Abstract
Aims/Introduction: Several experimental studies have shown that ezetimibe improves steatosis and insulin resistance in the liver. This suggests that ezetimibe may improve glucose metabolism, as well as lipid metabolism, by inhibiting hepatic lipid accumulation. Therefore, we compared HbA1c levels after 3 months ezetimibe treatment with baseline levels in patients with type 2 diabetes and examined the factors associated with reductions in HbA1c following ezetimibe administration.
Materials and Methods: Lipid profiles, hepatic function, and HbA1c were assessed before and after 3 months treatment with 10 mg/day ezetimibe in 96 patients with type 2 diabetes and hypercholesterolemia. Regression analysis was used to investigate associations between metabolite levels and the percentage change in HbA1c.
Results: Low‐density lipoprotein–cholesterol was significantly lower after 3 months treatment compared with baseline, and HbA1c decreased in approximately 50% of patients. Univariate linear regression analyses showed that changes in HbA1c were significantly associated with serum alanine aminotransferase (ALT), the aspartate aminotransferase (AST)/ALT ratio, and age. Two‐tailed chi‐square tests revealed that serum ALT ≥35 IU/L and an AST/ALT ratio <1.0 were significantly associated with decreases in HbA1c following ezetimibe administration.
Conclusions: The results of the present study indicate that ezetimibe may improve glucose metabolism. Serum ALT levels and the AST/ALT ratio were useful predictors of a glucose metabolism response to ezetimibe. This trial was registered with UMIN (no. UMIN000005307). (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2011.00147.x, 2011)
Keywords: Ezetimibe, Hepatic insulin sensitivity, Liver steatosis
Introduction
The 9‐year interim report of the Japan Diabetes Complications Study, which investigated risk factors for complications in Japanese individuals with type 2 diabetes, revealed that high levels of low‐density lipoprotein cholesterol (LDL‐C) were the most significant risk factor for coronary heart disease1. That study showed that strict blood glucose control in addition to aggressive lipid control was important in the care of Japanese patients with type 2 diabetes.
The recent introduction of ezetimibe into clinical use, together with a growing body of evidence relating cholesterol absorption to the risk of cardiovascular events2, has raised awareness of the importance of controlling cholesterol absorption for the prevention of cardiovascular events. Some investigators have reported an increase in intestinal cholesterol absorption in individuals with type 2 diabetes3. Therefore, it is possible that ezetimibe, which, as an inhibitor of cholesterol transporters in the small intestine, selectively inhibits cholesterol absorption, may be effective for lipid control in type 2 diabetes. In terms of the effects of ezetimibe on steatosis and insulin sensitivity in the liver, recent studies have shown that ezetimibe has the potential to improve not only lipid metabolism, but also glucose metabolism by inhibiting the accumulation of lipids in the liver4–7. However, no studies have investigated the clinical effects of ezetimibe on the relationship between glucose metabolism and liver function in individuals with type 2 diabetes.
Therefore, in the present study, we compared HbA1c levels after 3 months ezetimibe treatment with baseline levels in patients with type 2 diabetes and examined the factors associated with reductions in HbA1c following ezetimibe administration. Consequently, we found that ezetimibe improved HbA1c in patients with type 2 diabetes and liver dysfunction, and that serum alanine aminotransferase (ALT) levels and the aspartate aminotransferase (AST)/ALT ratio at baseline are useful predictors for improvements in HbA1c.
