Highlights
-
•
DTI was collected for 34 male adolescents, ages 15–17.
-
•
Aerobic fitness related to white matter connectivity in frontal and motor tracts.
-
•
HF had higher tractography streamline counts in CST and Fminor compared to LF.
-
•
A negative relationship was seen between VO2 peak and FA in the L CST.
-
•
Exercise is an important environmental factor to consider during neurodevelopment.
Abbreviations: AD, axial diffusion; AF, arcuate fasciculus; ATR, anterior thalamic radiations; BMI, body mass index; CST, corticospinal tract; DTI, diffusion tensor imaging; DWI, diffusion-weighted images; FA, fractional anisotropy; FACT, fiber assignment by continuous tracking; FDR, false discovery rate; Fmajor, forceps major; Fminor, forceps minor; FSL, FMRIB Software Library; HF, high-fit; IFO, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; IQ, general intelligence; L, left; LF, low-fit; LME, linear mixed-effects; M, mean; MNI, Montreal Neurological Institute; PDS, Pubertal Development Scale; PLQ, Personal Lifestyle Questionnaire; R, right; RD, radial diffusion; ROI, region of interest; SE, standard error; SES, socioeconomic status; TE, echo time; TI, inversion time; TR, repetition time; UNC, uncinate fasciculus; VO2 peak, peak aerobic uptake; YAAQ, Youth Adolescent Activity Questionnaire
Keywords: Adolescence, Aerobic exercise, White matter, Diffusion tensor imaging, Tractography
Abstract
Exercise has been shown to have positive effects on the brain and behavior throughout various stages of the lifespan. However, little is known about the impact of exercise on neurodevelopment during the adolescent years, particularly with regard to white matter microstructure, as assessed by diffusion tensor imaging (DTI). Both tract-based spatial statistics (TBSS) and tractography-based along-tract statistics were utilized to examine the relationship between white matter microstructure and aerobic exercise in adolescent males, ages 15–18. Furthermore, we examined the data by both (1) grouping individuals based on aerobic fitness self-reports (high fit (HF) vs. low fit (LF)), and (2) using VO2 peak as a continuous variable across the entire sample. Results showed that HF youth had an overall higher number of streamline counts compared to LF peers, which was driven by group differences in corticospinal tract (CST) and anterior corpus callosum (Fminor). In addition, VO2 peak was negatively related to FA in the left CST. Together, these results suggest that aerobic fitness relates to white matter connectivity and microstructure in tracts carrying frontal and motor fibers during adolescence. Furthermore, the current study highlights the importance of considering the environmental factor of aerobic exercise when examining adolescent brain development.
1. Introduction
The adolescent brain undergoes significant changes (Giedd et al., 1996, Giedd et al., 1999, Dahl, 2004, Casey et al., 2008), and this period of neurodevelopment may be particularly sensitive for environmental factors to impart their effects on brain and behavior (Andersen, 2003, Masten, 2004, Marco et al., 2011). Thus, it is important to identify environmental factors that may influence typical adolescent neurodevelopment. Aerobic exercise is defined as sustained activity that stimulates heart and lung function, resulting in improved bodily oxygen consumption, and includes a number of physical activities, such as running, walking, swimming, and cycling (Armstrong et al., 2007). Given the widespread epidemic of an increasing sedentary lifestyle for children and adolescents in Western countries, it has become of increasing interest to understand how aerobic exercise may influence not only the body but also the brain. In fact, aerobic exercise is an environmental factor that has been shown to substantially impact gray matter brain structure in children, adults, and elderly (for review see Hillman et al., 2008, van Praag, 2009). Furthermore, we have shown that aerobic exercise also relates to structure and function in the adolescent brain (Herting et al., 2012b, Herting et al., 2013). However, no study to date has assessed if aerobic fitness also relates to white matter microstructure during adolescence. White matter is primarily comprised of glial cells and myelinated neurons. Myelination leads to efficient neural transmission throughout the brain, and it is thought to contribute to enhanced processing speed and cognitive function seen to occur during childhood and adolescence (Casey et al., 2008). Thus, determining how exercise affects white matter connectivity may be particularly important for further understanding the developing adolescent brain.
In recent years, MRI advancements, such as diffusion tensor imaging (DTI), have allowed for in vivo assessment of white matter microstructure and connectivity in the human brain. DTI exploits characteristics of water diffusion in the brain to make inferences about white matter fiber microstructure (Basser, 1995). Primary metrics of DTI include fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD). Together these variables characterize different components of water diffusion, including restricted, or anisotropic, diffusion (FA), diffusion along the primary eigenvector (AD), and diffusion perpendicular to the primary eigenvector (RD). These diffusion characteristics are thought to reflect different neurobiological components of white matter microstructure, with higher FA and AD, and/or lower RD values, likely representing increased axon caliber, myelination, and/or fiber organization in white matter pathways (Beaulieu, 2002, Alexander et al., 2007). Beyond quantifying diffusion patterns, DTI data can also be utilized to perform tractography, a three-dimensional modeling technique to estimate fiber tracts (Mori et al., 1999). This technique provides useful information by virtually separating different white matter tracts for each individual, and can be combined with basic DTI metrics (i.e., FA, RD, and AD) to provide individual volumes of interest for the assessment of white matter microstructure (Colby et al., 2012).
