Abstract
G protein-coupled receptor (GPCR) signaling is precisely regulated. After activation, GPCRs are desensitized, internalized and either recycled to the cell surface or sorted to lysosomes for degradation. The main route for GPCR lysosomal sorting requires ubiquitination and the endosomal-sorting complex required for transport (ESCRT). Four distinct ESCRT adaptor protein complexes act sequentially to bind and sort ubiquitinated cargo to lysosomes. Several studies now indicate that alternate pathways exist for GPCR lysosomal sorting that require only some components of the ESCRT and autophagy machinery. While direct GPCR ubiquitination is not required for alternate lysosomal sorting, new evidence suggests that ubiquitin may function indirectly to modulate adaptor protein activity. Here, we discuss the atypical regulation of GPCR lysosomal sorting by ubiquitination.
Introduction
G protein-coupled receptors (GPCRs) are a large and diverse family of signaling receptors that control vast physiological responses. The temporal and spatial regulation of GPCR signaling is critical for proper cellular and organ function. Indeed, dysregulation of GPCR signaling has been implicated in neurological dysfunctions, cardiovascular disorders, cancer progression and numerous other diseases [1-3]. Signaling by GPCRs is rapidly desensitized by phosphorylation and β-arrestin binding, which uncouples the receptor from heterotrimeric G proteins and promotes receptor internalization from the plasma membrane. Once internalized, agonist activated GPCRs are sorted at endosomal membranes by adaptor proteins and either recycled back to the cell surface or targeted to lysosomes for degradation. In addition to desensitization, intracellular trafficking of GPCRs has important roles in signal termination, propagation and resensitization. Many GPCRs require posttranslational modification with ubiquitin and interaction with ubiquitin-binding domains (UBDs) of the endosomal-sorting complex required for transport (ESCRT) machinery for lysosomal sorting. However, not all GPCRs require direct ubiquitination or all components of the ESCRT machinery for degradation in the lysosome, suggesting that alternate sorting pathways exist. Here, we highlight recent work on two alternative pathways for GPCR lysosomal sorting that are regulated by the G protein-coupled receptor associated sorting protein-1 (GASP-1) and ALG-interacting protein X (ALIX).
Ubiquitin- and ESCRT-dependent sorting of GPCRs
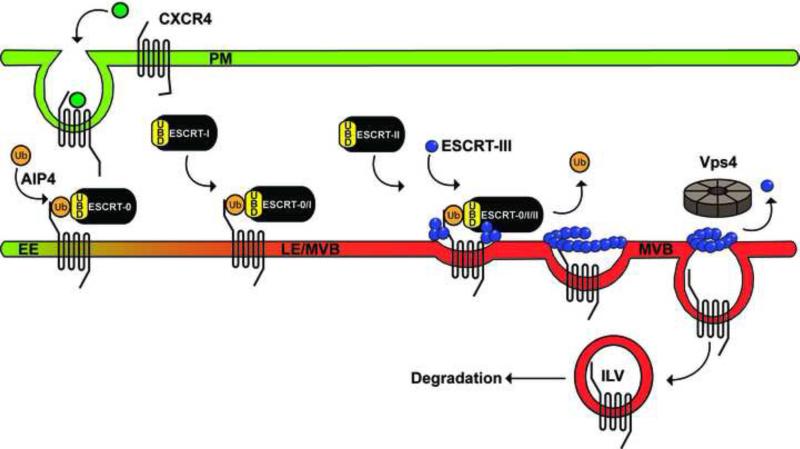
Many but not all mammalian GPCRs require direct ubiquitination for lysosomal sorting via the highly conserved ESCRT pathway [4]. Ubiquitin is a 76-amino acid protein that is covalently attached to lysine residues of substrate proteins by ubiquitin ligases. Ubiquitin-conjugated proteins bind non-covalently to UBDs, a large structurally diverse class of protein modules [5]. The ESCRTs comprise four distinct complexes, three of which contain components with UBDs, indicating that they bind, capture and sort ubiquitinated proteins from early endosomes to late endosomes/multivesicular bodies (MVBs), where cargo proteins are incorporated into intraluminal vesicles (ILVs) of MVBs and degraded (Figure 1) [6]. The ESCRT-mediated GPCR lysosomal sorting pathway is best characterized for the chemokine CXCR4 receptor and protease-activated receptor-2 (PAR2) (Figure 1) [7-10]. However, several new studies provide evidence that question the absolute requirement for receptor ubiquitination and the canonical ESCRTs in lysosomal sorting of GPCRs and suggest that other pathways exist.
