Abstract
Aims/Introduction: Serum glycated albumin (GA) and glycated hemoglobin (HbA1c) are influenced by plasma glucose levels, and are used for monitoring chronic glycemic control in diabetic patients. Both glycated proteins are known to be influenced by various factors other than plasma glucose levels. In the present study, we examined the effects of hypertriglyceridemia on them.
Materials and Methods: The present study comprised 273 non‐diabetic men. They were grouped into men with normotriglyceridemia (serum triglyceride [TG] <150 mg/dL) and those with hypertriglyceridemia (serum TG ≥150 mg/dL).
Results: Body mass index (BMI) and high sensitivity C‐reactive protein (hs‐CRP) were significantly higher in the 160 men with hypertriglyceridemia than the 113 men with normotriglyceridemia. In men with hypertriglyceridemia, as compared with those with normotriglyceridemia, fasting plasma glucose, 2‐h plasma glucose after 75 g oral glucose tolerance test, and HbA1c were significantly higher. By contrast, serum GA was significantly lower in men with hypertriglyceridemia. BMI‐adjusted serum GA was also significantly lower in these men. In a multivariate analysis, serum TG was an inverse explanatory variable for serum GA.
Conclusions: Serum GA is low in relation to plasma glucose levels in men with hypertriglyceridemia. This might be caused by increased albumin metabolism associated with hypertriglyceridemic state. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00049.x, 2010)
Keywords: Glycated albumin, Glycated hemoglobin, Triglyceride
Introduction
Glycation of proteins is increased in diabetic patients compared with non‐diabetic subjects. Some glycated proteins have been shown to play a role in the development and progression of chronic diabetic complications1. Among various glycated proteins, glycated hemoglobin (HbA1c) is widely used as a clinical parameter of chronic glycemic control2,3. From the results of the Diabetes Control and Complications Trial (DCTT), it is that HbA1c be maintained at <7.0% in order to prevent the development and progression of diabetic complications4. As the average lifespan of erythrocytes is approximately 120 days, the HbA1c level reflects the glycemic control state over the past 1–2 months. HbA1c values are affected not only by plasma glucose (PG) levels, but also by the shortened lifespan of erythrocytes (e.g., hemolytic anemia, renal anemia and hepatic cirrhosis) and variant hemoglobin. Thus, under these conditions, HbA1c gives erroneous values and is unsuitable as a glycemic control marker5,6.
Serum glycated albumin (GA) has recently become used as another glycemic control marker. As the half‐life of serum albumin is shorter than that of erythrocytes (17 days), GA reflects shorter‐term glycemic control (about 2 weeks) as compared with HbA1c. Thus, serum GA has been positioned as a more useful indicator than HbA1c for assessing shorter‐term changes in glycemic control7. HbA1c is inadequate for the evaluation of glycemic control states in patients who receive hemodialysis for chronic renal insufficiency and pregnant women, because its levels are affected by anemia. In contrast, serum GA is not affected by hemoglobin metabolism8 and thus it has been shown to be useful as a glycemic control marker in these conditions9–11. However, serum GA does not accurately reflect glycemic control in disorders influencing serum albumin metabolism (e.g., nephrotic syndrome, hepatic cirrhosis and thyroid dysfunction)12–14.
Serum GA has been shown to set low in relation to PG levels in obese subjects14–16. We previously reported a significant inverse correlation between body mass index (BMI) and serum GA in non‐diabetic subjects, independent of PG levels17. High sensitivity C‐reactive protein (hs‐CRP), which was elevated in obese subjects, also showed an inverse correlation with serum GA levels. It shows that chronic microinflammation leads to increased albumin catabolism18 and thereby set serum GA lower in relation to PG levels17. Furthermore, we have shown that in smoking subjects and hyperuricemic subjects, both of whom showed elevated hs‐CRP, serum GA was low relative to PG levels19,20. Thus, we speculated that the same mechanism might be involved in lower serum GA levels relative to PG levels in obese subjects, smoking subjects and hyperuricemic subjects.