While no study has examined these relationships in youth, recent studies in healthy adults (≥21 years) and elderly samples (≥55 years) suggest aerobic exercise may influence white matter microstructural properties (Marks et al., 2011, Johnson et al., 2012, Voss et al., 2012, Tseng et al., 2013). However, one of the challenges facing aerobic exercise studies is accurately quantifying aerobic fitness in humans (Etnier et al., 2006, Armstrong et al., 2008). One approach is dichotomizing individuals into groups based on their history of aerobic training (frequency, type of exercise, etc.). However, self-reports of aerobic training can be biased by perception (Armstrong et al., 2008). Furthermore, an important factor in exercise training is the intensity with which the activity is performed. That is, aerobic quantity does not necessarily reflect aerobic intensity, and an increase in an individual's oxygen utilization of the body requires high intensity training (Midgley et al., 2006). In this regard, an individual's ability to utilize oxygen during exercise can be objectively measured by their body's maximum aerobic capacity, or VO2 peak (Armstrong et al., 2007). To date, adult and elderly studies have utilized both approaches. Positive relationships have been detected between cardiovascular fitness (as indexed by VO2) and FA in cingulum white matter (Marks et al., 2011), as well as in portions of the corpus callosum carrying premotor and prefrontal cortex fibers (Johnson et al., 2012). Similarly, using training experience as a dichotomous variable, a recent study showed higher FA in regions associated with motor function in previous Master athletes versus age-matched elderly controls (Tseng et al., 2013). However, Voss and colleagues (Voss et al., 2012) recently implemented an aerobic fitness intervention study to assess how aerobic exercise affects white matter microstructure. Interestingly, no significant differences were seen for FA, RD, or AD between groups, but greater improvements in aerobic fitness predicted larger increases in FA values in the prefrontal, parietal, and temporal cortex of a walking intervention group, but not a control (stretching) group (Voss et al., 2012). Together these findings reflect that exercise modality is not only important, but that the magnitude of fitness on white matter microstructure may matter.
Thus, the goal of the current study was to examine how aerobic fitness relates to white matter connectivity and microstructure in male youth, ages 15–18. To accomplish this, we employed DTI and assessed relationships between aerobic fitness and WM microstructure using tract-based spatial statistics and tractography-based along-tract statistics. Furthermore, given the aforementioned limitations in quantifying aerobic fitness (Etnier et al., 2006, Armstrong et al., 2008), we examined the data by both (1) grouping individuals based on aerobic exercise self-report (high fit (HF) vs. low fit (LF)), as well as (2) examining the relationship between VO2 peak and white matter microstructure across the entire sample. Based on research in adults and elderly (Marks et al., 2011, Johnson et al., 2012, Voss et al., 2012), we hypothesized that HF youth would have higher FA (driven by lower RD) in white matter tracts carrying premotor and frontal cortical white matter fibers when compared to their LF peers. Based on Voss et al. (2012), we also hypothesized these relationships would be largely accounted for by aerobic fitness (e.g. VO2 peak) and not overall differences in general activity between the groups.
2. Materials and methods
2.1. Participants
Participants were recruited from the larger Portland, Oregon community as part of an ongoing adolescent neurodevelopment study. Informed written parent consent and child assent were obtained for all participants, and procedures were approved Oregon Health & Science University's Institutional Review Board. Inclusionary criteria for youth included being of the male sex, 15–18 years of age, and meeting either high or low-fit criteria based on aerobic physical activity self-report (see Section 2.2). The male sex was specifically chosen, as sex differences are reported in white matter microstructure across adolescence (Szeszko et al., 2003, Silveri et al., 2006, Schmithorst et al., 2008, Perrin et al., 2009, Herting et al., 2012a), as well as differences in activity levels (Riddoch et al., 2004) and aerobic capacity (Krahenbuhl et al., 1985). To eliminate potential sex-related confounds, we chose to first examine these relationships in male adolescents alone. Exclusionary criteria included current DSM-IV psychiatric diagnoses [Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001, Hoven et al., 2005)]; significant substance use (>10 lifetime alcoholic drinks or 2 drinks per occasion, >5 uses of marijuana, >4 cigarettes per day, or any other drug use) [Brief Lifetime Customary Drinking and Drug Use Record (Brown et al., 1998)]; history of psychotic disorders in biological parents [Family History Assessment Module (Rice et al., 1995)]; major medical condition or significant head trauma [Structured Clinical Interview (Brown et al., 1994)]; left-handedness [Edinburgh Handedness Inventory (Oldfield, 1971)], or irremovable metal. Youth and parents were each compensated for completing behavioral tests and MRI scanning. Participants in this study have been used in previously published studies examining exercise and brain structure and function (Herting et al., 2012b, Herting et al., 2013).
2.2. High and low-fit criteria based on self-report
A modified version of the Youth Adolescent Activity Questionnaire (YAAQ) was administered to youth to assess participation in exercise over the past year. The YAAQ asks detailed questions about participation in different types of physical activity across all four seasons of the year, as well as the number of hours per week spent doing each activity (Wolf et al., 1994). Both highly aerobic (e.g. basketball, soccer, track, swimming, etc.) and less aerobic physical activities (e.g. baseball, weight-lifting, etc.) were included on the YAAQ. Based on hours of highly aerobic physical activity reported by the youth on the YAAQ, HF youth were defined as those participating in an average of ≥10 h per week of regular, organized highly aerobic physical activity, purposely performed to allow for improvement or maintenance of aerobic fitness across one or more seasons, within the past year. LF youth were defined as those individuals that had participated in ≤1.5 h of highly aerobic physical activity per week over the past year. HF youth were asked to participate in the study during the season in which they were most physically active based on their YAAQ self-report. These criteria were set forth, as significant increases in aerobic fitness have been seen in adolescents who participated in ≥10 h of aerobic exercise per week (Brown et al., 1972, Weber et al., 1976, Lussier et al., 1977), and relatively extreme categorizations (≥10 h versus ≤1.5 h per week) maximize the likelihood of detecting group differences.
2.3. Objective measures of daytime activity levels and aerobic fitness
Daytime activity levels were assessed via ambulatory actigraphy using an Actiwatch (Mini Mitter Company, Bend, OR, USA). The procedures for data collection and scoring were implemented as previously published (Long et al., 2008). Specifically, the watch was worn on the nondominant wrist for 24 h per day for 7 days to provide continuous monitoring of subject's activity levels (except during the aerobic fitness test and MRI scan in which they were instructed to remove it). Counts were stored on the device in 1 min epochs, beginning on the subject's first visit to the lab. Data were extracted using Mini Mitter's Actiware software. Daytime activity scores that were obtained from the data included mean activity level and peak activity level. Mean activity level was calculated as the mean number of activity counts per 1 min epoch during each daytime wake period. Peak activity level was calculated as the highest number of 1 min epochs per day.
