Abstract
Aims/Introduction: The therapeutic effectiveness against type 1 diabetes mellitus (DM) of the novel immunomodulator FTY720 (fingolimod), alone and in combination with insulin glargine, was examined in the non‐obese diabetic (NOD) mouse model.
Materials and Methods: Female NOD mice that had developed DM spontaneously were divided into four groups: (i) an FTY720 (0.1 mg/kg, p.o., twice weekly)‐treated group; (ii) an insulin glargine (1.0 IU, s.c., once daily)‐treated group; (iii) a combination FTY720 + insulin glargine (0.1–1.0 IU, s.c., once daily)‐treated group; and (iv) a placebo (vehicle)‐treated group. Treatment was initiated at the time of onset of DM and continued for 70 days or until death. The therapeutic efficacy of FTY720, insulin glargine and FTY720 + insulin glargine was evaluated by measuring the ratio of insulin‐positive β‐cells/total islet area, the extent of islet inflammation (insulitis score), blood glucose levels, and serum C‐peptide levels.
Results: Therapeutic administration of FTY720 to NOD mice with hyperglycemia (i.e. overt DM) significantly prolonged survival (P < 0.05 vs placebo). In the placebo group, all mice died within 63 days on the onset of DM; in contrast, 45% of FTY720‐treated mice survived during the observation period (up to 70 days after the onset of DM). Therapeutic administration of FTY720 in combination with insulin glargine to NOD mice with hyperglycemia further improved survival (P < 0.05) compared with either FTY720 or insulin glargine alone (i.e. 85% of FTY720 + insulin glargine‐treated mice survived to the end of the observation period). The efficacy of FTY720 in combination with insulin glargine was confirmed by histochemical, immunohistochemical and endocrinologic observations.
Conclusions: Combination therapy with FTY720 plus insulin glargine is a promising candidate for the treatment of DM and may allow for a reduction in the frequency of insulin self‐injections. (J Diabetes Invest, doi:10.1111/j.2040‐1124.2011.00160.x, 2011)
Keywords: FTY720, Non‐obese diabetic mouse, Type 1 diabetes mellitus
Introduction
The novel immunomodulator FTY720 (fingolimod) is a synthetic structural analogue of myriocin (ISP‐I), a metabolite of Isaria cinclarii1,2. It was developed by Tetsuro Fujita (F), of our group, in collaboration with Taito Co. (T; Mitsui Sugar, Tokyo, Japan) and Yoshitomi Pharmaceutical Industries Ltd (Y; Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) in Japan. FTY720 has been reported to be effective not only in preclinical transplantation models3, but also in several models of immunological diseases, including rheumatoid arthritis4, myasthenia gravis5, multiple sclerosis6, and atopic dermatitis7. The mechanism of action of FTY720 differs from that of established immunosuppressants, such as tacrolimus hydrate and cyclosporin. Specifically, FTY720 blocks sphingosine 1‐phosphate (S1P) signaling by inducing internalization and intracellular partial degradation of the receptors8–10. As a result, FTY720 suppresses the immune response by sequestering circulating mature lymphocytes from the blood and peripheral tissues to secondary lymphoid tissues and the thymus11,12.
The non‐obese diabetic (NOD) mouse is an excellent animal model of spontaneous type 1 diabetes mellitus (DM) that exhibits many of the characteristics of human DM13. As in human DM, in NOD mice with DM, autoreactive T cells attack islet β‐cells, resulting in depletion of insulin secretion. In general, in humans DM develops in childhood and is treated with intensive insulin therapy consisting of four or more daily insulin injections. Insulin self‐injections impose a considerable burden on patients and it is difficult to achieve effective blood glucose control, especially during the period of development of secondary sexual characteristics. If surviving islet β‐cells can be protected from autoimmune destruction at an early stage of DM, it should at least be possible to reduce the frequency of insulin injections needed as part of intensive insulin therapy.
In the present study, we examined the efficacy of FTY720 alone and in combination with once‐daily injections of insulin glargine. The results indicate that: (i) therapeutic administration of FTY720 improves the survival of NOD mice with overt DM; and (ii) therapeutic administration of FTY720 in combination with insulin glargine further improves the survival of NOD mice.