Hypertriglyceridemia is also known to be a condition associated with elevated hs‐CRP21. Therefore, we hypothesized that serum GA is low in relation to PG levels in hypertriglyceridemic subjects and analyzed their serum GA levels, in comparison with HbA1c.
Materials and methods
Subjects
The present study comprised 273 non‐diabetic men recruited from subjects undergoing routine medical examinations at Kinki Central Hospital, Hyogo, Japan, between July and August 2008. Subjects with diabetes were excluded, because measurement of glycated protein is greatly affected in them. A 75‐g oral glucose tolerance test (OGTT) was carried out in all subjects, and glucose tolerance status was evaluated according to American Diabetes Association criteria22. Subjects being treated with medications for dyslipidemia were excluded. Mean age was 48.4 ± 6.4 years, and mean body mass index (BMI) was 24.5 ± 2.9 kg/m2. Subjects with serum triglyceride (TG) <150 mg/dL were defined as those with normotriglyceridemia, and ≥150 mg/dL as those with hypertriglyceridemia. The institutional committee approved the protocol of this study and all participants gave their written informed consent.
Laboratory Methods
Height, bodyweight and waist circumstance were measured on the same day at the health examination. Blood pressure was measured with an automatic sphyngmanometer in the sitting position after 5 min of rest. Fasting blood was collected from subjects between 9.00 and 11.00 h, and was centrifuged immediately for measurements. Plasma glucose, serum uric acid, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol and serum TG were determined by means of standard laboratory assays. HbA1c was measured with ADAMS‐A1c HA‐8160 (Arkray, Kyoto, Japan), by high performance liquid chromatography16,23. Inter‐assay coefficient variations were 0.85 and 0.67%, respectively, as determined in representative blood samples (5.3 and 10.4% HbA1c). Serum GA was determined by Hitachi 7600 autoanalyzer (Hitachi Instruments Service, Tokyo, Japan), by the enzymatic method using albumin‐specific proteinase, ketoamine oxidase and albumin assay reagent (Lucica GA‐L; Asahi Kasei Pharma, Tokyo, Japan)16,24. Inter‐assay coefficient variations were 1.38 and 1.32%, respectively, as determined in representative serum samples (13.3 and 34.9% GA). We previously reported that BMI negatively regulates serum GA levels16,17. Therefore, we calculated the BMI‐adjusted serum GA levels using the data from non‐diabetic subjects17. High sensitivity‐CRP was determined by means of latex‐enhanced immunonephelometrics on a BNII Analyzer (Dade Behring, Marburg, Germany), as described previously25.
Statistical Analysis
All data are shown as means ± SD. To correct for skewed distributions, plasma hs‐CRP concentrations were logarithmically transformed. For statistical analyses, unpaired Student’s t‐test was used to compare the two groups. To analyze the effects of explanatory variables on HbA1c, GA or hs‐CRP, univariate regression analysis as well as stepwise multivariate regression analysis was carried out using StatView software (Version 5.0 for Windows, Abacus Concepts, Berkeley, CA, USA). In the stepwise multiple regression analysis, the F‐value for the inclusion of the variables was set at 4.0. A P‐value of <0.05 was considered to be statistically significant.
Results
HbA1c, but not serum GA, showed a significant positive association with fasting plasma glucose (FPG) and OGTT 2 h‐PG. HbA1c also showed a significant positive association with bodyweight, BMI, waist circumstance, hs‐CRP and serum TG, whereas serum GA showed a significant inverse association with these variables. In addition, HbA1c was inversely but serum GA was positively associated with HDL cholesterol (Table 1).
