Abstract
Aims/Introduction: Reduced insulin sensitivity and secretion are important in the pathogenesis of type 2 diabetes. Their relationships to prediabetes, impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) have been previously studied with the oral glucose tolerance test (OGTT). We investigated whether or not baseline measures of insulin secretion and sensitivity obtained from fasting blood specimens were related to the development of prediabetes and how these measures compared with those based on the OGTT.
Materials and Methods: In 152 Japanese subjects with normal glucose tolerance, we measured baseline plasma glucose and insulin after an overnight fast and during a 75 g OGTT, insulin resistance index (homeostasis model assessment [HOMA‐IR]), and insulin secretion (insulinogenic index [30 min insulin − fasting insulin] ÷ [30 min glucose − fasting glucose] or HOMA‐β).
Results: At a 5–6 year (mean 5.7 years) follow‐up examination, we confirmed 36 cases of prediabetes. After adjusting for age, sex, family history of diabetes, body mass index, and 2‐h plasma glucose, the odds ratio comparing the lowest tertile (≤0.82) of insulinogenic index with the highest tertile (≥1.43) was 6.98 (95% confidence interval, 1.96–24.85) and was 10.72 (2.08–55.3) comparing the lowest tertile (≤76.3) of HOMA‐β with the highest tertile (≥122.1), whereas the respective odds ratios of HOMA‐IR were 3.74 (1.03–13.57) and 10.89 (1.93–61.41) comparing the highest tertile (≥1.95) with the lowest tertile (≤1.25).
Conclusions: Lower insulin secretion and sensitivity are independent risk factors for prediabetes. Clinically practical identification of those at risk for prediabetes is obtainable from HOMA‐β and HOMA‐IR, both of which are measured in fasting state. (J Diabetes Invest, doi: 10.1111.j.2040‐1124.2010.00041.x, 2010)
Keywords: Glucose intolerance, Insulin resistance, Epidemiology
Introduction
The prevalence of type 2 diabetes is rapidly growing worldwide1,2. Because impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are associated with a higher risk for future type 2 diabetes3–6, they are also called prediabetes. In addition, IGT has been reported to be associated with an increased risk for cardiovascular disease, ophthalmic diabetic complications, and mortality7–10. Therefore, it may be important to detect subjects at high risk of development of prediabetes in order to prevent or delay onset of type 2 diabetes and its associated complications.
Although higher insulin resistance and lower insulin secretion are known to be key pathogenic factors in type 2 diabetes, only a few prospective studies have reported whether or not these are prospectively associated with an increased risk of prediabetes11–13. All have reported an association between lower insulin secretion and risk of IGT or IFG, but all also used the oral glucose tolerance test (OGTT) or a variant of it to assess insulin secretion. Because the OGTT is both time‐ and cost‐consuming, a fasting test might be preferable when screening for those at risk for prediabetes among normal glucose tolerance (NGT) individuals. Homeostasis model assessment of β‐cell function (HOMA‐β) requires only a single measurement of insulin and glucose in the fasting state, but its ability to predict prediabetes has not been thoroughly studied. We therefore prospectively examined the relationship of insulin secretion, estimated as HOMA‐β and insulinogenic index (Δinsulin [0–30 min] ÷Δglucose [0–30 min]), and insulin resistance, measured as HOMA‐IR, to the risk of IGT and/or IFG in Japanese men and women.
Materials and methods
Study Population
The study population consisted of native Japanese men and women with NGT enrolled between 1997 and 2003 at the Institute for Adult Diseases (IAD), Asahi Life Foundation, Tokyo, Japan. Subjects were excluded if they had diseases that might affect their ability to participate or influence glucose metabolism (endocrine, hepatic, pancreatic, pulmonary, neoplastic, neuromuscular or psychiatric diseases) or if they were receiving medications that could influence glucose metabolism, such as glucocorticoids.