Materials and Methods
Animals and Ethics
Female NOD mice bred under specific pathogen‐free conditions were purchased from CLEA Japan (Tokyo, Japan). The mice were given γ‐ray‐irradiated food (CRF‐1; Oriental Bio, Kyoto, Japan) and distilled water for injection (Ohtsuka Pharmaceutical, Tokyo, Japan). The protocols of the present study were approved by the institutional animal care committee of Setsunan University. Throughout the experimental procedures, every effort was made to minimize the number of animals used and their suffering. The maintenance of specific pathogen‐free status in the laboratory was routinely confirmed by means of the conventional falling bacteria test.
Drugs
2‐Amino‐2‐[2‐(4‐octylphenyl)ethyl]propane‐1,3‐diol hydrochloride (FTY720; Fingolimod) was kindly provided by Yoshitomi Pharmaceutical Industries. Insulin glargine (LANTUS) was purchased from Sanofi‐Aventis (Paris, France).
Study Protocol
Female NOD mice (aged 13–59 weeks) that had developed DM spontaneously were divided into four groups: (i) the FTY720 group (n = 11), given FTY720 in water (0.1 mg/kg, p.o., twice a week); (ii) the insulin group (n = 13), given insulin glargine (1.0 IU, s.c., once daily); (iii) the combination therapy group (n = 13), given FTY720 plus insulin glargine (to prevent hypoglycemia, blood glucose levels 2 h after insulin injection were controlled to 70–130 mg/dL by adjusting the initial insulin dose (0.1–1.0 IU, s.c., once daily) and the insulin dose thus identified was maintained for the duration of the observation period); and (iv) the placebo group (n = 11), given vehicle (water) alone. Treatment was initiated at the time of DM onset and continued for 70 days or until death. The onset of DM was defined as glucosuria followed by hyperglycemia >250 mg/dL for more than three consecutive days14,15. Urinary glucose was examined weekly using Keto‐Diastix (Bayer Yakuhin, Osaka, Japan) and blood glucose levels were determined using a Glucocard diameter α (Arkray, Kyoto, Japan) after overnight fasting.
Immunolabeling of Pancreatic Sections and Quantification of Islet Cell Mass
At the end of the observation period, mice were anesthetized with sodium pentobarbital and the pancreas excised. Tissues were fixed in 10% buffered formalin solution (Wako Pure Chemical Industries, Osaka, Japan) and embedded in paraffin. Then, sections (5 μm) were cut from each sample at 200 μm intervals. Five pancreatic sections from each mouse were stained for insulin using monoclonal rat anti‐insulin antibody (R&D Systems, Minneapolis, MN, USA) and peroxidase‐conjugated monoclonal mouse anti‐rat IgG2a antibody (Invitrogen, Carlsbad, CA, USA). Samples were visualized using diaminobenzidine16. The ratio of insulin‐positive β‐cells/total islet area17 was determined using ImageJ software (http://rsbweb.nih.gov/ij/, accessed 9 December 2009).
Extent of Insulitis
The extent of insulitis was evaluated in sections stained with hematoxylin–eosin using Mayer’s hematoxylin solution (Wako Pure Chemical Industries) according to standard methods and scored using the following criteria15: Grade 0, no mononuclear cell infiltration; Grade 1, mononuclear cell infiltration around the islet but no intra‐islet infiltration; Grade 2, mononuclear cell infiltration in and around the islet, but intra‐islet infiltration in less than one‐third of the islet area; Grade 3, intra‐islet mononuclear cell infiltration in one‐third to half of the islet area; Grade 4, extensive intra‐islet infiltration occupying more than half of the islet area.
Serum C‐peptide
Blood samples were drawn from the tail vein of overnight‐fasted mice and serum C‐peptide levels were determined using a Mouse C‐Peptide ELISA KIT (U‐type, AKRCP‐03; Shibayagi, Gunma, Japan).
Statistical Analysis
Unless noted otherwise, data are presented as the mean ± SD. The significance of differences in the ratio of insulin‐positive β‐cells/total islet area and insulitis scores was evaluated using the Mann–Whitney U‐test, whereas the significance of differences in the incidence of DM and survival rate was evaluated by using the log rank test. P < 0.05 was considered significant.
Results
Therapeutic Effect of FTY720 on Established DM in NOD Mice
The NOD mice that had developed DM spontaneously were divided into an FTY720‐treated group (n = 11) and a placebo‐treated group (n = 11). The two groups were matched for blood glucose levels at the beginning of treatment (419 ± 98 and 434 ± 126 mg/dL in the FTY720‐ and placebo‐treated groups, respectively). Survival curves in the two groups are shown in Figure 1. In the placebo group, all mice died within 63 days after the start of treatment, whereas five mice (45%) in the FTY720 group survived throughout the observation period (up to 70 days after the beginning of treatment). There was a significant difference in survival rate between the two groups (P < 0.05, log rank test).