Materials and Methods
Subjects and Study Design
The study included 96 individuals with type 2 diabetes and hypercholesterolemia (57 men and 39 women) who visited our medical institutions as outpatients. Patients were excluded from the study if: they were using a new antidiabetic agent or a new antihypertensive agent, or had changed doses and/or the types of agents used, between 2 months prior to the study and the end of the study; their current or past alcohol intake was >20 g/day; or they had serological evidence of viral hepatitis, hemochromatosis, autoimmune liver disease, or non‐alcoholic steatohepatitis (NASH). The diagnosis of NASH was based on the following criteria8,9: (i) an intake of <20 g/day ethanol; (ii) biopsy‐proven steatohepatitis, steatosis, inflammatory infiltrates, and ballooning degeneration with or without Mallory bodies or pericellular/perivenular fibrosis; and (iii) appropriate exclusion of other liver diseases. Patients using statins or fibrates at a dose that did not change in the 3 months prior to the study were eligible for inclusion. The subjects’ lifestyle, including diet, excise and habits, did not change during the study. All subjects were informed of the objectives of the research and provided consent prior to participating in the study. The study was approved by the Ethics Committee of Kumamoto University (approval no. 413).
Following screening, subjects were divided into three groups and treated with ezetimibe alone (monotherapy group; n = 53), ezetimibe plus a statin (statin group; n = 11), or ezetimibe plus a fibrate (fibrate group; n = 32). Ezetimibe was administered at a dose of 10 mg/day in all patients. Baseline values of LDL‐C, high‐density lipoprotein–cholesterol (HDL‐C), the LDL/HDL ratio, triglycerides (TG), serum AST, serum ALT, serum γ‐glutamyltransferase (GGT), the AST/ALT ratio, and HbA1c were compared with values after 3 months treatment. Serum lipids and liver function were determined using enzymatic methods; LDL‐C was measured directly using a homogeneous assay (Deteriner LDL‐C; Kyowa Medex, Tokyo, Japan). HbA1c was measured by high‐performance liquid chromatography. In the present study, according to the guidelines of the Japan Diabetes Society (JDS)10, the value for HbA1c (%) was estimated as an NGSP equivalent value (%) calculated using the formula HbA1c (%) = HbA1c (JDS) (%) + 0.4%, where HbA1c (NGSP) (%) = 1.019 × HbA1c (JDS) (%) + 0.30. The coefficient of variation for measurements of HbA1c was 2–3%10.
To identify predictors of reductions in HbA1c following ezetimibe administration, subjects were divided into groups according to whether HbA1c decreased during the 3‐month study and the clinical parameters at baseline were compared between groups. Pearson’s correlation coefficient analysis was used to determine univariate correlations between improvements in HbA1c and individual baseline characteristics.
Statistical Analysis
Data are expressed as the mean ± SD. Changes in clinical parameters following ezetimibe treatment were evaluated using paired t‐tests. Differences in baseline clinical parameters between subjects with and without a decrease in HbA1c were evaluated using unpaired t‐tests. Pearson’s product–moment correlation coefficient analysis was used to determine the statistical significance of the regression line. Values of P < 0.05 were considered significant. Two‐tailed chi‐square tests were used to identify factors associated with the glucose metabolism‐improving effects of ezetimibe and odds ratios (OR) were calculated by cross‐tabulation. Data analyses were performed using SPSS software version 11.5 for Windows (SPSS, Chicago, IL, USA).
Results
The baseline characteristics for all subjects are given in Table 1. Sixty‐two were on oral antidiabetic agents, whereas 12 were receiving insulin therapy. In the present study, 11 subjects were treated with a statin and 32 were treated with a fibrate.
Table 1. Baseline characteristics of the subjects.
| n (men/women) | 96 (57/39) |
| Age (years) | 61.5 ± 11.4 |
| BMI (kg/m2) | 25.5 ± 4.2 |
| LDL‐C (mg/dL) | 164 ± 36 |
| HDL‐C (mg/dL) | 55 ± 14 |
| LDL/HDL ratio | 3.2 ± 1.1 |
| TG (mg/dL) | 198 ± 117 |
| HbA1c (%) | 6.7 ± 1.0 |
| AST (IU/L) | 25 ± 13 |
| ALT (IU/L) | 27 ± 19 |
| AST/ALT ratio | 1.1 ± 0.4 |
| GGT (IU/L) | 50 ± 53 |
| Antidiabetic therapy (n) | |
| Diet only | 28 |
| Sulfonylurea | 39 |
| α‐Glucosidase inhibitor | 19 |
| Biguanide | 18 |
| Thiazolidine | 18 |
| Glinide | 8 |
| Insulin | 12 |
| Antihyperlipidemic therapy (n) | |
| Pravastatin | 2 |
| Rosuvastatin | 5 |
| Atorvastatin | 3 |
| Pitavastatin | 1 |
| Bezafibrate | 31 |
| Fenofibrate | 1 |
Data are the mean ± SD or the number of subjects in each group, as indicated. BMI, body massindex; LDL‐C, low‐density lipoprotein–cholesterol; HDL‐C, high‐densitylipoprotein–cholesterol; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ‐glutamyltransferase.