Figure 1. Ubiquitin- and ESCRT-dependent sorting of CXCR4.
In the absence of agonist, CXCR4 resides at the plasma membrane (PM). After agonist stimulation, CXCR4 is ubiquitinated by the NEDD4-family member AIP4 E3 ubiquitin ligase and sorted from early endosomes (EE) to late endosomes (LE)/multivesicular bodies (MVBs) by the ESCRT machinery, which binds to CXCR4 via ubiquitin-binding domains (UBDs) and function sequentially to sort ubiquitinated CXCR4 to intralumenal vesicles (ILVs) of MVBs for degradation. The AAA-ATPase Vps4 disassembles and recycles ESCRT-III and is essential for ESCRT function and CXCR4 degradation.
GASP-1-mediated GPCR lysosomal sorting
GASP-1 regulates lysosomal sorting of a subset of GPCRs through a non-conventional pathway that requires some but not all components of the canonical ESCRT and autophagy machinery. In addition, GASP-1-mediated lysosomal sorting occurs independent of GPCR ubiquitination. GASP-1 was discovered in a yeast two-hybrid screen using the cytoplasmic tail of the δ-opioid receptor (DOR) [11]. GASP-1 binds directly to the C-tail domain of DOR as well as to many other GPCRs [12], but only regulates degradation of GPCRs that are efficiently targeted to the lysosome including DOR [11], cannabinoid 1 receptor (CB1R) [13], the cannabinoid related GPR55 [14], the D2 and D3 dopamine receptors [15,16] and the virally encoded chemokine receptor US28 [17].
The GASP-1 protein lacks obvious functional domains and is expressed predominantly in the central nervous system [18]. The GASP-1 C-terminal and middle region appear to mediate interaction with the C-tail domain of GPCRs [11,12,19], however little is known about how this interaction is regulated. A function for GASP-1 in agonist-induced lysosomal sorting of GPCRs was initially assessed by ectopic expression of a dominant-inhibitory GASP-1 C-terminus and RNAi-mediated depletion of the endogenous GASP-1 protein in cultured cells [11,16]. In more recent work, genetic deletion of GASP-1 in mice suggest a function in dopamine responsiveness that appears to be linked to dysregulated trafficking of the D2 class of dopamine receptors [15] and analgesic tolerance associated with altered CB1R trafficking [13,20]. Thus, the regulation of GPCR intracellular trafficking by GASP-1 appears to have important in vivo functional relevance.
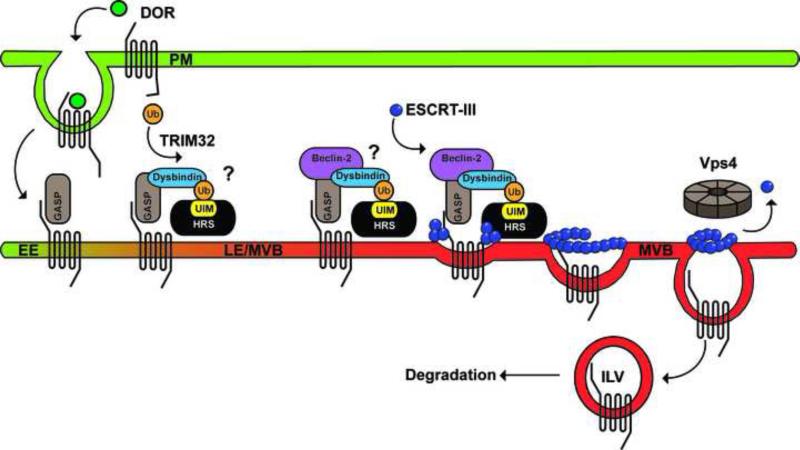
A role for GASP-1 in lysosomal sorting of DOR is most clear. DOR is extensively ubiquitinated and efficiently sorted to MVBs/lysosomes for degradation via a GASP-1 dependent pathway (Figure 2) [11,21,22]. However, lysosomal degradation of DOR does not require direct ubiquitination or Tsg101 (ESCRT-I), despite its dependence on HRS (ESCRT-0) and the AAAATPase Vps4, a critical regulator of ESCRT function [22,23]. The non-ubiquitinated calcitonin receptor-like receptor, also sorts to lysosomes for degradation through an HRS-dependent pathway [24]. Thus, direct ubiquitination of GPCRs is not the only signal essential for ESCRT-mediated sorting into lysosomes. It is possible that DOR-associated proteins are ubiquitinated and provide the signal necessary for engagement of ubiquitin-binding ESCRT components. New studies showed that GASP-1 interacts with dysbindin, a component of the BLOC-1 complex [25], and that dysbindin regulates lysosomal degradation of DOR and D2 dopamine receptors possibly through interaction with HRS, an ESCRT-0 component (Figure 2) [26]. Dysbindin is also ubiquitinated by the RING-domain containing E3 ubiquitin ligase TRIM32 [27], raising the possibility that ubiquitination of dysbindin is important for GASP-1-mediated interaction with ESCRTs and GPCR lysosomal sorting.