Figure 1.
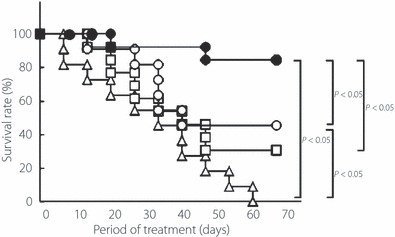
Effect of FTY720 alone or in combination with insulin glargine on survival rates of NOD mice with overt type 1 diabetes mellitus (DM). Female NOD mice with hyperglycemia (i.e. with overt DM) were treated with: 0.1 mg/kg, p.o., FTY720 twice a week (n = 11; ○); 1.0 IU, s.c., insulin glargine once daily, (n = 13; □); 0.1 mg/kg, p.o., FTY720 twice a week plus insulin glargine (for dosing, refer to Materials and Methods; n = 13; •); or vehicle (n = 11; △). Survival was monitored for 70 days after start of treatment. The significance of differences in survival was evaluated using the log rank test.
To examine insulin secretory function, fasting serum C‐peptide levels were determined. Serum samples were obtained from a separate group as described in the Materials and Methods. C‐Peptide levels after 5 weeks treatment were significantly higher in the FTY720 group than in the placebo‐treated group (261 ± 35 vs 100 ± 56 pg/mL, respectively; n = 3 in both groups; Figure 2). In addition, fasting blood glucose levels after 5 weeks treatment were significantly lower in the FTY720 group than in the placebo‐treated group (434 ± 43 vs >600 mg/dL, respectively; n = 3 in both groups; Figure 3). The FTY720 and placebo groups had been matched for blood glucose levels (454 ± 154 vs 461 ± 143 mg/dL, respectively) and age (31 ± 7 vs 32 ± 8 weeks, respectively) at the beginning of treatment. Thus, the results suggest that FTY720 prolongs the survival of animals with overt DM by protecting β‐cells from autoimmune destruction, thereby maintaining insulin secretory function.
Figure 2.
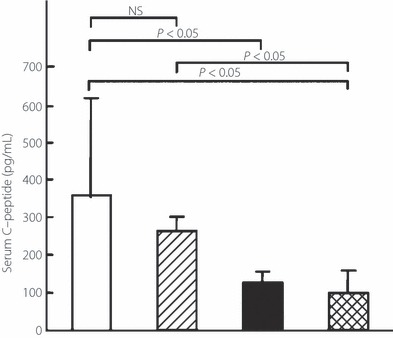
Effect of therapeutic administration of FTY720 alone or in combination with insulin glargine on insulin secretory function (serum C‐peptide levels) in NOD mice with overt type 1 diabetes mellitus (DM). Female NOD mice with hyperglycemia (i.e. overt DM) were treated with: 0.1 mg/kg, p.o., FTY720 twice a week plus insulin glargine (for dosing, refer to Materials and Methods; n = 6; □); 0.1 mg/kg, p.o., FTY720 twice a week (n = 3;  ); 1.0 IU, s.c., insulin glargine once daily, (n = 3; ▮); and vehicle (n = 3;
); 1.0 IU, s.c., insulin glargine once daily, (n = 3; ▮); and vehicle (n = 3;  ). Serum C‐peptide levels after 5 weeks of treatment in the four groups were determined by ELISA. Data are the mean ± SD. The significance of differences in serum C‐peptide levels was determined by the Mann–Whitney U‐test.
). Serum C‐peptide levels after 5 weeks of treatment in the four groups were determined by ELISA. Data are the mean ± SD. The significance of differences in serum C‐peptide levels was determined by the Mann–Whitney U‐test.
Figure 3.
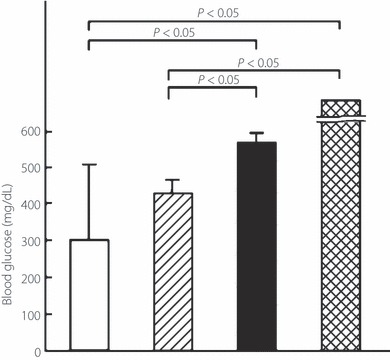
Effect of therapeutic administration of FTY720 alone or in combination with insulin glargine on fasting blood glucose levels in NOD mice with overt type 1 diabetes mellitus (DM). Female NOD mice with hyperglycemia (i.e. overt DM) were treated with: 0.1 mg/kg, p.o., FTY720 twice a week plus insulin glargine (for dosing, refer to Materials and Methods; n = 6; □); 0.1 mg/kg, p.o., FTY720 twice a week (n = 3;  ); 1.0 IU, s.c., insulin glargine once daily, (n = 3; ▮); and vehicle (n = 3;
); 1.0 IU, s.c., insulin glargine once daily, (n = 3; ▮); and vehicle (n = 3;  ). Fasting blood glucose levels after 5 weeks treatment were determined in mice after overnight fasting. Data are the mean ± SD. The significance of differences in blood glucose levels was determined by the Mann–Whitney U‐test.
