Abstract
Abstract
Concept of Diabetes Mellitus:
Diabetes mellitus is a group of diseases associated with various metabolic disorders, the main feature of which is chronic hyperglycemia due to insufficient insulin action. Its pathogenesis involves both genetic and environmental factors. The long‐term persistence of metabolic disorders can cause susceptibility to specific complications and also foster arteriosclerosis. Diabetes mellitus is associated with a broad range of clinical presentations, from being asymptomatic to ketoacidosis or coma, depending on the degree of metabolic disorder.
Classification (Tables 1 and 2, and Figure 1):
The classification of glucose metabolism disorders is principally derived from etiology, and includes staging of pathophysiology based on the degree of deficiency of insulin action. These disorders are classified into four groups: (i) type 1 diabetes mellitus; (ii) type 2 diabetes mellitus; (iii) diabetes mellitus due to other specific mechanisms or diseases; and (iv) gestational diabetes mellitus. Type 1 diabetes is characterized by destruction of pancreatic β‐cells. Type 2 diabetes is characterized by combinations of decreased insulin secretion and decreased insulin sensitivity (insulin resistance). Glucose metabolism disorders in category (iii) are divided into two subgroups; subgroup A is diabetes in which a genetic abnormality has been identified, and subgroup B is diabetes associated with other pathologic disorders or clinical conditions. The staging of glucose metabolism includes normal, borderline and diabetic stages depending on the degree of hyperglycemia occurring as a result of the lack of insulin action or clinical condition. The diabetic stage is then subdivided into three substages: non‐insulin‐ requiring, insulin‐requiring for glycemic control, and insulin‐dependent for survival. The two former conditions are called non‐insulin‐dependent diabetes and the latter is known as insulin‐dependent diabetes. In each individual, these stages may vary according to the deterioration or the improvement of the metabolic state, either spontaneously or by treatment.
Diagnosis (Tables 3–7 and Figure 2):
Categories of the State of Glycemia: Confirmation of chronic hyperglycemia is essential for the diagnosis of diabetes mellitus. When plasma glucose levels are used to determine the categories of glycemia, patients are classified as having a diabetic type if they meet one of the following criteria: (i) fasting plasma glucose level of ≥126 mg/dL (≥7.0 mmol/L); (ii) 2‐h value of ≥200 mg/dL (≥11.1 mmol/L) in 75 g oral glucose tolerance test (OGTT); or (iii) casual plasma glucose level of ≥200 mg/dL (≥11.1 mmol/L). Normal type is defined as fasting plasma glucose level of <110 mg/dL (<6.1 mmol/L) and 2‐h value of <140 mg/dL (<7.8 mmol/L) in OGTT. Borderline type (neither diabetic nor normal type) is defined as falling between the diabetic and normal values. According to the current revision, in addition to the earlier listed plasma glucose values, hemoglobin A1c (HbA1c) has been given a more prominent position as one of the diagnostic criteria. That is, (iv) HbA1c≥6.5% is now also considered to indicate diabetic type. The value of HbA1c, which is equivalent to the internationally used HbA1c (%) (HbA1c [NGSP]) defined by the NGSP (National Glycohemoglobin Standardization Program), is expressed by adding 0.4% to the HbA1c (JDS) (%) defined by the Japan Diabetes Society (JDS).
Subjects with borderline type have a high rate of developing diabetes mellitus, and correspond to the combination of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) noted by the American Diabetes Association (ADA) and WHO. Although borderline cases show few of the specific complications of diabetes mellitus, the risk of arteriosclerosis is higher than those of normal type. When HbA1c is 6.0–6.4%, suspected diabetes mellitus cannot be excluded, and when HbA1c of 5.6–5.9% is included, it forms a group with a high risk for developing diabetes mellitus in the future, even if they do not have it currently.
Clinical Diagnosis:
-
1
If any of the criteria for diabetic type (i) through to (iv) is observed at the initial examination, the patient is judged to be ‘diabetic type’. Re‐examination is conducted on another day, and if ‘diabetic type’ is reconfirmed, diabetes mellitus is diagnosed. However, a diagnosis cannot be made only by the re‐examination of HbA1c alone. Moreover, if the plasma glucose values (any of criteria [i], [ii], or [iii]) and the HbA1c (criterion [iv]) in the same blood sample both indicate diabetic type, diabetes mellitus is diagnosed based on the initial examination alone. If HbA1c is used, it is essential that the plasma glucose level (criteria [i], [ii] or [iii]) also indicates diabetic type for a diagnosis of diabetes mellitus. When diabetes mellitus is suspected, HbA1c should be measured at the same time as examination for plasma glucose.
-
2
If the plasma glucose level indicates diabetic type (any of [i], [ii], or [iii]) and either of the following conditions exists, diabetes mellitus can be diagnosed immediately at the initial examination.
-
•
The presence of typical symptoms of diabetes mellitus (thirst, polydipsia, polyuria, weight loss)
-
•
The presence of definite diabetic retinopathy
-
•
-
3
If it can be confirmed that the above conditions 1 or 2 existed in the past, diabetes mellitus can be diagnosed or suspected regardless of the current test results.
-
4
If the diagnosis of diabetes cannot be established by these procedures, the patient is followed up and re‐examined after an appropriate interval.
-
5
The physician should assess not only the presence or absence of diabetes, but also its etiology and glycemic stage, and the presence and absence of diabetic complications or associated conditions.
Epidemiological Study: For the purpose of estimating the frequency of diabetes mellitus, ‘diabetes mellitus’ can be substituted for the determination of ‘diabetic type’ from a single examination. In this case, HbA1c≥6.5% alone can be defined as ‘diabetes mellitus’.
Health Screening: It is important not to misdiagnose diabetes mellitus, and thus clinical information such as family history and obesity should be referred to at the time of screening in addition to an index for plasma glucose level.
Gestational Diabetes Mellitus: There are two hyperglycemic disorders in pregnancy: (i) gestational diabetes mellitus (GDM); and (ii) diabetes mellitus. GDM is diagnosed if one or more of the following criteria is met in a 75 g OGTT during pregnancy:
-
1
Fasting plasma glucose level of ≥92 mg/dL (5.1 mmol/L)
-
2
1‐h value of ≥180 mg/dL (10.0 mmol/L)
-
3
2‐h value of ≥153 mg/dL (8.5 mmol/L)
However, diabetes mellitus that is diagnosed by the clinical diagnosis of diabetes mellitus defined earlier is excluded from GDM. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00074.x, 2010)
Keywords: Diabetes mellitus, Clinical diagnosis, HbA1c
Review of the History of Diagnostic Criteria for Diabetes Mellitus by the Japan Diabetes Society and International Background
The Japan Diabetes Society (JDS) has published reports on the diagnostic criteria for diabetes mellitus three times2–4. In 2009, a minor revision was made regarding the normal range of fasting plasma glucose level5.
In 1970, the JDS’s first committee proposed reference values for plasma glucose determination in the oral glucose tolerance tests (OGTT)2. At that time, glucose tolerance was assessed using the OGTT, and the JDS took the position that the diagnosis of diabetes mellitus should be carried out comprehensively, and should include an evaluation of glucose tolerance. This is the position that diabetes mellitus is not defined by hyperglycemia alone. The classification according to the OGTT includes normal, borderline and diabetic types. This position is still maintained today.
In 1979, the American National Diabetes Data Group published diagnostic criteria based on the 75 g OGTT and classifications such as insulin‐dependent diabetes mellitus and non‐insulin‐dependent diabetes mellitus6. At that time, mild glucose intolerance was categorized as impaired glucose tolerance (IGT). In 1980, the World Health Organization (WHO) Expert Committee issued a report based on this definition7. In light of this, the JDS established a second committee and published criteria using the 75 g OGTT3. The policy of classifying OGTT by types continued.
In 1997, the American Diabetes Association (ADA) reviewed the plasma glucose reference values for the diagnosis of diabetes mellitus, and a fasting plasma glucose level ≥126 mg/dL (7.0 mmol/L) and an OGTT 2‐h value ≥200 mg/dL (11.1 mmol/L) were regarded as diagnostic for diabetes mellitus8. The report at that time also recommended making a diagnosis using the fasting plasma glucose level without OGTT in routine clinical practice. Because IGT, which is defined by the 2‐h plasma glucose level, cannot be determined without conducting an OGTT, a fasting plasma glucose level between normal and diabetes mellitus values was defined as impaired fasting glucose (IFG) instead. The WHO expert committee issued a similar proposal in 1999, although continuing to recognize the necessity of OGTT in clinical practice9.
Meanwhile, the JDS had established a third committee on diagnosis and classification in 1995, and had begun updating opinions from an academic panel. They considered the new reports from the ADA and the WHO, and a report on classification and diagnostic criteria for diabetes mellitus was issued in 1999, which has been used until this revision4. Etiological classification was emphasized, and diabetes mellitus was divided into type 1, type 2, other types, and gestational diabetes mellitus, together with classification according to pathophysiological stage. The confirmation of chronic hyperglycemia was required for a diagnosis, and diabetic type was defined as a fasting plasma glucose level ≥126 mg/dL (≥7.0 mmol/L), 2‐h OGTT value ≥200 mg/dL (≥11.1 mmol/L) or casual plasma glucose level ≥200 mg/dL (≥11.1 mmol/L). Normal type was defined as a fasting plasma glucose <110 mg/dL (<6.1 mmol/L) and 2‐h OGTT <140 mg/dL (<7.8 mmol/L), with a diagnosis of borderline type between these two. Clinical diagnosis of diabetes mellitus requires observation of a diabetic type at least twice in tests on different days. However, diabetes mellitus could be diagnosed from a single finding of diabetic type hyperglycemia if (i) there are symptoms of diabetes mellitus; (ii) the hemoglobin A1c (HbA1c) is ≥6.9%; or (iii) there is diabetic retinopathy. However, when conducting an epidemiological survey, researchers may consider a single confirmation of diabetic type hyperglycemia as diabetes mellitus.