Figure 2. GASP-1 mediated lysosomal sorting of DOR.
Upon ligand stimulation, DOR is internalized from the plasma membrane (PM) to early endosomes (EE), where GASP-1 binds directly to the C-tail domain of DOR and facilitates lysosomal degradation via ESCRT-III and Vps4. Dysbindin also binds to GASP-1 and HRS, a component of ESCRT-0, and mediates lysosomal degradation of DOR. A recent study now indicates that Beclin-2, a component of the autophagy machinery, has a distinct function in lysosomal degradation of GPCRs including DOR that requires interaction with GASP-1. Although ubiquitination of DOR is not required for lysosomal degradation, it is possible that ubiquitination of dysbindin mediated by the RING-domain E3 ligase TRIM32 or some other component of this complex mediates interaction with HRS via its ubiquitin-interacting motif (UIM), but this has not been tested. In addition, the order and timing of GASP-1, Beclin-2 and HRS recruitment to DOR remains unclear.
A more recent study reported a surprising function for a new autophagy-associated protein Beclin-2 in the regulation of GPCR lysosomal sorting via interaction with GASP-1. An interaction between Beclin-2 and GASP-1 was discovered in a yeast two-hybrid screen designed to assess potential autophagy independent functions of Beclin-2 [28]. A function for Beclin-2 in lysosomal degradation of DOR and CB1R, but not CXCR4 was demonstrated by siRNA-mediated depletion. The interaction between Beclin-2 and GASP-1 is also required for GPCR degradation (Figure 2). In mouse embryonic fibroblasts derived from Beclin-2 knockouts, endogenous CB1R and D2 dopamine receptor expression was elevated [28]. Moreover, Beclin-2 heterozygous deficient mice exhibited increased expression of CB1R and altered food intake, obesity and insulin resistance, suggesting a potential causal relationship. This works reveals a critical role for Beclin-2 in GASP-1-mediated sorting of GPCRs from early endosomes to lysosomes. However, many questions remain as to how Beclin-2, dysbindin and GASP-1 engage components of the ESCRT machinery to ultimately sort GPCRs into ILVs of MVBs for degradation.
ALIX and GPCR lysosomal sorting
ALIX is a ubiquitously expressed, multivalent adaptor protein that couples membrane-bound proteins to ESCRTs. ALIX has three functional domains, an N-terminal Bro1 domain, a central V-domain and a C-terminal proline-rich region, that bind to other adaptor proteins and ubiquitin ligases [29-32]. In addition to serving as an adaptor for ESCRTs during cytokinesis [33], ALIX facilitates budding of enveloped viruses via interaction with charged MVB protein 4 (CHMP4), an ESCRT-III complex subunit that lacks UBDs [34,35]. However, unlike its yeast counterpart Bro1 [36], a role for ALIX in lysosomal sorting of mammalian host cell proteins remained obscure.