Aerobic fitness was objectively measured by peak aerobic uptake (VO2 peak) for each participant, as it is the gold standard method for assessing aerobic fitness (Armstrong et al., 2007). VO2 peak was measured using the same computerized indirect calorimetry system (VMax Series, V6200 Autobox) during a Bruce Protocol (Bruce et al., 1973). VO2 peak values were only considered valid if the participant delivered maximal effort on the test, as defined by one of the following the physiological criteria (Armstrong et al., 2008): (1) oxygen consumption remained at a steady state despite an increase in workload, as evidenced by a plateau in oxygen consumption, (2) heart rate reached ≥200 beats per minute, (3) the respiratory exchange ratio was ≥1.0; and/or the subjective criteria of reporting a 10 on the perceived exertion scale. Lean body mass (LBM) was determined just prior to aerobic testing by conducting a bioelectrical impedance test on each subject using the Body Composition Analyzer (Model 310e; Biodynamics Corp.), allowing for peak oxygen consumption to be expressed in mL/kg LBM/min.
2.4. Lifestyle assessment
In physical fitness studies, there is often concern that other non-exercise related factors might account for group differences that may coexist with exercise. In the current study, we assessed these variables, and to the best of our ability, matched groups. Groups were assessed for general intelligence [IQ; 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)], socioeconomic status (SES) [Hollingshead Index of Social Position and household income (Hollingshead, 1975)], body mass index (BMI) [Center for Disease Control and Prevention Child and Teen Calculator (Center for Disease Control, 2011)], pubertal status [Pubertal Development Scale (PDS) (Petersen et al., 1988)], personal lifestyle habits involving nutrition, safety, relaxation, health promotion, and substance use [Revised Personal Lifestyle Questionnaire (PLQ) (Mahon et al., 2003)], and extracurricular activities [number, frequency, and type].
2.5. Imaging acquisition and preprocessing
Images were acquired on a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a twelve-channel head coil. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1 weighted MPRAGE scanning sequence (TI = 900 ms, flip angle = 10°, TE = 3.58 ms, TR = 2300 ms, acquisition matrix = 256 × 240, resolution = 1 mm3). Diffusion-weighted images (DWI) were acquired oblique to the AC–PC plane using a high-angular resolution echo planar imaging sequence (TR = 9500 ms, TE = 95 ms, field of view = 240 mm2, 72 slices, slice thickness = 2 mm). Gradient encoding pulses were applied in 30 directions with a b-value of 1000 s/mm2, 2 diffusion-weighted acquisitions were collected, with 6 b0 (non-diffusion weighted) images per acquisition.
Each raw diffusion weighted acquisition for each subject was preprocessed separately according to standard protocols available in FSL (http://www.fmrib.ox.ac.uk/fsl) and TrackVis (http://www.trackvis.org). Briefly, eddy current effects, magnetic field inhomogeneities, and head motion were corrected using FMRIB's Diffusion Toolbox and Utility for Geometrically Unwarping EPIs (Jenkinson, 2003). Affine registration was applied to align the corrected DWI data to the averaged b0 volume for each run. Using FSL, a six-parameter tensor model of diffusion was then fit to the raw data to give voxelwise maps of the 3 principle diffusion directions, as well as the magnitudes of diffusion along these three axes.
2.6. Tract-based spatial statistics (TBSS)
Tract-based spatial statistics (TBSS, version 1.1) was utilized to conduct voxel-wise analyses (Smith et al., 2006, Smith et al., 2007). First, individual FA maps from the 2 DTI runs were aligned and averaged for each subject. Next, using individual's averaged FA maps, a common registration target image was identified from the data and affine aligned to standard MNI space. Each participant's FA map was then nonlinearly registered to this common target using FMRIB's Non-linear Image Registration Tool (Andersson et al., 2007). Aligned FA images were averaged to create a group-wise mean FA map and a white matter skeleton, representing only the major tracts common across all participants. A mean FA threshold of 0.3 was applied to the white matter skeleton to reduce partial volume effects and each participant's aligned FA image projected onto the white matter skeleton to run voxelwise group-level statistics.
2.7. Tractography and along-tract statistics
Whole-brain brute-force, atlas-based tractography was performed in Diffusion Toolkit v0.6 (http://www.trackvis.org/dtk), using the previously published Fiber Assignment by Continuous Tracking (FACT) algorithm (Mori et al., 1999). This process generates deterministic streamlines by iteratively moving from voxel to voxel along the direction of maximal diffusion, while using the following constraints: (1) a whole-brain mask, (2) an FA threshold of 0.2 to prevent spurious fibers, and (3) a tract-dependent turning angle threshold of 60°, to prevent biologically implausible fibers. Successful tracts were defined as those with ≥1 streamline. Atlas-based white matter tracts were identified using a multi-ROI approach established by Wakana et al. (2007). This included performing a twelve-mode affine transformation to co-register the subjects’ DTI images to standardized (MNI) space, applying white matter atlas-based ROIs, and assigning fibers that intersected these ROIs to a given white matter tract. The atlas-based ROIs were based on Wakana's standardized dataset and were identical for each subject. Fourteen atlas-based white matter tracts were used for the current study, including: forceps major (Fmajor), forceps minor (Fminor), as well as the arcuate fasciculus (AF), anterior thalamic radiations (ATR), corticospinal tract (CST), and inferior fronto-occipital fasciculus (IFO), inferior longitudinal fasciculus (ILF), and uncinate fasciculus (UNC) for each hemisphere (right = R; left = L).
The along-tract mapping toolbox (Colby et al., 2012) was utilized to assess FA at multiple locations parameterized along the tract lengths. This toolbox was implemented via MATLAB (MATLAB) and R software (Team, 2008), and included: (1) reorientation of streamlines toward a common origin, (2) re-parameterization of streamlines with cubic B-splines, (3) resampling of streamlines into a discrete number of evenly spread vertices along the length of the tract, (4) resampling the metric volume (e.g. FA map) at these new streamline vertices, and (5) collapsing metrics values (i.e. FA, RD, and AD) across streamlines at each group of vertices to obtain mean scalar estimates (mean (M) and standard error (SE)) along the length of the tract (Colby et al., 2012). For tracts created, along-tract statistics were then averaged across the successfully estimated white matter tracts from the 2 DTI runs for each subject (see Table 1 for break down by group). Importantly, there was no significant difference in the number of successful tracts (i.e. tracts with ≥1 streamline) mapped for each atlas-based ROI between groups. However, some tracts were unpowered, with as little as 50% of subjects from one or both groups not having successful tracts for either DTI run (i.e. R and L AF; R and L IFO). Thus, to eliminate unnecessary testing on extremely low-powered fiber tracts, statistical analyses were performed only for tracts for which at least 60% of subjects from each group had successful tracking for both DTI runs.