In 2003, the ADA lowered the upper limit of normal fasting plasma glucose from 110 mg/dL (6.1 mmol/L) to 100 mg/dL (5.5 mmol/L)10. The principal reason for this reduction was that IGT was often overlooked in conventional testing based on fasting plasma glucose level alone. However, the WHO Expert Committee (2006) decided to retain the conventional determination criterion for fasting plasma glucose level, because a large number of people would be added as having abnormal glycemic control if the fasting plasma glucose level criterion were lowered, and because the population that would be newly determined to have IFG does not have a very high risk of macrovascular disorders11.
The JDS established a diagnostic criteria exploratory committee on diabetes mellitus and glucose metabolism disorders that examined this question, and found that impaired glucose tolerance is common in the group with fasting plasma glucose levels of 100 mg/dL (5.5 mmol/L) to 109 mg/dL (6.0 mmol/L). In 2008, this segment was called ‘high‐normal’ within the range of normal fasting plasma glucose5.
Furthermore, the JDS newly established a committee for diagnostic criteria that reviewed the current diagnostic criteria and examined the practical use of HbA1c. The standardization of HbA1c measurement in Japan was examined in the early stage12, and in the 1999 committee report on the classification and diagnostic criteria for diabetes mellitus, the JDS took global initiative by including HbA1c as an additional tool for diabetes mellitus diagnosis4. In Japan, since 1997 the HbA1c has been used to estimate the number of patients with diabetes mellitus in national surveys of diabetes mellitus by the government, and HbA1c is used in national health examinations and health guidance that were initiated in 2008.
Although HbA1c has become widely used as an index for the treatment of diabetes mellitus globally, it hadn’t been used as an index for diagnosis. The main reason for this is that HbA1c measurement had not been sufficiently standardized8. Thereafter, the standardization of HbA1c measurement was investigated by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and an international expert committee formed by members of the ADA, the European Diabetes Association, and the International Diabetes Federation recommended the use of the HbA1c values of the National Glycohemoglobin Standardization Program (NGSP) for the diagnosis of diabetes mellitus in June 200913. HbA1c has several advantages in that it is a suitable index of chronic hyperglycemia, a characteristic of diabetes mellitus, blood samples can be taken without concern about the effects of meals, it shows less day‐to‐day changes than the plasma glucose level, and its relation to the risk of retinopathy is equivalent to that of the plasma glucose level. However, there are also several issues concerning the application of HbA1c for the diagnosis of diabetes mellitus, including that HbA1c is affected by red blood cell turnover in addition to plasma glucose, and that there are differences between the NGSP values that have been used in the USA, Europe, Asia and many other countries for HbA1c (NGSP) and the JDS values used in Japan for HbA1c (JDS).
As described earlier, the fasting plasma glucose level is recommended in the USA for diagnosis of diabetes mellitus because it is simple and easy, while OGTT is recommended in Japan for a more accurate diagnosis. Although HbA1c is widely used as an index for treatment or for epidemiological data, this test alone has not generally been used to diagnose diabetes mellitus. The ADA’s expert committee therefore examined the validity of using HbA1c for diagnosis, and in a 1997 report recommended against the use of HbA1c for diagnosing diabetes mellitus. This position was taken mainly because of the lack of progress in standardizing the test8. In an additional 2003 report, although HbA1c standardization became possible by applying NGSP, it was still considered that there were disadvantages when using it for diagnosis10. However, according to a 2009 report, evaluation of HbA1c as a diagnostic tool was improved, as the accuracy and precision of HbA1c measurement were shown to be similar to those of plasma glucose levels13. HbA1c has the further advantage that blood sampling is less burdensome and simpler for the patient, considering that the specimens are comparatively stable after collection and that there has been international progress in standardizing HbA1c measurement.
In addition, HbA1c is a more stable index than the fasting plasma glucose level, and is now considered to be a superior index of chronic hyperglycemia compared to the plasma glucose level, which varies during the day. Based on this, the relationship between HbA1c and diabetic retinopathy specific to diabetes mellitus (moderate non‐proliferative diabetic retinopathy or worse) was investigated using a large epidemiological database. Specifically, 48,331 patients aged 20–79 years in nine countries were surveyed, and a diagnosis of diabetes mellitus based on HbA1c was proposed because of the higher frequency of retinopathy with HbA1c (NGSP) ≥6.5%13. Thus, in January 2010, the American Diabetes Association proposed new diagnostic criteria for diabetes mellitus with HbA1c in parallel with three plasma glucose indices14.
As described later, HbA1c (JDS) is approximately 0.4% lower than HbA1c (NGSP)15, and this difference was not considered in the international standardization of plasma glucose control targets. Standardization and precision control of HbA1c measurement has been successfully practiced in Japan, but because most other countries use the NGSP values at present, it is judged appropriate to shift to a new internationally standardized HbA1c by adding 0.4% to the conventional HbA1c (JDS) value.
Concept of Diabetes Mellitus
Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia due to insufficient insulin action. The common feature of this group of diseases is a deficiency of insulin action, which leads to abnormalities in almost the entire metabolic system, including carbohydrate, lipid and protein metabolism. The mechanism for a lack of insulin action in this group of diseases includes insufficient insulin secretion (absolute or relative) and decreased insulin sensitivity (insulin resistance) in organs (cells) on which insulin acts.
The causes of diabetes mellitus are various, including both genetic and environmental factors. Insufficient insulin secretion can occur in association with destruction of pancreatic islet β‐cells or due to dysfunction within the pancreatic β‐cells themselves. Besides the decrease in insulin supply, decreased insulin sensitivity can contribute to relative insufficient insulin action. In either case, the principal mechanism for development of diabetes is decreased functional pancreatic β‐cell mass that results in failure to provide adequate insulin action on the organs. The associated metabolic disorders can be improved by various therapeutic means to ameliorate insufficient insulin action.
If the metabolic abnormality is mild, patients may be asymptomatic, and thus may neglect it for a long time. However, in a metabolic state with markedly high plasma glucose levels, thirst, polydipsia, polyuria and weight loss can be seen. In the most extreme cases, ketoacidosis or a marked hyperosmotic, hyperglycemic state occurs, which can lead to disturbance of consciousness, coma and even death if no effective treatment is provided.
With long duration of diabetic metabolism, diabetes‐specific complications, chiefly involving small vessels (retinopathy, nephropathy and neuropathy), may ensue and lead to serious outcomes, such as visual disturbance, renal failure and gangrene. Diabetes accelerates and exacerbates the occurrence of arteriosclerosis, increasing the risks for myocardial infarction, stroke and occlusive artery disease of the lower extremities. These complications constitute the major causes of morbidity and mortality in diabetic patients.
Classification
Etiological Classification and Pathophysiological Stages
Etiology and pathophysiological stages (or states) should be assessed separately for each patient. Before development of overt diabetes, patients will pass stages at which different degrees of deficiency of insulin action exist. The abnormal glucose metabolism not only progresses, but may regress spontaneously or in response to treatment. For example, islet autoantibodies are occasionally detected before recognition of hyperglycemia, suggesting that the autoimmune process of type 1 diabetes has already begun. Obese diabetic patients may be improved to borderline type or even to normal glucose tolerance after weight reduction with diet therapy. The horizontal axis in Figure 1 shows the degree of deficiency of insulin action associated with a disorder of glucose metabolism. The patients are judged to have diabetes when hyperglycemia has exceeded a certain level, which is presumed to confer risk for specific complications. The diabetic area is divided into three stages, (i) in which insulin treatment is not needed; (ii) in which insulin injections are required for glycemic control; and (iii) in which insulin treatment is indispensable to prevent ketosis and to sustain life.
Figure 1.
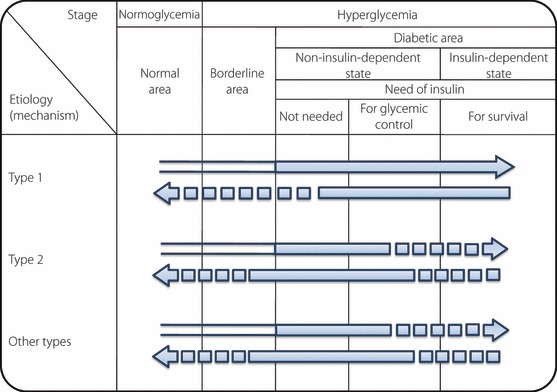
A scheme of the relationship between etiology (mechanism) and patho‐physiological stages (states) of diabetes mellitus. Arrows pointing right represent worsening of glucose metabolism disorders (including onset of diabetes mellitus). Among the arrow lines,  indicates the condition classified as ‘diabetes mellitus’. Arrows pointing left represent improvement in the glucose metabolism disorder. The broken lines indicate events of low frequency. For example, in type 2 diabetes mellitus, infection can lead to ketoacidosis and require temporary insulin treatment for survival. Also, once diabetes mellitus has developed, it is treated as diabetes mellitus regardless of improvement in glucose metabolism, therefore, the arrow lines pointing left are filled in black. In such cases, a broken line is used, because complete normalization of glucose metabolism is rare.
indicates the condition classified as ‘diabetes mellitus’. Arrows pointing left represent improvement in the glucose metabolism disorder. The broken lines indicate events of low frequency. For example, in type 2 diabetes mellitus, infection can lead to ketoacidosis and require temporary insulin treatment for survival. Also, once diabetes mellitus has developed, it is treated as diabetes mellitus regardless of improvement in glucose metabolism, therefore, the arrow lines pointing left are filled in black. In such cases, a broken line is used, because complete normalization of glucose metabolism is rare.
The terms type 1 and type 2 are used for classification based on etiology. The terms insulin‐dependent and non‐insulin‐dependent are used for pathophysiological staging of diabetes mellitus regardless of the etiology. In this case, failure to administer insulin in an insulin‐dependent condition can lead to ketosis and can be life threatening. Patients whose conditions do not require insulin treatment for prevention of ketosis or for survival, but require insulin for glycemic control are considered to be in a non‐insulin‐dependent state.