The clinical parameters of the subjects in the monotherapy, statin, and fibrate groups before and after treatment are given in Table 2. Although HDL‐C did not change significantly, both LDL‐C and the LDL/HDL ratio decreased significantly in each group after 3 months treatment with ezetimibe. Specifically, LDL‐C decreased by 17.8% in the monotherapy group, by 33.3% in the statin group, and by 20.3% in the fibrate group following ezetimibe treatment. There was no significant change in TG in the monotherapy group, but TG levels did decrease significantly in the statin and fibrate groups. Serum AST, ALT, and GGT levels did not increase significantly in any of the groups, and a significant decrease in serum ALT levels was seen in the fibrate group. Although HbA1c decreased in 49% of the entire cohort (n = 47), the decrease was only significant in the fibrate group.
Table 2. Effects of 3 months ezetimibe therapy on clinical parameters.
| Ezetimibe monotherapy (n = 53) | Ezetimibe | Total (n = 96) | ||||||
|---|---|---|---|---|---|---|---|---|
| + Statin (n = 11) | + Fibrate (n = 32) | |||||||
| Baseline | After 3 months | Baseline | After 3 months | Baseline | After 3 months | Baseline | After 3 months | |
| Age (years) | 61.8 ± 11.7 | – | 63.0 ± 12.5 | – | 60.6 ± 10.8 | – | 61.5 ± 11.4 | – |
| BMI (kg/m2) | 24.9 ± 4.4 | 24.8 ± 4.3 | 26.7 ± 3.3 | 26.9 ± 3.2 | 26.1 ± 4.2 | 26.4 ± 4.3 | 25.5 ± 4.2 | 25.5 ± 4.2 |
| LDL‐C (mg/dL) | 157 ± 38 | 128 ± 31** | 165 ± 43 | 111 ± 40** | 173 ± 27 | 139 ± 32** | 164 ± 36 | 129 ± 33** |
| HDL‐C (mg/dL) | 59 ± 15 | 57 ± 15 | 52 ± 11 | 51 ± 13 | 50 ± 10 | 53 ± 11 | 55 ± 14 | 55 ± 14 |
| LDL/HDL ratio | 2.9 ± 1.0 | 2.4 ± 0.8** | 3.3 ± 1.2 | 2.3 ± 1.0** | 3.6 ± 1.1 | 2.9 ± 0.9** | 3.2 ± 1.1 | 2.5 ± 0.9** |
| TG (mg/dL) | 148 ± 74 | 140 ± 68 | 213 ± 93 | 143 ± 101* | 276 ± 140 | 172 ± 97** | 198 ± 117 | 152 ± 84** |
| HbA1c (%) | 6.8 ± 0.8 | 6.7 ± 0.9 | 7.2 ± 1.3 | 7.2 ± 1.4 | 6.5 ± 1.1 | 6.4 ± 0.9** | 6.7 ± 1.0 | 6.7 ± 1.0 |
| AST (IU/L) | 23 ± 7.0 | 24 ± 9 | 20 ± 5 | 20 ± 8 | 31 ± 20 | 31 ± 15 | 25 ± 13 | 26 ± 12 |
| ALT (IU/L) | 24 ± 18 | 26 ± 22 | 23 ± 10 | 23 ± 12 | 33 ± 21 | 28 ± 16* | 27 ± 19 | 26 ± 19 |
| AST/ALT ratio | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.99 ± 0.34 | 0.94 ± 0.26 | 1.1 ± 0.60 | 1.2 ± 0.5 | 1.1 ± 0.4 | 1.1 ± 0.4 |
| GGT (IU/L) | 37 ± 37 | 40 ± 44 | 67 ± 90 | 62 ± 96 | 64 ± 56 | 56 ± 55 | 50 ± 53 | 49 ± 56 |
Data are the mean ± SD. *P < 0.05, **P < 0.01 compared with baseline (paired t‐test). BMI, body mass index; LDL‐C, low‐density lipoprotein–cholesterol; HDL‐C, high‐density lipoprotein–cholesterol; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ‐glutamyltransferase.