Of 281 NGT subjects in the original baseline cohort, 85 subjects could not be contacted or refused to further participate, 15 moved and three died. Of the remaining 178 subjects, 14 were excluded because they were examined more than 7 years after their baseline examination, leaving 164 subjects who completed a 5–6‐year (mean 5.7 ± 0.6 years) follow‐up examination. For the current analysis, 12 subjects who developed diabetes during the follow‐up period were also excluded. The study population for analyses therefore included 152 participants. The protocol for this research was reviewed by the institutional review board of Asahi Life Institute, and conforms to the provisions of the Declaration of Helsinki. Signed informed consent was obtained from all participants.
Data Collection
All evaluations were carried out at IAD, Asahi Life Foundation. The clinical examination consisted of a medical history, physical examination, anthropometric measurements and self‐administered questionnaires. Plasma glucose and insulin were measured after an overnight 12‐h fast and during a 75‐g OGTT at 30, 60 and 120 min. Participants were classified as NGT, IFG, IGT or type 2 diabetes based on the American Diabetes Association 1997 criteria14. Plasma glucose was measured by an automated glucose oxidase method. Plasma insulin was measured by radioimmunoassay (RIA) from December 1997 to October 1998 at IAD and from October 1998 to November 2000 by solid phase RIA at SRL Inc (Tokyo, Japan). The conversion formula based on a comparison of the two assays on duplicate samples was SRL RIA insulin = 1.232 × IAD insulin (R2 = 0.948). From November 2000, insulin was measured by enzyme immunoassay (EIA) at SRL. The conversion formula was SRL RIA insulin = 1.040 × SRL EIA insulin 0.706 (R2 = 0.992). All the data are converted to SRL RIA insulin. Insulin sensitivity was estimated by homeostasis model assessment (HOMA‐IR): (fasting glucose [mg/dL]) × [fasting insulin {μU/mL}] ÷ 405)15. To assess insulin secretion, we used the insulinogenic index (30–0 min insulin [μU/mL]) ÷ (30–0 min plasma glucose [mg/dL]), which provides a measurement of early insulin release during the OGTT, and HOMA‐β (fasting plasma insulin [μU/mL] × 360 ÷ [fasting plasma glucose {mg/dL} – 63])15. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared.
Diagnosis of NGT, IFG, IGT and Type 2 Diabetes
Type 2 diabetes was diagnosed if fasting plasma glucose (FPG) was ≥126 mg/dL or 2‐h glucose was ≥200 mg/dL or the subject was taking oral hypoglycemic medication or insulin. Isolated IGT was diagnosed if the subject had no history of diabetes and FPG was <110 mg/dL, but 2‐h glucose was ≥140 and <200 mg/dL. Isolated IFG was diagnosed if the subject had no history of diabetes and FPG was ≥110 and <126 mg/dL, but 2‐h glucose was <140 mg/dL. Subjects who met the criteria for both IGT and IFG (FPG of ≥110 and <126 mg/dL, and 2‐h glucose of ≥140 and <200 mg/dL) were classified as IGT/IFG. Subjects with no history of diabetes, FPG < 110 mg/dL, and 2‐h glucose <140 mg/dL were classified as NGT. These criteria, based on the earlier 1997 ADA criteria14 and currently used by the Japan Diabetes Society, were applied at baseline and follow up.
Statistical Analyses
We used multiple logistic regression analysis to estimate the odds ratio (OR) for incidence of prediabetes in relation to baseline variables. The presence of effect modification was tested by the insertion of first‐order interaction terms into appropriate regression models. Multicollinearity was assessed using the variance inflation factor (VIF). A value of VIF exceeding 10 is regarded as indicating serious multicollinearity and values above 4.0 might be a cause for concern. We calculated the 95% confidence interval (CI) for each OR. The areas under the receiver operator characteristic (ROC) curves for each multiple logistic regression model, which included the insulinogenic index or HOMA‐β, were calculated and statistically compared. P‐values presented are two‐tailed. We carried out statistical analyses using the PASW Statistics version 17.0 (SPSS, Chicago, IL, USA) software package and Stata SE, version 10.0 (StataCorp, College Station, TX, USA).