Etiological Classification
Etiological classifications of diabetes mellitus and glucose metabolism disorders are shown in Table 1, and use the terms type 1 and type 2. In recent years, various forms of diabetes with genetic abnormalities have been identified, and are treated as a separate category. An individual patient may have diabetes mellitus resulting from multiple etiological causes. Those that cannot be classified at the present time are called unclassifiable.
Table 1. Etiological classification of diabetes mellitus and glucose metabolism disorders.
| I. Type 1 (destruction of pancreatic β‐cells, usually leading to absolute insulin deficiency) |
| A. Autoimmune |
| B. Idiopathic |
| II. Type 2 (ranging from predominantly insulin secretory defect, to predominantly insulin resistance with varying degrees of insulin secretory defect) |
| III. Due to other specific mechanisms or diseases (see Table 2 for details) |
| A. Those in which specific mutations have been identified as a cause of genetic susceptibility |
| (1) Genetic abnormalities of pancreatic β‐cell function |
| (2) Genetic abnormalities of insulin action |
| B. Those associated with other diseases or conditions |
| (1) Diseases of exocrine pancreas |
| (2) Endocrine diseases |
| (3) Liver disease |
| (4) Drug‐ or chemical‐induced |
| (5) Infections |
| (6) Rare forms of immune‐mediated diabetes |
| (7) Various genetic syndromes often associated with diabetes |
| IV. Gestational diabetes mellitus |
Note: Those that cannot at present be classified as any of the above are called unclassifiable.
The occurrence of diabetes‐specific complications has not been confirmed in some of these conditions.
Type 1 Diabetes Mellitus
Diabetes mellitus is caused by insulin deficiency due to destruction of pancreatic β‐cells principally via an autoimmune reaction, which is itself triggered by different factors. Type 1 diabetes develops in association with certain hereditary factors, such as Human Leukocyte Antigen (HLA) alleles, plus inducements/environmental factors, such as a virus infection. Diabetes resulting as one of the manifestations of other autoimmune disorders is not rare. As destruction of pancreatic β‐cells progresses, an absolute deficiency in insulin often occurs. It is typically regarded as developing rapidly in young people, but it can occur in any age group.
In many cases, autoantibodies against islet antigens (islet‐associated antibodies) are verifiable in the early phase of the disease, and because pancreatic β‐cell destruction involves autoimmune mechanisms, these are called ‘autoimmune’ type 1 diabetes mellitus. There are also cases that reach an insulin‐dependent state without verifiable autoantibodies, and these are called ‘idiopathic’ type 1 diabetes mellitus. However, patients who are dependent on insulin therapy and are autoantibody negative, but due to an identified cause, such as a genetic abnormality, and those with temporary insulin dependence, such as soft drink ketosis, are not included in the idiopathic category. Depending on the manner of onset and progression, it is classified as fulminant, acute or slowly progressive16–20.
Type 2 Diabetes Mellitus
Diabetes mellitus develops in association with multiple genetic factors that lead to decreased insulin secretion or insulin resistance augmented by lifestyle habits, such as overeating (especially high fat diet), lack of exercise and resultant obesity, as environmental factors and results in insufficient insulin action. It is presumed that most cases involve multiple genetic factors, with some of these now elucidated21,22. Decreased insulin secretion and decreased insulin sensitivity are both involved in the onset of type 2 diabetes mellitus, but the proportion of their involvement differs according to the patient. Non‐insulin‐dependent diabetes mellitus is mostly of this type. Pancreatic β‐cell function is retained to a certain degree, and insulin injections are rarely required for survival. However, complications, such as infections, can lead to ketoacidosis temporarily. Insulin secretion is particularly decreased in the early secretory response after a glucose load. Obesity or a history of obesity is common.
Onset is commonly regarded to be in middle age or later, but this type of diabetes mellitus has recently been shown to be increasing in children and young people23. The nature of type 2 diabetes mellitus is clearly not uniform, but it might possibly be further divided according to the presence or absence of obesity and differences in the degree of involvement of decreased insulin secretion and decreased insulin sensitivity.
Other Types of Diabetes Mellitus Due to Specific Causes:
These are divided into two groups (Table 2).
Table 2. Diabetes mellitus and glucose metabolism disorders due to other specific mechanisms and diseases.
| A. Those in which specific mutations have been identified as a cause of genetic susceptibility | B. Those associated with other diseases or conditions |
| (1) Genetic abnormalities of pancreatic β‐cell function Insulin gene (abnormal insulinemia, abnormal proinsulinemia, neonatal diabetes mellitus) HNF 4α gene (MODY1) Glucokinase gene (MODY2) HNF 1α gene (MODY3) IPF‐1 gene (MODY4) HNF 1β gene (MODY5) Mitochondria DNA (MIDD) NeuroD1 gene (MODY6) Kir6.2 gene (neonatal diabetes mellitus) SUR1 gene (neonatal diabetes mellitus) Amylin Others (2) Genetic abnormalities of insulin action Insulin receptor gene (type A insulin resistance, leprechaunism, Rabson–Mendenhall syndrome etc.) Others | (1) Diseases of exocrine pancreas Pancreatitis Trauma/pancreatectomy Neoplasm Hemochromatosis Others (2) Endocrine diseases Cushing’s syndrome Acromegaly Pheochromocytoma Glucagonoma Aldosteronism Hyperthyroidism Somatostatinoma Others (3) Liver disease Chronic hepatitis Liver cirrhosis Others (4) Drug‐ or chemical‐induced Glucocorticoids Interferon Others (5) Infections Congenital rubella Cytomegalovirus Others (6) Rare forms of immune‐mediated diabetes Anti‐insulin receptor antibodies Stiffman syndrome Insulin autoimmune syndrome Others (7) Various genetic syndromes often associated with diabetes Down syndrome Prader‐Willi syndrome Turner syndrome Klinefelter syndrome Werner syndrome Wolfram syndrome Ceruloplasmin deficiency Lipoatrophic diabetes mellitus Myotonic dystrophy Friedreich ataxia Laurence‐Moon‐Biedl syndrome Others |
The occurrence of diabetes‐specific complications has not been confirmed in some of these conditions.
-
(A)
Diabetes mellitus with identified genetic abnormalities: With the recent progress in genetic technology, several single genetic abnormalities have now been identified as causing diabetes mellitus24–29. These are divided into (i) genetic abnormalities related to pancreatic β‐cell function; and (ii) genetic abnormalities relevant to mechanisms of insulin action. Each group can be further divided according to the type of genetic abnormality. For example, (i) includes defects in the insulin gene itself and Maturity Onset Diabetes of the Young (MODY)24,25. MODY 1 through to 6 correspond to genetic abnormalities of HNF‐4α, glucokinase, HNF 1α, IPF‐1 (PDX‐1), HNF‐1β and NeuroD1/Beta230, respectively. Mitochondrial genetic abnormalities26 and amylin genetic abnormalities27 are also included in (i). Recently, in neonatal diabetes, genetic abnormalities in Kir6.2 and SUR1, which form the KATP channel in pancreatic β‐cells, have been identified31,32. Genetic abnormalities. such as those in insulin receptors. are included in (ii)28.
-
(B)
Various types of diabetes associated with other disorders and conditions: Some disorders, syndromes and conditions can be accompanied by a diabetic stage, and these have conventionally been called secondary diabetes. They include diabetes associated with pancreatic disease, endocrine disease, liver disease, drug use, exposure to chemical substances, viral infections and various genetic syndromes.
Gestational Diabetes Mellitus
Glucose metabolism disorder that is first discovered or develops during pregnancy, excluding clinically diagnosed diabetes mellitus (details described later). The etiology is presumably based on common pathogenic mechanisms with type 1 and type 2, with pregnancy triggering the manifestation of a glucose metabolism disorder. It is debated whether gestational diabetes mellitus (GDM) should be treated as an independent etiological classification, but due to its clinical importance, the need for special consideration and different features from diabetes in the absence of pregnancy, it is treated as a separate category. This is because pregnancy itself worsens glucose metabolism, and diagnosis and control require special considerations that are different from those in the absence of pregnancy, and even a comparatively mild disorder in glucose metabolism during pregnancy can exert significant influence on the infant and mother. In addition, glucose metabolism disorders during pregnancy often return to normal after delivery, but the risk of developing diabetes in the future is increased in women who have disorders of glucose metabolism during pregnancy.
Useful Findings for the Classification of Diabetes Mellitus
For etiological classification of diabetes, the following clinical information is useful:
-
1
Detailed information about the family history of diabetes and the mode of inheritance.
-
2
Age of onset and the course of diabetes.
-
3
Physical characteristics, such as obesity, history of weight changes in the past, hearing disturbance (mitochondrial DNA abnormality) and acanthosis nigricans (severe insulin resistance).
-
4
For diagnosing type 1 diabetes mellitus, examination of islet‐associated antibodies such as GAD antibody, IA‐2 antibody, insulin autoantibody (IAA; present before the use of insulin), islet cell antibody (ICA) and ZnT8 antibody. The presence of any of these autoantibodies suggests type 1 diabetes.
-
5
Examination of HLA antigens. Disease‐susceptible HLA related to type 1 diabetes in Japanese patients are DR4 and DR9, while disease‐resistant HLA is DR2; as DR4 and DR9 are also frequently present in healthy individuals, a diagnosis of type 1 diabetes cannot be made only if these antigen types are shown; this condition should be considered with an ancillary diagnosis that patients having no DR4 or DR9 and those having DR2 are unlikely to have type 1 diabetes, that major disease‐susceptible haplotypes of type 1 diabetes in Japanese patients at the gene level (DNA typing) are DRB1*0405‐DQB1*0401 and DRB1*0901‐DQB1*0303, and that the manner in which these haplotypes are combined has a bearing on the mode of development of the disease.
-
6
Tests for insulin secretion and insulin resistance (fasting plasma insulin and C‐peptide levels, insulin response to glucose loading, and, in particular cases, hyperinsulinemic euglycemic clamp or minimal model etc.).