Therefore, to investigate the characteristics of individuals in whom HbA1c decreased after ezetimibe treatment, the subjects in each group were divided into two subgroups: either responders (i.e. HbA1c decreased after 3 months treatment) or non‐responders (i.e. HbA1c did not decrease after 3 months treatment). We then compared the baseline characteristics between these subgroups. In those patients who responded to ezetimibe treatment, HbA1c decreased from 6.9 ± 0.8 to 6.5 ± 0.7% (P < 0.001) in the monotherapy group, from 7.1 ± 1.3 to 6.6 ± 0.6% (P = 0.27) in the statin group, and from 6.8 ± 1.1 to 6.5 ± 1.0% (P < 0.001) in the fibrate group. Comparisons of baseline parameters between the responders and non‐responders within each treatment group are given in Table 3. In the monotherapy group, age and the AST/ALT ratio were significantly lower <¼ while serum AST and ALT levels were significantly higher, in HbA1c responders than in non‐responders. In the statin group, HDL‐C was significantly lower in HbA1c responders than in non‐responders. In the fibrate group, age and the AST/ALT ratio were significantly lower, whereas HbA1c, ALT, and GGT were significantly higher, in the HbA1c responders than non‐responders.
Table 3. Comparisons of baseline clinical parameters between subjects with (+) or without (−) a decrease in HbA1c following 3 months treatment with ezetimibe.
| Ezetimibe monotherapy (n = 53) | Ezetimibe | Total (n = 96) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + Statin (n = 11) | + Fibrate (n = 32) | |||||||||||
| HbA1c | HbA1c | P‐value | HbA1c | HbA1c | P‐value | HbA1c | HbA1c | P‐value | HbA1c | HbA1c | P‐value | |
| decrease (+) | decrease (−) | decrease (+) | decrease (−) | decrease (+) | decrease (−) | decrease (+) | decrease (−) | |||||
| (n = 23) | (n = 30) | (n = 5) | (n = 6) | (n = 19) | (n = 13) | (n = 47) | (n = 49) | |||||
| Age (years) | 57.4 ± 12.1 | 65.2 ± 10.3 | 0.009 | 59.4 ± 12.5 | 66.0 ± 12.8 | 0.21 | 56.1 ± 10.0 | 67.2 ± 8.4 | 0.001 | 57.1 ± 11.1 | 65.8 ± 10.0 | < 0.001 |
| BMI (kg/m2) | 25.1 ± 5.0 | 24.8 ± 4.0 | 0.39 | 28.1 ± 1.2 | 25.5 ± 4.1 | 0.10 | 26.3 ± 4.6 | 26.0 ± 3.8 | 0.41 | 25.9 ± 4.6 | 25.2 ± 3.9 | 0.20 |
| LDL‐C (mg/dL) | 162 ± 26 | 157 ± 45 | 0.22 | 158 ± 48 | 170 ± 41 | 0.32 | 170 ± 27 | 179 ± 26 | 0.19 | 165 ± 29 | 162 ± 41 | 0.37 |