). Fasting blood glucose levels after 5 weeks treatment were determined in mice after overnight fasting. Data are the mean ± SD. The significance of differences in blood glucose levels was determined by the Mann–Whitney U‐test.
Therapeutic Effect of FTY720 in Combination with Insulin Glargine on Established DM in NOD Mice
Although therapeutic administration of FTY720 prolonged the survival of mice with overt DM, as described above, six of 11 mice still died. Therefore, we next examined the effect of FTY720 in combination with insulin glargine, which is widely used to maintain basal insulin levels, on survival of NOD mice with DM.
The NOD mice with hyperglycemia (i.e. overt DM) were divided into an insulin group and a combination therapy group (n = 13 in each). The two groups were matched for age (25 ± 5 vs 26 ± 9 weeks, respectively) and blood glucose levels (450 ± 65 vs 488 ± 79 mg/dL, respectively) at the beginning of treatment. Survival curves in the two groups are shown in Figure 1. Four mice (31%) in the insulin group and 11 mice (85%) in the combination therapy group survived throughout the observation period. The differences in survival rates between the combination therapy group and the insulin‐, FTY720‐, and placebo‐treated groups were significant (P < 0.05, log rank test).
To confirm the therapeutic efficacy of the combination therapy histochemically and endocrinologically, the ratio of insulin‐positive β‐cells/total islet area, the extent of islet inflammation (insulitis score) at the end of the observation period, and the insulin‐secretory function (i.e. serum C‐peptide levels after 5 weeks treatment) were examined. The ratio of insulin‐positive β‐cells/total islet area in the combination therapy group (n = 3) was similar to that in age‐matched NOD mice with normoglycemia and mice in the FTY720 group (0.60 ± 0.24, 0.69 ± 0.18, and 0.57 ± 0.16, respectively), but was significantly higher than that in the insulin group (0.25 ± 0.18; Figure 4a). The insulitis score in the combination therapy group (n = 3) was similar to that in age‐matched NOD mice with normoglycemia and mice in the FTY720 group (1.5 ± 0.1, 1.4 ± 0.3, and 1.0 ± 1.0, respectively), but was significantly lower than that in the insulin group (2.1 ± 0.4; Figure 4b). Serum C‐peptide levels in the combination therapy group (n = 6) were significantly higher than those in the placebo‐treated group with hyperglycemia and insulin‐treated group (363 ± 279, 100 ± 56, and 123 ± 23 pg/mL, respectively; n = 3 in each; Figure 2). However, fasting blood glucose levels after 5 weeks treatment in the combination therapy group (n = 6) were significantly lower than in the placebo‐ and insulin‐treated groups (305 ± 212, >600, and 597 ± 36 mg/dL, respectively; n = 3 in each; Figure 3).
Figure 4.
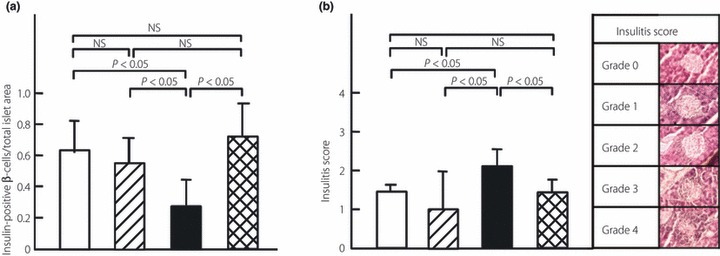
Effect of therapeutic administration of FTY720 alone or in combination with insulin glargine on (a) the ratio of insulin‐positive β‐cells/total islet area and (b) the extent of insulitis in NOD mice with overt type 1 diabetes mellitus (DM). Representative pictures illustrating the different insulitis scores are also shown (b). Female NOD mice with hyperglycemia (i.e. with overt DM) were treated for 70 days with: 0.1 mg/kg, p.o., FTY720 twice a week plus insulin glargine (for dosing, refer to Materials and Methods; n = 4; □); 0.1 mg/kg, p.o., FTY720 twice a week (n = 4;  ); 1.0 IU, s.c., insulin glargine once daily, (n = 4; ▮). Pancreas sections were obtained after the observation period (70 days) from mice in each of the three groups, as well as from age‐matched NOD mice with normoglycemia (n = 4;
); 1.0 IU, s.c., insulin glargine once daily, (n = 4; ▮). Pancreas sections were obtained after the observation period (70 days) from mice in each of the three groups, as well as from age‐matched NOD mice with normoglycemia (n = 4;  ) and stained with monoclonal rat anti‐insulin antibody or with hematoxylin–eosin. Data are the mean ± SD. The significance of differences was determined by the Mann–Whitney U‐test.