-
7
DNA analysis may give a definite diagnosis in special cases belonging to A(1) and A(2) in Table 2.
However, the etiological classification of diabetes mellitus based on these data is not immediately required for treatment.
To assess the pathophysiological stage of diabetes, clinical information (history of the disease, glycemic level and its stability, ketosis‐proneness, and response to diet and drug therapy), plasma insulin assays (fasting and after glucose load, and after intravenous glucagon), and C‐peptide assays in plasma and urine will help to evaluate the degree of insulin deficiency.
Diagnosis
The diagnosis of diabetes mellitus is the process of confirming that the subject conforms to the disease concept described earlier. Confirmation of chronic hyperglycemia is essential for a diagnosis of diabetes mellitus. Table 3 shows the criteria for fasting plasma glucose levels, 75 g OGTT 2‐h plasma glucose levels, casual plasma glucose level and HbA1c measurements. Fasting plasma glucose is measured before breakfast under fasting conditions, at least 10 h from the night before (water may be consumed). OGTT is described later. Temporal relation of diet to blood sampling time is disregarded in the casual plasma glucose test.
Table 3. Criteria of fasting plasma glucose levels and 75 g oral glucose tolerance test 2‐h value.
| Normal range | Diabetic range | |
|---|---|---|
| Fasting value | <110 mg/dL (6.1 mmol/L) | ≥126 mg/dL (7.0 mmol/L) |
| 75 g OGTT 2‐h value | <140 mg/dL (7.8 mmol/L) | ≥200 mg/dL (11.1 mmol/L) |
| Evaluation of OGTT | Normal type: If both values belong to normal range | *Diabetic type: If any of the two values falls into diabetic range |
| Borderline type Neither normal nor diabetic types | ||
*Casual plasma glucose ≥200 mg/dL (≥11.1 mmol/L) and HbA1c≥6.5% are also regarded as to indicate diabetic type.
Even for normal type, if 1‐h value is 180 mg/dL (10.0 mmol/L), the risk of progression to diabetes mellitus is greater than for <180 mg/dL (10.0 mmol/L) and should be treated as with borderline type (follow‐up observation, etc.). Fasting plasma glucose level of 100–109 mg/dL (5.5–6.0 mmol/L) is called ‘high‐normal’: within the range of normal fasting plasma glucose.
Plasma glucose level after glucose load in oral glucose tolerance test (OGTT) is not included in casual plasma glucose levels. The value for HbA1c (%) is indicated with 0.4% added to HbA1c (JDS) (%).
The plasma glucose is often elevated temporarily in cases of severe stress (for example, infections, myocardial infarction, stroke and surgery). Therefore, excepting the situation of a severe metabolic disturbance necessitating immediate treatment, the evaluation of hyperglycemia should be made after resolution of such stressful conditions.
We first describe the procedures for diagnosis in the clinical setting, and then epidemiological study and health screening.
Clinical Diagnosis
Clinical diagnosis involves not only the presence or absence of diabetes mellitus, but requires a comprehensive understanding of the etiology, stage, degree of glucose metabolism disorder, and the presence and degree of complications. In the present report, plasma glucose and HbA1c levels are described as ‘type’, as in past reports of the JDS. This is based on the position that determination of test results and diagnosis of the disease (or a group of diseases) are different.
Procedures for the Diagnosis of Diabetes Mellitus
-
1
At initial examination, if any of the following is observed, it is determined to be ‘diabetic type’: (i) fasting plasma glucose level ≥126 mg/dL (≥7.0 mmol/L); (ii) 75 g OGTT 2‐h value ≥200 mg/dL (≥11.1 mmol/L); (iii) casual plasma glucose level ≥200 mg/dL (≥11.1 mmol/L); or (iv) HbA1c≥6.5%. Re‐examination is conducted at another date, and diabetes mellitus is diagnosed if ‘diabetic type’ is reconfirmed. However, a diagnosis cannot be made only on the basis of a repeat HbA1c examination. If the same blood sample is confirmed to be a diabetic type both by the plasma glucose level and HbA1c (any of [i] to [iii] plus [iv]), then diabetes mellitus can be diagnosed from the initial examination. When HbA1c is used, it is essential that the plasma glucose level (any of [i] to [iii]) also indicates diabetic type for a diagnosis of diabetes mellitus (Tables 4 and 5 and Figure 2).
-
2
If the plasma glucose level indicates diabetic type (any of [i] to [iii]) and either of the following conditions exists, diabetes mellitus can be diagnosed, even at the initial examination.
-
•
The presence of typical symptoms of diabetes mellitus (thirst, polydipsia, polyuria, weight loss).
-
•
The presence of definite diabetic retinopathy.
-
•
-
3
If it can be confirmed that either of the conditions 1 or 2 existed in the past, diabetes mellitus must be diagnosed or suspected, even if the present test values do not meet these conditions.
-
4
If diabetes mellitus is suspected, but the diagnosis cannot be made by conditions 1–3, diabetes mellitus should be suspected, and plasma glucose level and HbA1c should be measured again within 3–6 months.
-
5
Points to keep in mind are that when fasting plasma glucose level is used for determination, it is important to confirm the fasting conditions. If the casual plasma glucose level is ≥200 mg/dL (≥11.1 mmol/L) at the initial examination, an alternative test method is desirable at the second time. As a rule, both plasma glucose level and HbA1c should be measured during examination. In case of the disorders and conditions shown in Table 5, in which HbA1c may be apparently low, the plasma glucose level should be used for diagnosis.
Table 4. Procedures for diagnosing diabetes mellitus.
| Clinical diagnosis (1) At initial examination, a ‘diabetic type’ is diagnosed if any of the following criteria are met: (i) fasting plasma glucose level ≥126 mg/dL (7.0 mmol/L), (ii) 75 g OGTT 2‐h value ≥200 mg/dL (11.1 mmol/L), (iii) casual plasma glucose level ≥200 mg/dL (11.1 mmol/L) or (iv) *HbA1c≥6.5%. Re‐examination is carried out at another date and diabetes mellitus is diagnosed if ‘diabetic type’ is confirmed again**. However, diagnosis cannot be made on the basis of a repeated HbA1c test alone. If the same blood sample is confirmed to be diabetic type by both plasma glucose and HbA1c levels (any of [i] to [iii] plus [iv]), then diabetes mellitus can be diagnosed from the initial test |
| (2) If plasma glucose level shows diabetic type (any of [i] to [iii]) and either of the following conditions exists, diabetes mellitus can be diagnosed immediately at the initial examination • The presence of typical symptoms of diabetes mellitus (thirst, polydipsia, polyuria, weight loss) • The presence of definite diabetic retinopathy |
| (3) If it can be confirmed that either of the above conditions 1 or 2 existed in the past, diabetes mellitus must be diagnosed or suspected even if present test values do not meet the above conditions |
| (4) If diabetes mellitus is suspected but the diagnosis cannot be made by the above (1) to (3), the patient should be followed‐up |
| (5) The following points should be kept in mind when selecting the method of determination in initial examination and re‐examination • If HbA1c is used at initial examination, another method of determination is required for diagnosis at re‐examination. As a rule, both plasma glucose level and HbA1c should be measured • If casual plasma glucose level is ≥200 mg/dL (11.1 mmol/L) at the initial test, a different test method is desirable for re‐examination • In the case of disorders and conditions in which HbA1c may be inappropriately low, plasma glucose level should be used for diagnosis (Table 5) |
| Epidemiological study For the purpose of estimating the frequency of diabetes mellitus, determination of ‘diabetic type’ from a single test can be considered to represent ‘diabetes mellitus’. Whenever possible, the criteria to be used are HbA1c≥6.5% or OGTT 2‐h value ≥200 mg/dL (11.1 mmol/L) |
| Health screening It is important to detect diabetes mellitus and identify high risk groups without overlooking anyone. Therefore, besides measuring plasma glucose and HbA1c, clinical information such as family history and obesity should be referred |
*The value for HbA1c (%) is indicated with 0.4% added to HbA1c (JDS) (%). **Hyperglycemia must be confirmed in a non‐stressful condition. OGTT, oral glucose tolerance test.
Table 5. Disorders and conditions associated with low HbA1c values.
| Anemia |
| Liver disease |
| Dialysis |
| Major hemorrhage |
| Blood transfusion |
| Chronic malaria |
| Hemoglobinopathy |
| Others |
Figure 2.
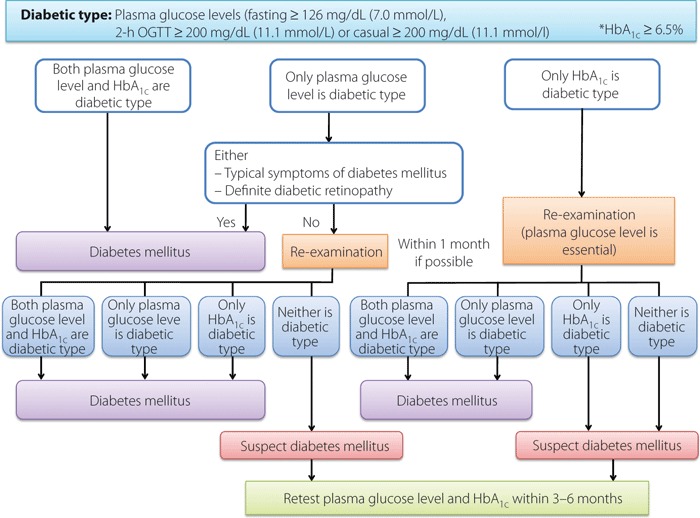
Flow chart outlining steps in the clinical diagnosis of diabetes mellitus. *The value for HbA1c (%) is indicated with 0.4% added to HbA1c (JDS) (%).