Table 1.
Number of subjects with successful tracts. Tractography analyses were performed on two DTI runs per subject. Successful tracts were defined as those with ≥1 streamline. For each tract of interest, the numbers of subjects are reported for successful and unsuccessful tracks generated, as well as the chi-square (and its associated p-value) test for differences in the proportion of successful tracts between groups. Both = number of subjects with successful tracts generated for one DTI runs; One = number of subjects with successful tracts generated for both DTI runs; None = number of subjects in which tracks could not be generated for either DTI run. Note: Only tracts with ≥60% of subjects per/group with successful tracts for both DTI runs were utilized. Tract abbreviations: see text (methods).
| Tracts | HF |
LF |
X2 | ||||
|---|---|---|---|---|---|---|---|
| Both | One | None | Both | One | None | ||
| L AF | 8 | 2 | 7 | 12 | 1 | 4 | (2) 2.0, p = .38 |
| R AF | 8 | 4 | 5 | 11 | 2 | 4 | (2) 1.3, p = .53 |
| L ATR | 17 | – | – | 17 | – | – | – |
| R ATR | 17 | – | – | 17 | – | – | – |
| L CST | 16 | – | 1 | 17 | – | – | (1) 1.0, p = .31 |
| R CST | 15 | 1 | 1 | 17 | – | – | (2) 2.1, p = .34 |
| Fmajor | 16 | 1 | – | 17 | – | – | (1) 1.0, p = .31 |
| Fminor | 17 | – | – | 17 | – | – | – |
| L IFO | 8 | 3 | 6 | 13 | 1 | 3 | (2) 3.2, p = .20 |
| R IFO | 9 | 4 | 4 | 12 | 1 | 4 | (2) 2.2, p = .33 |
| L ILF | 16 | 1 | – | 16 | – | 1 | (2) 2.0 p = .37 |
| R ILF | 16 | 1 | – | 15 | 1 | 1 | (2) 1.0, p = .60 |
| L UNC | 17 | – | – | 17 | – | – | – |
| R UNC | 16 | 1 | – | 16 | – | 1 | (2) 2.0, p = .37 |
2.8. Statistical analyses
All statistical analyses for participant demographics were carried out using PASW 18 (Chicago, IL) and R software (Team, 2008). Normality was verified on all variables, and transformations were used when appropriate. When data continued to violate normality, nonparametric tests were employed. Independent t-tests were used to examine participant demographics, aerobic fitness, daytime activity levels, BMI, as well as lifestyle differences between the two groups, whereas Pearson's r was used to determine correlations between aerobic fitness (VO2 peak) and other theoretical demographic confounds, including PDS, SES, and daytime activity levels. Significant group differences or fitness-related correlations for any demographic or lifestyle variables were then included as covariates in all further analyses. Furthermore, follow-up analyses, including daytime activity levels as a covariate were performed to address if general motor or activity levels contributed to the results.
TBSS data was analyzed using FSL's randomize software (Smith et al., 2004). Models included general linear regression, including group or VO2 peak as the independent variables, controlling for SES and puberty levels as covariates, when appropriate. Statistical thresholding included cluster-based correction for multiple comparisons using the default threshold-free cluster enhancement (TFCE) for DTI data as implemented within randomize (Smith et al., 2009).
Along-tract statistical analyses were performed using R software (Team, 2008). First, streamline number per each tract and hemisphere was examined using linear mixed-effects (LME), with fixed between-subject effects including overall intercept, tract, hemisphere, and group (HF and LF), controlling for significant group differences in SES and puberty, when appropriate. Next, cross-sectional scalar estimates obtained along a tract were examined using LME modeling for each tract and hemisphere, with fixed between-subject effects including overall intercept, position along tract (dummy coded), group (HF and LF), and group by position interactions, controlling for significant group differences in SES and puberty. Similar LME models were run using VO2 peak as a continuous variable, rather than group, to more directly assess the relationship between aerobic fitness and white matter. To reduce family-wise Type 1 error rate, Bonferroni correction was applied to LME F-tests (p < .005 (.05/10 tracts)). Furthermore, an analogous approach to FSL's TFCE was implemented in MATLAB to enhance the initial statistic from each model using the intensity of the data point and information from its neighboring voxels, followed by applying a maximum-statistic permutation method to the enhanced statistic to correct for multiple comparisons (Smith et al., 2009). Specifically, the following algorithm was applied in MATLAB:
with ‘i’ being the data (FA scalar value at one point), ‘h’ representing height of the statistic, and ‘e’ as the neighborhood information surrounding that point (e.g., extent of the cluster). As suggested for white matter (Smith et al., 2009), TFCE parameters used were E = 0.5 and H = 2. The maximum-statistic permutation included permutating of the independent variable of interest, fitting the model again, and recording the maximum statistic across all comparisons. This process was repeated multiple times to empirically build up a distribution of the maximum test statistic under the null, against which the original results were compared to obtained corrected p-values.
To better characterize white matter differences, FA, AD and RD values were extracted for the significant regions generated from the group by position tract analyses. Independent t-tests or general linear regression were then performed to assess group differences or VO2 peak relationships with AD and RD for these regions.