Results
Among 152 subjects with NGT at baseline who had follow‐up examinations at 5–6 years (mean 5.7 ± 0.6 years), we confirmed 36 cases of IGT and/or IFG (26 isolated IGT, 6 isolated IFG and 4 IGT/IFG). The baseline anthropometric and metabolic characteristics of the 152 subjects are presented in Table 1. Subjects who developed prediabetes during the follow up tended to have higher mean levels of BMI, HOMA‐IR, and fasting and 2‐h plasma glucose, and a higher prevalence of family history of diabetes than those who did not. There were no statistically significant differences in HOMA‐β and insulinogenic index levels between those who developed prediabetes and those who did not. The baseline characteristics of subjects who participated in follow up versus those who did not were not significantly different (data not shown).
Table 1. Characteristics of study subjects at baseline according to whether prediabetes developed after 5–6 year follow up.
| Characteristics | Total (n = 152) | Glucose tolerance status at follow up | P | |
|---|---|---|---|---|
| NGT (n = 116) | Prediabetes (n = 36) | |||
| Age (years) | 58.8 ± 6.6 | 58.3 ± 6.3 | 60.3 ± 7.4 | 0.119 |
| Female sex (%) | 53.3 | 52.6 | 55.6 | 0.757 |
| Body mass index (kg/m2) | 23.0 ± 2.9 | 22.7 ± 2.7 | 24.0 ± 3.5 | 0.022 |
| Family history of diabetes (%) | 11.2% (17/152) | 7.8% (9/116) | 22.2% (8/36) | 0.016 |
| Fasting plasma glucose, (mg/dL) | 89.7 ± 7.1 | 88.6 ± 6.6 | 93.0 ± 7.5 | 0.001 |
| 2‐h plasma glucose (mg/dL) | 106.5 ± 20.2 | 103.6 ± 20.9 | 116.1 ± 14.4 | 0.001 |
| Fasting plasma insulin (μU/mL) | 7.78 ± 3.69 | 7.53 ± 3.74 | 8.58 ± 3.48 | 0.137 |
| HOMA‐IR | 1.73 ± 0.86 | 1.66 ± 0.85 | 1.98 ± 0.85 | 0.046 |
| Insulinogenic index | 1.55 ± 2.00 | 1.68 ± 2.22 | 1.11 ± 0.88 | 0.132 |
| HOMA‐β | 110.2 ± 56.2 | 111.1 ± 58.7 | 107.2 ± 47.5 | 0.718 |
Variables are expressed as mean ± SD or percentages.
IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGT, normal glucose tolerance.
Because beta‐cell secretion is affected by prevailing insulin resistance, we took this into consideration in several different ways. To examine the relationship of HOMA‐β and insulinogenic index to incidence of prediabetes by the levels of HOMA‐IR, we stratified subjects according to tertiles of HOMA‐IR (Table 2). In all of these strata, the highest tertile of HOMA‐β and insulinogenic index had the lowest incidence of prediabetes. In contrast, after subjects were stratified according to tertiles of HOMA‐β or insulinogenic index, higher levels of HOMA‐IR were associated with greater incidence of IGT and/or IFG (Table 2).
Table 2. Incidence of prediabetes according to homeostasis model assessment of β‐cell function, insulinogenic index and HOMA‐IR.
| HOMA‐IR | ||||
|---|---|---|---|---|
| Total | Low (≤1.25) | Medium (1.25–1.95) | High (≥1.95) | |
| Total | 12.0% (6/50) | 25.0% (13/52) | 34.0% (17/50) | |
| Insulinogenic index range | ||||
| Low (≤0.82) | 31.4% (16/51) | 8.7% (2/23) | 50.0% (10/20) | 50.0% (4/8) |
| Medium (0.82–1.43) | 26.0% (13/50) | 21.1% (4/19) | 16.7% (2/12) | 36.8% (7/19) |
| High (≥1.43) | 13.7% (7/51) | 0% (0/8) | 5% (1/20) | 26.1% (6/23) |
| HOMA‐β | ||||
| Low (≤76.25) | 24.0% (12/50) | 13.2% (5/38) | 60.0% (6/10) | 50.0% (1/2) |
| Medium (76.25–122.13) | 26.9% (14/52) | 10.0% (1/10) | 17.2% (5/29) | 61.5% (8/13) |
| High (≥122.13) | 20.0% (10/50) | 0% (0/2) | 15.4% (2/13) | 22.9% (8/35) |
HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance.