OGTT and the Criteria for OGTT
-
1
Procedure of OGTT: OGTT evaluates the rate of glucose disposal after an oral glucose challenge, and is the most sensitive test to detect a mild disturbance of glucose metabolism. When the results of the fasting plasma glucose level, casual plasma glucose level or HbA1c measurement are not definite, OGTT results provide solid information for diagnosing diabetes mellitus. In clinical practice, OGTT is recommended for confirming glucose tolerance in cases as shown in Table 6, except when overt symptoms of diabetes, marked hyperglycemia or ketosis are present. In fact, extensive analyses in Japan have made it clear that when the fasting plasma glucose level is 100 mg/dL (5.5 mmol/L) or higher, or the HbA1c is 5.6% or higher, these include (i) a group in which diabetes mellitus must be suspected; and (ii) a group in which, if the disease is not present now, is at a high risk for developing diabetes mellitus in the future. OGTT is an important diagnostic tool allowing physicians to avoid the risk of overlooking these patients5,33,34. In particular, OGTT is strongly recommended in the case of (i) and should be carried out if possible in case of (ii).
When conducting OGTT, the following conditions are necessary to obtain an accurate result. After consuming meals containing at least 150 g of carbohydrates for at least 3 days, patients receive 75 g (as anhydride) of glucose or its equivalent of 250–350 mL carbohydrate solution orally in the morning under fasting conditions. Blood is then sampled over time to measure the plasma glucose levels. The solution should be consumed within 5 min, and evaluation should be timed from the time that the patient starts to drink. The patient should be fasted for 10–14 h from the day before until the test. Nothing, except for water, can be consumed until the test is completed, and the patient should remain as quiet as possible and may not smoke during the test. Measuring urinary glucose at the same time is useful to estimate the urinary glucose excretion threshold. For a diagnosis of diabetes mellitus, at least fasting and 2‐h plasma glucose levels are measured. Starvation or low carbohydrate intake may induce glucose intolerance. In patients who have undergone a gastrectomy, the plasma glucose level may rise dramatically soon after the glucose load.
At the least, fasting and 2‐h blood samples should be taken. In clinical cases, it is recommended also to measure plasma glucose at 30 and 60 min to enhance the accuracy of diagnosis. The measurement of plasma insulin can help to predict the risk for future development of diabetes.
Table 6. Situations where a 75‐g oral glucose tolerance test is recommended.
| Strongly recommended (suspicion of present diabetes mellitus cannot be ruled out) Fasting plasma glucose level is 110–125 mg/dL (6.1–6.9 mmol/L) Casual plasma glucose level is 140–199 mg/dL (7.8–11.0 mmol/L) *HbA1c is 6.0–6.4% (excluding those having overt symptoms of diabetes mellitus) |
| Testing is desirable (high risk of developing diabetes mellitus in the future; Testing is especially advisable for patients with risk factors for arteriosclerosis such as hypertension, dyslipidemia and obesity.) Fasting plasma glucose level is 100–109 mg/dL (5.5–6.0 mmol/L) *HbA1c is 5.6–5.9% Strong family history of diabetes mellitus or present obesity regardless of above criteria |
*The value for HbA1c (%) is indicated with 0.4% added to HbA1c (JDS) (%).
-
2
Cut‐off Levels for OGTT:Table 3 shows the criteria for diabetes mellitus according to OGTT. As in past JDS committee reports, the results are classified as normal, borderline and diabetic types2–4. The normal range and the diabetic range of fasting plasma glucose levels and OGTT 2‐h values are shown in Table 3.
-
•
Diabetic type: Either a fasting plasma glucose level of 126 mg/dL (7.0 mmol/L) or higher, or an OGTT 2‐h value of 200 mg/dL (11.1 mmol/L) or higher is called diabetic type.
-
•
Normal type: Normal type is defined as a range of glycemia that is unlikely to develop into diabetes after several years of observation. A fasting plasma glucose level below 110 mg/dL (6.1 mmol/L) and an OGTT 2‐h value below 140 mg/dL (7.8 mmol/L) are called normal type. In a previous JDS report, normal type was considered as ‘patients mostly do not develop diabetes mellitus even after several years of follow up’, and the plasma glucose criteria were thus set at lower levels2,3. However, in the 1999 JDS report, the upper limit of normal type was set at the same value as the lower limit of the WHO IGT reference value. This was partly in respect to the international reference value, and partly because the progression rate among the Japanese data from normal type to diabetic type was as low as 0.6–1.0%4.
-
•
Borderline type: This category is defined when the pattern of OGTT is neither diabetic nor normal type. Borderline type includes heterogeneous conditions: a subject in transition to developing diabetes, diabetes in remission, insulin resistance syndrome and temporary deterioration of glucose tolerance due to stress in an essentially healthy individual. Subjects with borderline type are at little risk of developing diabetes‐specific microangiopathy, but at increased risk of progressing to diabetes and to developing macroangiopathy. The fasting plasma glucose level that defines IFG is set at 100–125 mg/dL (5.5–6.9 mmol/L)10 by the American Diabetes Association and at 110–125 mg/dL (6.1–6.9 mmol/L)11 by the WHO. The JDS called the range of 100–109 mg/dL (5.5–6.0 mmol/L) ‘high‐normal’, because those with a fasting plasma glucose level of 100 mg/dL (5.5 mmol/L) or higher are often borderline type or diabetic type according to OGTT. However, because the judgment of normal type is made in conjunction with the OGTT 2‐h value, the fasting plasma glucose criterion below 110 mg/dL (6.1 mmol/L) is retained5. The JDS borderline type corresponds to a combination of IGT and a narrowly defined IFG (elevated fasting plasma glucose level only, not IGT). Individually, IGT and IFG are often incongruent35,36.
Subjects with a 100 mg/dL (5.5 mmol/L) or higher fasting value and a 1‐h value of 180 mg/dL (10.0 mmol/L) or higher (steep hyperglycemia) should be followed similarly to borderline type, even if they belong to normal type, because it is known that such individuals are at higher risk of developing diabetes. Diabetic patients have a decreased early insulin response to glucose with a low insulinogenic index, a 0.4 (μU/mL per mg/dL) or lower ΔIRI/ΔPG (the ratio of increment of plasma insulin to that of glucose at 30 min after oral glucose load), and borderline types showing low insulinogenic index have been reported to have a high risk of progression into diabetes mellitus, and all of these are thought to be fundamental characteristics of diabetes mellitus37–40.
Epidemiological Study
The purpose of epidemiological study is to estimate the prevalence and incidence of diabetes mellitus or glucose metabolism disorders in a population and to examine their risk factors. In this case, repeated examination of plasma glucose is usually difficult. While the reproducibility of fasting plasma glucose level or OGTT results in each individual is not good, for each population, the plasma glucose level distribution and the mean values are reasonably reproducible. Consequently, when estimating the prevalence of diabetes mellitus, the determination of ‘diabetic type’: based on a single test can be considered as representative of ‘diabetes mellitus’ (Table 4). Because it is difficult to verify that the subject actually observed the fasting time for the fasting blood sample, HbA1c≥6.5% is used as the standard as far as possible. When using the fasting plasma glucose level, OGTT 2‐h value or HbA1c, it is always necessary to clarify the diagnostic method in the survey report, because the prevalence of ‘diabetes mellitus’ and individual subjects diagnosed as having ‘diabetes mellitus’ vary according to the methods. When presenting the survey results, it is desirable to include not only the prevalence of ‘diabetes mellitus’ and borderline glucose metabolism disorders, but also the distribution data on plasma glucose levels and HbA1c in the study population.
Health Screening
The purpose of health screening is to detect diabetes mellitus and its high risk groups. Therefore, in addition to measuring plasma glucose and HbA1c, information such as family history, body weight history, pregnancy and birth history, present status of obesity, blood pressure and findings concerning complications is gathered, and subjects at great risk of developing diabetes mellitus should be identified. The diagnosis of subjects thus screened then follows the same procedure as for clinical diagnosis.
Since April 2008 in Japan, national health examinations and health guidance have been conducted for health insurance subscribers aged 40–74 years. The basic idea of the new health screening system is to find those who need healthcare guidance to prevent harmful lifestyle habits, focusing on visceral fat obesity. Subjects who receive healthcare guidance are those with a fasting plasma glucose level of 100 mg/dL (5.5 mmol/L; lower limit of high‐ normal) or higher, which corresponds to an OGTT 2‐h value of 140 mg/dL (7.8 mmol/L) (lower limit of borderline type), together with HbA1c≥5.6%. From the standpoint of diabetes prevention, test data are to be handled as follows, even in those failing to meet the Japanese criteria for waist circumference and body mass index (Table 6).
-
1
If the fasting plasma glucose level or HbA1c corresponds to the values that recommend further examination (fasting plasma glucose ≥126 mg/dL [7.0 mmol/L] or HbA1c≥6.5%), the subject is immediately examined at a healthcare facility, because diabetes mellitus is strongly suspected.
-
2
If the fasting plasma glucose level is 110–125 mg/dL (6.1–6.9 mmol/L) or HbA1c is 6.0–6.4%, OGTT should be performed wherever feasible. If the result is borderline type, follow up or lifestyle guidance is conducted. If the result is diabetic type, the subject should be examined at a healthcare facility.
-
3
If the fasting plasma glucose level is 100–109 mg/dL (5.5–6.0 mmol/L) or HbA1c is 5.6–5.9%, because the risk of developing diabetes mellitus or arteriosclerosis is probably greater than in those with lower values, other risk factors (family history, obesity, hypertension, dyslipidemia, etc.) are taken into consideration, and provision of information, follow‐up observation or OGTT should be carried out.
Elderly People and Children
Elderly People
Diagnostic procedures for diabetes mellitus are performed as usual in the elderly with the same reference ranges as described earlier. Because OGTT 2‐h plasma glucose levels tend to be more elevated compared to fasting plasma glucose levels in elderly people, it is advisable to confirm an increased HbA1c at diagnosis. In elderly people showing only a slightly higher level above the reference range, even though the condition is diabetic type, it is preferable not to administer pharmacotherapy, but to monitor the progress by providing lifestyle guidance alone as in the borderline cases.