| HDL‐C (mg/dL) | 58 ± 13 | 59 ± 17 | 0.40 | 44 ± 6 | 58 ± 10 | 0.01 | 51 ± 10 | 50 ± 9 | 0.37 | 54 ± 12 | 56 ± 15 | 0.17 |
| LDL/HDL ratio | 2.9 ± 0.8 | 2.8 ± 1.2 | 0.38 | 3.7 ± 1.3 | 3.0 ± 1.1 | 0.19 | 3.6 ± 1.2 | 3.8 ± 1.1 | 0.32 | 3.2 ± 1.0 | 3.1 ± 1.2 | 0.28 |
| TG (mg/dL) | 166 ± 77 | 135 ± 70 | 0.07 | 232 ± 100 | 198 ± 93 | 0.28 | 253 ± 129 | 309 ± 154 | 0.14 | 208 ± 110 | 189 ± 125 | 0.21 |
| HbA1c (%) | 6.9 ± 0.8 | 6.6 ± 0.9 | 0.17 | 7.1 ± 1.3 | 7.2 ± 1.3 | 0.44 | 6.8 ± 1.1 | 6.0 ± 0.8 | 0.01 | 6.9 ± 1.0 | 6.6 ± 1.0 | 0.049 |
| AST (IU/L) | 25 ± 8 | 21 ± 5 | 0.02 | 19 ± 5 | 20 ± 6 | 0.39 | 29 ± 10 | 34 ± 29 | 0.25 | 26 ± 9 | 24 ± 16 | 0.28 |
| ALT (IU/L) | 32 ± 25 | 19 ± 7 | 0.004 | 24 ± 12 | 22 ± 10 | 0.42 | 39 ± 20 | 25 ± 21 | 0.03 | 34 ± 22 | 21 ± 13 | < 0.001 |
| AST/ALT ratio | 1.0 ± 0.4 | 1.2 ± 0.3 | 0.003 | 1.0 ± 0.4 | 1.0 ± 0.3 | 0.44 | 0.8 ± 0.2 | 1.6 ± 0.7 | < 0.001 | 0.9 ± 0.3 | 1.3 ± 0.5 | < 0.001 |
| GGT (IU/L) | 46 ± 53 | 30 ± 15 | 0.07 | 48 ± 30 | 86 ± 128 | 0.27 | 79 ± 64 | 44 ± 33 | 0.04 | 60 ± 57 | 40 ± 47 | 0.04 |
Data are the mean ± SD. BMI, body mass index; LDL‐C, low‐density lipoprotein–cholesterol; HDL‐C, high‐density lipoprotein–cholesterol; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ‐glutamyltransferase.
Simple linear regression analyses including all individuals showed that baseline ALT was significantly correlated with the percentage change in HbA1c, calculated as (post‐treatment value – baseline value) × 100/baseline value (r = –0.329; P = 0.001; Figure 1a), as was the AST/ALT ratio (r = 0.306, P = 0.003; Figure 1b). A significant correlation was also noted between the percentage change in serum ALT levels and percentage change in HbA1c (r = 0.307; P = 0.002; Figure 1c), as well as between the percentage change in the AST/ALT ratio and the percentage change in HbA1c (r = –0.221; P = 0.03; Figure 1d). Table 4 lists the correlations between other parameters and the percentage change in HbA1c, as determined by linear regression analysis. The percentage change in HbA1c was significantly correlated with age.
Figure 1.
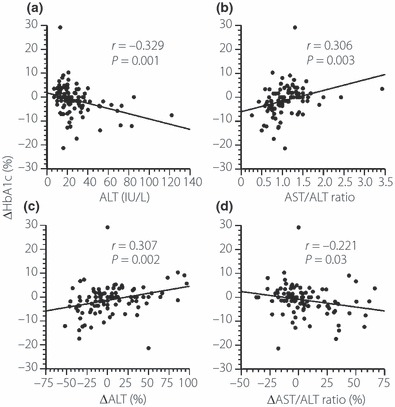
Correlations between the percentage change in HbA1c and (a) serum alanine aminotransferase (ALT) at baseline, (b) the aspartate aminotransferase (AST)/ALT ratio at baseline, (c) percentage changes in serum ALT and (d) percentage changes in the AST/ALT ratio.