We also carried out regression analysis to adjust for HOMA‐IR and clarify the independent effects of insulinogenic index or HOMA‐β on the incidence of IGT and/or IFG in two multivariate regression models (Table 3). Because insulinogenic index and HOMA‐IR showed a nonlinear association with the incidence of prediabetes, to simplify the models we included insulinogenic index and HOMA‐IR categorized by tertiles. After adjusting for age, sex, family history of type 2 diabetes, BMI and 120‐min plasma glucose level, lower insulinogenic index and higher HOMA‐IR were independently associated with the risk of developing prediabetes (Model 1, Table 3). Likewise, when HOMA‐β was substituted for insulinogenic index, lower HOMA‐β and higher HOMA‐IR were also independently associated with an increased risk of incidence of prediabetes (Model 2, Table 3). Although HOMA‐β and HOMA‐IR both include fasting glucose and fasting insulin, they were independent risk factors when assessed by VIF. We examined the significance of the interaction terms between HOMA‐IR and insulinogenic index or HOMA‐β, but there was no significant improvement in fit. The interaction of sex with HOMA‐IR, insulinogenic index, or HOMA‐β was not significant. There was no multicollinearity in all models we examined.
Table 3. Multivariate models of the incidence of prediabetes in relation to baseline insulin secretion and resistance.
| Variables in the model | Multiple‐adjusted odds ratio (95% CI) | P |
|---|---|---|
| Model 1 | ||
| Insulinogenic Index | ||
| Tertile 1 (≤0.82) | 6.98 (1.96–24.85) | 0.003 |
| Tertile 2 (0.82‐1.43) | 3.73 (1.13–12.25) | 0.030 |
| Tertile 3 (≥1.43) | 1.00 | |
| HOMA‐IR | ||
| Tertile 1 (≤1.25) | 1.00 | |
| Tertile 2 (1.25–1.95) | 2.13 (0.64–7.07) | 0.215 |
| Tertile 3 (≥1.95) | 3.74 (1.03–13.57) | 0.045 |
| Female | 1.12 (0.47–2.69) | 0.801 |
| Age | 1.05 (0.99–1.13) | 0.132 |
| Body mass index | 1.20 (1.01–1.43) | 0.037 |
| 2‐h glucose | 1.03 (1.00–1.06) | 0.027 |
| Family history of diabetes | 4.37 (1.26–15.23) | 0.021 |
| Model 2 | ||
| HOMA‐β | ||
| Tertile 1 (≤76.25) | 10.72 (2.08–55.32) | 0.005 |
| Tertile 2 (76.25–122.13) | 4.05 (1.19–13.84) | 0.026 |
| Tertile 3 (≥122.13) | 1.00 | |
| HOMA‐IR | ||
| Tertile 1 (≤1.25) | 1.00 | |
| Tertile 2 (1.25–1.95) | 3.69 (0.87–15.59) | 0.076 |
| Tertile 3 (≥1.945) | 10.89 (1.93–61.41) | 0.007 |
| Female | 1.32 (0.54–3.18) | 0.543 |
| Age | 1.05 (0.98–1.12) | 0.149 |
| Body mass index | 1.20 (1.01–1.42) | 0.038 |
| 2‐h glucose | 1.03 (1.01–1.06) | 0.015 |
| Family history of diabetes | 2.87 (0.81–10.17) | 0.103 |
HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance.