Table 1. Univariate regression analyses on HbA1c and serum glycated albumin in 273 non‐diabetic men.
| HbA1c | GA | |||
|---|---|---|---|---|
| R | P | R | P | |
| Bodyweight | 0.287 | <0.0001 | −0.241 | <0.0001 |
| BMI | 0.308 | <0.0001 | −0.288 | <0.0001 |
| Waist circumstance | 0.295 | <0.0001 | −0.284 | <0.0001 |
| hs‐CRP (log transformed) | 0.178 | 0.0033 | −0.226 | 0.0002 |
| Systolic blood pressure | 0.148 | 0.0146 | −0.039 | 0.5184 |
| Diastolic blood pressure | 0.189 | 0.0017 | 0.021 | 0.7336 |
| Serum TG | 0.125 | 0.0392 | −0.271 | <0.0001 |
| LDL cholesterol | 0.180 | 0.0028 | −0.118 | 0.0523 |
| HDL cholesterol | −0.153 | 0.0115 | 0.205 | 0.0007 |
| FPG | 0.480 | <0.0001 | 0.117 | 0.0531 |
| OGTT 2‐h PG | 0.476 | <0.0001 | 0.090 | 0.1361 |
| HbA1c | – | – | 0.248 | <0.0001 |
| Serum GA | 0.248 | <0.0001 | – | – |
| BMI‐adjusted serum GA | 0.356 | <0.0001 | – | – |
BMI, body mass index; FPG, fasting plasma glucose; GA, glycated albumin; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; hs‐CRP, high sensitivity C‐reactive protein; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test; PG, plasma glucose; TG, triglyceride.
There were 113 men with normotriglyceridemia and 160 with hypertriglyceridemia. Age did not significantly differ between both groups, but BMI and waist circumference were significantly higher in men with hypertriglyceridemia (BMI 25.6 ± 2.9 vs 23.7 ± 2.6 kg/m2, P < 0.0001; waist circumference 89.0 ± 7.8 vs 84.0 ± 7.7 cm, P < 0.0001). In men with hypertriglyceridemia, hs‐CRP was significantly higher than in those with normotriglyceridemia (−0.4 ± 0.5 vs−1.6 ± 0.4 log‐mg/L, P = 0.0007). There was a significant positive correlation between serum TG and hs‐CRP (R = 0.169, P = 0.0053; Figure 1).
Figure 1.
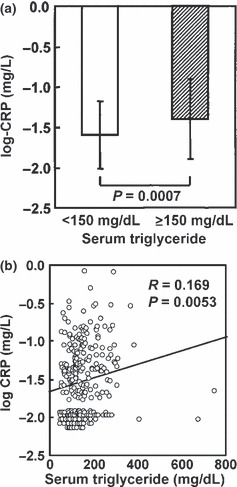
(a) High sensitivity C‐reactive protein levels in 113 men with normotriglyceridemia (serum triglyceride <150 mg/dL; open column) and 160 men with hypertriglyceridemia (serum triglyceride ≥150 mg/dL; closed column) and (b) their correlation with serum triglyceride.
In men with hypertriglyceridemia, as compared with men with normotriglyceridemia, FPG (97 ± 8 vs 95 ± 8 mg/dL, P = 0.0195), OGTT 2‐h PG (128 ± 28 vs 117 ± 29 mg/dL, P = 0.0018) and HbA1c (5.3 ± 0.3 vs 5.2 ± 0.3%, P = 0.0177) were significantly higher (Figure 2). In contrast, serum GA was significantly lower in men with hypertriglyceridemia (13.7 ± 0.9 vs 14.3 ± 1.2%, P < 0.0001; Figure 3a). BMI‐adjusted serum GA was also significantly lower in men with hypertriglyceridemia, as compared with men with normotriglyceridemia (14.3 ± 1.1 vs 14.7 ± 1.1%, P = 0.0045; Figure 3b). In men with normotriglyceridemia, HbA1c (R = 0.405, P < 0.0001) and serum GA (R = 0.207, P = 0.0277) showed a significant positive association with FPG, whereas in men with hypertriglyceridemia, HbA1c (R = 0.512, P < 0.0001), but not serum GA (R = 0.128, P = 0.1059), showed significant positive association with FPG.
Figure 2.