) and stained with monoclonal rat anti‐insulin antibody or with hematoxylin–eosin. Data are the mean ± SD. The significance of differences was determined by the Mann–Whitney U‐test.
Discussion
The novel immunomodulator FTY720 (Fingolimod) has a number of attractive properties. The mechanism of its immunosuppressive effect differs from those of established immunosuppressants, as described in the Introduction. At therapeutic doses, FTY720 does not affect T or B cell responses in vitro or in vivo3,7. Because FTY720 treatment allows for the preservation of many aspects of immune function, including the total number of lymphocytes, the capacity for lymphocyte activation in lymph nodes and tissues, the capacity for generating antibodies, and innate immune responses18,19, there is only a limited increase in susceptibility to infectious disease, including herpes virus infections, urinary tract infections etc.20. Furthermore, immune memory function is not impaired3. Recently, FTY720 was approved by the Food and Drug Administration (USA) for the treatment of multiple sclerosis (http://www.gilenya.com, accessed 30 September 2010).
Prophylactic administration of FTY720 completely suppressed the development of DM in NOD mice (data not shown) and therapeutic administration of FTY720 prolonged the survival of NOD mice with overt DM. These results were confirmed histochemically and endocrinologically in terms of the ratio of insulin‐positive β‐cells/total islet area and serum C‐peptide levels, and are in agreement with previous findings21,22. Our data clearly show that FTY720 protects β‐cells against autoimmune destruction and maintains insulin secretory function. However, because the therapeutic effectiveness of FTY720 alone in animals with overt DM was limited, it was necessary to develop a more effective regimen for the treatment of DM. Itoh and Maki23 reported that surgical removal of 90% of pancreatic tissue before the onset of insulitis induces a long‐term diabetes‐free condition in NOD mice and that pancreatectomy after the development of moderate insulitis has no effect on the course of DM. Together with our findings, these results led us to examine combination therapy with FTY720 plus a once‐daily injection of a long‐acting insulin formulation (insulin glargine). The combination therapy significantly improved survival, with 85% of NOD mice with overt DM surviving to the end of the observation period. The ratio of insulin‐positive β‐cells/total islet area and the insulitis score in the combination therapy group were almost equal to those in age‐matched normoglycemic NOD mice, whereas the ratio in the combination therapy group was higher and the insulitis score was lower than in the insulin group. Serum C‐peptide levels in the combination therapy group were significantly higher than in the placebo and insulin groups and, accordingly, blood glucose levels in the combination therapy group were lower than those in the placebo and insulin groups. These results indicate that β‐cells are protected by FTY720 against autoimmune destruction, and that the remaining insulin secretory function is able to control blood glucose levels. The principles underlying the combination therapy are as follows: (i) insulin glargine is expected to compensate for the decreased basal insulin secretion; and (ii) surviving β‐cells, which have been protected by FTY720, are expected to exhibit glucose‐stimulated insulin secretion. These two components correspond to the basal and bolus insulin injections, respectively, in intensive insulin therapy. The next step will be to develop criteria to identify those individuals for whom the combination therapy would be appropriate.
In conclusion, the results of the present study suggest that the combination of FTY720 plus insulin glargine is a promising candidate for the treatment of DM at an early phase, when there is still residual β‐cell function. This approach may allow for a reduction in the frequency of insulin self‐injections as part of the usual intensive insulin treatment regimen. Accordingly, the problems associated with intensive insulin therapy (i.e. the burden on patients and the difficulty of achieving good blood glucose control) may be ameliorated.
Acknowledgement
This work was supported in part by a Grant‐in‐Aid for Young Scientists (Start‐up) (19890249) from the Japan Society for the Promotion of Science. The authors declare no conflicts of interest.