3. Results
3.1. Participant characteristics
One participant's parent (LF) chose not to disclose total household income, and one subject (HF) did not complete the PLQ, resulting in pairwise missing data for these measures. Participant characteristics can be found in Table 2. The groups were matched on age and IQ, and displayed similar self-reports of lifestyle behaviors (nutrition, relaxation, health promotion, safety, substance use, BMI, frequency and number of extracurricular activities). Mean activity levels and peak activity levels were higher in HF compared to LF youth, although only peak activity levels reached statistical significance. VO2 peak testing was used to objectively measure aerobic fitness, and as expected, was significantly different between the groups, confirming better aerobic fitness in HF youth. Although both groups came from households that made above the national income average, the HF had an overall higher SES (reflected by lower scores on the Hollingshead) and median household income, as reported by their parents. Self-report of pubertal maturation was also different between the groups, with HF being less mature compared to LF youth. A significant correlation was found between VO2 peak and puberty (r(32) = −.36, p = .03). VO2 peak did not significantly relate to SES (r(32) = −.21, p = .23), mean activity levels (r(32) = .12, p = .51), or peak activity levels (r(32) = .15, p = .39). To account for these associations, follow-up analyses were performed covarying for SES and puberty in subsequent group-level analyses, as well as covarying for puberty for VO2 peak regression analyses.
Table 2.
Participant characteristics by group. Means and standard deviations unless otherwise noted. hrs/wk = hours per week. HF = high-fit and LF = low-fit, as defined by self-report on the YAAQ.
| Demographics | HF | LF | |
|---|---|---|---|
| N | 17 | 17 | |
| Age | 16.6 (.8) | 16.2 (.8) | t(32) = 1.36, p = .19 |
| % Caucasian | 82.4 | 82.4 | |
| IQa | 117.1 (11.8) | 118.0 (8.1) | t(26.1) = .26, p = .79 |
| SESb | 18.3 (5.9) | 26.5 (12.9)* | t(22.6) = 2.39, p = .03 |
| Median household incomeb (thousands) | 130 | 90h | |
| Pubertyc | 3.06 (.4) | 3.3 (.3)* | U = 80, z = 2.24, p = .026 |
| Daytime activity levels and aerobic fitness | |||
| Mean activity leveld | 435349.8 (95458) | 373182.1 (95392.71) | t(32) = −1.90, p = .07 |
| Peak activity leveld | 3711.4 (780.5) | 3046.1 (589.91)** | t(29.8) = −2.80, p = .009 |
| Aerobic activity (hrs/wk over past year)e | 11.3 (3.4) | .26 (.5)** | t(16.6) = 11.33, p < .001 |
| Aerobic activity (hrs/wk in season scanned)e | 12.6 (3.8) | .23 (.5)** | t(16.5) = 13.39, p < .001 |
| VO2 peak (mL/kg LBM/min) | 77.7 (10.5) | 67.0 (7.4)** | t(32) = 3.41, p = .002 |
| Body composition | |||
| BMIf | 21.6 (2.9) | 22.4 (4.4) | t(25.72) = .67, p = .51 |
| Lifestyle | |||
| Nutritiong,h | 12.0 (1.0) | 12.4 (1.5) | t(31) = .77, p = .45 |
| Relaxationg,h | 15.2 (2.2) | 15.4 (2.2) | t(31) = .22, p = .83 |
| Health promotiong,h | 13.7 (1.3) | 13.0 (2.3) | U = 117, z = .70, p = .51 |
| Safetyg,h | 14.2 (1.3) | 15.0 (1.2) | U = 86.5, z = 1.8, p = .07 |
| Substance useg,h | 11.6 (0.5) | 11.3 (1.0) | U = 122, z = .58, p = .63 |
| Extracurricular activities | |||
| Frequency | 4 (0) | 3.5 (1.0) | U = 110.5, z = 2.09, p = .25 |
| Number | 2.9 (1.3) | 2.3 (1.3) | U = 109, z = 1.28, p = .23 |
Wechsler Abbreviated Scale of Intelligence.
Hollingshead Index of Social Position; lower values reflect higher SES.
Pubertal Development Scale.
Actiwatch data.
Youth Adolescent Activity Questionnaire.
Body Mass Index.
Personal Lifestyle Questionnaire.
n = 16 due to missing data.
Denotes p < .05.
Denotes p < .01
3.2. TBSS
No significant voxel-based group differences in FA were found between HF and LF youth, nor did aerobic fitness (VO2 peak) predict FA values across the sample. These findings were similar after controlling for SES and puberty between groups, as well as covarying for puberty in VO2 peak analyses.
3.3. Along-tract statistics
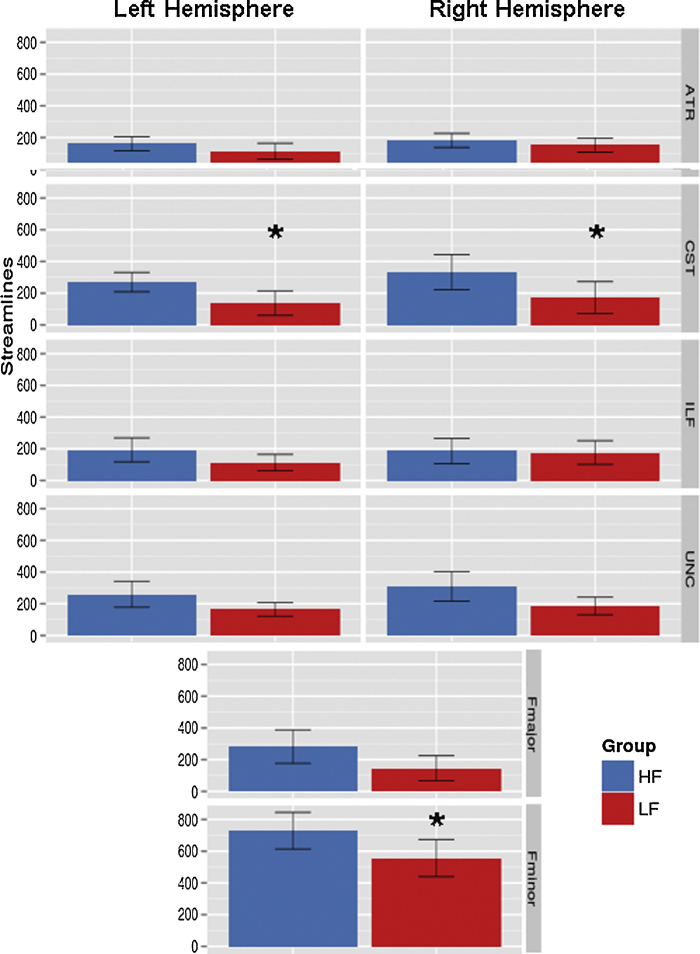
For group analyses, models including covariates (SES and puberty) showed that neither covariate significantly related to FA in the 10 tracts of interest (all p's > .05). Furthermore, similar results were obtained with and without including these covariates. Thus, to conserve power, these variables were excluded from the final model. No significant differences were seen for streamline counts between hemispheres within each group, but streamline numbers did vary between tracts (Fig. 1). An overall significant main effect of group was seen, with LF youth having significantly fewer streamlines than their HF peers (t = 6.10, df = 466, p = 2.27e−9). To further elucidate if group differences in streamline number varied across the 10 tracts, a follow-up post hoc LME was performed, with fixed effects including overall intercept, tract, group, and a group-by-tract interaction term. This analysis revealed that group differences in streamline count were significant for only the CST (t = 2.17, df = 460, p = .03) and Fminor (t = 2.17, df = 460, p = .03). Group differences in streamline count remained significant after controlling for daytime activity levels between the groups (p's < 2.25e−6). After correcting for multiple comparisons, there were no significant group differences in FA for any of the 10 tracts of interest.