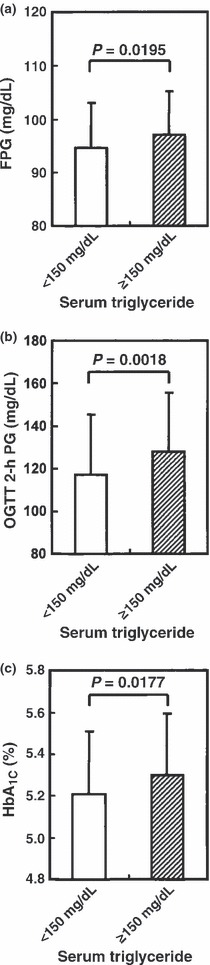
(a) Fasting plasma glucose (FPG), (b) oral glucose tolerance test (OGTT) 2‐h plasma glucose (PG) and (c) glycated hemoglobin (HbA1c) levels in 113 men with normotriglyceridemia (serum triglyceride <150 mg/dL; open column) and 160 men with hypertriglyceridemia (serum triglyceride ≥150 mg/dL; closed column).
Figure 3.
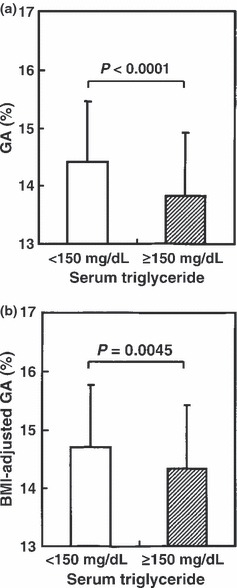
(a) Serum glucose albumin (GA) and (b) body mass index (BMI)‐adjusted serum GA levels in 113 men with normotriglyceridemia (serum triglyceride <150 mg/dL; open column) and 160 men with hypertriglyceridemia (serum triglyceride ≥150 mg/dL; closed column).
Stepwise multivariate regression analyses were carried out with HbA1c and serum GA as objective variables. The results showed that serum GA and HbA1c were significant positive explanatory variables for each other. Fasting plasma glucose, OGTT 2h‐PG, BMI and LDL cholesterol were significant positive variables for HbA1c (Table 2), whereas serum TG, in addition to BMI and serum uric acid, was a significant negative explanatory variable for serum GA (Table 3).
Table 2. Stepwise multivariate regression analyses on glycated hemoglobin levels in 273 non‐diabetic men.
| β | F | P | |
|---|---|---|---|
| Serum GA | 0.274 | 29.8 | <0.0001 |
| OGTT 2‐h PG | 0.272 | 26.7 | <0.0001 |
| FPG | 0.268 | 25.9 | <0.0001 |
| BMI | 0.228 | 19.0 | <0.0001 |
| LDL cholesterol | 0.136 | 8.2 | 0.0096 |
An objective variable is glycated hemoglobin (HbA1c) and explanatory variables are age (years), body mass index (BMI; kg/m2), serum glucose albumin (GA; %), fasting plasma glucose (FPG; mg/dL), oral glucose tolerance test (OGTT) 2‐h plasma glucose (PG; mg/dL), serum triglyceride (TG; mg/dL), low‐density lipoprotein (LDL) cholesterol (mg/dL), high‐density lipoprotein (HDL) cholesterol (mg/dL), systolic blood pressure (mmHg), diastolic blood pressure (mmHg) and serum uric acid (mg/dL). R2 = 0.421, F = 38.8 and P < 0.0001.
Table 3. Stepwise multivariate regression analyses on serum glycated albumin levels in 273 non‐diabetic men.
| β | F | P | |
|---|---|---|---|
| HbA1c | 0.307 | 28.7 | <0.0001 |
| BMI | −0.268 | 19.9 | <0.0001 |
| Age | 0.182 | 11.4 | 0.0020 |
| Serum uric acid | −0.126 | 5.0 | 0.0225 |
| Serum TG | −0.131 | 5.0 | 0.0243 |
| HDL cholesterol | 0.116 | 4.3 | 0.0826 |
An objective variable is serum glycated albumin and explanatory variables are age (years), body mass index (BMI; kg/m2), glycated hemoglobin (HbA1c; %), fasting plasma glucose (FPG; mg/dL), oral glucose tolerance test (OGTT) 2‐h plasma glucose (PG; mg/dL), serum triglyceride (TG; mg/dL), low‐density lipoprotein (LDL) cholesterol (mg/dL), high‐density lipoprotein (HDL) cholesterol (mg/dL), systolic blood pressure (mmHg), diastolic blood pressure (mmHg) and serum uric acid (mg/dL). R2 = 0.300, F = 19.0 and P < 0.0001.