Table 4. Correlations between baseline clinical characteristics and percentage changes in HbA1c.
| r | P‐value | |
|---|---|---|
| Age | 0.2877 | 0.005 |
| BMI | −0.1422 | 0.18 |
| LDL‐C | 0.0448 | 0.66 |
| ΔLDL‐C (%) | 0.0448 | 0.66 |
| HDL‐C | 0.1211 | 0.24 |
| LDL/HDL ratio | −0.0171 | 0.87 |
| TG | −0.0373 | 0.72 |
| HbA1c | −0.1968 | 0.06 |
| AST | −0.1225 | 0.23 |
| GGT | −0.1991 | 0.06 |
Correlations were determined using Pearson’s product–moment correlation analysis. BMI, body mass index; LDL‐C, low‐density lipoprotein–cholesterol; HDL‐C, high‐density lipoprotein–cholesterol; TG, triglycerides; AST, aspartate aminotransferase; GGT, γ‐glutamyltransferase.
When the subjects were divided into groups on the basis of serum ALT levels (i.e. ≥35 and <35 IU/L, a criterion proposed for the screening of fatty liver disease in Japanese subjects11), HbA1c decreased in 75.0% of individuals with serum ALT levels ≥35 IU/L (Figure 2a). Similarly, when the subjects were divided on the basis of the AST/ALT ratio (i.e. ≥1 and <1)12–15, HbA1c decreased in 75.0% of those with an AST/ALT ratio <1 (Figure 2b).
Figure 2.
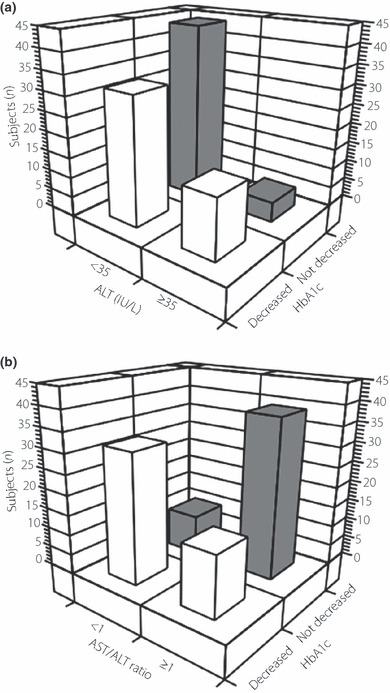
Distribution of subjects stratified according changes in HbA1c and (a) serum alanine aminotransferase (ALT) at baseline or (b) the aspartate aminotransferase (AST)/ALT ratio at baseline.
To identify factors associated with reductions in HbA1c following ezetimibe treatment, we applied two‐tailed chi‐square tests to each parameter and calculated OR by cross‐tabulation. Serum ALT ≥35 IU/L (OR 4.125; 95% confidence interval [CI] 1.36–12.51; P = 0.02; sensitivity 0.319; specificity 0.898) and an AST/ALT ratio <1 (OR 8.320; 95% CI 3.29–21.02; P < 0.001; sensitivity 0.681, specificity 0.796) were significantly associated reductions in HbA1c following 3 months ezetimibe treatment.
Discussion
In the present study, ezetimibe treatment improved HbA1c in patients with type 2 diabetes and liver dysfunction, in addition to improving lipid profiles. These findings suggest that improvements in lipid profiles could reduce insulin resistance, and possibly other risk factors, for diabetes. These findings are supported by several other studies16–18.