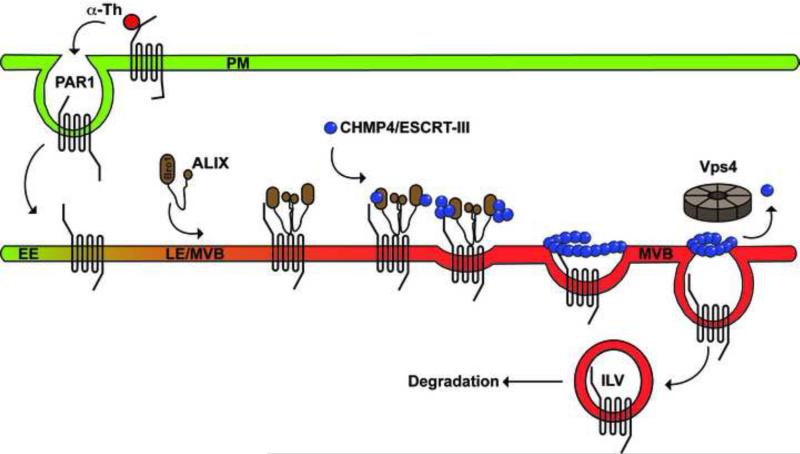
New discoveries now indicate that ALIX mediates lysosomal sorting of protease-activated receptor-1 (PAR1), a GPCR for thrombin, via a non-canonical pathway that requires ESCRT-III (Figure 3) [10]. PAR1 is ubiquitinated following activation, but ubiquitination is not necessary or sufficient for lysosomal degradation of the receptor [37]. In addition, neither HRS nor Tsg101 are essential for PAR1 degradation [38], suggesting that this receptor can bypass the requirement for both ubiquitination and ubiquitin-binding ESCRT components. This pathway is mechanistically distinct from GASP-1-dependent GPCR lysosomal sorting, which requires HRS and Vps4, but not Tsg101 [22]. However, ESCRT-III and Vps4 are essential for PAR1 degradation [10], indicating that some components are conserved in the GASP-1 and ALIX regulated lysosomal sorting pathways.
Figure 3. ALIX-mediated lysosomal degradation of PAR1.
PAR1 is rapidly internalized from the plasma membrane (PM) following agonist stimulation and sorted from early endosomes (EE) to late endosomes (LE)/multivesicular bodies (MVBs) independent of ubiquitination and ubiquitin-binding components of the ESCRT machinery. PAR1 is targeted to lysosomes via interaction with ALIX, which binds directly to a YPX3L motif localized within the second intracellular loop of PAR1, and recruits CHMP4/ESCRT-III to facilitate receptor sorting into intraluminal vesicles (ILVs) of MVBs for degradation.
The ALIX Bro1 domain has a curved structure that cradles CHMP4 within its concave surface [31]. Intriguingly, an ALIX mutant lacking the Bro1 domain was insufficient to restore PAR1 degradation in siRNA knockdown/rescue experiments [10], indicating that ALIX interaction with CHMP4 is critical for lysosomal sorting of PAR1 (Figure 3). In addition, depletion of ALIX by siRNA prevented CHMP4 interaction with activated PAR1. The central V-domain of ALIX is known to bind to highly conserved YPXnL motifs [31,39] and interacts with an YPX3L motif within the second intracellular loop (ICL2) of PAR1 [10]. Mutation of the critical tyrosine residue within the YPX3L motif disrupts PAR1-ALIX interaction and perturbs receptor degradation. While the related PAR2 lacks the YPXnL sorting sequence and is not regulated by ALIX, a subset of other class A GPCRs with conserved YPXnL motifs within ICL2 were identified [10]. These findings suggest that ALIX directs an alternative GPCR lysosomal sorting pathway that is not dependent on direct GPCR ubiquitination or ubiquitin-binding ESCRT components but requires ESCRT-III function.