The areas under the ROC curves for each multiple logistic regression model, which included the insulinogenic index (Model 1 of Table 3) or HOMA‐β (Model 2 of Table 3), were calculated to compare which model was a better predictor. Model 2, which included HOMA‐β, had almost the same areas under the ROC curves as Model 1, which included insulinogenic index (Figure 1; 0.797 and 0.800 are the areas under the ROC curves, respectively, P = 0.920).
Figure 1.
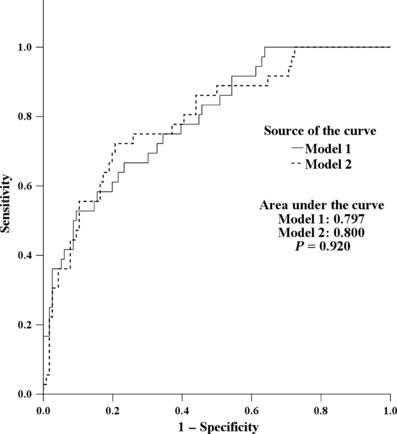
Receiver operator characteristic (ROC) curve and areas under the ROC curves for Models 1 and 2.
Discussion
In the present study, we showed that even among NGT subjects, both HOMA‐IR and lower insulin secretion evaluated by insulinogenic index or HOMA‐β were associated with an increased risk of IGT and/or IFG. This finding was independent of age, sex, family history of type 2 diabetes, and 120‐min plasma glucose (OGTT). The multivariate model that included HOMA‐β had almost the same predictive power as the model that included insulinogenic index.
A few previous studies have shown that insulin resistance and abnormal insulin secretion are both risk factors for the development of IGT11–13. Haffner et al. showed in the San Antonio Heart Study that decreased insulin secretion, assessed by low insulinogenic index using OGTT data, and increased insulin resistance, assessed by fasting serum insulin, predicted the development of IGT. Hayashi et al.13 showed in an analysis of prospective OGTT data from the Japanese American Community Diabetes Study that both HOMA‐IR and insulinogenic index were independent risk factors for incident IGT, even after adjusting for visceral adiposity as measured by computed tomography. Faerch et al. reported that prospective data from the Inter 99 Study showed reduced insulin secretion was present before the development of IFG and low insulin sensitivity was present before the development of IGT. However, there was no adjustment for BMI, insulin secretion, insulin sensitivity and other factors11. To our knowledge, ours is the first prospective study to show a significant relationship of insulin resistance and impaired β‐cell function, both assessed by using only a fasting measurement, to incident IGT and/or IFG.
In the present study, 23.7% of the participants developed IGT and/or IFG during a mean 5.7 years of follow up. This rate is higher than shown in several previous studies12,16,17, but is lower than in another report18. There are several possible reasons for these discrepancies, such as differences in age, BMI, race/ethnicity, study design (hospital‐based or population‐based), criteria for abnormal glucose tolerance and follow‐up interval.
A limitation of the present study is that IGT and IFG could not be analyzed separately because of the low number of incident cases of IFG. These are different conditions11,19,20 and might be associated with differences in the types or severity of complications associated with hyperglycemia21. Although the present study was carried out in Japanese, it is likely that similar results would be obtained in other ethnic groups, because the pathogenesis of glucose intolerance has been reported to be similar among four different ethnic groups: Caucasians, Blacks, Hispanics and Asians22. HOMA‐β and HOMA‐IR are known to be useful in assessing insulin secretion and resistance when the fasting glucose level is not very high. Thus, our finding HOMA‐β and HOMA‐IR to be significant risk factors for prediabetes might be considered reliable, because the study subjects were NGT at baseline with normal fasting glucose.
Finally, there was a relatively low follow‐up rate, although a comparison of baseline data showed no significant differences between those who dropped out and those who did not.