Children
In children, type 1 diabetes usually presents with overt symptoms and with marked hyperglycemia, leaving little problem in diagnosis. However, if type 1 diabetes mellitus is diagnosed in an asymptomatic child at a school health screening, it can be difficult to determine the exact etiology of the disease. Slowly progressive type 1 diabetes mellitus is not rare, even in children, and measurement of autoantibodies, such as GAD antibody or IA‐2 antibody, and monitoring of C‐peptide are useful to differentiate type 1 diabetes from other types. In Japan, 20–30% of childhood‐onset type 2 diabetes mellitus is non‐obese, and sometimes differentiating this from slowly progressive type 1 can be difficult. Although those who develop type 1 diabetes mellitus with onset in infancy or childhood quite often show negative results of islet‐associated antibodies, endogenous insulin secretion of these patients is usually depleted from an early stage. If an OGTT is needed to diagnose diabetes mellitus, the glucose load should be actual body weight (in kilograms) × 1.75 g (maximum 75 g). The classifications of hyperglycemia and the diagnosis of diabetes mellitus are the same as for adults.
Diabetes mellitus with onset in newborns under 6 months‐of‐age and infants often has a specific pathogenesis, such as a single genetic abnormality, and is classified as neonatal diabetes.
Gestational Diabetes Mellitus
Glucose metabolism disorders occurring during pregnancy can be classified as pregnancy with pre‐existing diabetes and hyperglycemic disorders in pregnancy, with the latter having two forms: GDM and overt diabetes diagnosed or developed during pregnancy.
The diagnosis of GDM is important because of the recognition that excessive fetal growth can occur even with mild glucose metabolism disorders, increasing the perinatal risk. In addition, even if the mother’s glucose metabolism disorder improves after birth, she has an increased risk of developing diabetes mellitus in the future. The definition of GDM has undergone many historical changes. In 2008, the results of an international randomized comparative study of the effects of mild hyperglycemia during pregnancy on the child, Hyperglycemia and Adverse Pregnancy Outcome Study (HAPO Study)41, were reported. The report from this study made recommendations on the definition, diagnostic criteria and screening for GDM based on evidence of an increase in perinatal complications42. Based on this, and considering consistency with international guidelines, the definition of GDM excluded overt diabetes and, following the International Association of Diabetes and Pregnancy Study Groups (IADPSG) Consensus Panel, revised the diagnostic criteria for GDM (Table 7). If diabetes mellitus was present before pregnancy, the risk of fetal anomaly is greater than that seen in GDM.
Table 7. Definition and diagnostic criteria of gestational diabetes mellitus.
| Definition of gestational diabetes mellitus Glucose metabolism disorder with first recognition or onset during pregnancy, but that has not developed into diabetes mellitus |
| Diagnostic criteria of gestational diabetes mellitus Diagnosed if one or more of the following criteria is met in a 75 g OGTT Fasting plasma glucose ≥92 mg/dL (5.1 mmol/L) 1‐h value ≥180 mg/dL (10.0 mmol/L) 2‐h value ≥153 mg/dL (8.5 mmol/L) However, diabetes mellitus that is diagnosed according to ‘Clinical diagnosis’ outlined in Table 4 is excluded from gestational diabetes mellitus |
(IADPSG Consensus Panel, Reference 42, partly modified with permission of Diabetes Care).
Risk factors for GDM include positive urinary glucose, family history of diabetes mellitus, obesity, excessive weight gain, having previously given birth to a large baby and aging. In order to ensure detection of GDM, a casual plasma glucose test is conducted at the first visit and at mid‐term of pregnancy if insulin resistance is elevated, and an OGTT is performed for those patients with a plasma glucose level of 100 mg/dL (5.5 mmol/L) or higher. A diagnosis of GDM is made if one or more of the following criteria is met: fasting plasma glucose ≥92 mg/dL (≥5.1 mmol/L), 1‐h value ≥180 mg/dL (≥10.0 mmol/L) or 2‐h value ≥153 mg/dL (≥8.5 mmol/L). However, women who are diagnosed with diabetes mellitus according to this clinical diagnosis are precluded from GDM.
Commentary
This committee considered the following points when preparing this report: (i) consistency with recent international reports; (ii) sufficient application of recent data obtained in Japan; (iii) succession of the basic concepts of the 1999 JDS committee report on diabetes mellitus; and (iv) respect for the opinions of the academic council. Points that could not be presented in the main text will be discussed here.
Worsening and Improving of Stages of Diabetes
Figure 1 is a two‐dimensional depiction of individual patient positioning, with the etiological classification of diabetes mellitus on the vertical axis and the degree of insulin deficiency on the horizontal axis. The ‘diabetic area’ on the horizontal axis indicates that hyperglycemia has exceeded a certain level or the degree of insulin deficiency has exceeded a certain level. The vertical axis of type 1 and type 2 shows the etiology (mechanisms), although the word ‘diabetes’ is not used here. This is because even if disease procession that can lead to diabetes mellitus is proceeding, it is not called ‘diabetes’ until hyperglycemia has reached a certain stage or degree.
The arrows in Figure 1 point in both directions, and the arrows pointing to the left indicate improvement in the diabetic condition, either naturally or due to treatment. In the figures of the American Diabetes Association14 and WHO11, the left and right arrows are depicted by a single line, but in Figure 1, the line pointing left is a separate and partially broken line. Improvement of definite diabetes mellitus to the point of normal glucose metabolism is not common, except in special cases, such as pheochromocytoma resection or soft drink ketosis43. The arrow pointing left can be said to represent the goal of diabetes mellitus treatment.
Even if diabetes mellitus diagnosed in a patient improves with treatment from diabetic type glucose tolerance to a borderline or normal type, unless the underlying disease process has definitely been eliminated, the patient must still be considered and treated as having diabetes and the subsequent course should be observed.
Diagnostic Criteria and Relevant Matters
The Position of HbA1c in the Diagnosis of Diabetes Mellitus
It is clinically useful to use HbA1c for making a diagnosis for the following reasons. The use of this parameter is scientifically relevant in that elevation of HbA1c serves as an indicator reflecting chronic hyperglycemia. Furthermore, HbA1c is specified as an indicator of glycemic control in the treatment guide for diabetes mellitus. Its use enhances continuity of diagnosis and treatment of diabetes, because day‐to‐day variations are less conspicuous and not affected by dietary conditions, and its use enables diagnosis of diabetes by one test even on a single occasion. However, because the distribution of HbA1c in diabetic types is broad, diabetes mellitus cannot be diagnosed by HbA1c alone (Figure 3), and HbA1c is affected by red blood cell turnover in addition to plasma glucose levels (Table 5)4,13.
Figure 3.
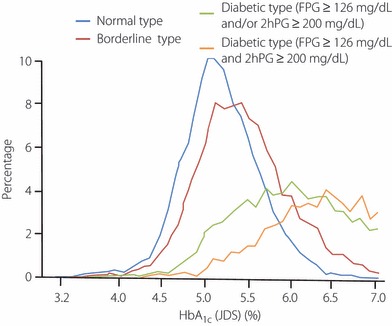
Distribution of HbA1c in groups with varying degree of glucose intolerance. Distribution of HbA1c (JDS) in 6720 normal types, 6296 borderline types and 5040 diabetic types. Among diabetic types, 2950 cases that had fasting plasma glucose (FPG) ≥126 mg/dL (7.0 mmol/L) and 2‐h oral glucose tolerance test (2hPG) ≥200 mg/dL (11.1 mmol/L) are shown separately (Chikako Ito, Reference 4, with the author’s permission).
In contrast, fasting plasma glucose and OGTT 2‐h values have long been used in the diagnosis of diabetes mellitus, and there is ample evidence that they reflect chronic hyperglycemia and that they are related to retinopathy, which is considered highly characteristic of diabetes mellitus8,44. In addition, these have come to be regarded at the most important findings for a diagnosis of diabetes mellitus. Therefore, this committee examined the relationship of fasting plasma glucose level and OGTT 2‐h value to HbA1c in Japanese people, and the relationship between HbA1c and retinopathy. Retinopathy was recorded by fundus camera photography and the findings were judged by an ophthalmologist.
When 6658 OGTT examinees aged under 60 years were reviewed, a very high correlation between fasting plasma glucose level and HbA1c was seen (r = 0.854), with a fasting plasma glucose level of 126 mg/dL (7.0 mmol/L) corresponding to a HbA1c (JDS) of 6.1%, calculated from the regression equation HbA1c (JDS) = 1.869 + 0.0333x (fasting plasma glucose level in mg/dL; Figure 4a). In the same manner, a correlation was identified between OGTT 2‐h value and HbA1c, (r = 0.809), with an OGTT 2‐h value of 200 mg/dL (11.1 mmol/L) corresponding to a HbA1c (JDS) of 6.0%, calculated from the regression equation HbA1c (JDS) = 3.553 + 0.0122x (OGTT 2‐h value; Figure 4b). Conversely, when the fasting plasma glucose level and OGTT 2‐h value corresponding to a HbA1c of 6.1% were sought from the regression equations, fasting plasma glucose level = −9.2 + 21.9x (HbA1c [JDS]) and the OGTT 2‐h value = −127.1 + 53.5x (HbA1c [JDS]), a fasting plasma glucose level of 124.4 mg/dL (6.91 mmol/L) and an OGTT 2‐h value of 199.3 mg/dL (11.07 mmol/L) corresponded to a HbA1c (JDS) of 6.1% (Figure 4c,d). These results indicate that a HbA1c (JDS) of 6.1% corresponds to the criteria for a diabetic range from the fasting plasma glucose level and OGTT 2‐h value45.
Figure 4.