On the basis of our results, markers of liver function and age differed significantly between HbA1c responders and non‐responders in the monotherapy and fibrate groups, but not in the statin group (which was comprised of a smaller number of subjects than the other two groups). We also found that decreases in HbA1c were significantly correlated with serum ALT, the AST/ALT ratio, and age in the entire cohort. Subjects with ALT levels ≥35 IU/L and those with an AST/ALT ratio <1 experienced significant reductions in HbA1c from 6.5 ± 1.0 to 6.3 ± 0.8% (P = 0.001) and from 6.9 ± 1.0 to 6.6 ± 1.0% (P < 0.001), respectively. These findings suggest that improvements in hepatic insulin sensitivity as a result of suppressed hepatic fat accumulation may underlie the improvements in HbA1c conferred by ezetimibe. This improvement may occur more readily in subjects with hepatic insulin resistance induced by non‐alcoholic fatty liver disease. According to a previous study11, when subjects were analyzed according to age, the prevalence of fatty liver among 23,148 Japanese men was almost unchanged in those aged between 30 and 60 years. Conversely, in 12,371 Japanese women, the prevalence of fatty liver increased with age. However, some authors have reported that fatty liver is more common in younger than older individuals19,20. Thus, the association between age and the prevalence of fatty liver is contentious. Moreover, no studies have investigated the correlation between age and the prevalence of fatty liver in subjects with type 2 diabetes. It is possible that the prevalence of fatty liver in younger individuals was higher than in older individuals in the present study. Although it was difficult to obtain reliable data regarding the duration of diabetes in these patients, it may be possible that the duration of diabetes and diet therapy was shorter in younger individuals than in older individuals. Future trials should investigate relationships between the prevalence of liver disease, the age of diabetic patients, and the duration of diabetes and diet therapy. Although it has been reported that the incidence of steatosis generally increases with increases in body mass index (BMI)11, the present study found no correlation between BMI and decreases in HbA1c. This may have been because only 12.5% of the subjects in the present study had a BMI ≥30 kg/m2, a level above which 80% of Japanese individuals have liver steatosis11.
In the present study, HbA1c decreased significantly after 3 months treatment in the fibrate group, but not in the other two groups, and 31 of the 32 subjects in the fibrate group were being treated with bezafibrate. Because bezafibrate is a non‐specific agonist of peroxisome proliferator‐activated receptor (PPAR) α and actually activates all three PPAR subtypes (i.e. α, γ, and δ), bezafibrate may directly improve insulin sensitivity21 and alleviate liver steatosis22. Therefore, combination therapy with ezetimibe and bezafibrate may confer greater improvements in glucose metabolism.
Certain limitations of the present study warrant consideration. First, because we did not measure markers of insulin sensitivity directly (e.g. using glucose clamp or the homeostasis model assessment of insulin resistance [HOMA‐IR]) or free fatty acids (FFAs) in the present study, we cannot provide direct evidence that ezetimibe improves insulin sensitivity or FFA profiles. However, it is not possible to routinely perform the glucose clamp in clinical practice. In addition, HOMA‐IR may not be a reliable method for determining insulin resistance in diabetic patients treated with antidiabetic agents, particularly in those treated with insulin. Second, we did not perform ultrasound echography, computed tomography, or liver biopsy to evaluate the progression of liver steatosis. However, because 43.8% of subjects (n = 42) in the present study had an AST/ALT ratio <1, it is likely that most had liver steatosis.
In conclusion, some of the subjects in the present study experienced improvements in HbA1c following 3 months treatment with ezetimibe. Serum ALT levels and the AST/ALT ratio were both predictive factors of the glucose metabolism response to ezetimibe, which appeared to reflect improvements in hepatic insulin sensitivity conferred by ezetimibe. Therefore, ezetimibe may not only improve lipid metabolism, but also liver steatosis and glucose metabolism in type 2 diabetic patients with steatosis, as indicated by serum ALT levels ≥35 IU/L or an AST/ALT ratio <1.
Acknowledgement
The authors thank to Dr Fumihiko Sakamoto (Tenjin Clinic, Kumamoto, Japan) for his assistance with patient referral to the present study. The authors declare no potential conflicts of interest relevant to this article.