In conclusion, we have provided evidence that lower insulinogenic index or HOMA‐β and higher HOMA‐IR are significant risk factors for the future development of prediabetes among Japanese with NGT. Because HOMA‐β can estimate insulin secretion in the fasting state, it is more practical than the insulinogenic index, which requires an OGTT. Further research is needed to identify whether or not the relationship of low insulin secretion and high insulin resistance with risk of IGT and IFG might differ between these two prediabetic states. Finally, further research might show whether intervention in people with NGT identified to be at high risk for prediabetes will prevent the development of future IGT and/or IFG.
Acknowledgements
We thank the participants for their contributions to this research. We also thank Dr Kinori Kosaka for discussing and sharing with us his thoughtful insight into the growing problem of diabetes in Japan, and Nobuhiro Tachibana, Shizumi Hasegawa, Takuro Sugano and Rieko Ichihashi for their assistance with data collection and management. There is no financial support or relationship that may pose conflict of interest.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414–1431 [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053 [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Fujimoto WY, Leonetti DL, et al. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000; 23: 465–471 [DOI] [PubMed] [Google Scholar]
- 4.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46: 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosaka K, Kuzuya T, Yoshinaga H, et al. A prospective study of health check examinees for the development of non‐insulin‐dependent diabetes mellitus: relationship of the incidence of diabetes with the initial insulinogenic index and degree of obesity. Diabet Med 1996; 13(9 Suppl 6): S120–S126 [PubMed] [Google Scholar]
- 6.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002; 346: 802–810 [DOI] [PubMed] [Google Scholar]
- 7.Fuller JH, Shipley MJ, Rose G, et al. Coronary‐heart‐disease risk and impaired glucose tolerance. The Whitehall study. Lancet 1980; 1: 1373–1376 [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Barrett‐Connor EL, Blunt BA, et al. Visual impairment and retinopathy in people with normal glucose tolerance, impaired glucose tolerance, and newly diagnosed NIDDM. Diabetes Care 1991; 14: 914–918 [DOI] [PubMed] [Google Scholar]
- 9.Shaw JE, Hodge AM, De Courten M, et al. Isolated post‐challenge hyperglycaemia confirmed as a risk factor for mortality. Diabetologia 1999; 42: 1050–1054 [DOI] [PubMed] [Google Scholar]
- 10.Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The funagata diabetes study. Diabetes Care 1999; 22: 920–924 [DOI] [PubMed] [Google Scholar]
- 11.Faerch K, Vaag A, Holst JJ, et al. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care 2009; 32: 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haffner SM, Miettinen H, Gaskill SP, et al. Decreased insulin action and insulin secretion predict the development of im‐paired glucose tolerance. Diabetologia 1996; 39: 1201–1207 [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care 2003; 26: 650–655 [DOI] [PubMed] [Google Scholar]
- 14.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183–1197 [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 16.Engberg S, Vistisen D, Lau C, et al. Progression to impaired glucose regulation and diabetes in the population‐based Inter99 study. Diabetes Care 2009; 32: 606–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiyohara Y, Shinohara A, Kato I, et al. Dietary factors and development of impaired glucose tolerance and diabetes in a general Japanese population: the hisayama study. J Epidemiol 2003; 13: 251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiltunen L, Kivela SL, Laara E, et al. Progression of normal glucose tolerance to impaired glucose tolerance or diabetes in the elderly. Diabetes Res Clin Pract 1997; 35: 99–106 [DOI] [PubMed] [Google Scholar]
- 19.Abdul‐Ghani MA, Williams K, DeFronzo R, et al. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006; 29: 1613–1618 [DOI] [PubMed] [Google Scholar]
- 20.Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006; 29: 1909–1914 [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG. Screening and diagnosis of prediabetes: where are we headed? Diabetes Obes Metab 2007; 1: 12–16 [DOI] [PubMed] [Google Scholar]
- 22.Jensen CC, Cnop M, Hull RL, et al. Beta‐cell function is a major contributor to oral glucose tolerance in high‐risk relatives of four ethnic groups in the U.S. Diabetes 2002; 51: 2170–2178 [DOI] [PubMed] [Google Scholar]