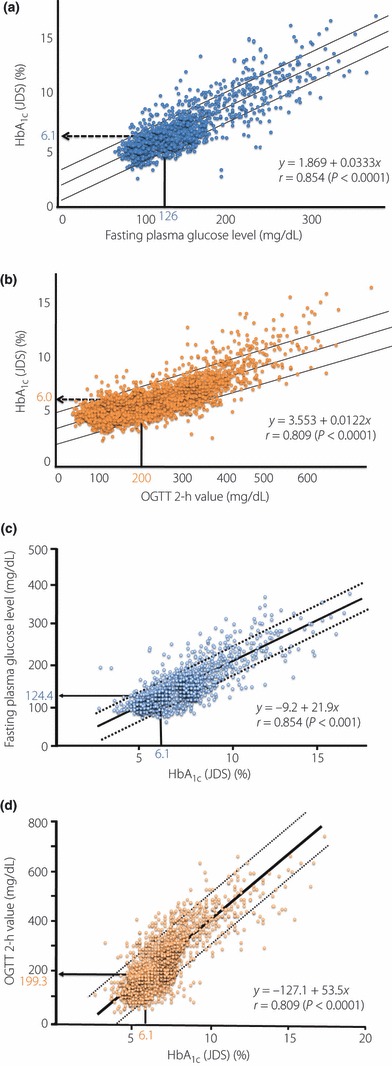
Relationship between HbA1c and fasting plasma glucose level and oral glucose tolerance test (OGTT) 2‐h values. (a) Relationship between fasting plasma glucose and HbA1c (JDS) (n = 6658). (b) Relationship between OGTT 2‐h value and HbA1c (JDS) (n = 6658). (c) Relationship between HbA1c (JDS) and fasting plasma glucose (n = 6658). (d) Relationship between HbA1c (JDS) and OGTT 2‐h value (n = 6658) (Chikako Ito, unpublished data and Reference 45, with permission from Diabetes Res Clin Pract).
Second, based on data covering 36,267 examinees, the prevalence of diabetic retinopathy (except for capillary microaneurysm alone) was compared by HbA1c and was found to be 0.06% for HbA1c (JDS) ≤4.5%. The prevalence of diabetic retinopathy increased with increasing HbA1c to become as high as 0.59% for HbA1c (JDS) between 6.1 and 6.5%; hence, it was considered relevant to set the cut‐off value of HbA1c (JDS) at 6.1% (Figure 5)44.
Figure 5.
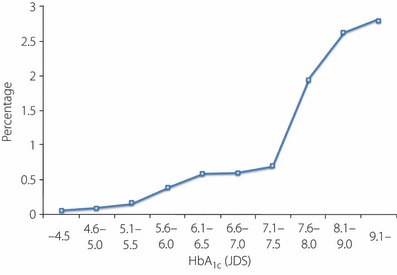
HbA1c (JDS) and frequency of diabetic retinopathy (n = 36,267). Excluding those with capillary microaneurysms alone (Chikako Ito, unpublished data).
The relationship between HbA1c and diabetic retinopathy (moderate non‐proliferative diabetic retinopathy and worse) is being studied in the USA with an extensive amount of epidemiological data. Specifically, a HbA1c (NGSP) ≥6.5% is being advocated as diagnostic for diabetes mellitus based on the high frequency of retinopathy at this level13. It has been pointed out in individual cases that there is a possibility of overlooking the disease if the diagnosis is made based on HbA1c alone46. A recent report regarding diagnostic exploration for the risk of diabetes indicates that, compared to the fasting plasma glucose level, HbA1c shows an equivalent relationship to the risk of developing diabetes mellitus and a stronger relationship with cardiovascular diseases and death47.
Concerning HbA1c15
-
1.
Definition of HbA1c
HbA1c was originally named as one of the chromatography peaks in the hemoglobin of healthy people as a trace component, but the IFCC redefined the substance as hemoglobin that is non‐enzymatically and stably bound to glucose, a valine on the hemoglobin’s β‐chain N‐terminal being glycosylated to formβ‐N1‐deoxyfructosyl Hb48–50.
-
2.
HbA1c measurement and precision control
Presently, the exact separation of the stable form β‐N1‐mono‐deoxyfructosyl Hb by high performance liquid chromatography using cation‐exchange resin is established as the standard method of HbA1c measurement. Other methods of measurement include immunological methods (latex agglutination immunoassay [inhibition assay], agglutination immunoassay [blocking assay] and turbidimetric immunoinhibition) and an enzymatic method. Each of these can accurately measure the stable form β‐N1‐ deoxyfructosyl Hb.
However, to maintain the precision and stability of the measured values, nationwide surveillance of differences in measured values between facilities and test methods, facility certification, and disclosure of information on assay reagents are necessary. Especially in some Point of Care (POC) devices for simplified HbA1c measurement, standardization is seen as insufficient and the use of such devices for diagnosis of diabetes mellitus cannot be recommended at this time.
-
3.
Notation for the international standardization of HbA1c
HbA1c described in the JDS values that are used in Japan, despite its leading position in the world in precision control and progressive standardization12,15, has the problem that it is approximately 0.4% lower than the HbA1c described in the NGSP values that are used by almost every other country. International standardization using a new notation with numeric values very different from the previous ones is being studied by the IFCC, including Japan, to solve the various problems facing HbA1c measurement. This notation (IFCC value), which exactly indicates the currently defined β‐N1‐deoxyfructosyl Hb, is approximately 1.5% lower than HbA1c (JDS)(%) and 1.9% lower than HbA1c (NGSP; %), and mistakes and confusion in clinical diagnosis might occur if it were immediately adopted in general medical practice. Therefore, the IFCC recommends use of the System International (SI) unit (mmol/mol) to indicate the IFCC value. However, shifting to that notation will probably take considerable time, and the JDS has decided that a notation that does not differ from HbA1c (NGSP) should be used in the current revision from the standpoint of emphasizing international standardization, as follows. The HbA1c (%) is estimated as a NGSP equivalent value (%) calculated by the formula HbA1c (%) = HbA1c (JDS; %) + 0.4%, considering the relational expression of HbA1c (JDS; %) measured by the previous Japanese standard materials and measurement methods and HbA1c (NGSP) (NGSP [%] = 1.019xJDS [%] + 0.30) and the coefficient of variance of 2–3% in the measurement of HbA1c.
Evidence for Setting Reference Values for Diabetic Type
Complications characteristic of diabetes mellitus are closely related to hyperglycemia. The degree of hyperglycemia at which complications will occur is the basis for setting plasma glucose reference standards to determine diabetes mellitus51. Fasting plasma glucose level, OGTT 2‐h value or HbA1c all show a relationship with retinopathy52.
The fasting plasma glucose level and OGTT 2‐h value that determine the ‘diabetic type’ in the 1999 JDS report are the same as the reference values that decide ‘diabetes mellitus’ in the reports of the ADA and the WHO. This was due to the importance of international consistency and, in the Japanese data, the mean fasting plasma glucose level corresponding to a 75 g OGTT 2‐h value of 200 mg/dL (11.1 mmol/L) is approximately 125 mg/dL (6.9 mmol/L) in patients under the of age 60 years45,53.
When viewed with reference to domestic and overseas cross‐sectional survey decile method data, in contrast, the risk of retinopathy apparently increases with a fasting plasma glucose level of 140 mg/dL (7.8 mmol/L), an OGTT 2‐h value of 230–240 mg/dL (12.8–13.3 mmol/L) or a HbA1c of 6.9%, and some studies claim that these levels should be used as the reference values for determining the diabetic type8,54. That is, the current reference values of fasting plasma glucose level ≥126 mg/dL (≥7.0 mmol/L) and OGTT 2‐h value ≥200 mg/dL (≥11.1 mmol/L) may be set too low from the point of view of apparent retinopathy risk. However, these reference standards have been adopted because: (i) reference values to determine diabetic type need to conform to international reference values as far as possible; and (ii) the aforementioned data by decile method are from a cross‐sectional survey, and it seems preferable to begin treatment before the risk of retinopathy markedly increases and to prevent hyperglycemia from reaching that level.
Casual plasma glucose level ≥200 mg/dL (≥11.1 mmol/L) was added to the determination of diabetic type. This is because plasma glucose measured 1.5–3 h after eating exceeding 200 mg/dL (11.1 mmol/L) usually reflects a more severe degree of glucose metabolism disorder than a 75 g OGTT 2‐h value of 200 mg/dL (11.1 mmol/L) or higher, and casual plasma glucose levels often do not reach 200 mg/dL (11.1 mmol/L), even if the OGTT indicates a diabetic type35. For these reasons, the combined use of HbA1c measurement and another method other than casual plasma glucose level is recommended for diabetes mellitus screening and early diagnosis.
Diagnosis of ‘Diabetes’ by Fasting Plasma Glucose Level and Oral Glucose Tolerance Test
Diabetic type can be determined by fasting plasma glucose level or OGTT 2‐h value or HbA1c. In the Japanese health screening data, determining the diabetic type from OGTT 2‐h value alone results in a greater frequency of diabetic type than by determination using the fasting plasma glucose level alone. However, the reverse can also be true depending on the country. Assessment results according to these two reference values in each individual are frequently discordant55. From a pathophysiological viewpoint, fasting plasma glucose level is primarily determined by glucose output from the liver, while OGTT 2‐h value is affected by the absorption rate of glucose from the gut, the utilization rate of glucose by muscle and other peripheral tissues, and changes in glucose handling by the liver. It is thus conceivable that fasting plasma glucose level and OGTT 2‐h value do not increase in parallel in some patients.
In the Japanese population, it is common for an increase in the OGTT 2‐h value to precede an increase in the fasting plasma glucose level. Therefore, to actively detect mild glucose metabolism disorders, a fasting plasma glucose level alone is insufficient, and performing the OGTT is important. Measuring insulin levels at the same time is very useful to understand the clinical condition and predict future onset of diabetes mellitus, and is strongly recommended.
Acknowledgements
We express sincere appreciation to Advisors of The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus: Hiroyasu Iso (Department of Social and Environmental Medicine, Osaka University Graduate School of Medicine), Yutaka Kiyohara (Department of Environmental Medicine, Graduate School of Medical Sciences, Kyushu University), Takeshi Kuzuya (Diabetes Center, Aino Institute for Aging Research), Kenji Shima (Department of Internal Medicine, Kawashima Hospital), Makoto Tominaga (Medical Corporation Yushikai Rehabilitation Hananoie Hospital) and Mitsuhiko Noda (Department of Diabetes and Metabolic Medicine, National Center for Global Health and Medicine) for their valuable comments and suggestions.