References
- 1.Sone H, Tanaka S, Iimuro S, et al. Long‐term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010; 53: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strandberg TE, Tilvis RS, Pitkala KH, et al. Cholesterol and glucose metabolism and recurrent cardiovascular events among the elderly: a prospective study. J Am Coll Cardiol 2006; 48: 708–714 [DOI] [PubMed] [Google Scholar]
- 3.Lally S, Tan CY, Owens D, et al. mRNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann Pick C1‐like 1, ABCG5 and G8 and microsomal triglyceride transfer protein. Diabetologia 2006; 49: 1008–1016 [DOI] [PubMed] [Google Scholar]
- 4.Davies JP, Scott C, Oishi K, et al. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet‐induced hypercholesterolemia. J Biol Chem 2005; 280: 12710–12720 [DOI] [PubMed] [Google Scholar]
- 5.Deushi M, Nomura M, Kawakami A, et al. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett 2007; 581: 5664–5670 [DOI] [PubMed] [Google Scholar]
- 6.Muraoka T, Aoki K, Iwasaki T, et al. Ezetimibe decreases SREBP‐1c expression in liver and reverses hepatic insulin resistance in mice fed a high‐fat diet. Metabolism 2010; 60: 617–628 [DOI] [PubMed] [Google Scholar]
- 7.Nozaki Y, Fujita K, Yoneda M, et al. Long‐term combination therapy of ezetimibe and acarbose for non‐alcoholic fatty liver disease. J Hepatol 2009; 51: 548–556 [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander‐Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003; 37: 1202–1219 [DOI] [PubMed] [Google Scholar]
- 9.American Gastroenterological Association . American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology 2002; 123: 1702–1704 [DOI] [PubMed] [Google Scholar]
- 10.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Diabetol Int 2010; 1: 2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima S, Watanabe N, Numata M, et al. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol 2003; 38: 954–961 [DOI] [PubMed] [Google Scholar]
- 12.Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis. Med Clin North Am 1996; 80: 1147–1166 [DOI] [PubMed] [Google Scholar]
- 13.Pinto HC, Baptista A, Camilo ME, et al. Nonalcoholic steatohepatitis. Clinicopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci 1996; 41: 172–179 [DOI] [PubMed] [Google Scholar]
- 14.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol 1999; 94: 1018–1022 [DOI] [PubMed] [Google Scholar]
- 15.Wallerstedt S, Olsson R, Waldenström J. The diagnostic significance of a high ASAT–ALAT (GOT–GPT) ratio in patients with very high serum aminotransferase levels. Acta Med Scand 1974; 195: 227–229 [PubMed] [Google Scholar]
- 16.Bajaj M, Suraamornkul S, Kashyap S, et al. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab 2004; 89: 4649–4655 [DOI] [PubMed] [Google Scholar]
- 17.Santomauro AT, Boden G, Silva ME, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999; 48: 1836–1841 [DOI] [PubMed] [Google Scholar]
- 18.Güçlü F, Ozmen B, Hekimsoy Z, et al. Effects of a statin group drug, pravastatin, on the insulin resistance in patients with metabolic syndrome. Biomed Pharmacother 2004; 58: 614–618 [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Fukatsu M, Suzuki S, et al. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol 2010; 25: 352–356 [DOI] [PubMed] [Google Scholar]
- 20.Lonardo A, Lombardini S, Scaglioni F, et al. Fatty liver, carotid disease and gallstones: a study of age‐related. World J Gastroenterol 2006; 12: 5826–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenenbaum T, Fisman EZ, Boyko V, et al. Attenuation of progression of insulin resistance in patients with coronary artery disease by bezafibrate. Arch Intern Med 2006; 166: 737–741 [DOI] [PubMed] [Google Scholar]
- 22.Inoue I, Noji S, Awata T, et al. Bezafibrate has an antioxidant effect: peroxisome proliferator‐activated receptor alpha is associated with Cu2+, Zn2+‐superoxide dismutase in the liver. Life Sci 1998; 63: 135–144 [DOI] [PubMed] [Google Scholar]