Fig. 1.
Tractography streamline counts. The number of streamlines (±95% confidence interval) is shown for each group. *Denotes LF youth have significantly less streamlines compared to HF youth (post hoc group-by-tract interaction result, p ≤ .05).
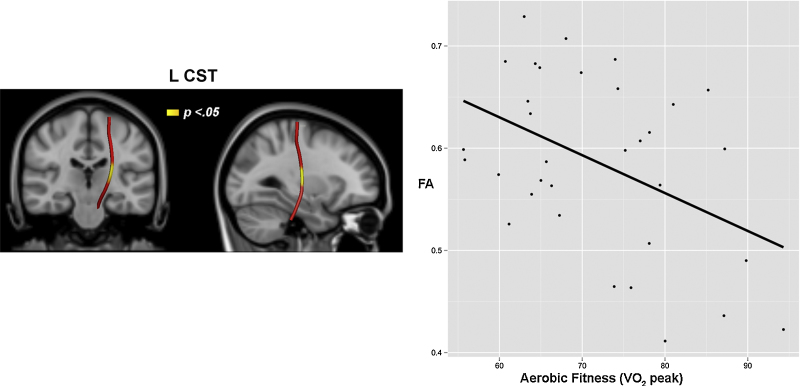
Aerobic fitness, as measured by VO2 peak did not relate to total streamline number nor streamline counts for any of the 10 tracts of interest (p's > .12). However, mixed-effect modeling results showed a significant negative relationship between aerobic fitness and FA in the L CST (tract positions 27 through 31) (Fig. 2). After controlling for daytime activity levels in the model, aerobic fitness and L CST FA findings remained significant for tract positions 28 through 31 (p's < .05), whereas position 27 became a trend (p = .059). Follow-up analyses showed that neither RD nor AD significantly related to aerobic fitness on their own (p's > .21), suggesting aerobic fitness relates to both decreased axial and increased radial diffusion in this region.
Fig. 2.
Visualization of along-tract statistics for FA. Significant negative VO2 peak relationships with FA are shown in yellow (p < .05, corrected for multiple comparisons) overlaid on a representative geometry of the L CST tract (red). Plot depicts the negative relationship between FA in this region and VO2 peak across the sample. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
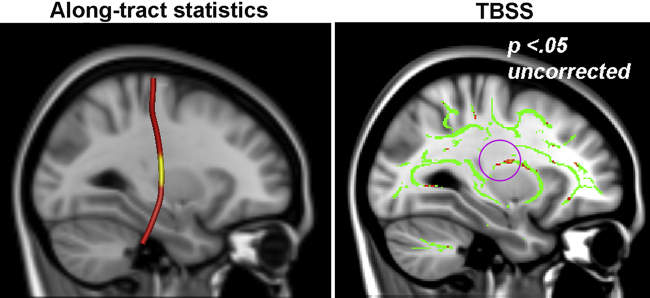
3.4. Exploratory comparison of left CST between TBSS and along-tract statistics
Given that the negative L CST along-tract finding was contrary to our hypothesis, we aimed to determine if this result was spurious by performing a post hoc exploratory examination of the uncorrected TBSS data. Thus, while TBSS results did not reach statistical significance, we examined uncorrected data to determine if similar patterns could be seen between aerobic fitness and microstructural properties of the L CST, as detected by tractography and along-tract data analyses. At a threshold of p < .05, uncorrected for multiple comparisons, a similar negative relationship was seen between VO2 peak and FA in the L CST portion of the TBSS white matter skeleton (t = 3.34; x = −29, y = −7, z = 17; 28 voxels). This comparison verifies that the significant negative correlation seen in the L CST using along-tract analyses is not merely an artifact of the method.
4. Discussion
To our knowledge, the current study is the first to examine how aerobic fitness relates to white matter microstructure in youth. Using tractography and along-tract statistics, our results showed HF youth had a greater number of streamlines, especially in the CST and Fminor, when compared to their LF peers. In addition, greater aerobic fitness, as indexed by higher VO2 peak, was related to lower FA values in the L CST. Together, these findings provide support that aerobic exercise relates to white matter structural connectivity during adolescence.
Collapsed across the 10 defined tracts of interest, HF youth had significantly greater streamline count per tract compared to their LF youth. However, follow-up analyses showed that these group differences were largely driven by streamline numbers in the CST and the anterior corpus callosum (Fminor). The CST is comprised of projections of sensorimotor and motor neurons and carries motor information to the spinal cord. Alternatively, Fminor fibers connect lateral and medial frontal regions via the genu of the corpus callosum. Given the length, curvature, and degree of branching that occurs within a fiber tract, the number of reconstructed streamlines does not directly translate to fiber count (Jones et al., 2012). However, lower streamline counts have been shown for pathways affected by lesions following stroke (Schaechter et al., 2008), suggesting tractography-based streamline counts can also capture important structural information. Thus, it is feasible that higher streamline counts in HF youth may reflect better axonal organization or bigger fiber bundles, which would ultimately lead to greater number of streamlines in the CST and Fminor. This idea may be supported by research showing that voluntary exercise induces increases in axonal outgrowth in the spinal cord in rodents (Molteni et al., 2004, Ghiani et al., 2007).