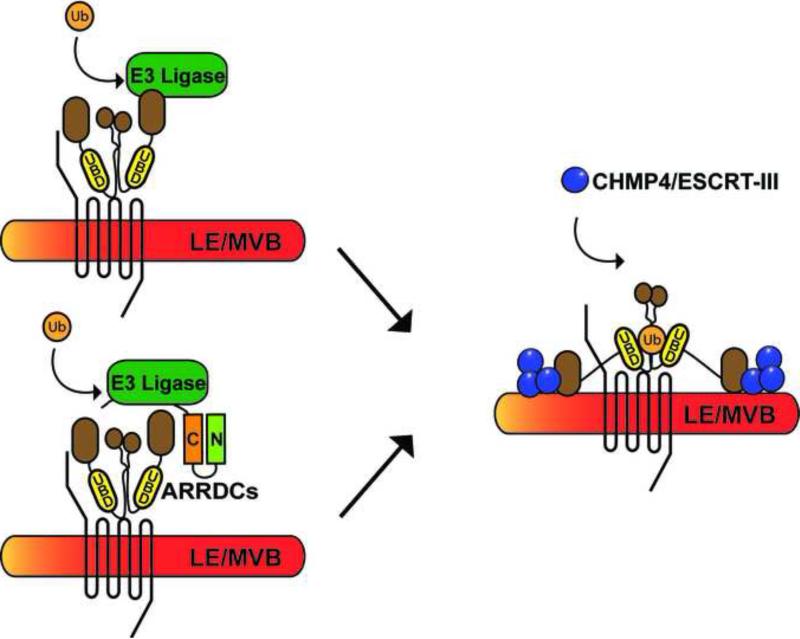
Unlike the canonical ESCRT-dependent lysosomal sorting pathway, the mechanisms that regulate ALIX-mediated GPCR degradation are not known. Recent findings now suggest that ubiquitin may directly modulate ALIX activity. The HECT-domain containing E3 ligase NEDD4-1 and the RING-E3 ligase Ozz-E3 were shown to bind to and ubiquitinate ALIX (Figure 4) [30,40]. Depletion of NEDD4-1 caused a decrease in ALIX ubiquitination and attenuated ALIX-dependent HIV-1 viral particle release [30], demonstrating that ubiquitin can regulate ALIX function. ALIX can also form a complex with E3 ubiquitin ligases indirectly via interaction with the arrestin-domain containing proteins (ARRDCs) (Figure 4). ARRDCs have arrestin-like N- and C- domains, followed by a C-terminus that contains consensus PY binding sites for HECT-domain E3 ubiquitin ligases [41]. Of the five human ARRDCs, ARRDC1 and ARRDC3 were shown to directly interact with ALIX and WWP1, WWP2, NEDD4-1 and Itch/AIP4 E3 ubiquitin ligases in a yeast two-hybrid screen [42]. ARRDC3 was previously shown to mediate ubiquitination of β2-adrenergic receptor (β2-AR) and vasopressin receptor-2 by recruitment of NEDD4-family E3 ubiquitin ligases [43,44]. However, a recent study suggests that ARRDC3-mediated ubiquitination of β2-AR may play a secondary role to β-arrestin-2 in ubiquitin-dependent lysosomal degradation [45]; instead ARRDC3 sorts the β2-AR/NEDD4 complex to a subpopulation of early endosomes. It is possible that ALIX or ARRDC binding partners recruit E3 ubiquitin ligases to facilitate lysosomal sorting of YPXnL-motif containing GPCRs, however the role of ARRDCs in the ubiquitination of ALIX, and the lysosomal sorting of YPXnL-motif GPCRs has yet to be determined.
Figure 4. Ubiquitin regulation of ALIX function.
Several new studies indicate that ALIX function may be regulated by ubiquitination. E3 ubiquitin ligases can bind to and directly ubiquitinated ALIX. It is also possible that the ARRDC adaptor proteins, which harbor PY motifs recruit HECT-domain containing E3 ligases to ALIX to facilitate ubiquitination but this has not been examined. Intriguingly, ALIX was recently shown to contain a bona fide ubiquitin-binding domain within the central V domain that is important for its function. This raises the possibility that ALIX ubiquitination and the UBD may coordinate its function by facilitating interaction with YPXnL motifs, interaction with cofactors or endosomal membranes enriched in LBPA. ALIX dimerization may also influence its function.
Three recent studies demonstrate that ALIX harbors a highly conserved ubiquitin-binding region within the V-domain that is distinct from the YPXnL binding site and regulates its activity [46-48]. Indeed, mutation of critical residues within the UBD of ALIX abrogates lentiviral budding [48]. Interestingly, while ubiquitination of HIV-1 gag is required for viral budding, it is not essential for interaction with ALIX [49], suggesting that the UBD of ALIX is unlikely to function solely as a binding site for ubiquitinated cargo, like its yeast homologue Bro1 [46]. Rather, the UBD may bind ubiquitinated residues within ALIX itself resulting in a conformational change that enhances ALIX binding to YPXnL motifs, interaction with cofactors or endosomal membranes (Figure 4). ALIX binds to lysobisphosphatidic acid (LBPA), a unique lipid enriched at late endosomal membranes [50], and is important for its function, but how LBPA affects ALIX activity is not known. Recent studies demonstrate that the Bro1 domain undergoes a calcium-dependent conformational change that facilitates recruitment of ALIX to LBPA-enriched membranes and promotes viral budding [51]. Ubiquitination of ALIX may also enhance or stabilize this conformation, anchoring ALIX to endosomal membranes during cargo sorting. Another possibility is that the ALIX UBD enhances interaction with adaptor proteins such as ARRDCs that become ubiquitinated during receptor sorting. ARRDC1 is ubiquitinated by the E3 ligase WWP1 [42], but the ubiquitination status of ARRDC3 has not been reported. The ALIX UBD may also facilitate ALIX dimerization. Dimerization of ALIX is mediated by the V-domain [29], and ALIX dimers are present at endosomal membranes (Figure 4) [51]. Taken together this new evidence suggest that ubiquitin is important for ALIX function, and may serve to indirectly regulate the ‘ubiquitin-independent’ lysosomal sorting of YPXnL containing GPCRs by modulating the activity of ALIX.