Discussion
Hypertriglyceridemia, in addition to diabetes, hypertension and hyperuricemia, is more prevalent in obese subjects26, and it has been proposed to be an element of metabolic syndrome27. In the present study, BMI and waist circumference were higher in men with hypertriglyceridemia than in those with normotriglyceridemia. This suggests that visceral fat accumulation was accelerated in men with hypertriglyceridemia. Thus, in these subjects, impaired glucose tolerance would be expected to be more prevalent. In the present study targeted non‐diabetic men, FPG, 2‐h PG and HbA1c, were higher in men with hypertriglyceridemia than those with normotriglyceridemia. In contrast to these results, serum GA was significantly lower in men with hypertriglyceridemia, suggesting that some factor(s) independent of PG levels affect serum GA in men with hypertriglyceridemia. As serum GA is shown to be inversely correlated with BMI15–17, we also determined BMI‐adjusted values. The results showed that BMI‐adjusted serum GA remained significantly lower in men with hypertriglyceridemia than in men with normotriglyceridemia. Multivariate analysis showed serum TG to be an independent negative factor for serum GA. The discrepant results between HbA1c and GA in men with hypertriglyceridemia raise a question as to which is a suitable glycemic index in these subjects. To reveal this, a continuous glucose monitoring system28 could clearly show the role of triglyceridemia on HbA1c and GA levels.
Hypertriglyceridemia is considered a risk factor for arteriosclerosis29,30, and patients with hypertriglyceridemia have been shown to have elevated hs‐CRP levels21. The present study also showed that hs‐CRP was higher in men with hypertriglyceridemia, compared with those with normotriglyceridemia, and serum TG was a significant positive explanatory variable for hs‐CRP. Furthermore, there has been a shown significant inverse association of hs‐CRP with serum GA, but not with HbA1c17,18. The present findings suggest that low serum GA levels are related to chronic inflammation associated with conditions showing hypertriglyceridemia.
In conclusion, the present study showed that serum TG levels influenced serum GA, but not HbA1c, independent of PG levels in non‐diabetic men. The present study was carried out in non‐diabetic men, and thus the results cannot be simply extrapolated in diabetic patients. However, if similar results are also observed in diabetic patients, caution is necessary for evaluating serum GA levels in diabetic patients with hypertriglyceridemia. Furthermore, most patients with type 2 diabetes are overweight or obese and have metabolic syndrome traits including hypertriglyceridemia, and thus serum GA levels might be set lower in relation to glycemia in these patients.
Acknowledgement
None of the authors have conflicts of interest to declare.