References
- 1.Fujita T, Inoue K, Yamamoto S, et al. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot 1994; 47: 208–215 [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi M, Adachi K, Kohara T, et al. Synthesis and immunosuppressive activity of 2‐substituted 2‐aminopropane‐1,3‐diols and 2‐aminoethanols. J Med Chem 2000; 43: 2949–2961 [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Pinschewer D, Chiba K, et al. FTY720: a novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol Sci 2000; 1: 49–52 [DOI] [PubMed] [Google Scholar]
- 4.Tsunemi S, Iwasaki T, Kitano S, et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin Immunol 2010; 136: 197–204 [DOI] [PubMed] [Google Scholar]
- 5.Kohno T, Tsuji T, Hirayama K, et al. A novel immunomodulator, FTY720, prevents development of experimental autoimmune myasthenia gravis in C57BL/6 mice. Biol Pharm Bull 2005; 28: 736–739 [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, Tsuji T, Fujita T, et al. Relapse of experimental autoimmune encephalomyelitis after discontinuation of FTY720 (fingolimod) treatment, but not after combination of FTY720 and pathogenic autoantigen. Biol Pharm Bull 2011; 34: 933–936 [DOI] [PubMed] [Google Scholar]
- 7.Kohno T, Tsuji T, Hirayama K, et al. A novel immunomodulator, FTY720, prevents spontaneous dermatitis in NC/Nga mice. Biol Pharm Bull 2004; 27: 1392–1396 [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1‐phosphate receptors. J Biol Chem 2002; 277: 21453–21457 [DOI] [PubMed] [Google Scholar]
- 9.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004; 427: 355–360 [DOI] [PubMed] [Google Scholar]
- 10.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down‐regulates sphingosine 1‐phosphate G‐protein‐coupled receptors. FASEB J 2004; 18: 551–553 [DOI] [PubMed] [Google Scholar]
- 11.Chiba K, Yanagawa Y, Masubuchi Y, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol 1998; 160: 5037–5044 [PubMed] [Google Scholar]
- 12.Yanagawa Y, Sugahara K, Kataoka H, et al. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J Immunol 1998; 160: 5493–5499 [PubMed] [Google Scholar]
- 13.Makino S, Kunimoto K, Muraoka Y, et al. Breeding of a non‐obese, diabetic strain of mice. Jikken Dobutsu 1980; 29: 1–13 (Japanese). [DOI] [PubMed] [Google Scholar]
- 14.Kaino Y, Ito T, Goto Y, et al. Lack of recurrence of insulin‐dependent diabetes mellitus in syngeneic and allogeneic islet‐transplanted diabetic biobreeding rats. Transplantation 1998; 65: 1543–1548 [DOI] [PubMed] [Google Scholar]
- 15.Kaino Y, Hirai H, Ito T, et al. Prevention of diabetes in non‐obese diabetic (NOD) mice by short‐term and high‐dose IGF‐I treatment. J Pediatr Endocrinol Metab 1998; 11: 267–272 [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Tokui Y, Yamagata K, et al. Continuous stimulation of human glucagon‐like peptide‐1 (7–36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia 2007; 50: 1900–1909 [DOI] [PubMed] [Google Scholar]
- 17.Mu J, Petrov A, Eiermann GJ, et al. Inhibition of DPP‐4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol 2009; 623: 148–154 [DOI] [PubMed] [Google Scholar]
- 18.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415 [DOI] [PubMed] [Google Scholar]
- 19.Mehling M, Brinkmann V, Antel J, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008; 71: 1261–1267 [DOI] [PubMed] [Google Scholar]
- 20.Kappos L, Radue EW, O’Connor P, et al. A placebo‐controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401 [DOI] [PubMed] [Google Scholar]
- 21.Maki T, Gottschalk R, Monaco AP. Prevention of autoimmune diabetes by FTY720 in nonobese diabetic mice. Transplantation 2002; 74: 1684–1686 [DOI] [PubMed] [Google Scholar]
- 22.Maki T, Gottschalk R, Ogawa N, et al. Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation 2005; 79: 1051–1055 [DOI] [PubMed] [Google Scholar]
- 23.Itoh A, Maki T. Protection of nonobese diabetic mice from autoimmune diabetes by reduction of islet mass before insulitis. Proc Natl Acad Sci USA 1996; 93: 11053–11056 [DOI] [PMC free article] [PubMed] [Google Scholar]


