 4.5 | 0.871 |
| Daily insulin dosage (U/day) | 40.6 ± 13.2 | 42.3 ± 19.4 | 0.751 |
Data are number of patients (categorized data) or mean ± SD (quantitative data).
Table 3. Changes in HbA1c and blood glucose levels according to change in daily insulin dosage after administration of miglitol.
| Insulin dose reduction group | Insulin dose increase or no change group | P‐value* | |||
|---|---|---|---|---|---|
| Baseline | End‐point | Baseline | End‐point | ||
| Insulin dose (U/day) | 40.6 ± 13.2 | 35.7 ± 13.5 | 42.3 ± 19.4 | 42.4 ± 19.3 | |
| Change | −4.9 ± 2.8 | +0.1 ± 0.4 | <0.001 | ||
| HbA1c (%) | 7.36 ± 0.65 | 7.39 ± 0.79 | 7.89 ± 0.98 | 7.78 ± 1.03 | |
| Change | +0.03 ± 0.54 | −0.11 ± 0.78 | 0.532 | ||
| Fasting blood glucose (mg/dL) | 211.5 ± 89.7 | 204.6 ± 89.0 | 221.6 ± 78.3 | 211.6 ± 89.2 | |
| Change | −6.9 ± 81.8 | −13.7 ± 75.4 | 0.786 | ||
| 0.5 h postprandial glucose (mg/dL) | 265.5 ± 90.5 | 192.9 ± 82.1 | 258.8 ± 80.9 | 204.1 ± 89.5 | |
| Change | −64.7 ± 60.7 | −61.0 ± 72.5 | 0.870 | ||
| 1 h postprandial glucose (mg/dL) | 270.0 ± 110.1 | 182.3 ± 93.6 | 277.6 ± 79.9 | 211.3 ± 89.9 | |
| Change | −82.5 ± 77.5 | −70.8 ± 89.9 | 0.676 | ||
| 2 h postprandial glucose (mg/dL) | 253.5 ± 116.8 | 191.6 ± 101.9 | 248.9 ± 93.5 | 226.2 ± 90.9 | |
| Change | −58.6 ± 95.5 | −28.5 ± 79.1 | 0.290 | ||
| Bodyweight (kg) | 59.7 ± 8.6 | 59.3 ± 8.6 | 61.7 ± 10.9 | 62.3 ± 11.4 | |
| Change | −0.5 ± 1.2 | +0.6 ± 1.2 | 0.006 | ||
Data are mean ± SD. *Two‐sample t‐test.
Discussion
In the present study, 43 T1DM patients receiving intensive insulin therapy were given miglitol in combination with the usual therapy for a period of 12 weeks. Although no significant reduction in HbA1c was noted under the combination treatment, postprandial elevation of plasma glucose was suppressed by the administration of miglitol. In addition, the administration of miglitol reduced the required insulin dose. In terms of adverse events, no serious hypoglycemia or gastrointestinal symptoms were observed, and the drug was well tolerated. Regarding the incidence of hypoglycemia, only one moderately severe case requiring the injection of glucagon was observed, whereas all the other symptoms were mild.
The DCCT study showed that strict control of plasma glucose levels is important in diabetes patients in order to prevent the development and progression of vascular disorders1,2. However, the results of UKPDS 358 showed that the correlation between the development of macroangiopathy and HbA1c level is not as strong as that existing in the case of microvascular disease. In contrast, the results of the DECODE/DECODA study3,4 and the Funagata Diabetes Study5 clearly showed postprandial hyperglycemia to be an independent risk factor in cardiovascular events, which suggests that strict control of plasma glucose levels, including not only HbA1c but also postprandial hyperglycemia, is important from the viewpoint of preventing the progression of macroangiopathy. In recent years, it has been reported that repeated sharp fluctuations in plasma glucose can induce apoptosis of vascular endothelial cells9, increase oxidative stress10 and promote the appearance of adhesion molecules11. The mechanism whereby repeated postprandial hyperglycemia triggers vascular disorders is being elucidated and results have shown that endothelial disorders caused by postprandial hyperglycemia can be improved by correcting the hyperglycemia with drug therapy. The DCCT/EDIC study, in which T1DM patients were enrolled, also confirmed that the incidence of cardiovascular events can be reduced by strictly controlling fluctuations in plasma glucose through intensive insulin therapy12. Based on these reports, we postulate that the inhibition of postprandial hyperglycemia and the flattening out of postprandial fluctuations in plasma glucose by the miglitol–insulin combination treatment in T1DM patients could prevent the occurrence and progression of macroangiopathy in such patients. Further studies are required to confirm this hypothesis.
One of the objectives of the combined use of oral diabetes drugs in diabetic patients treated with insulin is to reduce the required dosage of insulin and lower the risk of hypoglycemia. Weight gain can also become a problem in patients receiving insulin therapy and the ability to reduce the risk of weight gain by lowering the dosage of insulin is thought to be another advantage. In 17/43 subjects in the present study, the combined use of miglitol made it possible to reduce the daily dosage of insulin. In order to assess the advantages and disadvantages of reducing insulin dosage, we used the data obtained from this study to carry out comparisons of baseline values and plasma glucose control status between subjects in whom insulin dosage was reduced and those in whom it was not. Comparison of the baseline values showed that the age was somewhat higher and the HbA1c values somewhat lower for the subjects for whom insulin was reduced. This result might suggest that insulin dose tends to be reduced for older patients with better glycemic control receiving miglitol treatment. In contrast, both the fasting and postprandial plasma glucose levels were about the same as for the other subjects. The data obtained at the end of treatment with miglitol showed that postprandial elevation of plasma glucose level was similarly inhibited in both the subjects in whom insulin dosage was reduced and those in whom it was not, and no deterioration in HbA1c values as a result of reduced insulin dosage was observed. These results suggest that good plasma glucose control is maintained even after reducing the dosage of insulin. Furthermore, bodyweight tended to decrease in the subjects receiving the reduced dosage of insulin, suggesting that plasma glucose can be controlled without triggering weight gain by adopting a combination treatment with miglitol that allows for reduction of the insulin dosage. Recently, we found that miglitol increases plasma GLP‐1 level after meals7. Considering that GLP‐1 suppresses appetite and glucagon secretion, the patients with a high response of GLP‐1 secretion by miglitol should show the decreased bodyweight by the reduced appetite and required insulin dose as the result of the reduction of glucagon secretion. Accordingly, the difference observed by the analysis in the present study between insulin dose reduction group and insulin dose increase or no change group might be derived at least in part from the response of GLP‐1. Several parameters, including GLP‐1 concentration, are to be measured to examine the details of this hypotheses in future research.