Despite group differences in streamline count, we did not replicate previous findings showing positive relationships between aerobic exercise and FA in frontal and motor regions in adult and elderly samples (Marks et al., 2011, Johnson et al., 2012, Voss et al., 2012, Tseng et al., 2013). In contrast to our hypothesis, aerobic fitness predicted lower FA values in the L CST across all subjects. The direction of this relationship may seem surprising and initially counterintuitive. However, DTI indices are not selective markers of specific neurobiological properties. That is, while in the literature lower FA values are often thought to reflect less myelination or axonal organization, other tissue properties such as glial cell number and increases in the number of crossing fibers may contribute to decreased FA in HF youth. In this regard, a recent animal study showed that voluntary exercise increases oligodendrocytes, which are the cells responsible for myelinating axons (Krityakiarana et al., 2010). Furthermore, exercise has also been shown to decrease myelin-associated glycoprotein expression in the spinal cord (Molteni et al., 2004, Ghiani et al., 2007). Given that inhibition of these myelin-associated glycoproteins allows for more axonal outgrowth, it may be feasible that aerobic exercise could potentially lead to less axonal organization and/or crossing fibers, as indexed by lower FA values. Additional tractography studies in humans, as well as empirical animal work regarding exercise-induced brain changes, are necessary to help confirm such possibilities.
While only indirect inferences can be made about the underlying tissue composition contributing to the current findings, the streamline differences between high and low fit-groups and VO2 peak associations with L CST FA are in agreement with previous research showing aerobic fitness to be related to white matter properties in motor and frontal regions in adults and elderly. Together, these findings suggest that aerobic fitness may have a specific impact on frontal and motor white matter at many stages across the lifespan. That said, results found using VO2 peak as a continuous independent variable were not synonymous with those seen when splitting the sample into two groups based on self-report of aerobic exercise fitness. Again, the purpose of examining both group (HF vs. LF) and VO2 peak was to help clarify and better characterize how aerobic exercise relates to white matter microstructure in adolescents. Notably, self-report may be a good indicator of aerobic exercise quantity, whereas VO2 peak may be influenced more by aerobic intensity. For this reason, aerobic exercise self-reports and VO2 peak, as well as each of their associations with white matter microstructure, may not show a 1-to-1 correspondence in adolescents. Differences in results seen between VO2 peak and aerobic training groups may stem from the differences in what each independent variable is able to capture about aerobic exercise training experience. Thus, more research is warranted to determine if perhaps the amount of aerobic exercise may lead to thicker white matter axon bundles, whereas aerobic exercise intensity may have larger effects on axonal outgrowth and glial cell numbers within whiter matter regions.
Overall, this study provides an important extension of previous research on how aerobic exercise relates to brain structure in adolescents. However, a number of limitations must be mentioned. There is often concern that other non-exercise, such as lifestyle factors that may coexist with exercise, and/or non-aerobic motor behaviors, may account for aerobic fitness findings. In the current study, we assessed a number of these variables via the lifestyle questionnaire, including nutrition and health promotion, and to the best of our ability, matched groups, as well as statistically controlled for any remaining group differences (SES and puberty). Furthermore, controlling for general daytime activity levels, as objectively measured by actigraphy, did not account for these relationships. However, the lifestyle measures were brief, so it is impossible to entirely rule-out non-exercise factors as potential confounds, as well as possibility that these difference may be premorbid and contribute to self-selection into aerobic exercise behaviors. Nonetheless, the current cross-sectional preliminary findings warrant the implementation of longitudinal aerobic exercise intervention study to confirm the influence of aerobic fitness on white matter microstructure in youth. In addition, while tractography and along-tract mapping have a number of advantages (spatial localization, creating individualized tracts), there are limitations to these techniques as well. In the current study, we tried to reduce these methodological issues by performing analyses on two DWI runs per subject and utilizing an additional whole-brain voxelwise analytic approach (e.g. TBSS). Patterns in the L CST were similar between the along-tract statistics and TBSS results when left uncorrected for multiple comparisons (Fig. 3), although the tractography and along-tract statistics approach yielded more statistically robust findings. However, tractography was not successful for a large portion of subjects for two of the tracts (AF and IFO), inhibiting our ability to assess FA along these pathways. Although aerobic fitness effects could not be differentiated from the null hypothesis when assessed in these regions via the TBSS skeleton analysis, it is feasible that we may have missed fully capturing the effect of aerobic fitness through our inability to examine these regions with along-tract statistics. Moving forward, using newer DTI collection methods, such as high angular resolution diffusion imaging acquisition schemes and image reconstruction based on alternative models of diffusion, may be useful to implement in future studies of aerobic exercise and white matter to better accommodate these aforementioned limitations of streamline-type tractography (Kuhnt et al., 2013).
Fig. 3.
Comparison of TBSS and along-tract statistics results. Visualization of uncorrected TBSS data also revealed a negative relationship between VO2 peak and FA in the L CST of the white matter skeleton (voxelwise thresholded only; p < .05) that mirrored corrected along-tract statistics results.
In summary, aerobic fitness relates to white matter connectivity and microstructure in adolescent male youth. Taken together with previous research, these findings suggest aerobic exercise may impact brain structure in frontal and motor regions, not only in adulthood and aging, but also in adolescence. Moving forward it will be important to clarify the functional implications for these associations, as well as to replicate these preliminary findings using an exercise intervention design to better infer causality. Given previous work showing that aerobic exercise relates to white matter microstructure in various age groups, future research is also needed to determine if aerobic exercise during each of these periods has long-lasting, additive effects throughout the lifespan.