References
- 1.Cohen MP. Nonenzymatic glycation: a central mechanism in diabetic microvasculopathy? J Diabet Complications 1998; 2: 214–217 [DOI] [PubMed] [Google Scholar]
- 2.Koenig RJ, Peterson CM, Jones RL, et al. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med 1976; 295: 417–420 [DOI] [PubMed] [Google Scholar]
- 3.Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science 1978; 20: 21–27 [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 5.Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med 2004; 21: 657–665 [DOI] [PubMed] [Google Scholar]
- 6.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001; 47: 153–163 [PubMed] [Google Scholar]
- 7.Takahashi S, Uchino H, Shimizu T, et al. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1C) in type 2 diabetic patients: usefulness of GA for evaluation of short‐term changes in glycemic control. Endocr J 2007; 54: 139–144 [DOI] [PubMed] [Google Scholar]
- 8.Guthrow CE, Morris MA, Day JF, et al. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci USA 1979; 76: 4258–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18: 896–903 [DOI] [PubMed] [Google Scholar]
- 10.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 2008; 7: 1062–1068 [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto K, Noguchi S, Morimoto Y, et al. A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care 2008; 31: 1945–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga M. Glycated albumin and fructosamine. Sogo Rinsho 2008; 57: 1922–1927 (Japanese). [Google Scholar]
- 13.Koga M, Kasayama S, Kanehara H, et al. CLD (chronic liver disease)‐HbA1c as a novel indicator for estimation of mean plasma glucose in the patients with chronic liver disease. Diabetes Res Clin Pract 2008; 81: 258–262 [DOI] [PubMed] [Google Scholar]
- 14.Koga M, Murai J, Saito H, et al. Effect of thyroid hormone on serum glycated albumin levels: study on non‐diabetic subjects. Diabetes Res Clin Pract 2009; 84: 141–144 [DOI] [PubMed] [Google Scholar]
- 15.Nishimura R, Kanda A, Sano H, et al. Glycated albumin is low in obese, non‐diabetic children. Diabetes Res Clin Pract 2006; 71: 334–338 [DOI] [PubMed] [Google Scholar]
- 16.Koga M, Matsumoto S, Saito H, et al. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J 2006; 53: 387–391 [DOI] [PubMed] [Google Scholar]
- 17.Koga M, Otsuki M, Matsumoto S, et al. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta 2007; 378: 48–52 [DOI] [PubMed] [Google Scholar]
- 18.Das I. Raised C‐reactive protein levels in serum from smokers. Clin Chim Acta 1985; 153: 9–13 [DOI] [PubMed] [Google Scholar]
- 19.Koga M, Saito H, Mukai M, et al. Serum glycated albumin levels are influenced by smoking status, independent of plasma glucose levels. Acta Diabetol 2009; 46: 141–144 [DOI] [PubMed] [Google Scholar]
- 20.Koga M, Murai J, Saito H, et al. Serum glycated albumin, but not glycated hemoglobin, is low in relation to glycemia in hyperuricemic men. Acta Diabetol, 2009. doi: 10.1007/s00592‐009‐0168‐6 [DOI] [PubMed] [Google Scholar]
- 21.Lundman P, Eriksson MJ, Silveira A, et al. Relation of hypertriglyceridemia to plasma concentrations of biochemical markers of inflammation and endothelial activation. Am J Cardiol 2003; 91: 1128–1131 [DOI] [PubMed] [Google Scholar]
- 22.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160–3167 [DOI] [PubMed] [Google Scholar]
- 23.Schnedl WJ, Lahousen T, Wallner SJ, et al. Silent hemoglobin variants and determination of HbA1C with the high‐resolution program of the HPLC HA‐8160 hemoglobin analyzer. Clin Biochem 2005; 38: 88–91 [DOI] [PubMed] [Google Scholar]
- 24.Kouzuma T, Uemastu Y, Usami T, et al. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta 2004; 346: 135–143 [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Kasayama S, Yamamoto H, et al. Strong association of C‐reactive protein with body mass index and 2‐h post‐challenge glucose in non‐diabetic, non‐smoker subjects without hypertension. Diabet Med 2004; 21: 581–585 [DOI] [PubMed] [Google Scholar]
- 26.Matsuura F, Yamashita S, Nakamura T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous for fat obesity. Metabolism 1998; 47: 929–933 [DOI] [PubMed] [Google Scholar]
- 27.Tsouli SG, Liberopoulos EN, Mikhailidis DP, et al. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism 2006; 55: 1293–1301 [DOI] [PubMed] [Google Scholar]
- 28.Currie CJ, Poole CD, Papo NL. An overview and commentary on retrospective, continuous glucose monitoring for the optimisation of care for people with diabetes. Curr Med Res Opin 2009; 25: 2389–2400 [DOI] [PubMed] [Google Scholar]
- 29.Bonora E. The metabolic syndrome and cardiovascular disease. Ann Med 2006; 38: 64–80 [DOI] [PubMed] [Google Scholar]
- 30.Fruchart JC, Nierman MC, Stroes ES, et al. New risk factors for atherosclerosis and patient risk assessment. Circulation 2004; 109(23 Suppl 1): III15–III19 [DOI] [PubMed] [Google Scholar]