The incidence of hypoglycemia in the present study was 88.4%, at a frequency of 4.11 times/person/30 days. There are numerous reports on the prevalence of hypoglycemia among patients with T1DM, as in Japanese (3.93 times/person/30 days)13 and overseas (3.46 times/person/30 days)14 studies of insulin glulisine and the overseas study of insulin aspart (3.7 times/person/30 days)15, and the incidence of hypoglycemia in the present study is not greatly different from these reference reports.
There are several limitations of the present study. This study was carried out as an open study, in which a control group was not set. Additionally, the adjustment of insulin dose in response to the blood glucose control was carried out by each doctor without a shared rule of insulin dose adjustment. Therefore, the discussion based on the comparison between control and treated groups, and the detailed estimation of the influence on insulin adjustment are not available in the present study. Considering that all the participating doctors are diabetes specialists in Japan, the data in the present study represents the effect of miglitol under the current control of diabetes specialists in Japan.
In the present study, important clinical data were gathered on the efficacy and safety of the co‐administration of miglitol in patients with T1DM on insulin therapy. The application of this combination therapy in treatment practice could be promising.
Acknowledgments
We thank the following physicians for their cooperation in this research: Nobuyuki Abe, Setsuko Abe, Kazuyuki Hamaguchi, Katsuhiro Tanaka and Rie Abe (Abe Clinic); Takashi Iizuka (Asahi Internal Medicine Department Clinic); Kazuo Mimura, Fumio Umeda, Yuji Tajiri, Mikako Kimura and Ryoko Takei (Fukuoka City Medical Association Hospital); Hideaki Jinnouchi, Tomio Jinnouchi, Ken‐ichi Koyama and Seiji Sakai (Jinnouchi Hospital); Ikuro Matsuba (Matsuba Clinic), Makoto Hayashi, Noriyoshi Yamakita, Toshihiro Murai, Akifumi Akai, Yasufumi Ito and Hiromichi Tanahashi (Matsunami General Hospital); Nobuichi Kuribayashi (Misaki Internal Medicine Clinic); and Takeshi Osonoi, Miyoko Saito and Naoko Takayanagi (Naka Memorial Clinic). This study is financially supported by Sanwa Kagaku Kenkyusyo Co. Ltd.
References
- 1.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial (DCCT) Research Group . Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995; 75: 894–903 [DOI] [PubMed] [Google Scholar]
- 3.The DECODE Study Group . Glucose tolerance and cardiovascular mortality: comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med 2001; 161: 397–405 [DOI] [PubMed] [Google Scholar]
- 4.Nakagami T, Qiao Q, Tuomilehto J, et al.Screen‐detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: DECODA study. Eur J Cardiovasc Prev Rehabil 2006; 13: 555–561 [DOI] [PubMed] [Google Scholar]
- 5.Tominaga M, Eguchi H, Manaka H, et al.Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999; 22: 920–924 [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation (IDF) . Guideline for Management of Post‐Meal Glucose. International Diabetes Federation (IDF), Brussels, 2007. [Google Scholar]
- 7.Arakawa M, Ebato C, Mita T, et al.Miglitol suppresses the postprandial increase in interleukin 6 and enhances active glucagon‐like peptide 1 secretion in viscerally obese subjects. Metabolism 2008; 57: 1299–1306 [DOI] [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, et al.Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J 2000; 321: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risso A, Mercuri F, Quagliaro L, et al.Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab 2001; 281: E924–E930 [DOI] [PubMed] [Google Scholar]
- 10.Quagliaro L, Piconi L, Assaloni R, et al.Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD (P) H‐oxidase activation. Diabetes 2003; 52: 2795–2804 [DOI] [PubMed] [Google Scholar]
- 11.Quagliaro L, Piconi L, Assaloni R, et al.Intermittent high glucose enhances ICAM‐1, VCAM‐1 and E‐selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 2005; 183: 259–267 [DOI] [PubMed] [Google Scholar]
- 12.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamori R, Kadowaki T, Ishii H, et al.Efficacy and safety of insulin glulisine in Japanese patients with type 1 diabetes mellitus. Diabetes Obes Metab 2009; 11: 891–899 [DOI] [PubMed] [Google Scholar]
- 14.Garg SK, Rosenstock J, Ways K. Optimized Basal‐bolus insulin regimens in type 1 diabetes: insulin glulisine versus regular human insulin in combination with Basal insulin glargine. Endocr Pract 2005; 11: 11–17 [DOI] [PubMed] [Google Scholar]
- 15.Bode B, Weinstein R, Bell D, et al.Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002; 25: 439–444 [DOI] [PubMed] [Google Scholar]