Conclusions
Ultimately, most if not all GPCRs are degraded in the lysosome and require some components of the ESCRT machinery. The challenge now is to determine how GASP-1 and ALIX engage with ubiquitin and ESCRTs to mediate GPCR lysosomal degradation. GASP-1 and ALIX selectively regulate individual members of GPCR subfamilies, particularly those that are efficiently sorted to lysosomes and not recycled, but the underlying basis for this is not known. Since signaling is tightly linked to GPCR intracellular trafficking, we hypothesize that such alternative pathways are likely critical for appropriate regulation of the temporal and spatial aspects of signaling and cellular responses. Thus, selectively modulating the lysosomal sorting pathway of certain GPCRs could have important implications for drug development.
Highlights.
Several GPCRs sort to lysosomes independent of ubiquitination and certain ESCRTs.
GPCRs sort to lysosomes via two alternative pathways mediated by GASP-1 and ALIX.
GASP-1 interacts with Beclin-2 and dysbindin to mediate GPCR lysosomal sorting.
ALIX binds to a GPCR YPXnL motif and ESCRT-III to facilitate lysosomal degradation
ALIX activity is regulated by binding to ubiquitin and LBPA-enriched membranes
Acknowledgements
M. R. D. is a San Diego IRACDA Fellow supported by National Institutes of Health K12 GM06852 and J.T. is supported by National Institutes of Health R01 GM090689.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchese A, Paing MM, Temple BRS, Trejo J. G Protein-coupled Receptor Sorting to Endosomes and Lysosomes. Annu. Rev. Pharmcol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 3.Mason JS, Bortolato A, Congreve M, Marshall FH. New insights from structural biology into the druggability of G protein-coupled receptors. Trends Pharmacol Sci. 2012 doi: 10.1016/j.tips.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Dores MR, Trejo J. Ubiquitination of GPCRs, Functional Implications and Drug Discovery. Mol. Pharm. 2012;82:563–570. doi: 10.1124/mol.112.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains - from structures to functions. Nat Rev Mol Cell Biol. 2009;10:659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchese A, Raiborg C, Santini F, Keen JT, Stenmark H, Benovic JL. The E3 Ubiquitin Ligase AIP4 Mediates Ubiquitination and Sorting of the G Protein-coupled Receptor CXCR4. Dev. Cell. 2003;5:709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 8.Hasdemir B, Bunnett NW, Cottrell GS. Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) mediates post-endocytic trafficking of protease-activated receptor 2 and calcitonin receptor-like receptor. J Biol Chem. 2007;282:29646–29657. doi: 10.1074/jbc.M702974200. [DOI] [PubMed] [Google Scholar]
- 9.Malerod L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 10••.Dores MR, Chen B, Lin H, Soh UJK, Paing MM, Montagne WA, Meerloo T, Trejo J. ALIX binds a YPX3L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J. Cell Biol. 2012;197:407–419. doi: 10.1083/jcb.201110031. [This study demonstrate a function for ALIX in lysosomal sorting of a mammalian GPCR via interaction with a YPX3L motif and recruitment of CHMP4/ESCRT-III.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 12.Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. A Library of 7TM Receptor C-terminal Tails. J. Biol. Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 13.Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid-1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 14.Kargl J, Balenga NA, Platzer W, Martini L, Whistler JL, Waldhoer M. The GPCR-associated sorting protein 1 regulates ligand-induced down-regulation of GPR55. Br. J. Pharm. 2012;165:2611–2619. doi: 10.1111/j.1476-5381.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Malliard WS, Armstrong R, Bonci A, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc. Natl. Acad. Sci. 2005;102:11521–11526. doi: 10.1073/pnas.0502418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson D, Whistler JL. Dopamine D(3) receptors are down-regulated following heterologous endocytosis by a specific interaction with G protein-coupled receptor-associated sorting protein-1. J. Biol. Chem. 2011;286:1598–1608. doi: 10.1074/jbc.M110.158345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschische P, Moser E, Thompson D, Vischer HF, Parzmair GP, Pommer V, Platzer W, Schwarzbraun T, Schaider H, Smit MJ, et al. The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28. Traffic. 