Conflicts of interest statement
All authors of the current manuscript have no conflict of interests to report.
Acknowledgments
This research was supported by the National Institutes of Health (F31AA019866 – Herting; R01 AA017664 – Nagel; K08 NS052147 – Nagel; R01 MH087563 – Sowell; R01 HD053893 – Sowell; F30 AA020431 – Colby, T32 GM008042 – Colby), the Dana Foundation (Nagel), the Oregon Clinical and Translational Research Institute, the OHSU Tartar Trust Research Fellowship (Herting), American Psychological Association Science Directorate's Dissertation Research Award (Herting), ARCS Foundation, Inc. Portland Chapter (Herting). A special thanks to Madison Stroup, Karen Hudson, Jill Waldman, Jenny Peraza, and Kristen Mackiewicz Seghete for their assistance in data collection and data entry. Thank you to Dr. Elliot for her help with aerobic fitness testing.
References
- Alexander A.L. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R. 2007. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2 from http://www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- Armstrong N. Aerobic fitness: what are we measuring? Med. Sci. Sports. 2007;50:5–25. doi: 10.1159/000101073. [DOI] [PubMed] [Google Scholar]
- Armstrong N. Oxford University Press, Inc.; New York: 2008. Paediatric Exercise Science and Medicine. [Google Scholar]
- Basser P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brown C.H. The effects of cross-country running on pre-adolescent girls. Med. Sci. Sports. 1972;4(1):1–5. [PubMed] [Google Scholar]
- Brown S.A. Correlates of success following treatment for adolescent substance abuse. Appl. Prev. Psychol. 1994;3:61–73. [Google Scholar]
- Brown S.A. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Bruce R.A. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973;85(4):546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Casey B.J. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control . 2011, September. Child and Teen BMI Calculator. From http://apps.nccd.cdc.gov/dnpabmi/ [Google Scholar]
- Colby J.B. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59(4):3227–3242. doi: 10.1016/j.neuroimage.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N.Y. Acad. Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Etnier J.L. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Rev. 2006;52(1):119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Ghiani C.A. Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia. 2007;55(9):966–975. doi: 10.1002/glia.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J. Comp. Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Herting M.M. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 2012;233(2):517–525. doi: 10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M. Differences in brain activity during a verbal associative memory encoding task in high- and low-fit adolescents. J. Cogn. Neurosci. 2013;25(4):595–612. doi: 10.1162/jocn_a_00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University; New Haven, CT: 1975. Four Factor Index of Social Status. [Google Scholar]
- Hoven C.W. Psychopathology among New York city public school children 6 months after September 11. Arch. Gen. Psychiatry. 2005;62(5):545–552. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn. Reson. Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Johnson N.F. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. White matter integrity, fiber count, and other fallacies: the do's and don’ts of diffusion MRI. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl G.S. Developmental aspects of maximal aerobic power in children. Exerc. Sport Sci. Rev. 1985;13(1):503–538. [PubMed] [Google Scholar]
- Krityakiarana W. Voluntary exercise increases oligodendrogenesis in spinal cord. Int. J. Neurosci. 2010;120(4):280–290. doi: 10.3109/00207450903222741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnt D. Fiber tractography based on diffusion tensor imaging compared with high-angular-resolution diffusion imaging with compressed sensing: initial experience. Neurosurgery. 2013;72(Suppl. 1):165–175. doi: 10.1227/NEU.0b013e318270d9fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long A.C. Brief report: using actigraphy to compare physical activity levels in adolescents with chronic pain and healthy adolescents. J. Pediatr. Psychol. 2008;33(6):660–665. doi: 10.1093/jpepsy/jsm136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C.P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Lussier L. Effects of an endurance training regimen on assessment of work capacity in prepubertal children. Ann. N. Y. Acad. Sci. 1977;301:734–747. doi: 10.1111/j.1749-6632.1977.tb38243.x. [DOI] [PubMed] [Google Scholar]
- Mahon N.E. The revised Personal Lifestyle Questionnaire for early adolescents. West. J. Nurs. Res. 2003;25(5):533–547. doi: 10.1177/0193945903253000. [DOI] [PubMed] [Google Scholar]
- Marco E.M. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox. Res. 2011;19(2):286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- Marks B.L. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br. J. Sports Med. 2011;45(15):1208–1215. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- Masten A.S. Regulatory processes, risk, and resilience in adolescent development. Ann. N. Y. Acad. Sci. 2004;1021:310–319. doi: 10.1196/annals.1308.036. [DOI] [PubMed] [Google Scholar]
- MATLAB. The MathWorks, Inc., Natick, MA.
- Midgley A.W. Is there an optimal training intensity for enhancing the maximal oxygen uptake of distance runners?: empirical research findings, current opinions, physiological rationale and practical recommendations. Sports Med. 2006;36(2):117–132. doi: 10.2165/00007256-200636020-00003. [DOI] [PubMed] [Google Scholar]
- Molteni R. Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 2004;101(22):8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perrin J.S. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45(4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Petersen A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rice J.P. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol. Clin. Exp. Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Riddoch C.J. Physical activity levels and patterns of 9- and 15-yr-old European children. Med. Sci. Sports Exerc. 2004;36(1):86–92. doi: 10.1249/01.MSS.0000106174.43932.92. [DOI] [PubMed] [Google Scholar]
- Schaechter J.D. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39(3):1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J. Developmental differences in white matter architecture between boys and girls. Hum. Brain Mapp. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri M.M. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn. Reson. Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat. Protoc. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport. 2003;14(18):2469–2473. doi: 10.1097/00001756-200312190-00035. [DOI] [PubMed] [Google Scholar]
- Team R.D.C. 2008. R: A Language and Environment for Statistical Computing. From http://www.R-project.org. [Google Scholar]
- Tseng B.Y. White matter integrity in physically fit older adults. Neuroimage. 2013;82C:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32(5):283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G. Growth and physical training with reference to heredity. J. Appl. Physiol. 1976;40(2):211–215. doi: 10.1152/jappl.1976.40.2.211. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corp.; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wolf A.M. Reproducibility and validity of a self-administered physical activity questionnaire. Int. J. Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]