We are also very grateful to the assistant authors: Iseki Takamoto (Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo) and Haruhiko Tanaka (Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo) for their devotion to writing and proofreading.
There are no financial supports or relationships that may pose a conflict of interest.
References
- 1.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 2010; 53: 450–467 (Japanese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuzuya N, Abe M, Ueda H, et al. Report of the committee on the diagnostic criteria of the oral glucose tolerance test for diabetes mellitus. J Jpn Diabetes Soc 1970; 13: 1–7 (Japanese). [Google Scholar]
- 3.Kosaka K, Akanuma Y, Goto Y, et al. Report of the committee on the diagnosis of diabetes mellitus. J Jpn Diabetes Soc 1982; 25: 859–866 (Japanese); The essence of this report appears in English; Diab Res Clin Pract 1994; 24 (Suppl.): S59–S62. [DOI] [PubMed] [Google Scholar]
- 4.Kuzuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 1999; 42: 385–404 (Japanese); This report appears in English; Diabetes Res Clin Pract 2002; 55: 65–85. [DOI] [PubMed] [Google Scholar]
- 5.Kadowaki T, Haneda M, Tominaga M, et al. Report of the Japan Diabetes Society’s Committee on the Diagnostic Criteria for Diabetes Mellitus and Glucose Metabolism Disorder—A New Category of Fasting Plasma Glucose Values: “high‐normal”. J Jpn Diabetes Soc 2008; 51: 281–283 (Japanese). [Google Scholar]
- 6.National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28: 1039–1057 [DOI] [PubMed] [Google Scholar]
- 7.WHO Expert Committee on Diabetes Mellitus . Second report. World Health Organ Tech Rep Ser 1980; 646: 1–80 [PubMed] [Google Scholar]
- 8.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183–1197 [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553 [DOI] [PubMed] [Google Scholar]
- 10.Genuth S, Alberti KG, Bennett P, et al. Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160–3167 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. World Health Org, 2006. [Google Scholar]
- 12.Shima K, Endo J, Oimomi M, et al. Interlaboratory difference in HbA1c measurement in Japan—the interim report of the committee on an interlaboratory standardization of HbA1c determination. J Jpn Diabetes Soc 1994; 37: 855–864 (Japanese). [Google Scholar]
- 13.International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl 1): S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwagi A, Kadowaki T, Haneda M, et al. Consensus and Statement on International Standardization of HbA1C in Japan: Committee Report on Diabetes Mellitus Laboratory Testing Standardization. J Jpn Diabetes Soc 2009; 52: 811–818 (Japanese). [Google Scholar]
- 16.Imagawa A, Hanafusa T, Miyagawa J, et al. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes‐related antibodies. Osaka IDDM Study Group. N Engl J Med 2000; 342: 301–307 [DOI] [PubMed] [Google Scholar]
- 17.Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 2003; 26: 2345–2352 [DOI] [PubMed] [Google Scholar]
- 18.Imagawa A. Discovery and establishment of fulminant type 1 diabetes mellitus and new classification of type 1 diabetes mellitus based on it. J Jpn Diabetes Soc 2004; 47: 796–797 (Japanese). [Google Scholar]
- 19.Kobayashi T, Sato Y, Akazawa S. The position of type 1 in the classification of diabetes mellitus. J Jpn Diabetes Soc 1998; 41: A11–A13 (Japanese). [Google Scholar]
- 20.Kobayashi T. Slowly Progressive IDDM In: Japan Diabetes Society (ed). Advances in Diabetes Study, Volume 30. Shindan to Chiryosha, Tokyo, 1996. (Japanese). [Google Scholar]
- 21.Yasuda K, Miyake K, Horikawa Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 2008; 40: 1092–1097 [DOI] [PubMed] [Google Scholar]
- 22.Unoki H, Takahashi A, Kawaguchi T, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 2008; 40: 1098–1102 [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa T, Owada M, Urakami T, et al. Increased incidence of non‐insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila) 1998; 37: 111–115 [DOI] [PubMed] [Google Scholar]
- 24.Fajans SS. Scope and heterogeneous nature of MODY. Diabetes Care 1990; 13: 49–64 [DOI] [PubMed] [Google Scholar]
- 25.Froguel P, Vaxillaire M, Velho G. Genetic and metabolic heterogeneity of maturity‐onset diabetes of the young. Diabetes Rev 1997; 5: 123–130 [Google Scholar]
- 26.Maassen JA, Kadowaki T. Maternally inherited diabetes and deafness: a new diabetes subtype. Diabetologia 1996; 39: 375–382 [DOI] [PubMed] [Google Scholar]
- 27.Sakagashira S, Sanke T, Hanabusa T, et al. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes 1996; 45: 1279–1281 [DOI] [PubMed] [Google Scholar]
- 28.Kasuga M, Kadowaki T. Insulin receptor disorders in Japan. Diabetes Res Clin Pract 1994; 24(Suppl): S145–S151 [DOI] [PubMed] [Google Scholar]
- 29.Nanjo K, Oka Y, Kadowaki T, et al. Recent aspects on diabetes mellitus associated with gene mutation in Japan. J Jpn Diabetes Soc 1998; 41: A29–A31 (Japanese). [Google Scholar]
- 30.Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet 1999; 23: 323–328 [DOI] [PubMed] [Google Scholar]
- 31.Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP‐sensitive potassium‐channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004; 350: 1838–1849 [DOI] [PubMed] [Google Scholar]
- 32.Babenko AP, Polak M, Cave H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 2006; 355: 456–466 [DOI] [PubMed] [Google Scholar]
- 33.Ito C. Classification of fasting plasma glucose levels and HbA1c. Health screen (Ningen Dock) 2008; 22: 874–877 (Japanese). [Google Scholar]
- 34.Tominaga M. Lifestyle habits as factors in developing diabetes mellitus – from results of Funagata research focused on prospective cohort study in Japan. J Jpn Diabetes Soc 2008; 51: 473–475 (Japanese). [Google Scholar]
- 35.Kosaka K. Various parameters used for the diagnosis of diabetes and for the epidemiological investigation—their characteristics, their mutual relationship and their application. J Jpn Diabetes Soc 1998; 41: A101–A105 (Japanese). [Google Scholar]
- 36.Sasaki A, Shimizu T, Hasegawa K. Study of diagnostic criteria for diabetes melliltus from a viewpoint of clinical epidemiology In: Kosaka K (ed). Diabetology 99. Shindan to Chiryo Sha, Tokyo, 1999; 97–105 (Japanese). [Google Scholar]
- 37.Kosaka K, Hagura R, Kuzuya T, et al. Insulin secretory response of diabetics during the period of improvement of glucose tolerance to normal range. Diabetologia 1974; 10: 775–782 [DOI] [PubMed] [Google Scholar]
- 38.Seino Y, Kurahachi H, Goto Y, et al. Comparative insulinogenic effects of glucose, arginine and glucagon in patients with diabetes mellitus, endocrine disorders and liver disease. Acta Diabetol Lat 1975; 12: 89–99 [DOI] [PubMed] [Google Scholar]
- 39.Seino Y, Kurahachi H, Goto Y, et al. The insulinogenic index in secondary diabetes. Horm Metab Res 1975; 12: 107–115 [Google Scholar]
- 40.Kosaka K, Hagura R, Kuzuya T. Insulin responses in equivocal and definite diabetes, with special reference to subjects who had mild glucose intolerance but later developed definite diabetes. Diabetes 1977; 26: 944–952 [DOI] [PubMed] [Google Scholar]
- 41.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002 [DOI] [PubMed] [Google Scholar]
- 42.IADPSG Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada K, Nonaka K. Diabetic ketoacidosis in young obese Japanese men. Diabetes Care 1996; 19: 671 [DOI] [PubMed] [Google Scholar]
- 44.Ito C, Maeda R, Ishida S, et al. Importance of OGTT for diagnosing diabetes mellitus based on prevalence and incidence of retinopathy. Diabetes Res Clin Pract 2000; 49: 181–186 [DOI] [PubMed] [Google Scholar]
- 45.Ito C, Maeda R, Ishida S, et al. Correlation among fasting plasma glucose, two‐hour plasma glucose levels in OGTT and HbA1c. Diabetes Res Clin Pract 2000; 50: 225–230 [DOI] [PubMed] [Google Scholar]
- 46.Cowie CC, Rust KF, Byrd‐Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988‐2006. Diabetes Care 2010; 33: 562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med 2002; 40: 78–89 [DOI] [PubMed] [Google Scholar]
- 49.Mosca A, Goodall I, Hoshino T, et al. Global standardization of glycated hemoglobin measurement: the position of the IFCC Working Group. Clin Chem Lab Med 2007; 45: 1077–1080 [DOI] [PubMed] [Google Scholar]
- 50.Nordin G, Dybkaer R. Recommendation for term and measurement unit for “HbA1c”. Clin Chem Lab Med 2007; 45: 1081–1082 [DOI] [PubMed] [Google Scholar]
- 51.Kuzuya T, Nakagawa S, Satoh J, et al. Answers by Council members of JDS to a questionnaire on the diagnostic criteria and classification of diabetes mellitus. J Jpn Diabetes Soc 1997; 40: 203–210 (Japanese). [Google Scholar]
- 52.McCance DR, Hanson RL, Charles MA, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 1994; 308: 1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito C. OGTT criteria seen from the relation of fasting and 2‐hPG and complications. J Jpn Diabetes Soc 1998; 41: A33–A36 (Japanese). [Google Scholar]
- 54.Kosaka K. Progress in diabetology. Diagnosis of diabetes mellitus. Critical matters on the new diagnositic criteria in the 1997 ADA report and 1998 WHO report. Shindan to Chiryo 2002; 90: 851–861 (Japanese). [Google Scholar]
- 55.DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group . Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. BMJ 1998; 317: 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]