2010;11:660–674. doi: 10.1111/j.1600-0854.2010.1045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonin F, Karcher P, Boeuf JJ-M, Matifas A, Kieffer BL. Identification of a novel family of G-protein-coupled receptor associated sorting proteins. J. Neurochem. 2004;89:766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 19.Bornert O, Moller TC, Boeuf J, Candusso MP, Wagner R, Martinez KL, Simonin F. Identification of a novel protein-protein interaction motif mediating interaction of GPCR-associated sorting proteins with G protein-coupled receptors. PloS one. 2013;8:e56336. doi: 10.1371/journal.pone.0056336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, et al. A molecular basis of analgesic tolerance to cannabinoids. J. Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly Synthesized Human Opioid Receptors Retained in the Endoplasmic Reticulum Are Retrotranslocated to the Cytosol, Deglycosylated, Ubiquitinated, and Degraded by the Proteasome. J. Biol. Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 22.Tanowitz M, Von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J. Biol. Chem. 2002;277:50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- 23.Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic. 2011;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem. 2007;282:12260–12271. doi: 10.1074/jbc.M606338200. [DOI] [PubMed] [Google Scholar]
- 25.Mullin AP, Gokhale A, Larimore J, Faundez V. Cell biology of the BLOC-1 complex subunit dysbindin, a schizophrenia susceptibility gene. Mol Neurobiol. 2011;44:53–64. doi: 10.1007/s12035-011-8183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PloS one. 2010;5:e9325. doi: 10.1371/journal.pone.0009325. [This work showed that dysbindin functions in GASP-1 mediated lysosomal degradation of GPCRs possibly through interaction with HRS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke M, Tinsley CL, Benson MA, Blake DJ. TRIM32 is an E3 ubiquitin ligase for dysbindin. Hum Molecular Genet. 2009;18:2344–2358. doi: 10.1093/hmg/ddp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.He C, Wei Y, Sun K, Li B, Dong X, Zou Z, Liu Y, Kinch LN, Khan S, Sinha S, et al. Beclin 2 functions in autophagy, degradation of g protein-coupled receptors, and metabolism. Cell. 2013;154:1085–1099. doi: 10.1016/j.cell.2013.07.035. [A new autophagy associated protein, Beclin-2 was shown to directly bind to and regulate GASP-1 mediated lysosomal degradation of GPCRs and has potential significnance to metabolic regulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pires R, Hartlieb B, Signor L, Schoehn G, Lata S, Roessle M, Moriscot C, Popov S, Hinz A, Jamin M, et al. A crescent-shaped ALIX dimer targets ESCRT-III CHMP4 filaments. Structure. 2009;17:843–856. doi: 10.1016/j.str.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sette P, Jadwin JA, Dussupt V, Bello NF, Bouamr F. The ESCRT-associated protein Alix recruits the ubiquitin ligase Nedd4-1 to facilitate HIV-1 release through the LYPXnL L domain motif. J Virol. 2010;84:8181–8192. doi: 10.1128/JVI.00634-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai Q, Fisher RD, Chung HY, Myszka DG, Sundquist WI, Hill CP. Structural and functional studies of ALIX interactions with YPX(n)L late domains of HIV-1 and EIAV. Nat Struct Mol Biol. 2008;15:43–49. doi: 10.1038/nsmb1319. [DOI] [PubMed] [Google Scholar]
- 32.Shi X, Betzi S, Lugari A, Opi S, Restouin A, Parrot I, Martinez J, Zimmermann P, Lecine P, Huang M, et al. Structural recognition mechanisms between human Src homology domain 3 (SH3) and ALG-2-interacting protein X (Alix). FEBS letters. 2012;586:1759–1764. doi: 10.1016/j.febslet.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci U S A. 2008;105:10541–10546. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson LA, Hurley JH. In vitro reconstitution of the ordered assembly of the endosomal sorting complex required for transport at membrane-bound HIV-1 Gag clusters. Proc Natl Acad Sci U S A. 2012;109:16928–16933. doi: 10.1073/pnas.1211759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odorizzi G, Katzmann DJ, Babst M, Audhya A, Emr SD. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J.Cell Sci. 2003116:1893–1903. doi: 10.1242/jcs.00395. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe BL, Marchese A, Trejo J. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J. Cell Biol. 2007;177:905–916. doi: 10.1083/jcb.200610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: Evidence for retromer, Hrs and Tsg101 independent functions of sorting nexins. Mol. Biol. Cell. 2006;17:1228–1238. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher RD, Chung HY, Zhai Q, Robinson H, Sundquist WI, Hill CP. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell. 2007;128:841–852. doi: 10.1016/j.cell.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 40.Bongiovanni A, Romancino DP, Campos Y, Paterniti G, Qiu X, Moshiach S, Di Felice V, Vergani N, Ustek D, d'Azzo A. Alix protein is substrate of Ozz-E3 ligase and modulates actin remodeling in skeletal muscle. J. Biol. Chem. 2012;287:12159–12171. doi: 10.1074/jbc.M111.297036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aubry L, Guetta D, Klein G. The arrestin fold: variations on a theme. Curr Genomics. 2009;10:133–142. doi: 10.2174/138920209787847014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Rauch S, Martin-Serrano J. Multiple interactions between the ESCRT machinery and arrestin-related proteins: implications for PPXY-dependent budding. J. Virol. 2011;85:3546–3556. doi: 10.1128/JVI.02045-10. [A yeast two-hybrid interaction assay was used to demonstrate that ARRDCs directly interact with ALIX and E3 ubiquitin ligases.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO Rep. 2010;11:605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Shea FF, Rowell JL, Li Y, Chang TH, Alvarez CE. Mammalian alpha arrestins link activated seven transmembrane receptors to Nedd4 family e3 ubiquitin ligases and interact with beta arrestins. PloS one. 2012;7:e50557. doi: 10.1371/journal.pone.0050557. [This work provides evidence that ARRDCs link E3 ubiquitin ligases to GPCRs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han SO, Kommaddi RP, Shenoy SK. Distinct roles for beta-arrestin2 and arrestin-domain-containing proteins in beta2 adrenergic receptor trafficking. EMBO Rep. 2013;14:164–171. doi: 10.1038/embor.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Pashkova N, Gakhar L, Winistorfer SC, Sunshine AB, Rich M, Dunham MJ, Yu L, Piper RC. The yeast Alix homolog Bro1 functions as a ubiquitin receptor for protein sorting into multivesicular endosomes. Dev. Cell. 2013;25:520–533. doi: 10.1016/j.devcel.2013.04.007. [The yeast Bro1 homologue of ALIX was shown to contain a ubiquitin-binding domain that binds to and sorts ubiquitinated cargo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Keren-Kaplan T, Attali I, Estrin M, Kuo LS, Farkash E, Jerabek-Willemsen M, Blutraich N, Artzi S, Peri A, Freed EO, et al. Structure-based in silico identification of ubiquitin- binding domains provides insights into the ALIX-V:ubiquitin complex and retrovirus budding. EMBO J. 2013;32:538–551. doi: 10.1038/emboj.2013.4. [A ubiquitin-binding domain in the ALIX V domain was indentified and shown to bind to mono-ubiquitin, which induces ALIX oligomerization and facilitates viral budding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Dowlatshahi DP, Sandrin V, Vivona S, Shaler TA, Kaiser SE, Melandri F, Sundquist WI, Kopito RR. ALIX is a Lys63-specific polyubiquitin binding protein that functions in retrovirus budding. Dev Cell. 2012;23:1247–1254. doi: 10.1016/j.devcel.2012.10.023. [This work used affinity capture/mass spectrometry to show that ALIX binds to K63-linked polyubiquitin to facilitate viral budding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sette P, Nagashima K, Piper RC, Bouamr F. Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding. Retrovirology. 2013;10:79. doi: 10.1186/1742-4690-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 51••.Bissig C, Lenoir M, Velluz MC, Kufareva I, Abagyan R, Overduin M, Gruenberg J. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell. 2013;25:364–373. doi: 10.1016/j.devcel.2013.04.003. [This study demonstrates that the calcium-bound Bro1 domain ALIX is critical for recruitment to LBPA-enriched endosomal membranes and viral budding.] [DOI] [PMC free article] [PubMed] [Google Scholar]